Abstract
The Centers for Disease Control and Prevention implemented the Pregnancy Flu Line during the influenza A(H1N1)pdm09 (pH1N1) pandemic and continued operation through the 2010–11 influenza season to collect reports of intensive care unit (ICU) admissions and deaths among pregnant women with influenza. The system documented the severe impact of influenza on pregnant women during both seasons with 181 ICU/survivals and 37 deaths reported during the 2009 fall pandemic wave and 69 ICU/survivals and 10 deaths reported in the subsequent influenza season (2010–11). A health department survey suggests PFL participants perceived public health benefits and minimum time burdens.
Keywords: Influenza, Human, Influenza A Virus, H1N1 Subtype, Pregnancy, Surveillance
Pregnant women are at increased risk for influenza-related severe illness including hospitalization and death [1,2,3,4]. Early data from the influenza A(H1N1)pdm09 (pH1N1) pandemic indicated that pregnant women were four times more likely to be hospitalized than the general population, and demonstrated an urgent need for ongoing, national surveillance for influenza in this vulnerable population [1]. In response, the Centers for Disease Control and Prevention (CDC) implemented the Pregnancy Flu Line (PFL) to monitor the impact of influenza on maternal and fetal/infant health and provide consultation to clinicians and health departments.
The PFL consisted of enhanced surveillance for influenza in pregnant women and a 24-hour phone line connecting clinicians and health departments with board-certified obstetricians pediatricians and nurses [4]. Early in the pH1N1 pandemic, before PFL implementation, states responded voluntarily to a one-time CDC request for reporting of pregnant women with laboratory-confirmed influenza illness with onset from April 15 – August 21, 2009; 91 women who were admitted to intensive care units (ICUs) and survived (ICU/survivals), and 38 deaths were reported during this time [3, 4] (Figure 1). The PFL was implemented on October 9, 2009 and accepted reports of pregnant women with severe illness (ICU admission or death) and laboratory-positive influenza during the “fall wave” of the pH1N1 pandemic (onset from August 21, 2009 – August 10, 2010) through the 2010–11 influenza season (onset from August 11, 2010 – August 31, 2011) (Figure 1). PFL staff communicated with health departments from 54 jurisdictions to request PFL case report forms (including demographic and clinical data). For the 2010–11 influenza season, the American Congress of Obstetricians and Gynecologists (ACOG) communicated a request for physicians to report severely ill pregnant women with influenza to state health department contacts which were provided. In both seasons, health departments in states, localities, and territories used existing surveillance infrastructure to identify cases; surveillance methods varied from passive reporting of cases from physicians to health departments to more intense case finding for jurisdictions that participated in the population-based Influenza Hospitalization Surveillance Network, which conducts surveillance for laboratory-confirmed influenza-related hospitalizations. Our objectives were to examine the characteristics of the cases reported to the PFL and to evaluate the PFL’s burdens and benefits for the health jurisdictions that participated in the system.
Figure 1.
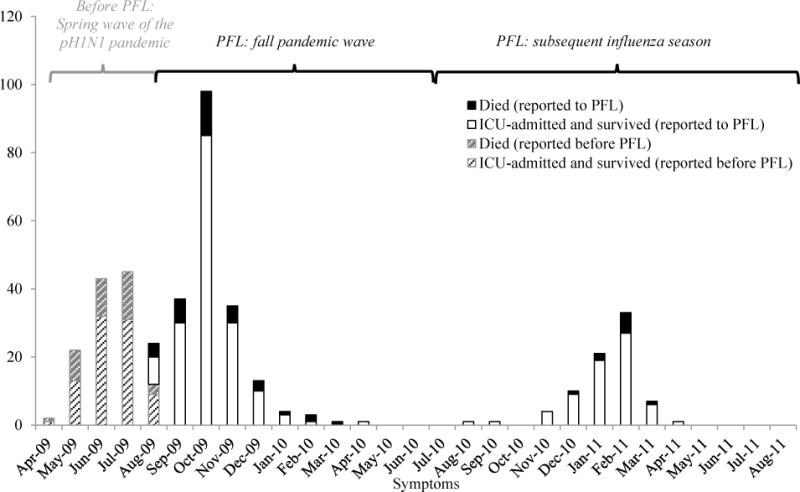
Number of deaths and ICU-only admissions with pregnancy and influenza reported to CDC by influenza onset datea
a missing onset dates for 5 ICU-admitted pregnant women reported during the pre-PFL time period, 14 ICU-admitted pregnant women reported to the PFL and 1 pregnant women who died reported to the PFL
METHODS
Cases reported to the PFL during the fall pH1N1 pandemic wave were compared to cases reported during the post-pandemic 2010–11 influenza season. PFL data from both seasons were also compared to pediatric deaths reported to the Influenza-Associated Pediatric Mortality Surveillance System [5] and to influenza-positive specimens submitted to U.S. laboratories collaborating with the World Health Organization/National Respiratory and Enteric Virus Surveillance System (WHO/NREVSS)1 [6]. We used chi-square tests to compare frequencies and non-parametric Wilcoxon signed-rank and Kolmogorov-Smirnov tests to compare differences in medians, both with a two-sided alpha of 0.05.
CDC conducted an evaluation of the PFL in September, 2011 to better understand possible season-specific variances in reporting and to examine the impact of the PFL on health department personnel. We interviewed CDC-funded influenza coordinators in 52/54 participating state, local, and territorial health departments. Coordinators were asked about jurisdictional recommendations/requirements for reporting of pregnant women with influenza, the time burden of adding PFL case-reporting to their official responsibilities, any programmatic changes made as a result of PFL participation, and whether they found the PFL 24-hour phone line beneficial.
These data were collected as part of the emergency pandemic response and were exempt from human subjects review.
RESULTS
More illness (181 ICU/survivals and 37 deaths) was reported to the PFL during the 2009 fall pandemic wave than in the subsequent influenza season (69 ICU/survivals, 10 deaths) (Figure 1). The proportion of deaths reported to the PFL between the two seasons (17% and 13%, respectively) was not statistically different (p>0.05). Pregnant women reported to the PFL initiated antiviral treatment earlier during the fall pandemic wave compared to the subsequent influenza season, although this difference in antiviral timing was only marginally significant (median of 4 days from onset of illness vs. 5 days, respectively; p=0.05). Nearly all persons were infected with pH1N1 during the fall pandemic wave [6]. During the subsequent influenza season, three influenza viruses predominated in national surveillance systems, however the PFL surveillance noted proportionally more influenza A(H1N1) than the other systems. Influenza A(H1N1) was characterized in 26% of pediatric deaths as noted in the Influenza-Associated Pediatric Mortality Surveillance System, 25% of all influenza-positive specimens submitted through WHO/NREVSS (Figure 2) and 55% of the severely ill pregnant women reported to the PFL (55% of ICU/survivals and 56% of deaths) with subtyping available (p<0.01); unsubtyped influenza A ranged from 17–22% in the three groups (Figure 2).
Figure 2.
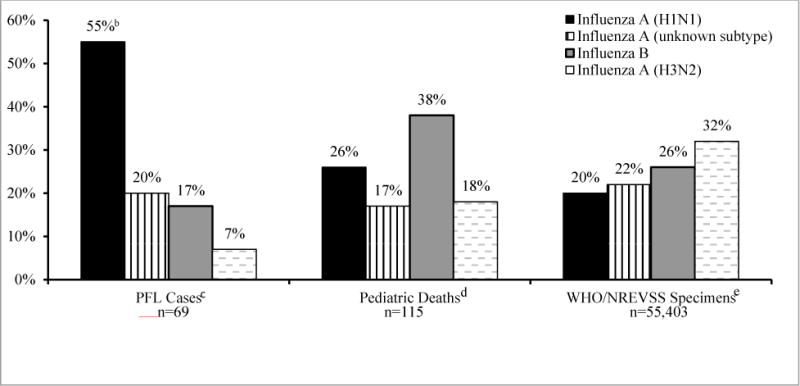
Influenza subtypes reported during the 2010–11 influenza seasona among severely ill pregnant women with influenza reported to the Pregnancy Flu Line (PFL), compared to subtypes among influenza pediatric deaths and WHO/NREVSS specimens
aFor PFL and pediatric deaths reported to the Influenza-Associated Pediatric Mortality Surveillance System, this reporting includes onset dates from August 11, 2010 through May 21, 2011. Reporting dates differ for influenza differ for influenza-positive specimens (not persons) submitted to U.S. laboratories collaborating with the World Health Organization/National Respiratory and Enteric Virus Surveillance System (WHO/NREVSS) and include onset dates from October 3, 2010 through May 21, 2011
bp <0.01 for Fisher’s exact test of proportion of PFL cases with influenza A (H1N1) compared to both pediatric deaths with influenza A (H1N1) and all ages with influenza A (H1N1)
cIncludes pregnant women who died or who were admitted to the intensive care unit and recovered
dSource: Influenza-Associated Pediatric Mortality Surveillance System (5)
eSource: U.S. laboratories collaborating with the World Health Organization/National Respiratory and Enteric Virus Surveillance System (WHO/NREVSS) (6); reporting dates are different than for the PFL and pediatric deaths as noted above
Of the 52 influenza coordinators who were interviewed for the PFL evaluation, most reported that their main sources for data collection were infection control practitioners at hospitals (48, 92%) and public health personnel at the state departments of health (39, 75%) or at district/local health departments (36, 69%). Fewer coordinators reported the support of the Influenza Hospitalization Surveillance Network (FluSurv-NET) personnel (13, 25%) or others such as CDC- and state-funded personnel at hospitals (18, 35%). The total time spent collecting information/reporting for the PFL by all persons involved was minimal, although the time spent was significantly greater during the pandemic (median of 8 hours/month [range: 0.5 to 180 hours]) than the subsequent influenza season (3 hours/month [range: 0–84 hours], p<0.05). Reporting practices also varied by jurisdiction and influenza season. Many jurisdictions required reporting of both influenza-related hospitalizations and deaths in pregnant women during the pandemic (23, 44%) while fewer required both reports during the subsequent influenza season (12, 23%). Other jurisdictions had mandatory reporting of hospitalizations only and but accepted reports of influenza-related deaths in pregnant women (8 [15%] during the pandemic and 5 [10%] subsequent season, respectively), while others had mandatory reporting of influenza-related deaths in pregnant women but also accepted reports of influenza-related hospitalizations (15 [29%] vs. 5 [10%] during the pandemic and subsequent influenza season, respectively). All jurisdictions responded that reporting of either hospitalization and/or deaths among pregnant women with influenza was required during the pandemic. Over half of jurisdictions (28, 54%) however indicated that reports of influenza-related hospitalization and/or deaths among pregnant women were not required during the subsequent influenza season. Two jurisdictions (4%) used other reporting requirements during the subsequent season.
Most influenza coordinators (33, 63%) reported that they found the PFL’s 24-hour phone line beneficial. In addition, many jurisdictions reported making programmatic changes as a result of their PFL participation, such as permanently adding pregnancy status to their influenza case report forms or laboratory reports (24, 46%) and many (30, 58%) also reported that they made other changes or realized other benefits as a result of their participation in the PFL.
DISCUSSION
As the first national influenza surveillance system among pregnant women spanning both pandemic and non-pandemic influenza seasons, the PFL improved understanding of severe influenza in pregnancy. The PFL findings suggest that during the same influenza season, different influenza viruses might predominate among pregnant women than among other groups (Figure 2). While this finding is subject to some limitations, it suggests the possibility of pregnancy-related differences in influenza severity related to influenza virus type, as has been suggested by other studies [7,8]. One recent study found pregnant women with pH1N1 to be twice as likely to be hospitalized as pregnant women with seasonal influenza [8]. Severity differences related to specific influenza type are also biologically plausible for pregnant women, especially during later trimesters when decreased maternal lung capacity might increase risk for severe disease from influenza virus types [2], such as pH1N1, that target the lower pulmonary lobe [9]. Complex immunologic changes associated with pregnancy [2] coupled with the exposure to H1N1, a novel virus for women of childbearing age [10], might increase risks for both initial infection and for increased severity upon infection. While causal pathways are not known, these data suggest the possibility of unique responses among pregnant women with influenza and the need for continued and detailed surveillance among this group.
Historically, CDC’s Pregnancy Mortality Surveillance System (PMSS), which collects data on pregnancy-related deaths from death certificates, has been used to estimate the number of deaths among pregnant women with influenza. PMSS data collected from 1998–2005 showed an average of approximately five deaths per year, ranging from 2 to 14 deaths annually [11]. The PFL received reports of ten influenza-related deaths among pregnant women during the 2010–11 influenza season alone. Both estimates likely underestimate the true burden of influenza-related deaths in the U.S. among pregnant women in non-pandemic influenza seasons. The PFL data are limited by low numbers (17/52 or 33%) of states with mandatory reporting requirements of influenza-related deaths among pregnant women during the 2010–11 season. PMSS data are limited by the variation in quality and completeness of vital records [12]. The true number of deaths among pregnant women with seasonal influenza and the seasonal variation in this number are difficult to measure given that influenza-related death in pregnancy is not a nationally reportable condition.
These data are subject to several limitations. First, the PFL only collected information on severely ill pregnant women, and this group likely is not representative of all pregnant women. Second, under-reporting and differential reporting might underestimate differences when comparing pregnancy-related influenza between the two seasons. Third, the PFL was designed initially for H1N1 reporting during the pandemic, and reporters could have been differentially disposed to report pregnant women with H1N1 infection compared to infections with other influenza virus types despite PFL communications requesting all influenza cases. Fourth, influenza subtype information was missing from all three systems and could have influenced our estimates of the proportions of deaths and specimens by subtype.
Health departments noted benefits and a minimum time burden with participation in the PFL, and PFL data provided new information about the unique burden of influenza on pregnant women and their infants. These findings suggest that similar efforts for monitoring disease in pregnant women might be useful in future emergency response situations and support the inclusion of pregnancy status in routine surveillance systems that capture influenza-related hospitalizations or deaths.
Acknowledgments
These findings were made possible by the dedicated work and collaboration of partners in state, local and territorial health departments across the U.S. We thank the influenza coordinators and supporting staff in all 54 reporting jurisdictions.
Financial support. This work was supported by the Centers for Disease Control and Prevention (contract 200-2010-35705).
Footnotes
Potential conflicts of interest. All authors: No reported conflicts.
Meetings where this information has been presented: Ailes EC, Newsome KB, Gilboa S, et al. Evaluation of CDC’s 2009–2011 Pregnancy Flu Line for Monitoring Pandemic and Seasonal Influenza. 2012 Epidemic Intelligence Service Conference, Atlanta GA. April 16–20.
Publisher's Disclaimer: DISCLAIMER
The findings and conclusions in this report are the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Note that WHO/NREVSS data refer to specimens and not persons.
Contributor Information
Elizabeth C. Ailes, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA; Epidemic Intelligence Service, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
Kimberly Newsome, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
Jennifer L. Williams, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA
Anne F. McIntyre, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA
Denise J. Jamieson, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA
Lyn Finelli, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
Margaret A. Honein, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA
References
- 1.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ, Ukeki TM. Effects of influenza on pregnant women and infants. American Journal of Obstetrics and Gynecology. 2012;207:S3–S8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 3.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newsome K, Williams J, Way S, et al. Maternal and infant outcomes among critically ill pregnant and postpartum women with laboratory-confirmed influenza during the 2009 H1N1 influenza pandemic. MMWR. 2011;60:1193–1196. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Influenza-associated pediatric mortality. Available at http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html (Accessed 07/16/2012)
- 6.Centers for Disease Control and Prevention. Flu view: US virologic surveillance. Available at http://www.cdc.gov/flu/weekly/weeklyarchives2010-2011/10-11summary.htm and http://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/09-10summary.htm (Accessed 07/16/2012)
- 7.Shiley KT, Nadolski G, Mickus T, et al. Differences in the epidemiological characteristics and clinical outcomes of pandemic (H1N1) 2009 influenza compared with seasonal influenza. Infection Control Hospital Epidemiology. 2010;31(7):676–682. doi: 10.1086/653204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen C, Desai S, Bredfeldt C, et al. A large population-based study of pandemic influenza A H1N1 diagnosis during pregnancy and maternal and neonatal outcomes. Journal of Infectious Diseases. 2012:1–9. doi: 10.1093/infdis/jis488. [DOI] [PubMed] [Google Scholar]
- 9.Van den Brand JMA, Stittelaar KJ, Van Amerongen G, et al. Comparison of temporal and spatial dynamics of seasonal H3N2, pandemic H1N1 and highly pathogenic avian influenza H5N1 virus infections in ferrets. PLoS ONE. 2012;7(8):e42343. doi: 10.1371/journal.pone.0042343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishman DN, Savage R, Jackson P. Older age and a reduced likelihood of 2009 H1N1 Virus Infection. New England Journal of Medicine. 2009;361:2000–2001. doi: 10.1056/NEJMc0907256. [DOI] [PubMed] [Google Scholar]
- 11.Callaghan WM, Chu SY, Jamieson DJ. Deaths from seasonal influenza among pregnant women in the United States, 1998–2005. Obstetrics and Gynecology. 2010;15:919–923. doi: 10.1097/AOG.0b013e3181d99d85. [DOI] [PubMed] [Google Scholar]
- 12.MacKay AP, Berg CJ, Duran C, et al. An assessment of pregnancy-related mortality in the United States. Paediatric and Perinatal Epidemiology. 2005;19(3):206–14. doi: 10.1111/j.1365-3016.2005.00653.x. [DOI] [PubMed] [Google Scholar]


