Abstract
Background. To investigate the relationship between hemagglutinin-inhibition (HI) antibody levels to the risk of influenza disease, we conducted a correlate of protection analysis using pooled data from previously published randomized trials.
Methods. Data on the occurrence of laboratory-confirmed influenza and HI levels pre- and postvaccination were analyzed from 4 datasets: 3 datasets included subjects aged <65 years who received inactivated trivalent influenza vaccine (TIV) or placebo, and 1 dataset included subjects aged ≥65 years who received AS03-adjuvanted TIV (AS03-TIV) or TIV. A logistic model was used to evaluate the relationship between the postvaccination titer of A/H3N2 HI antibodies and occurrence of A/H3N2 disease. We then built a receiver-operating characteristic curve to identify a potential cutoff titer between protection and no protection.
Results. The baseline odds ratio of A/H3N2 disease was higher for subjects aged ≥65 years than <65 years and higher in seasons of strong epidemic intensity than moderate or low intensity. Including age and epidemic intensity as covariates, a 4-fold increase in titer was associated with a 2-fold decrease in the risk of A/H3N2 disease.
Conclusions. The modeling exercise confirmed a relationship between A/H3N2 disease and HI responses, but it did not allow an evaluation of the predictive power of the HI response.
Keywords: A/H3N2, influenza, modeling, serologic correlates, vaccine
Inactivated and recombinant protein influenza vaccines are licensed based in part on immunogenicity data because regulatory authorities assume postvaccination hemagglutination-inhibition (HI) antibody titers above a defined threshold are predictive of clinical benefit.
An HI titer threshold of 1:40 is generally recognized as corresponding to a 50% reduction in the risk of influenza, and this is based on a challenge study in adults conducted by Hobson et al [1] in 1972. However, in the literature, there is no consensus on the definition of “protection”, with some studies defining protection as a predefined risk reduction (usually 50%) and other studies defining protection as the titer levels that provide the best separation between influenza cases and noncases [2–5].
To identify and validate any immunological threshold as a correlate of protection, it would be desirable for influenza efficacy vaccine trials to be adequately and consistently designed to allow correlate of protection (COP) analyses, either within a single trial or after pooling of data across different trials. The findings of such analyses would increase the clinical relevance of subsequent studies based on serologic endpoints generated by the same laboratory and assay protocol. Therefore, we pooled data from several trials to assess the relevance of HI antibody levels for protection.
In this study, we describe a COP analysis of pooled data from randomized trials of seasonal influenza vaccines including 7730 subjects [6–9]. In 1 trial, A/H3N2 was the most common influenza virus detected overall; therefore, this COP analysis focused on A/H3N2 [9]. This multitrial approach supports the search for an immunological measurement that is predictive of vaccine efficacy across various settings.
METHODS
Materials and Methods
The analysis was based on 2 trials in subjects aged 18–64 years, 1 trial in subjects aged 18–49 years, and 1 study in subjects aged ≥65 years. In each of these efficacy studies, immunogenicity analyses were performed on per-protocol immunogenicity subcohorts (including subjects who met eligibility criteria, complied with the protocol, received any dose of either vaccine or placebo, and for whom data were available for a given endpoint). An overview of the trials is as follows. (1) Beran et al [6] performed a randomized, double-blind, placebo-controlled study of the efficacy of trivalent influenza vaccine (TIV) against culture-confirmed influenza in healthy adults aged 18–64 years. A total of 5103 and 2549 subjects received TIV or placebo, respectively, during the 2006–2007 season in Czech Republic and Finland. The immunogenicity subcohort in our analysis included 291 and 148 subjects in the TIV and placebo groups, respectively (ClinicalTrials.gov NCT00363870). (2) Beran et al [7] performed a randomized, double-blind, placebo-controlled study of the efficacy of TIV against culture-confirmed influenza in healthy adults aged 18–64 years. A total of 4137 and 2066 subjects received TIV or placebo, respectively, during the 2005–2006 season in the Czech Republic. The immunogenicity subcohort in our analysis included 632 and 315 subjects in the TIV and placebo groups, respectively (ClinicalTrials.gov NCT00197223). (3) Jackson et al [8] performed a randomized, double-blind, placebo-controlled efficacy study of TIV against culture-confirmed influenza in healthy adults aged 18–49 years. In this study, a total of 3783 and 3828 subjects received TIV or placebo, respectively, during the 2005–2006 and 2006–2007 seasons in the United States. The immunogenicity subcohort in our analysis included 1298 and 216 subjects in the TIV and placebo groups, respectively (ClinicalTrials.gov NCT00216242). (4) The Influence65 trial was a randomized, observer-blinded study of the relative efficacy of AS03-TIV vs TIV against polymerase chain reaction (PCR)-confirmed influenza in healthy adults aged ≥65 years. The study included 43 695 subjects from 15 countries who received AS03-TIV or TIV during the 2008–2009 and 2009–2010 seasons. The immunogenicity subset included 2422 and 2408 subjects in the AS03-TIV and TIV groups, respectively, and this analysis included immunogenicity data from the 2008–2009 season (ClinicalTrials.gov NCT00753272) [9].
In the trials including adults aged 18–64 years and 18–49 years, eligible subjects were healthy. In the Influence65 trial including adults aged ≥65 years, subjects with comorbidities were eligible for inclusion if they were ambulatory, their health was stable, and they were without acute illness at the time of vaccination. Inclusion and exclusion criteria and ethics statements for the trials in this analysis have been previously published [6–9].
All vaccines were manufactured by GSK Vaccines. In the placebo-controlled studies, subjects were randomized to receive (1) 1 0.5 mL dose of TIV containing 15 μg each of the 3 hemagglutinin antigens (HAs) recommended by the World Health Organization (WHO) for the given influenza season or (2) saline placebo. In the Influence65 trial, subjects were randomized to receive one 0.7 mL dose of AS03-TIV or 0.5 mL TIV. Details of the vaccines used in each study have been previously described [6–9].
Case Definitions and Laboratory Methods
There were differences among the trials, but they all followed the same general protocol. During the study periods, subjects were monitored for influenza-like illness (ILI) by active surveillance (telephone contact/study center visit/home visits by study personnel) and by passive surveillance (whereby subjects notified the study center if they experienced ILI symptoms). Case definitions in each study have been previously described [6–9]. Nasal and throat swabs were obtained from subjects reporting ILI for the laboratory identification of influenza viruses as previously described [6–8].
In all studies, serum samples were taken before vaccination and 21 days after vaccination for the assessment of serum antibodies to each vaccine-homologous HA. All testing was performed at a GSK's laboratory (Laval, Canada and Dresden, Germany) according to an established method [10]. The high-sensitivity HI assay used 25 µL serum and 25 µL antigen from a solution concentrated at 4 HA units/50 µL (ie, 2 HA units). The erythrocyte (chicken) concentration was 0.45%. Hemagglutination-inhibition assay-based antibody responses were described as the antilog of the arithmetic mean of the log10-transformed titers (geometric mean titers [GMTs]). A titer of 1:5 was assigned to samples with a value below a cutoff titer of 1:10.
Statistical Analysis
The following variables were considered in our analyses: gender, age, seasonal influenza vaccination history within 2 previous years, A/H3N2 infection status by the end of the study season, pre- (Day 0) and postvaccination (Day 21) HI titers against A/H3N2, prevaccination immunity state (ie, A/H3N2 HI titer ≥1:40), A/H3N2 epidemic intensity (“strong intensity” or “low/moderate intensity”), and A/H3N2 disease occurrence. In Beran et al [6, 7] and Jackson et al [8], the epidemic intensity was based on the WHO influenza surveillance (FluNet) database and by evaluating the magnitude of the epidemics in the corresponding countries at the time the studies were conducted. In the Influence65 trial, epidemic intensity was based on national surveillance data and attack rates in the study, as assessed by the Adjudication Steering Committee for the influenza peak season, including experts in the field of influenza and influenza vaccination who were independent of the study investigators and the study sponsor.
In the descriptive analysis, the distribution of variables was characterized. For continuous variables, the number of observations, mean, standard deviation, and minimum and maximum values were computed. For HI titers, GMTs and their coefficient of variation were also calculated after a log10 transformation. Frequency statistics, including counts and proportions were obtained for the categorical variables. A preliminary graphical analysis was performed to assess each covariate versus A/H3N2 attack rates. The proportion of subjects with laboratory-confirmed A/H3N2 influenza was calculated for each dilution factor of the postvaccination HI response against A/H3N2.
A logistic regression model was used to assess the effect of covariates on A/H3N2 disease occurrence. The following covariates were considered: prevaccination immunity state (titer ≥1:40 defined as protected), Day 21 postvaccination A/H3N2 log10 titers, gender, history of vaccination (vaccination 1 and 2 years before study start), vaccine received, and age (Influence65 trial ≥65 years vs other trials <65 years). The epidemic intensity was also included in the model as a potential effect modifier because the protection level associated to a particular HI titer could depend on the level of exposure [11]. A manual stepwise variable selection was performed based on the Bayesian information criterion to select the best combination of covariates to describe the disease occurrence. One of the objectives of our analysis was to try to identify the HI titer that best separates the subjects who were protected from those who were not. A receiver-operating characteristic (ROC) curve, presenting the sensitivity against one minus the specificity at various A/H3N2 HI titer cutoff values, was derived. Sensitivity was defined as the proportion of subjects with a postvaccination titer below the cutoff value among those with confirmed A/H3N2 influenza; specificity was defined as the proportion of subjects with a postvaccination titer equal to or greater than the cutoff value among those without confirmed A/H3N2 influenza. The Youden index, identifying the lowest titer at which the sum of the specificity and sensitivity was maximum, was used to assess such a threshold [12]. The ROC area under the curve quantifies the overall ability of the test to discriminate between those individuals with A/H3N2 disease and those without disease; it represents the probability that a randomly selected influenza case will have a lower result (estimation from the model taking into account all selected covariates) than a randomly selected subject without disease. Because the noncases were a mixture of protected subjects and not sufficiently exposed or nonprotected subjects, we also derived a cutoff postvaccination titer value giving more weight to the cases detected, which we defined as the HI titer cutoff values for the detection of A/H3N2 influenza with 90% sensitivity.
RESULTS
Descriptive Analysis
An overview of subjects included in the analysis is shown in Figure 1. The demographic characteristics and GMTs for subjects included in the analysis by trial are shown in Table 1. Pre- and postvaccination GMTs in subjects who received TIV or AS03-TIV were 14.03–17.4 and 172.3–285.6, respectively, and in subjects who received placebo were 14.12 and 14.14, respectively. Prevaccination, 5405 (71.1%) subjects had an antibody titer against A/H3N2 that was <1:40 (Supplementary Data 1) and 2309 (29.9%) had a titer that was ≥1:40. Sixteen subjects aged ≥65 years did not have prevaccination titer data available.
Figure 1.
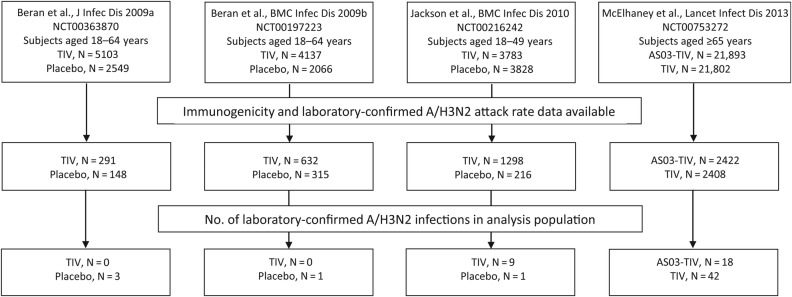
Overview of analysis population (descriptive analysis). Abbreviation: TIV, inactivated trivalent influenza vaccine.
Table 1.
Demographic Characteristics and A/H3N2 HI Antibody Titers Prevaccination (Day 0) and Postvaccination (Day 21) (Descriptive Analysis)
| Adults aged 18–64 years [6–8] |
Adults aged ≥65 years [9] |
|||
|---|---|---|---|---|
| TIV N = 2221 | Placebo N = 679 | AS03-TIV N = 2422 | TIV N = 2408 | |
| Mean age (SD) range, years | 35.78 (12.2) 18–64 | 34.53 (11.3) 18–64 | 73.2 (6.0) 65–95 | 73.4 (6.3) 65–100 |
| Vaccination history, n (%) | ||||
| 1 yr | 243 (10.9%) | 81 (11.9%) | 1647/2197 (75.0%) | 1650/2199 (75.0%) |
| 2 yr | 187 (8.4%) | 32 (4.7%) | 1569/2119 (74.0%) | 1578/2127 (74.2%) |
| GMT, (range) | ||||
| Day 0 | 14.03 (5–1810) | 14.12 (5–640) | 17.4 (5–1810) | 17.4 (5–1280) |
| Day 21 | 178.61 (5–7240) | 14.14 (5–905) | 285.6 (5–20480) | 172.3 (5–20480) |
Abbreviations: AS03, tocopherol-based oil-in-water Adjuvant System; GMT, geometric mean titer; HI, hemagglutination inhibition; n, number of subjects fulfilling definition; N, number of subjects in group; SD, standard deviation; TIV, inactivated trivalent influenza vaccine.
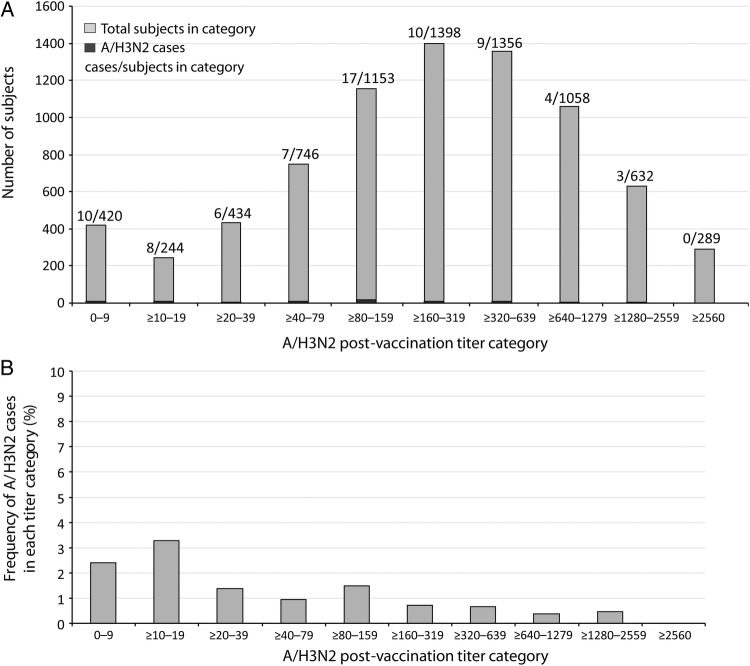
The A/H3N2 disease rates by age and epidemic intensity are shown in Table 2. The frequency of A/H3N2 cases and postvaccination HI titers against A/H3N2 are shown in Figure 2, among which 1098 and 6632 subjects, respectively, had postvaccination titers of <1:40 and ≥1:40. Of 1098 of 7730 (14.2%) subjects with postvaccination HI titers of <1:40, 24 of 1098 (2.2%) subjects had confirmed A/H3N2 disease; among 6632 of 7730 (85.8%) subjects with postvaccination titers of ≥1:40, 50 of 6632 (0.75%) had confirmed A/H3N2 disease.
Table 2.
A/H3N2 Infection Rates by Age and Epidemic Intensity in Subjects Pooled From 4 Vaccine Efficacy Trials (Per-protocol Immunogenicity Subcohorts) (Descriptive Analysis)
Figure 2.
Number of subjects in each titer category and number of A/H3N2 cases (A) and proportion of subjects in each titer category with laboratory-confirmed A/H3N2 infection (B) (descriptive analysis).
Logistic Regression Analysis
Table 3 shows the parameter estimates used in the selected models, which includes postvaccination HI titers, epidemic intensity, and age as covariates. The odds ratio for A/H3N2 disease in high versus moderate or low epidemic intensity was 3.4 (95% confidence interval [CI], 2.1–5.6). The odds ratio of A/H3N2 disease in subjects aged ≥65 years vs <65 years was 3.5 (95% CI, 1.9–6.3). Including postvaccination HI titer and age as covariates, the odds ratio for A/H3N2 disease in subjects aged ≥65 years vs <65 years was 4.2 (95% CI, 2.3–7.8).
Table 3.
Estimates of the Logistic Regression Model Parameters
| Parameter Estimate | P Value | Odds Ratio | 95% Confidence Interval on the Odds Ratio | |
|---|---|---|---|---|
| Baseline risk for the reference categorya | −3.922 | <.0001 | ||
| Postvaccination log titer | −1.1199 | <.0001 | 0.3263 | .2311, .4607 |
| Epidemic intensity | 1.2366 | <.0001 | 3.4439 | 2.0995, 5.6491 |
| Age as covariatesb | 1.2388 | .0001 | 3.4515 | 1.8863, 6.3154 |
a Null postvaccination titer, low moderate epidemic intensity, and <65 years.
b Trial (Influence65 trial [≥65 years] vs other trials [<65 years])/age (≥65 vs <65).
In the full model, including epidemic intensity and age category, a 4-fold increase in postvaccination HI titer was associated with a 49.0% decrease in the risk of infection, and including only age category as a covariate, a 4-fold increase in titer was also associated with a 49.4% decrease in the risk of infection. Consistency of the HI response across the HI range was an assumption of the statistical model, which appeared acceptable based on the cases and HI titers observed (Figure 3).
Figure 3.
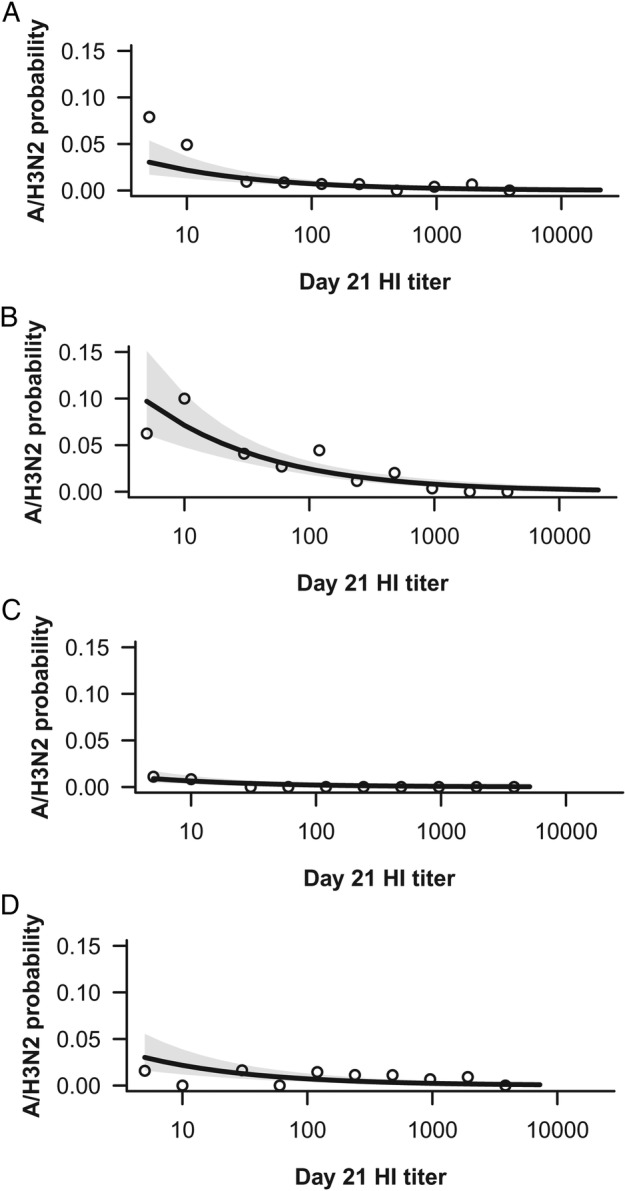
A/H3N2 HI antibody titer and estimated risk of A/H3N2 influenza disease in subjects aged ≥65 years in an epidemic of low to moderate intensity (A) or high intensity (B), and in subjects aged <65 years in an epidemic of low to moderate intensity (C) or high intensity (D). Dots represent the observed proportions of cases and shading shows 95% confidence interval (logistic regression). Abbreviation: HI, hemagglutination inhibition
The area under the curve for the ROC including age and season strength as covariates was estimated at 0.7735 (Supplementary Data 2). The Youden index HI titer cutoffs and the 90% sensitivity cutoff values are shown in Table 4.
Table 4.
Youden Index Cutoff and 90% Sensitivity Cutoff Values for A/H3N2 Hemagglutination Inhibition Antibody Titers (Logistic Regression)
|
Age |
Season Strength |
Youden Index Cutoff Titer | 90% Sensitivity Cutoff Titer |
|---|---|---|---|
| <65 yr | Low/moderate | 1:5 | 1:28 |
| <65 yr | High | 1:40 | 1:453 |
| ≥65 yr | Low/moderate | 1:40 | 1:453 |
| ≥65 yr | High | 1:640 | 1:5120 |
DISCUSSION
In agreement with previous reports, we found a correlation between HI titers and the risk of A/H3N2 disease [1, 13]. Including age and epidemic intensity as covariates, we showed that a 4-fold increase in titer was associated with a 2-fold decrease in the risk of A/H3N2 disease, with a similar difference in risk observed when including only age as a covariate. The Youden index cutoff values in a season of high epidemic intensity were 1:40 and 1:640 in subjects aged <65 years and ≥65 years, respectively. Although we found that age did not appear to affect the serological response to vaccination based on GMTs, older subjects seemed to have a greater risk of infection at similar titers compared with younger subjects.
Although age defined the population studied in each trial included in the analysis, case detection methods and circulating viruses may affect the susceptibility of a population to influenza disease. In addition, because these factors differed between the trials, the effect of age on HI titers as a COP must be interpreted with caution. Indeed, there are several factors related to the differences among the trials that are confounded in our analysis: the nature of the comparison (placebo or active treatment, adjuvanted or not), influenza case definitions, laboratory methods for viral detection (culture or PCR), HI measurements, and the age of participants (18–64 years, 18–49 years, ≥65 years). In addition, cell-mediated immunity contributes to protection against influenza, but the reliability of the HI titer as an index of both humoral and cellular immunity is unknown and likely to differ with advancing age. We believe that the most important differences among the trials were the differences in the population studied reflected by age, which is a surrogate for unmeasured differences in immunity based on past exposure, and differences in the definition of influenza disease.
Influenza occurrence depends upon the immunity of a given population as well as their exposure to circulating viruses. Because different countries have different vaccination policies that influence the transmission and exposure to viruses (eg, vaccination of children), we included epidemic intensity as a covariate based on surveillance in each country as an indicator of exposure. However, exposure may also change the level of antibody needed to prevent illness of any severity [11]. This is an important concept because in adults, most illnesses are relatively mild, but the occurrence of severe illness resulting in hospitalization and adverse outcomes increases with advancing age. In this pooled analysis, we did not have a systematic prospective classification of moderate to severe illness, and because the level of antibody correlating with protection against moderate to severe illness may be less than that needed to protect against mild illness, our analysis may be confounded. In addition, regional and seasonal variability of circulating viruses and variations in the severity of influenza illness are difficult to account for in a COP analysis.
An objective of our analysis was to try to identify the HI titer that best separates the 2 subject groups—protected and not protected. By considering a ROC approach, selecting a cutoff point involves a trade-off between sensitivity and specificity. The Youden index gives the same weight to both sensitivity and specificity because it defines the cutoff point as the titer value that maximizes the sum of the sensitivity and specificity [12]. The Youden index method depends upon the separability of the protected and nonprotected populations. However, the rate of infection among subjects with low titers may be strongly associated with the chance of exposure and disease prevalence, which vary among seasons and locations as well as social behavior. Therefore, the HI titer density curves for subjects who were not infected are a mixture of subjects who were protected and those who were possibly unprotected but also unexposed. The methodology we used relies on the belief that false negatives are likely to occur, and thus sensitivity (true cases) should determine the cutoff value. We reported the cutoff for 90% sensitivity, which was 1:453 in subjects <65 years and 1:5120 in subjects aged ≥65 years in a season of high epidemic intensity. This means that subject with a higher prevaccination risk (ie, older subjects) will need higher antibody titers to have the same level of protection as subjects with a lower prevaccination risk such that our model provides varying cutoff points for protection.
Further methods used to assess influenza vaccine protection include the scaled logistic regression model suggested by Dunning [14] in which the probability of the subject developing influenza is the probability that the subject is susceptible to influenza multiplied by the probability that susceptible individuals develop disease. In addition, Li et al [15] developed a dichotomization method based on the maximization of the correlation between the 2 populations and the dichotomous variable. In the noncases population, the methods included a parameter defining the probability that the observation arises from the case population (unprotected but not exposed).
In our logistic regression model, we found that the risk of disease was higher for older than younger subjects (prevaccination risk) and higher in a season of high epidemic intensity than moderate or low epidemic intensity. Subjects with a greater risk (ie, older subject and/or in a strong epidemic intensity) will need higher antibody titers to have the same level of protection as subjects with a lower risk (prevaccination or linked to the season), meaning that our model provides varying cutoff points for protection. We did not find a significant interaction between postvaccination titers and subject-related covariates, although the study lacks power. However, we found a similar relationship to that reported in the literature: a four-fold increase in postvaccination titers was associated with a 2-fold decrease in the risk of infection.
CONCLUSIONS
The statistical modeling exercise confirmed that there is a relationship between the occurrence of A/H3N2 disease and HI antibody responses but did not allow us to evaluate the predictive power of the HI response. An alternative to performing pooled analyses is to conduct influenza vaccine efficacy trials that are designed to provide data for COP analyses. In addition to accounting for age and epidemic intensity, optimizing the COP model would involve collecting serum samples from all participants, rather than from a subcohort, identifying influenza disease based on a consistent case definition, and using a consistent laboratory method for viral subtyping. The analysis should also take into account influenza disease severity and the level of antigenic mismatch between the infecting virus and the vaccine strains.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We are indebted to the participating study volunteers and their parents, clinicians, nurses, laboratory technicians at the study sites, and authors from the primary publications, especially P. Van Belle, A. Trofa, A. Karvonen, E. Kaliskova, N. Lindblad, and M. Peeters. We are also grateful to all Influence65 study group members and the sponsor's project staff for support and contributions throughout the study, especially M. Albanese, N. Della-Vecchia, M. Dupelle, S. Fannoy, N. Legare, L. Pesche, M. Ribot, S. Papagiannis, J. Roger, M. Lanoue, and V. Wansard for participation in clinical testing. We thank V. Dodeur, L. Hollinger, K. Peeters (freelance, Spain, on behalf of GSK Vaccines), and W. Talbott for involvement in the study coordination and study management. Finally, we thank A. Moon (Moon Medical Communications Ltd, UK) for providing medical writing services and B. Dumont (Business and Decision Life Sciences, on behalf of GSK Vaccines, Rixensart, Belgium) for editorial assistance and manuscript coordination.
Author contributions. All authors had full access to the data. All authors participated in the implementation of the study including substantial contributions to conception and design, the gathering of the data, or analysis and interpretation of the data. All authors were involved in critically revising the manuscript for important intellectual content and approved the submitted manuscript.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis and funded the development and the publishing of the present manuscript.
Potential conflicts of interest. J.-M. D., L. O., F. T., B. L. I., and W. D. are employees of the GSK group of companies and report ownership of stock options and/or restricted shares. At the time of the study, A. B. reports grants from GSK group of companies to their institutions for the conduct of the study, grants from the IAP Research network P7/06 of the Belgian State (Belgian Science Policy), and reports grants from the “Projet d'Actions de Recherche Concertées” 11/16–039 of the “Communautée Française de Belgique”, allowed by the Académie Universitaire Louvain. A. B. also reports she is now an employee of the GSK group of companies. G. L.-R., L. J., M. G., and O. L. report grants from GSK group of companies to their institutions for the conduct of the study. J. E. M. reports grants from GSK group of companies to their institutions for the conduct of the study, was reimbursed for travel and accommodation related to activities of the publication steering committee, and reports having received personal fees from Sanofi for Data Monitoring Board and Advisory Board, outside the submitted work. M. E. reports grants from GSK group of companies to her institutions for the conduct of the study and travel grant for meeting of publication steering committee and grants from Baxter and BMBF, outside the submitted work. T. V. reports grants from GSK group of companies to their institutions for the conduct of the study, personal fees for membership on Advisory Boards, and travel support to present results. C. L. reports a grant to support a PhD student (A. B.) under her supervision on this article.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett PN, Berezuk G, Fritsch S et al. . Efficacy, safety, and immunogenicity of a Vero-cell-culture-derived trivalent influenza vaccine: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2011; 377:751–9. [DOI] [PubMed] [Google Scholar]
- 3.Black S, Nicolay U, Vesikari T et al. . Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081–5. [DOI] [PubMed] [Google Scholar]
- 4.Siber GR. Methods for estimating serological correlates of protection. Dev Biol Stand 1997; 89:283–96. [PubMed] [Google Scholar]
- 5.Skowronski DM, Moser FS, Janjua NZ et al. . H3N2v and other influenza epidemic risk based on age-specific estimates of sero-protection and contact network interactions. PLoS One 2013; 8:e54015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beran J, Vesikari T, Wertzova V et al. . Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: a prospective, randomized, placebo-controlled trial. J Infect Dis 2009; 200:1861–9. [DOI] [PubMed] [Google Scholar]
- 7.Beran J, Wertzova V, Honegr K et al. . Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example. BMC Infect Dis 2009; 9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson LA, Gaglani MJ, Keyserling HL et al. . Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo-controlled trial over two influenza seasons. BMC Infect Dis 2010; 10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McElhaney JE, Beran J, Devaster JM et al. . AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis 2013; 13:485–96. [DOI] [PubMed] [Google Scholar]
- 10.Hehme N, Künzel W, Petschke F et al. . Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin Drug Invest 2002; 22:751–69. [Google Scholar]
- 11.Tsang TK, Cauchemez S, Perera RA et al. . Association between antibody titers and protection against influenza virus infection within households. J Infect Dis 2014; 210:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly MJ, Dunstan FD, Lloyd K, Fone DL. Evaluating cutpoints for the MHI-5 and MCS using the GHQ-12: a comparison of five different methods. BMC Psychiatry 2008; 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coudeville L, Bailleux F, Riche B et al. . Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 2010; 10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunning AJ. A model for immunological correlates of protection. Stat Med 2006; 25:1485–97. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Parnes M, Chan IS. Determining the cutoff based on a continuous variable to define two populations with application to vaccines. J Biopharm Stat 2013; 23:662–80. [DOI] [PubMed] [Google Scholar]