Abstract
While viral antigens in HPV-related oropharyngeal cancer (HPVOPC) are attractive targets for immunotherapy, the effects of existing standard-of-care therapies on immune responses to HPV are poorly understood. We serially sampled blood from stage III–IV OPC patients undergoing concomitant chemoradiotherapy (CRT) with or without induction chemotherapy. Circulating immunocytes including CD4+ and CD8+ T cells, regulatory T cells (Treg), and myeloid-derived suppressor cells (MDSC) were profiled by flow cytometry. Antigen-specific T cell responses were measured in response to HPV16 E6 and E7 peptide pools. The role of PD-1 signaling in treatment-related immunosuppression was functionally defined by performing HPV-specific T cell assays in the presence of blocking antibody. While HPV-specific T cell responses were present in 13/18 patients prior to treatment, 10/13 patients lost these responses within 3 months after CRT. CRT decreased circulating T cells and markedly elevated MDSC. PD-1 expression on CD4+ T cells increased by nearly 2.5-fold after CRT, and ex-vivo culture with PD-1 blocking antibody enhanced HPV-specific T cell responses in 8/18 samples tested. CRT suppresses circulating immune responses in HPVOPC patients by unfavorably altering effector:suppressor immunocyte ratios and upregulating PD-1 expression on CD4+ T cells. These data strongly support testing of PD-1-blocking agents in combination with standard-of-care CRT for HPVOPC.
INTRODUCTION
Squamous cell carcinoma is the most frequently occurring malignant tumor of the head and neck and a major cause of morbidity and mortality worldwide. While head and neck squamous cell carcinoma (HNSCC) related to environmental carcinogens (tobacco, alcohol) has declined in the US, incidence of HNSCC related to the human papilloma virus (HPV), primarily oropharyneal cancer (OPC) is rapidly increasing (1). HPV-mediated OPC (HPVOPC) is a different disease from classical environmentally-related HNSCC, with distinct epidemiology and natural history including a more favorable prognosis (2–4). The carcinogenic mechanism of HPVOPC is also distinct from tobacco/alcohol-associated HNSCC, driven by expression of viral antigens such as the E6 and E7 oncoproteins which bind and inactivate p53 and RB tumor suppressor genes respectively to transform oropharyngeal epithelial cells (5).
The viral antigens in HPVOPC provide compelling targets for immune-based therapy, a strategy both for reducing the roughly 20% recurrence rate seen with existing treatment (5), and for de-escalating chemoradiotherapy, which is associated with significant short- and long-term toxicity (6). Tumor-mediated immunosuppression by upregulation of signaling through negative costimulatory molecules such as CTLA4 and PD-1, release of soluble mediators, and induction of suppressive/regulatory immunocytes including myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg) (7,8) is an important mechanism driving tumor progression and resistance to host immunity. Tumor-mediated immunosuppression is also understood to be a major barrier to successful cancer immunotherapy. Thus, understanding how conventional chemoradiation interacts with the endogenous immune response to HPV will provide insight into the mechanism of current anti-tumor therapies in OPC, and may accelerate development of immune-based prognostic biomarkers and therapeutic approaches. This latter goal is particularly relevant given recent interest in combining immunotherapeutic approaches with standard-of-care chemotherapy and radiation.
In the present study, we hypothesize that platinum-based concomitant chemoradiation, with or without taxane-platinum-5FU (TPF) induction chemotherapy profoundly alters circulating immunocytes and HPV-specific T cell responses in HPVOPC patients. To test this hypothesis we performed serial blood sampling of 22 OPC patients at multiple time points before and after chemoradiotherapy, and analyzed both the profile of effector and suppressor immunocytes by multicolor flow cytometry, and HPV-specific T cell responses to pooled HPV E6 and E7 peptides. We found that CRT led to globally unfavorable changes in circulating immunity, including increased numbers of circulating MDSC, significant decrease in CD8+ T cells and CD8:MDSC and CD8:Treg ratios, and loss of HPV-specific T cell responses. We further demonstrated that CRT dramatically upregulates PD-1 expression on circulating CD4+ T cells and that chemoradiation-induced immunosuppression is potentially reversible by PD-1 blockade.
MATERIALS AND METHODS
Human subjects
We included 22 patients with biopsy-proven stage III–IV (T1-3, N0-2b, M0) squamous cell cancer of the oropharynx who were scheduled to be treated with standard-of-care concomitant chemoradiotherapy with or without induction chemotherapy. 20 patients were HPV-positive by PCR testing, and 2 HPV negative. CLIA-approved PCR-based HPV testing was performed as part of routine management of OPC patients at ISMMS. All patients were treated with 7 weeks of platinum-based (cisplatin or carboplatin) concomitant chemoradiotherapy using intensity-modulated radiotherapy (IMRT). A subset of patients also received induction chemotherapy using the taxane-platinum-5FU (TPF) regimen (9), which at ISMMS is offered to patients with advanced (stage III–IV) disease without medical contraindications to therapy. A minority of patients receiving TPF induction [7015, 7016, 7019, 7026 and 7032] was cross-enrolled in another clinical trial and thus received cabazitaxel instead of taxotere as part of the induction regimen. Induction chemotherapy regimens were 3 cycles of taxane, cisplatin, 5-fluorouracil prior to 7 weeks of chemoradiotherapy. All patients underwent baseline blood sampling prior to treatment and serial blood sampling after the completion of induction chemotherapy and/or chemoradiation. All patients were enrolled under the ISMMS IRB-approved human subjects protocol GCO# 10-1219 (PI Sikora).
Sample collection and processing
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh heparinized blood by Ficoll–Hypaque density gradient centrifugation and cryopreserved.
MDSC and T cell staining and immunophenotyping
Thawed PBMCs were surface stained for MDSC (CD33, CD11b, HLADR, CD14) and T cells (CD4, CD8, CD25, CD127, CD45RO, PD-1) [BD Pharmingen] for 1hour at 4°C. The cells were washed with FACS buffer (2%FBS-PBS). The data were acquired with an BD LSR Fortessa flow cytometer and analyzed using TreeStar Flowjo software.
Assessment of HPV- specific T cell responses by cytokine release
PBMCs were cultured in medium consisting of RPMI 1640 (Invitrogen, CA) supplemented with 25 mM HEPES buffer (Invitrogen), 2 mM L-glutamine, 1% nonessential amino-acids, 1mM sodium pyruvate, 50 units/ml penicillin, 50 μg/ml streptomycin (all from Sigma Aldrich, MO) and 10% normal human serum AB (GemCell, TX) with or without pooled HPV16 E6 and E7 peptides (20nM) for 11 days. At Day 2, 50U/ml IL-2 (Peprotech, NJ) and 50U/ml IL-7 (R&D Systems, MN) were added. On Day 8, cells were washed and cultured in medium without serum. On Day 11, cells were restimulated with HPV16 E6 and E7 peptide pool (1μM) or control peptide pool for 48h, and cytokines levels in the supernatants assessed by the BeadLyte cytokine assay kit (Upstate, MA) as per the manufacturer’s protocol. Overlapping 15-mer peptides spanning HPV16 E6 and HPV16 E7 proteins were purchased from Mimotopes (Victoria, Australia). A positive response was defined as >100 pg/mL IFN-γ release and > 3-fold greater than control peptide.
Assessment of HPV-specific T cell responses by ELISPOT
PBMCs were pre-sensitized with peptide pools from HPV16 E6 and E7 (20nM) (Sigma Aldrich, MO) and cultured in RPMI 1640 medium (Invitrogen, CA) supplemented with Glutamax (2 mM, Invitrogen), penicillin (50 U/ml), streptomycin (50 μg/ml), HEPES buffer (10 mM, all from Life Technologies, CA) and 10% normal human serum AB (GemCell, TX) in the presence of anti-PD-1 mAb (J116, 10 μg/ml) or control isotype IgGκ (P3.6.2.8.1, 10 μg/ml) (both from Affimetrix, CA). On day 2 and 8, IL-2 (Roche, Basel, Switzerland) and IL-7 (R&D Systems, MN) were added to a final concentration of 10 U/ml and 20 ng/ml, respectively. On day 5, half of the medium was replaced with complete medium containing IL-2 (20 U/ml) and IL-7 (40 ng/ml), and anti-PD-1 mAb (20 μg/ml) or control isotype IgG κ (20 μg/ml). On day 11, ELISPOT assays were performed to determine Ag-specific effector cells. Flat-bottom, 96-well nitrocellulose plates (MultiScreen-HA; Millipore, Bedford, MA) were coated with IFN-γ mAb (2 μg/ml, 1-D1K; Mabtech, Stockholm, Sweden), incubated overnight at 4°C, washed with RPMI 1640, and blocked with 10% human AB-type serum for 2 h at 37°C. Peptide pool-presensitized PBMC (5 × 104 or 1 × 104 cells) were added to wells and tested for reactivity to the HPV peptide pool (1 μM) or control DMSO, in the presence of anti-PD-1 mAb (10 μg/ml) or of control IgG (10 μg/ml). Following 20 h incubation, plates were washed thoroughly with water containing 0.05% Tween 20, and anti-IFN-γ mAb (0.2 μ/ml, 7-B6-1-biotin; Mabtech) was added to each well. After incubation for 2 h at 37°C, plates were washed and developed with streptavidin-alkaline phosphatase (1 μg/ml; Roche) for 1 h at room temperature. After washing, substrate (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium; Sigma-Aldrich, St. Louis, MO) was added and incubated for 7 min. Plate membranes displayed dark-violet spots that were scanned and counted using C.T.L. ImmunoSpot analyzer and software (Cellular Technologies, Cleveland, OH).
PD-L1/PD-L2 staining
PBMC were irradiated (30 Gy) (J.L. Shepherd and Associates Mark I Model 68A Irradiator Cesium-137 Source) and checked by flow cytometry for PD-L1 and PD-L2 expression following 4 days in culture in the presence of complete medium (RPMI + 10% SAB), with IL-2 (10 U/ml) added at day 2. For each sample, isotype controls were used to determine the autofluorescence of lymphocyte and antigen-presenting cells (APC) populations (selected based on SSC and FSC). PD-L1/PD-L2 expression were calculated as the difference of MFI between PD-L1 or PD-L2 stained samples and isotype control stained samples.
Statistical Methods
For most figures, aggregated data is presented using mean values to represent the central tendency, and standard error of the mean (SEM) to represent variability. Two-tailed paired or unpaired T tests were used to determine significance of differences, except for categorical data which was analyzed by Fisher’s exact test. P value ≤ 0.05 was used as the cutoff for significance.
RESULTS
Chemoradiation has profound and divergent effects on circulating immunocytes
To profile the effect of chemoradiotherapy on effector and suppressor immunocyte populations in oropharyngeal cancer (OPC) we collected blood from 20 HPV-positive and 2 HPV-negative patients with stage III–IV OPC undergoing standard-of-care chemoradiation before, during, and after treatment for up to one year (Figure 1A). The demographics of the patient population (Table 1) are representative of our non-surgical OPC population: predominantly middle-aged males with tongue base tumors, and a clinical stage distribution biased towards advanced nodal-stage (N2-3) disease. The two HPV-negative patients were included in the immunophenotyping results presented in Figure 2 only; all other data shown is limited to the HPV-positive patient population.
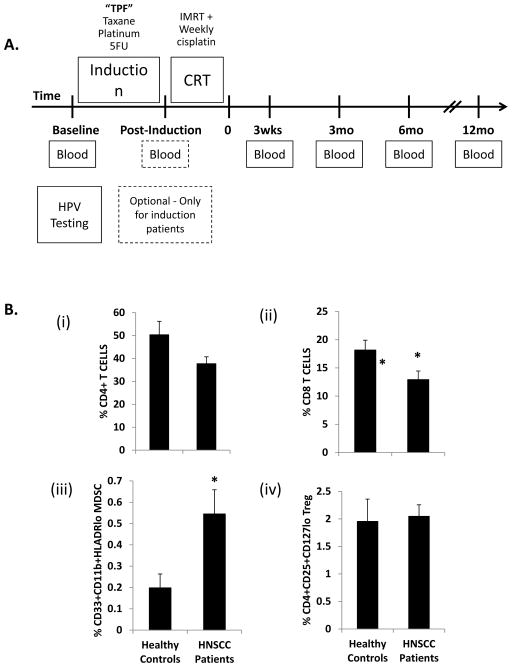
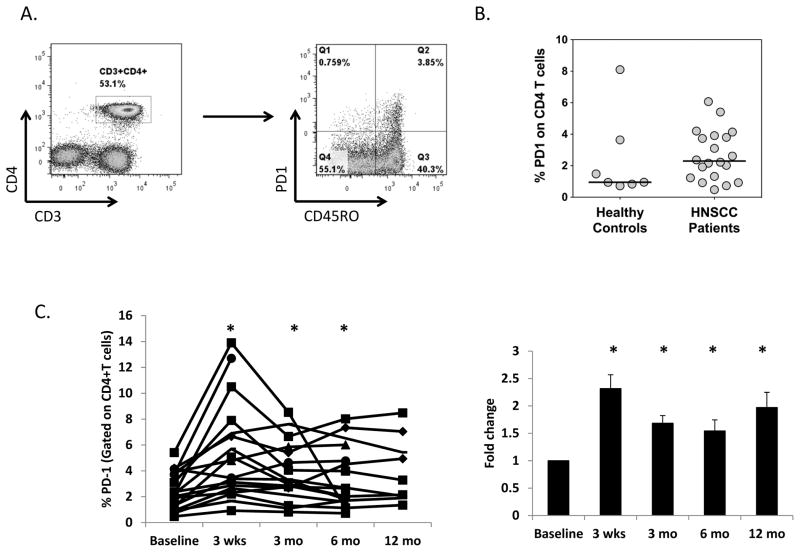
FIGURE 1. Comparison of effector and regulatory immunocyte populations in healthy control and HPV+ HNSCC patients at baseline.
(A) Outline of study design. Patients with biopsy-proven HPV+ squamous cell cancer of the oropharynx underwent blood sampling at baseline prior to treatment and at multiple time points after the completion of induction chemotherapy and/or chemoradiation therapy. Patients treated with induction chemotherapy received 3 cycles of TPF (taxane, cisplatin, 5-fluorouracil) prior to concomitant chemoradiation. All patients received a standard course of 7 weeks of concomitant chemoradiation therapy with intensity-modulated radiation therapy (IMRT) and platinum-based chemotherapy. (B) PBMC were harvested from healthy donors (N=7) or study patients (N=19), and flow cytometry performed to determine the relative number of circulating: (i). CD4+ T cells; (ii). CD8+ T cells; (iii). CD33+CD11b+HLADRlo myeloid derived suppressor cells (MDSC); and (iv). CD4+CD25+CD127lo regulatory T cells (Treg). Cancer patients had a significant increase in circulating MDSC, and significant decrease in CD8+ T cells compared to healthy controls. * = p<0.05.
Table 1.
Demographics
| Characteristic | Summary |
|---|---|
| Age (Avg) | 58.8 |
| Gender | |
| Male (%) | 19/22 (86.4%) |
| Female (%) | 3/22 (13.6) |
| HPV Status | |
| Positive (%) | 20/22 (90.9%) |
| p16 status | 20/20 positive |
| Negative (%) | 2/22 (9.1%) |
| p16 status | 2/2 negative |
| Subsite | |
| Tonsil | 7/22 (31.8%) |
| Tongue Base | 15/22 (68.2%) |
| T | |
| T1 | 4/22 (18.2%) |
| T2 | 12/22 (54.5%) |
| T3 | 4/22 (18.2%) |
| T4 | 2/22 (9.1%) |
| N | |
| N1 | 4/22 (18.2%) |
| N2a | 2/22 (9.1%) |
| N2b | 8/22 (36.4%) |
| N2c | 5/22 (22.7%) |
| N3a | 3/22 (13.6%) |
| Stage | |
| III | 4/22 (18.2%) |
| IV | 18/22 (81.8%) |
| CRT alone | 8/22 (36.4%) |
| Induction + CRT | 14/22 (63.6%) |
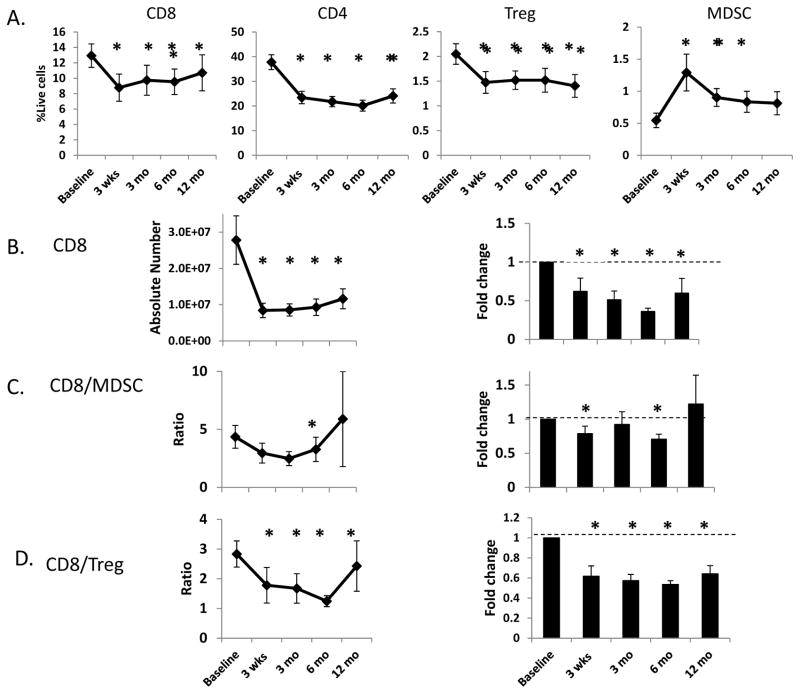
FIGURE 2. Effect of chemoradiation on circulating immunocytes.
A. Time course of changes in the relative number (expressed as % of all live cells) of effector (CD4+ and CD8+ T cells) and suppressor (CD11b+CD33+MHClo MDSC and CD4+CD127lo Treg) immunocytes before and after treatment. B.–D. Treatment-induced changes in the absolute number CD8+ T cells (B.), and the CD8+/MDSC (C.) and CD8+/Treg (D.) ratios based on absolute numbers (left column) and expressed as the -fold change of these ratios with respect to baseline (right column). * = p<0.05 with respect to baseline.
The balance between effector (such as CD8+ cytotoxic T cells and CD4+ “helper” T cells) and suppressor (such as Treg and MDSC) immunocytes is a critical determinant of effective anti-tumor activity. We sought to determine the effect of chemoradiation on peripheral immunocyte levels, and to compare both pre- and post-treatment levels to those of cancer-free control patients (average age 44 years; 86% male; neither age nor sex distribution were significantly different from the OPC patient population). At pre-treatment baseline, HPVOPC patients had a trend towards decreased numbers of CD4+ T cells and significantly decreased CD8+ T cells as compared to cancer-free controls [Figure 1B (i) and (ii); gating scheme shown in Figure S1]. Although we did not observe increased numbers of circulating Tregs at baseline, MDSC levels were significantly increased in HPVOPC patients [Figure 1 B (iii) and (iv)]. Overall, these results suggest that the pre-treatment peripheral effector/suppressor balance in OPC patients is already skewed towards immunosuppression.
Following CRT, we observed that both CD4+ and CD8+ T cell levels decreased sharply as, to a lesser degree, did CD4+CD127lo Treg levels (Figure 2A). In contrast to the ablative effect of chemoradiation on lymphoid populations, CD11b+CD33+HLADRlo MDSC levels strikingly increased (nearly 3-fold) immediately after completion of CRT (Figure 2A). Following chemoradiotherapy we also observed a sharp reduction in the absolute number of CD8+ T cells, and significantly decreased CD8+:MDSC and CD8+:Treg effector/suppressor ratios which remained suppressed even at 6 months post-treatment (Figure 2B, C, and D; also Figure S2). Taken together, our peripheral immunophenotyping data strongly support an overall immunosuppressive effect of chemoradiation on the systemic immunocyte milieu in OPC patients.
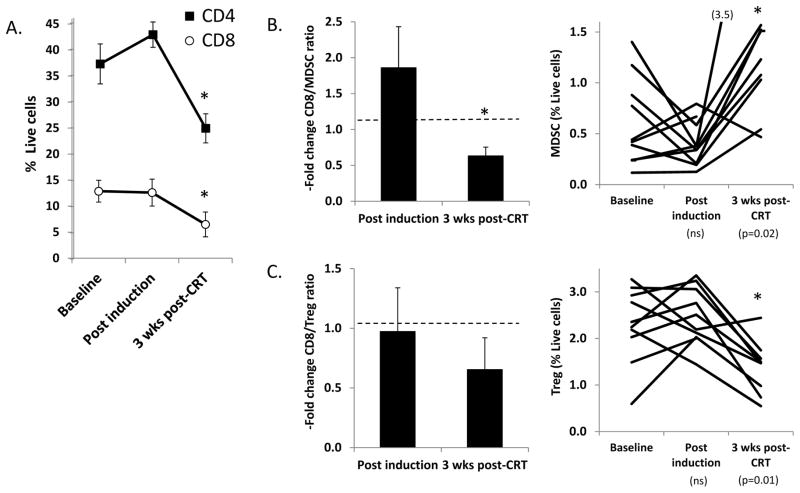
When we examined the subset of patients who received TPF induction chemotherapy, we again found evidence of post-treatment immunosuppression following completion of CRT (Figure 3). However, several interesting trends consistent with immune activation were observed in the interim post-induction blood samples. There were trends towards increased numbers of CD8+ T cells (Fig. 3A) and increased CD8/MDSC ratio (Fig. 3B), as well as a trend towards decreasing MDSC numbers which although not statistically significant in aggregate was driven by a sharp decrease in MDSC in 5/10 patient specimens (Fig. 3B). The Treg/MDSC ratio was not significantly altered after induction. While these favorable trends in CD8+ T cell and MDSC number missed statistical significance (likely because of the smaller sample size in the induction group), they are consistent with a potentially immunostimulatory effect of chemotherapy, as has been previously described for 5-FU (10) and the combination of taxol plus platinum chemotherapy (11).
FIGURE 3. Comparison of circulating immunocytes in patients treated with induction chemotherapy.
Changes in the circulating immunocytes after induction and completion of CRT. A. Relative number (expressed as % live cells) of CD4+ and CD8+ T cells B. Fold changes in CD8+/MDSC ratio (left) and relative MDSC number (right). C. Fold changes in the CD8+/Treg (left) and relative Treg number (right).
Chemoradiation suppresses pre-existing circulating HPV-specific T cell responses
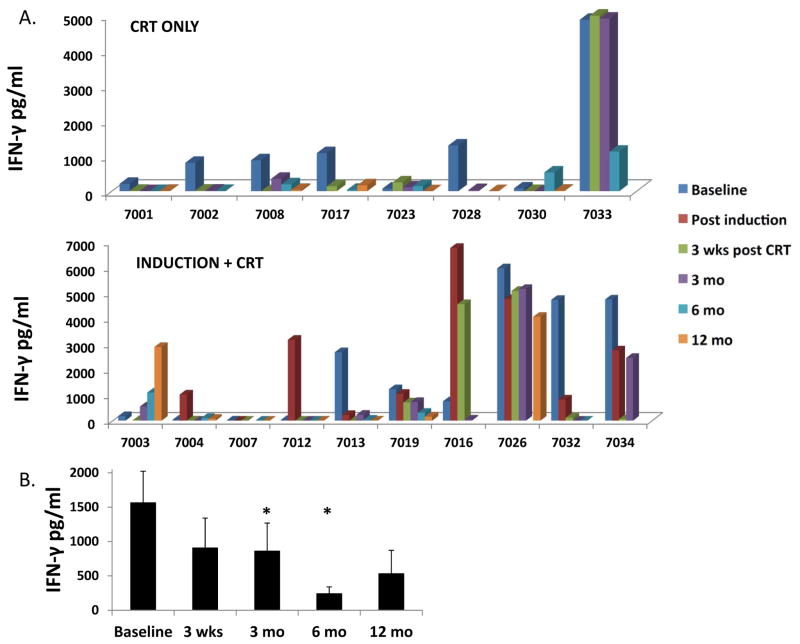
To determine the effect of chemoradiation on HPV-specific responses in HPVOPC, we analyzed HPV E6/E7-specific T cell responses before and after treatment by determining the level of IFN-γ production after ex vivo stimulation of PBMC with pooled HPV16 E6/E7 peptides. We found that 13/18 tested patients had measurable HPV-specific T cell responses (IFN-γ release in response to pooled HPV peptides) at pretreatment baseline (Figure 4A); an additional 2 patients who lacked baseline responses acquired them transiently after induction chemotherapy (7004 and 7012). Of 13 patients with pre-existing responses, 10/13 (77%) lost these responses by 3 months after completion of CRT (loss defined as <100 pg/mL IFN-γ at 3 weeks and/or 3 months post-treatment). The fraction of CRT-only and induction plus CRT patients losing pre-existing responses was roughly similar (5/6 and 5/7 respectively). Interestingly, 4/10 patients who lost responses regained these responses at some point during follow-up (#7008, #7003, #7013, and #7034) although in at least two cases (#7008, #7013) this was transient. When data was analyzed in aggregate, a significant decrease in average IFN-γ level with respect to baseline was seen at 3 and 6 months after CRT, and a borderline (p=0.05) decrease at 3 weeks post-CRT (Figure 4B). While the relatively low numbers of HPV-specific cells obtained from cryopreserved PBMC was rarely enough to perform additional studies in defined T cell subsets, in the small number of patients samples where we could analyze intracellular IFN-γ production we saw concordance with the levels measured in supernatants from unfractionated PBMC, particularly for CD4+ T cells (supplemental Figure S3).
FIGURE 4. Effect of chemoradiation on circulating HPV-specific T cell responses.
A. Batched analysis of cryopreserved PBMC was performed to determine the level of IFN-γ production after in vitro stimulation with pooled HPV16 E6/E7 peptides at baseline and post-treatment time points. A. Values are shown for patients who received chemoradiation only (upper panel) and from patients who received induction chemotherapy followed by chemoradiation (lower panel). In these graphs, very low or zero values are represented by a flat colored square, whereas missing time points are represented by a blank. All values reflect IFN-γ production from HPV peptide-stimulated PBMC minus the value of PBMC stimulated with irrelevant control peptide. B. Aggregated IFN-γ release data representing averaged data from all patients. * = p<0.05 with respect to baseline.
These data indicate that most patients had brisk anti-HPV T cell responses at baseline, but lost these responses following concomitant CRT. Our finding that two patients (7004 and 7012) acquired new HPV-specific responses, and that one patient (7016) with modest baseline response had a nearly 10-fold increase in response following induction therapy, is consistent with the favorable trends in immunocyte populations seen in the post-induction samples (shown in Figure 3). Together with the immunophenotyping data, these data suggest a predominantly suppressive effect of CRT and a potentially favorable effect of TPF chemotherapy alone on immune function.
PD-1 expression on CD4+ T cells increases after chemoradiotherapy and in vitro radiation upregulates PD-L2 expression on leukocytes
Many patients lost HPV-specific T cell responses after chemoradiation, suggesting the induction of specific immunosuppressive mechanisms. PD-1 is a costimulatory/checkpoint molecule which has been shown to negatively regulate T cell responses, and both PD-1 and its ligand PD-L1 have been demonstrated to play a role in limiting the immune response to HPV+ and HPV-negative HNSCC (12). The PD-1 ligand PD-L2 has also been shown to be expressed in cervical cancer (13), but has not previously been examined in HPVOPC. Both PD-L1 and PD-L2 can be expressed by tumor cells or by infiltrating and circulating leukocytes, and can potentially play a role in tumor-mediated and radiation-induced immune dysfunction.
PD-1 expression at baseline was observed on a minority (2–6%) of circulating CD4+ T cells (Figure 5), primarily on the CD45RO memory CD4+ population (Figure 5A). There was a trend towards elevated PD-1 expression on CD4+ T cells from HPVOPC patients compared to normal controls (median 2.3% and 1.0% of CD4+ cells respectively; Figure 5B). We observed that PD-1 expression levels on CD4+ T cells were increased nearly 2.5-fold at 3 weeks after completion of chemoradiation, decreasing somewhat by 3 months after completion of therapy, but remaining significantly elevated for up to 1 year (Figure 5C). When analysis was limited to CD45RO+ CD4+ T cells, we also found PD-1 expression to be increased at 3 weeks post-therapy (data not shown). While there was also a weaker and non-significant trend towards increased PD-1 on circulating CD8+ T cells (Figure S4), PD-1 expression on Treg cells was relatively low and not modulated by therapy (data not shown). Interestingly, we found that baseline PD-1 expression on CD4+ T cells is correlated with expression at 3 months and 6 months post-treatment (Figure S5) suggesting that PD-1 expression at baseline can predict post-treatment levels and potentially be used to select patients who could benefit from combining PD-1/ligand blocking therapy with CRT.
FIGURE 5. Effect of chemoradiotherapy on PD-1 expression on CD4+ T cells.
A. Representative FACS plots showing gating scheme of PD-1 and CD45RO expression on CD4+ T cells, gated on all live cells. B. Expression of PD-1 on CD4+ T cells from healthy control donors (N=7) and HPVOPC patients (N=19). C. Percent PD-1+ expressing CD4+ T cells for individual patients (left) and aggregated -fold change with respect to baseline (right). * = p<0.05 with respect to baseline.
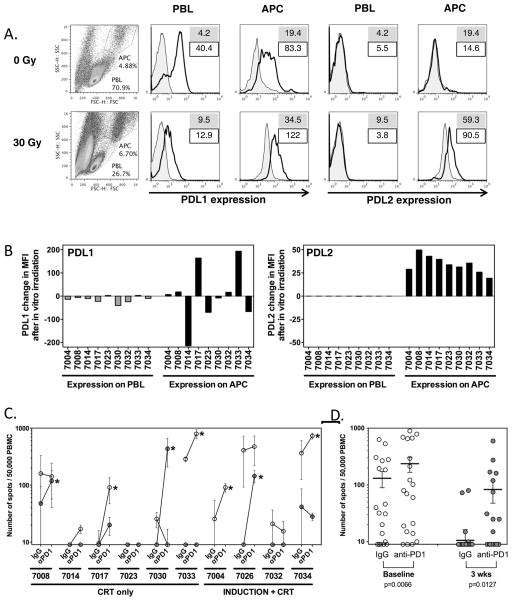
Since little is known about the potential effects of chemotherapy and radiation on PD-L1 and PD-L2 expression, we explored the possibility that chemoradiotherapy could also upregulate PD-1 ligand expression on tumor and/or leukocytes. While we found strong PD-L1 but nearly no PD-L2 expression on patient PBMC at baseline, we observed modest upregulation of PD-L2 on APC after CRT in 5/5 patients examined (data not shown), leading us to hypothesize that there may be direct effects of CRT on PD-1 ligand expression. To further determine whether radiation may directly modulate PD-L1 and PD-L2 expression, we exposed baseline patient PBMC samples to ex-vivo radiation and measured PD-L1 and PD-L2 levels after 4 days in vitro culture. While no clear trends were seen for PD-L1 expression, PD-L2 was modestly but consistently upregulated on APC (Figure 6A-B), suggesting that radiation could directly modulate PD-L2 expression in HPVOPC patients. Taken together, our data suggest that loss of HPV-specific immune response after CRT in our patient population could potentially be driven by a combination of increased expression levels of PD-1 on CD4+ T cells and PD-L2 on APC.
FIGURE 6. Modulation of PD1 ligand expression and effect of PD1 blockade. A–B. Effect of ex-vivo irradiation on PD-L1 and PD-L2 levels on patient PBMC.
A. Representative flow cytometry of PBMC for PD-L1 and PD-L2. Cells were gated independently for lymphocytes and APC based on forward and side scatter. Numbers on histogram plots indicate MFI of isotype control (filled) and PD-L1/PD-L2 mAb (line), respectively. B. Changes in PD-L1 and PD-L2 expression on lymphocytes (PBL) and antigen-presenting cells (APC) from PBMC of patients following in vitro irradiation. The changes in mean fluorescence intensity (MFI) were calculated based on MFI of PDL1 and PDL2 by flow cytometry after subtraction of MFI from respective isotype control, and results were expressed as the differences of these control-adjusted MFI levels before and after in vitro irradiation. Gating of lymphocytes and monocytic/granulocytic populations as above. While radiation increased the overall number of dead cells, viability of PBMC in the APC and PBL gates was >90%. Decrease in PDL1 on PBL and increase in PDL2 on APC after in vitro irradiation were found significant by paired Wilcoxon t test (p=0.0185 and <0.0001 respectively). C. Effect of PD1 blockade on HPV-specific T cell responses. HPV-specific T cell responses measured by ELISPOT in ten patients at baseline and 3 weeks after treatment, in the presence or absence of PD1 blockade. PBMC from individual time points were sensitized with a pool of overlapping long peptides from HPV E6 and E7, cultured in the presence of anti-PD1 mAb or control IgG for 11 days, and assayed for reactivity against the same HPV peptide pool by ELISPOT. Spot numbers indicate HPV-specific IFN-γ secreting T cells out of 50,000 cells tested, following removal of non-specific responses (DMSO control, typically <20 spots). Open and grey symbols indicate responses from samples at baseline and 3 weeks after treatment, respectively. Asterisks indicate samples with a greater than 2x increase in response following PD1 blockade. D. Dot plots showing mean (bar), and standard error of individual replicates from samples of all patients cultured with IgG vs anti-PD1. There were significantly more spots following PD1 blockade both at baseline and 3 weeks after treatment, as shown by paired Wilcoxon t test (p=0.0066 and 0.0127 respectively).
Ex-vivo PD-1 blockade enhances HPV-specific T cell responses and reverses chemoradiation-induced hyporesponsiveness
Dysregulated PD-1/ligand interaction is a mechanism of immune dysfunction amenable to targeting by clinically available anti-PD-1 or anti-PD-L1 blocking antibodies. We sought to determine whether blocking PD-1 signaling could enhance HPV-specific T cell responses in baseline patient PBMC samples and/or restore responsiveness in post-CRT samples where pre-existing baseline responses had been lost. HPV16 E6 and E7-stimulated PBMC were cultured in the presence of anti-PD-1 blocking mAb for 11 days prior to peptide restimulation and ELISPOT analysis of HPV-reactive T cells (Figure 6C-D). Consistent with our observation that PD-1, and PD-L1 are expressed on PBMC in baseline samples and that PD-1 and PD-L2 are elevated following CRT, we observed a >2-fold increase in the number of HPV-reactive T cells in presence of anti-PD-1 in 4/9 (44%) pre-treatment samples and 4/9 (44%) post-treatment samples (8/18 total samples). This confirms that dysregulated PD-1 signaling contributes to both tumor-induced (baseline) and chemotherapy-induced (post-treatment) immune dysfunction in HPVOPC patients, and provides proof-of-principle that therapeutic PD-1/ligand blocking antibodies could restore T cell responsiveness to HPV antigens and reverse CRT-induced immune suppression.
DISCUSSION
The impact of chemoradiation on immune function is widely agreed to be significant, but there is little agreement as to whether this impact skews primarily in the direction of immunosuppression or enhanced anti-tumor immunity. This is an important question, because there is increasing interest in combining radiotherapy and chemoradiotherapy with checkpoint inhibitors and other immune-based therapeutic approaches. This is particularly true for HPVOPC, where standard-of-care chemoradiotherapy is relatively effective and thus likely to be combined with, rather than replaced by, immunotherapy. In the present study we find evidence that systemic immunosuppression predominates in patients with HPVOPC treated with concomitant platinum-based CRT, and that unfavorable skewing of effector:suppressor immunocyte ratios and dysregulated PD-1/ligand signaling are significant mechanisms of CRT-induced immune dysfunction.
The potentially suppressive effects of radiation to the head and neck on systemic immunity, and the impact of global immune function on prognosis in HNSCC, have long been described (14–19), and recently described again with reference to HPV status (20). Conversely, radiation and chemoradiation have been suggested to have an enhancing effect on the immune response to both HPV-negative (21) and HPV-positive (22) head and neck cancer. Intact host immunity has also proposed to be critical for efficacy of conventional radiation and chemoradiation therapy for HPVOPC (23,24) suggesting immune-dependent mechanisms of action. Such a beneficial role for (chemo)radiation in host immune response to HPVOPC would be consistent with recent clinical literature describing immune-stimulating effects of chemotherapy and radiation in melanoma (25,26) and esophageal squamous cell carcinoma (27). However, the results of clinical studies in HPV+ cervical and oropharyngeal cancers have been mixed, with some studies showing evidence of post-treatment immune activation (28,29) and others immune suppression (30–32). It is possible that different treatment regimens may have differing effects on anti-tumor immunity, as has been shown in at least one study which found an immune-enhancing effect in the draining nodes after low-dose XRT but an immunosuppressive effect of high-dose XRT (50 Gy – consistent with standard-of-care clinical practice (32). However, an important difference between in vivo mouse models of radiation and XRT for head and neck cancer patients is that most of the existing mouse models employ relatively few fractions of radiation, whereas head and neck cancer patients are treated in many small fractions over the course of weeks. Thus it is important to analyze potential immune effects of chemoradiation by directly studying the effect of specific treatment regimens on host immune function in cancer patients undergoing standard-of-care treatment.
We sought to perform such a study of HPVOPC patients treated at a single institution with concomitant platinum-based chemoradiotherapy, with and without induction chemotherapy. At pre-treatment baseline HPVOPC patients had paradoxical evidence of peripheral immune activation (measurable HPV-specific T cell responses, which in many cases were quite strong), and immunosuppression (decreased CD8+ T cells and elevated levels of circulating MDSC as compared to normal controls [Figure 1B]; and elevated PD-1 expression on CD+ T cells [Figure 5B]). This is consistent with the widely accepted model of immunity in HPV-related cancers, in which a vigorous antigen-specific peripheral immune response is thwarted by immunosuppressive mechanisms upregulated in the tumor immune microenvironment. Such mechanisms found in the microenvironment of HPVOPC include induction of immunoregulatory immunocytes (MDSC, Treg, alternatively-activated macrophages) and negative costimulatory molecules (PD-1, CTLA4), as well as other immunosuppressive mechanisms common to many solid tumors (33). Thus host immunity to HPVOPC is characterized by a balance between immune activation and suppression that results in a vigorous but ineffective immune response incapable of controlling tumor growth. We next sought to determine whether chemoradiotherapy tends to drive this balance in the direction of enhanced immunity or towards worsening immunosuppression.
At the level of immunophenotyping of peripheral immune cells, nearly all changes observed after CRT were in the direction of immune suppression: decreased CD8+ and CD4+ T cells, and increased MDSC (Figure 2A). Even the modest decline in Treg cells observed was offset by a much greater decline in absolute CD8+ T cell number, leading to unfavorable ratios of CD8+ T cells with Treg as well as MDSC. The final effects of treatment on peripheral immune populations were very similar for patients treated with CRT alone and with induction chemotherapy + CRT. These results provide more granular detail about the significant post-radiation leukopenia previously reported in head and neck cancer patients (14,20), and suggest that interventions which enhance the radioresistance of CD8+ T cells (34) or which deplete Treg and myeloid suppressor populations could potentially restore immune homeostasis.
When we examined HPV-specific T cell responses, the overwhelming trend observed was loss of pre-existing (baseline) responses by 3 months – and often as early as 3 weeks – following completion of therapy. These immune-suppressing effects of CRT seemed to be intrinsic to the treatment itself, since there was no significant association with clinical stage, tumor subsite, initial strength of HPV response, or taxane (taxotere vs. cabazitaxel). The rapid loss of responses in most patients suggests active immunosuppression, rather than extinction of the response due to antigen loss associated with decreased tumor mass after effective treatment; however we will further explore this hypothesis in a parallel study of the immune response in surgically-treated patients to compare CRT with the effects of surgical ablation. We also hypothesize, based on the favorable trends in immunocyte populations following induction chemotherapy, and the markedly increased post-induction HPV-specific responses seen in several patients, that the immunosuppressive effects of CRT are primarily due to radiation, rather than chemotherapy. In fact, our data are suggestive of potentially beneficial immune effects of TPF chemotherapy alone, which we intend to explore in follow-up studies.
A functional role for PD-1 signaling in CRT-induced immunosuppression is supported by the strong upregulation of PD-1 on CD4+ T cells at 3 weeks after completion of therapy (Figure 5), which remained elevated as long as 12 months following CRT, and the ability of anti-PD-1 blocking mAb to restore HPV-specific T cell responses in a subset of HPVOPC patients (Figure 6). The PD-1 ligand PD-L1 is overexpressed in HPVOPC tumors (12,35) and tumor-infiltrating APC (12) and PD-1 is expressed on HPVOPC-infiltrating CD8+ (12) and CD4+ (36) T cells. Thus our findings are consistent with ample prior evidence suggesting that PD-1 is an important mechanism of HPVOPC immune escape. In fact, expression of PD-1 and other immune checkpoint molecules has been shown to be higher on tumor-infiltrating than circulating T cells (37), and cryopreservation has been shown to decrease PD-1 and PD-L1 expression on PBMC, so it is likely that our findings, derived from observations of cryopreserved circulating immunocyte populations, understate the impact of CRT as a driver of PD-1 expression and concomitant immune suppression.
Further support for this model comes from the observation that ex vivo radiation alone is sufficient to upregulate PD-L2 expression on patient APC (Figure 6B), suggesting that PD-1 ligand expression may also be modulated by CRT. While the role of PD-L2 in cancer is not as well defined as that of PD-L1, available evidence suggests it can play a role in tumor-associated immunosuppression (38). PD-L2 expression on APC has been shown to limit CD8+ T cell expansion and cytokine proliferation as well as Th1 cytokine production and tumor killing by human CTL (39). PD-L2 blockade has been shown to have anti-tumor activity in a mouse model of pancreatic cancer (40), and PD-L2 expression predicts poor prognosis in lung (41) and esophageal (42) cancer, suggesting that it may contribute to immune escape. With regard to HPV-related neoplasia, PD-L2 is expressed by benign respiratory papillomas (43) and advanced stage cervical cancer (13). We also observed a small but consistent increase in PD-L2 levels in post-treatment APC populations, which may be indicative of CRT-induced upregulation. Prior evidence of transient direct radiation effects on PD-1 and ligand upregulation has been observed in mouse models of breast cancer and melanoma in which radiation upregulated CD8 T cell PD-1 expression and anti-PD-1 mAb synergized with radiotherapy in treatment of established tumors (44). In another set of studies, radiation upregulated tumor PD-L1 expression and anti-PD-L1 following radiotherapy led to improved tumor control (45,46). An additional study in a mouse glioblastoma model found that anti-PD-1 mAb enhanced anti-tumor efficacy of stereotactic radiotherapy (47). Thus there is prior biological plausibility for modulation of PD-1 and receptor expression by (chemo)radiotherapy, and the potentially beneficial effects of combining radiotherapy with clinically available antibodies targeting PD-1 and its ligands. The present data in patient samples add to this preclinical evidence and provide further support for targeting PD-1 signaling in HPVOPC, and testing PD-1/PD-L1 blockade in combination with chemoradiotherapy.
Our study has several limitations. The most obvious limitation is that our analyses are limited to circulating/systemic immunity, rather than direct interrogation of the tumor microenvironment itself. While many immune processes are anticipated to be regulated similarly in the tumor and circulating compartments, and while systemic immunity has obvious relevance to long-term immune surveillance and risk of relapse, we anticipate that many of our findings would be even more striking were we to sample the tumor immune microenvironment. While our focus on CRT patients, the vast majority of which have a complete initial response to treatment, prohibits a classical pre/post-treatment window-of-opportunity trial approach, we anticipate that follow-up studies will incorporate a mid-treatment tumor biopsy to interrogate this immunologically important compartment. A further limitation is the relatively small clinical series studied (N=22 patients), which makes it difficult to study potentially interesting but statistically non-significant differences between, for example, patients treated with induction chemotherapy versus CRT alone, or patients who maintained and those who lost circulating HPV-specific immune responses. Nevertheless, even with this relatively modest sample size there was sufficient consistency of results to find statistically significant and reproducible treatment-induced changes in immunocyte populations, PD-1 expression, and HPV-specific responses.
Our study also has considerable strengths which add significantly to our understanding of the effects of chemoradiotherapy on immune responses to HPVOPC and other HPV-related cancers. We provide detailed analyses of treatment-induced changes in what is to date the largest immune-focused series of HPV-positive OPC patients treated with a single modality (chemoradiation), and identify specific mechanisms of treatment-induced immunosuppresion (effector:Treg and effector:MDSC imbalance, and PD-1 upregulation) potentially amenable to therapeutic intervention. In particular, the present data provide further evidence of the emerging role of PD-1 signaling as a major mechanism of HPVOPC immune escape, and strongly support the development of clinical trials testing the integration of PD-1/PD-L1 blocking antibodies with standard of care chemoradiation in HPVOPC.
Supplementary Material
Acknowledgments
A.S. and F.P. were supported in part by grants from the National Institutes of Health (NIH) National Institute of Dental and Craniofacial Research (5R03DE021741-02) and National Cancer Institute (1K08CA154963-01A1). S.G and N.I. were supported in part by a Cancer Vaccine Collaborative grant funded by the Cancer Research Institute and Ludwig Institute for Cancer Research Ltd. A.C. was supported by a scholarly fellowship from the Doris Duke foundation.
Footnotes
CONFLICT OF INTEREST DISCLOSURE STATEMENT:
The corresponding author is the recipient of an unrestricted industry grant from Advaxis Pharmaceuticals for an investigator-initiated immunotherapy trial for HPV-positive oropharyngeal cancer.
References
- 1.Näsman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer J Int Cancer. 2009;125:362–6. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 4.Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2011;22:1071–7. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Münger K, Scheffner M, Huibregtse JM, Howley PM. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 1992;12:197–217. [PubMed] [Google Scholar]
- 6.Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:3582–9. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frumento G, Piazza T, Di Carlo E, Ferrini S. Targeting tumor-related immunosuppression for cancer immunotherapy. Endocr Metab Immune Disord Drug Targets. 2006;6:233–7. doi: 10.2174/187153006778250019. [DOI] [PubMed] [Google Scholar]
- 8.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 9.Posner M, Vermorken JB. Induction therapy in the modern era of combined-modality therapy for locally advanced head and neck cancer. Semin Oncol. 2008;35:221–8. doi: 10.1053/j.seminoncol.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Feng Q-M, Wang Y, Shi J, Ge H-L, Di W. The immunologic aspects in advanced ovarian cancer patients treated with paclitaxel and carboplatin chemotherapy. Cancer Immunol Immunother CII. 2010;59:279–91. doi: 10.1007/s00262-009-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–41. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15:6341–7. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 14.Verastegui EL, Morales RB, Barrera-Franco JL, Poitevin AC, Hadden J. Long-term immune dysfunction after radiotherapy to the head and neck area. Int Immunopharmacol. 2003;3:1093–104. doi: 10.1016/S1567-5769(03)00013-4. [DOI] [PubMed] [Google Scholar]
- 15.Tisch M, Heimlich F, Daniel V, Opelz G, Maier H. Cellular immune defect caused by postsurgical radiation therapy in patients with head and neck cancer. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 1998;119:412–7. doi: 10.1016/S0194-5998(98)70092-0. [DOI] [PubMed] [Google Scholar]
- 16.Gray WC, Chretien PB, Suter CM, Revie DR, Tomazic VT, Blanchard CL, et al. Effects of radiation therapy on T-lymphocyte subpopulations in patients with head and neck cancer. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 1985;93:650–60. doi: 10.1177/019459988509300515. [DOI] [PubMed] [Google Scholar]
- 17.Wara WM, Ammann AJ, Wara DW. Effect of thymosin and irradiation on immune modulation in head and neck and esophageal cancer patients. Cancer Treat Rep. 1978;62:1775–8. [PubMed] [Google Scholar]
- 18.Olkowski ZL, McLaren JR, Skeen MJ. Effects of combined immunotherapy with levamisole and Bacillus Calmette-Guérin on immunocompetence of patients with squamous cell carcinoma of the cervix, head and neck, and lung undergoing radiation therapy. Cancer Treat Rep. 1978;62:1651–61. [PubMed] [Google Scholar]
- 19.Stefano S, Kerman R, Abbate J. Serial studies of immunocompetence in head and neck cancer patients undergoing radiation therapy. AJR Am J Roentgenol. 1976;126:880–6. doi: 10.2214/ajr.126.4.880. [DOI] [PubMed] [Google Scholar]
- 20.Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2013 doi: 10.1002/hed.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabachnyk M, Distel LVR, Büttner M, Grabenbauer GG, Nkenke E, Fietkau R, et al. Radiochemotherapy induces a favourable tumour infiltrating inflammatory cell profile in head and neck cancer. Oral Oncol. 2012;48:594–601. doi: 10.1016/j.oraloncology.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Vu HL, Sikora AG, Fu S, Kao J. HPV-induced oropharyngeal cancer, immune response and response to therapy. Cancer Lett. 2010;288:149–55. doi: 10.1016/j.canlet.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Vermeer DW, Spanos WC, Vermeer PD, Bruns AM, Lee KM, Lee JH. Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of human papillomavirus-positive cancer. Int J Cancer J Int Cancer. 2013;133:120–9. doi: 10.1002/ijc.28015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–46. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 25.Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol. 2012;2:95. doi: 10.3389/fonc.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85:293–5. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72:3967–76. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]
- 28.Delgado FG, Martínez E, Céspedes MA, Bravo MM, Navas MC, Cómbita Rojas AL. Increase of human papillomavirus-16 E7-specific T helper type 1 response in peripheral blood of cervical cancer patients after radiotherapy. Immunology. 2009;126:523–34. doi: 10.1111/j.1365-2567.2008.02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fattorossi A, Battaglia A, Ferrandina G, Coronetta F, Legge F, Salutari V, et al. Neoadjuvant therapy changes the lymphocyte composition of tumor-draining lymph nodes in cervical carcinoma. Cancer. 2004;100:1418–28. doi: 10.1002/cncr.20130. [DOI] [PubMed] [Google Scholar]
- 30.Al-Taei S, Banner R, Powell N, Evans M, Palaniappan N, Tabi Z, et al. Decreased HPV-specific T cell responses and accumulation of immunosuppressive influences in oropharyngeal cancer patients following radical therapy. Cancer Immunol Immunother CII. 2013;62:1821–30. doi: 10.1007/s00262-013-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qinfeng S, Depu W, Xiaofeng Y, Shah W, Hongwei C, Yili W. In situ observation of the effects of local irradiation on cytotoxic and regulatory T lymphocytes in cervical cancer tissue. Radiat Res. 2013;179:584–9. doi: 10.1667/RR3155.1. [DOI] [PubMed] [Google Scholar]
- 32.Battaglia A, Buzzonetti A, Martinelli E, Fanelli M, Petrillo M, Ferrandina G, et al. Selective changes in the immune profile of tumor-draining lymph nodes after different neoadjuvant chemoradiation regimens for locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2010;76:1546–53. doi: 10.1016/j.ijrobp.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Tong CCL, Kao J, Sikora AG. Recognizing and reversing the immunosuppressive tumor microenvironment of head and neck cancer. Immunol Res. 2012;54:266–74. doi: 10.1007/s12026-012-8306-6. [DOI] [PubMed] [Google Scholar]
- 34.Saavedra MM, Henríquez-Hernández LA, Lara PC, Pinar B, Rodríguez-Gallego C, Lloret M. Amifostine modulates radio-induced apoptosis of peripheral blood lymphocytes in head and neck cancer patients. J Radiat Res (Tokyo) 2010;51:603–7. doi: 10.1269/jrr.10030. [DOI] [PubMed] [Google Scholar]
- 35.Ukpo OC, Thorstad WL, Lewis JS. B7-H1 expression model for immune evasion in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2013;7:113–21. doi: 10.1007/s12105-012-0406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–38. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 37.Jie H-B, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–35. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol. 2012;2012:656340. doi: 10.1155/2012/656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesterhuis WJ, Punt CJA, Hato SV, Eleveld-Trancikova D, Jansen BJH, Nierkens S, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2011;121:3100–8. doi: 10.1172/JCI43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okudaira K, Hokari R, Tsuzuki Y, Okada Y, Komoto S, Watanabe C, et al. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35:741–9. doi: 10.3892/ijo_00000387. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Wang L, Li Y, Pan Y, Wang R, Hu H, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. OncoTargets Ther. 2014;7:567–73. doi: 10.2147/OTT.S59959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11:2947–53. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 43.Hatam LJ, Devoti JA, Rosenthal DW, Lam F, Abramson AL, Steinberg BM, et al. Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD-1/PD-L1/L2 expression. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18:1925–35. doi: 10.1158/1078-0432.CCR-11-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163–74. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 45.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol Baltim Md 1950. 2013;190:5874–81. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.