Abstract
We describe CD4 counts at 6-month intervals for 5 years after combination antiretroviral therapy initiation among 12 879 antiretroviral-naive human immunodeficiency virus-infected adults from Latin America and the Caribbean. Median CD4 counts increased from 154 cells/mm3 at baseline (interquartile range [IQR], 60–251) to 413 cells/mm3 (IQR, 234–598) by year 5.
Keywords: cART, cohort, combination antiretroviral therapy, HIV/AIDS, immune recovery, inverse probability of censoring weights, Latin America and the Caribbean
Combination antiretroviral therapy (cART) reduces human immunodeficiency virus (HIV)-related morbidity and mortality through suppression of plasma HIV RNA, thus allowing immune restoration. In clinical trials, approximately 80% of HIV-infected patients achieve undetectable plasma HIV RNA within 6 months of cART initiation [1]. Patients typically experience marked increases in CD4 counts (CD4) during the first 2 years of therapy, followed by smaller but consistent increases through 3–5 years of treatment. Observational studies show that improvements in CD4 may persist up to 7–10 years after initiation of cART in high- and middle-income countries [2, 3]. A recent review suggested that patients from low-income countries who remained in follow-up with access to treatment may have comparable responses to those from high-income countries [4]. These encouraging results likely reflect the best-case scenario as most published studies from low- and middle-income countries included only patients alive and in care and therefore did not adequately address mortality and loss to follow-up (LTFU) when estimating immune response [4]. In the present study, we estimated CD4 up to 5 years after cART initiation for clinical cohorts in 7 low- to middle-income countries in Latin America and the Caribbean while correctly accounting for missing data and LTFU, and we quantified the relevance of CD4 at cART initiation and at 6 months for predicting CD4 at year 5.
METHODS
Study Population and Outcome Definition
The Caribbean, Central, and South America Network for HIV Epidemiology (The Caribbean, Central and South America Network for HIV Epidemiology [CCASAnet]) is a consortium of cohorts from 7 countries (Argentina, Brazil, Chile, Haiti, Honduras, Mexico, and Peru) to study HIV outcomes [5]. For this study, cART-naive HIV-infected adults (≥18 years at cART initiation) initiating cART at CCASAnet sites on or after January 1, 2000 to December 31, 2011 were included; follow-up was extended to December 31, 2012 to allow patients at least 1 year of follow-up. Included patients had to have a CD4 at cART initiation (baseline), defined as the CD4 closest to cART initiation up to 6 months prior; for missing values, the CD4 closest to cART initiation up to 3 months after was used.
The outcome of interest was the median CD4 during 6-month periods in the first 5 years of cART (ie, for 10 periods). For each period, CD4 encompassed a 6-month window (closest measurement within 3 months before or after 6, 12, 18, etc. months post-cART initiation).
For patients who died, end of follow-up was defined as the date of death, and CD4 for periods occurring after death were recorded as the lowest-rank (worst) CD4. The rationale for including deceased patients after their death date was that an analysis including only living patients would overestimate CD4 during the first 5 years. Therefore, our results reflect the observed median CD4 among all patients initiating cART, including those who died. This analysis choice is similar to that used by others [6]. For those not known to have died, end of follow-up was determined by the last alive date or the end of the 5-year period. Any unavailable CD4 for a specific 6-month period between cART initiation and end of follow-up (defined by end of study, date of death, or last alive date) was defined as missing.
Statistical Analyses
We describe the inverse probability of censoring weighted (IPCW) semi-annual CD4 after cART initiation as represented by median and interquartile ranges (IQRs). The inverse probability of censoring weighted semi-annual CD4 were defined as those adjusted for missing and LTFU using IPCW [6, 7].
The probability of having a missing CD4 and of being LTFU were modeled separately by site using pooled logistic regression. Weights were obtained using fitted models that included demographic and clinical factors thought to correlate with missingness, LTFU, and CD4, namely study site, age, gender, mode of HIV transmission, initial ART regimen, pretreatment clinical stage, clinical trial participation, date of treatment initiation, and pretreatment CD4 count. Final weights were obtained by multiplying weights obtained with each model; weights were truncated at the 99th percentiles (Supplementary Figure 1).
Estimated median CD4 and IQRs were visualized graphically by stratification variables; to enhance presentation, we provide data animations accessible online. Inverse probability of censoring weighted median regression was used to quantify the predictive value of baseline CD4 in estimating median CD4 at year 5 while adjusting for covariates. For the subset of patients who survived and were not LTFU 6 months after cART initiation, we quantified the ability of CD4 in the first 6-month period and of CD4 change during the first 6 months to predict CD4 at year 5 in addition to baseline CD4. Restricted cubic splines were used to relax linearity assumptions. R statistical software (www.r-project.org version 2.15.2) was used for all analyses. Analysis scripts are posted at http://biostat.mc.vanderbilt.edu/wiki/main/archivedanalyses.
RESULTS
Among the study population of 12 879 patients, the median age was 36 years (IQR, 30–44 years), 59.1% (7617) were male, 25.7% (3311) had pre-cART acquired immune deficiency syndrome diagnosis, and 89.4% (11 513) started a nonnucleoside reverse transcriptase inhibitors-based regimen. Median follow-up was 4.2 years (IQR, 2.5–6.7 years), 1818 (14.1%) were LTFU, and 1279 patients (9.9%) died. Among those patients alive and in care at 5 years (n = 5211), 56.3% were still on their initial cART regimen, whereas 43.7% had made at least 1 regimen modification (ie, changed at least 1 drug).
Overall, median CD4 increased from a baseline value of 154 cells/mm3 (IQR, 60–251 cells/mm3) to 259 cells/mm3 (IQR, 158–385 cells/mm3) at 6 months and to 413 cells/mm3 (IQR, 234–598 cells/mm3) by year 5 (Figure 1A). Although CD4 continued to increase throughout the 5 years, the first 2 years after cART initiation showed a steeper increase compared to the latter 3 years. Median CD4 at year 5 was 376 cells/mm3 (IQR, 196–544 cells/mm3), 514 cells/mm3 (IQR, 342–692 cells/mm3), and 625 cells/mm3 (IQR, 416–833 cells/mm3) for those with baseline CD4 <200, 200–349, and ≥350 cells/mm3, respectively (Figure 1B). A supplemental website displays animated figures showing CD4 response, death, and LTFU over time according to baseline CD4 and other strata (http://biostat.mc.vanderbilt.edu/ccasanet/dataviz/cd4_trajectory.htm). In addition, Supplementary Figure 2 compares our IPCW estimates with those we would have obtained had we not accounted for death and LTFU: observed median CD4 was 256 cells/mm3 (IQR, 155–383 cells/mm3) at 6 months and 400 cells/mm3 (IQR, 170–593 cells/mm3) at year 5. Among the study population with regular viral load monitoring (at least 1 measurement per year; n = 4561) and suppressed viral load (all measurements >6 months after ART initiation <400 copies/mL; n = 3521) observed median CD4 was 296 cells/mm3 (IQR, 177–435 cell/mm3) at 6 months and 484 cells/mm3 (IQR, 339–670 cells/mm3) at year 5 (Supplementary Figure 3).
Figure 1.
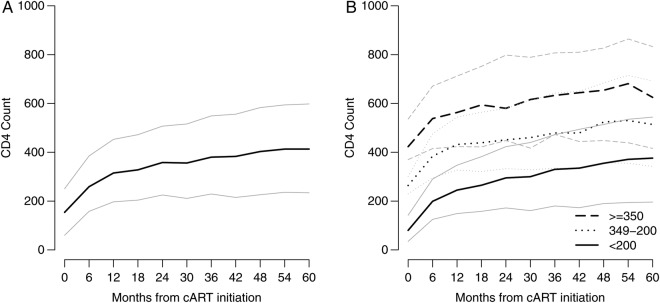
Estimated median and interquartile range CD4 count (CD4) by months from combination antiretroviral therapy (cART) initiation for the study population (A) and stratified by baseline CD4 (B). Dark lines designate the estimated medians, and lighter lines designate the estimated 25th and 75th percentiles.
After adjusting for study site, age, gender, mode of HIV transmission, pretreatment clinical stage, clinical trial participation, initial ART regimen, and date of treatment initiation, we observed an approximately linear positive association between baseline CD4 and median CD4 at year 5 (Supplementary Figure 4). Study site was also associated with CD4 recovery, with sites in Argentina, Brazil, Chile, Mexico, and Peru showing somewhat higher predicted median CD4 at year 5 compared with sites in Haiti and Honduras. Median CD4 at year 5 was 53 cells/mm3 higher for women than for men (95% confidence interval [CI], 32–74 cells/mm3) and 43 cells/mm3 higher for men who reported having sex with other men compared with those reporting heterosexual sex as their mode of HIV transmission (95% CI, 22–65 cells/mm3). In addition, older patients had a lower median CD4 increase compared with those starting cART in their thirties (P < .001). For the subset of individuals who survived and were not lost by 6 months (n = 12 056; 93.6%), CD4 at 6 months was found to significantly predict CD4 at 5 years (P < .001; Supplementary Figure 5), whereas CD4 at cART initiation no longer offered predictive value after adjusting for CD4 at 6 months and the other variables. In contrast, when we included both baseline CD4 and CD4 change during the first 6 months in the model, in addition to the other covariates, both offered significant predictive value (P < .001 for both; Supplementary Figure 6).
DISCUSSION
In this study, we estimated CD4 up to 5 years after cART initiation in more than 12,000 patients followed in HIV clinical cohorts in Latin America and the Caribbean. We found that CD4 continued to improve over 5 years. These results are consistent with the growing literature that report CD4 at cART initiation is a determining factor for improved immune response, and provide further support for early initiation of cART [8]. We found that baseline CD4 significantly predicted patients' immune response at 5 years. In addition, when including both baseline CD4 and CD4 at 6 months in the model, we found that CD4 at 6 months significantly predicted patients' immune response at 5 years. In this model, CD4 at 6 months was a better predictor of CD4 at 5 years because it captured not only the starting point (CD4 at baseline) but also CD4 response during the first 6 months. Accordingly, the model that included both baseline CD4 and CD4 change during the first 6 months showed that both variables were highly predictive of immune response at 5 years. Although HIV RNA measurement is the earliest and most sensitive indicator of cART efficacy, CD4 at cART initiation and 6 months thereafter is readily available in most middle- and low-income countries and may facilitate patient care through early assessment of immune response.
There have been few studies of CD4 response to cART in Latin America and the Caribbean. Our observed CD4 response from a median of 154 cells/mm3 at cART initiation to 259 at 6 months to 413 cells/mm3 after 5 years is generally consistent with that observed in a large multicohort collaboration that included Latin American patients (7%) as well as those from African and Asian sites (median of 114, 230, and 395 cells/mm3 at cART initiation, 6 months, and 5 years, respectively; n = 19 967) [9]. In addition, a study of 5115 cART initiators in Chile reported a CD4 change from a median of 102 cells/mm3 at cART initiation to 244 and 301 cells/mm3 1 and 5 years after cART initiation, respectively [10]. Neither study accounted for LTFU and death thus likely provided overly optimistic estimates of CD4 response among all cART initiators; however, compared with data from our study, these estimates do not appear notably optimistic - in fact, the median CD4 at 5 years in the Chilean study was actually surprisingly low. Of note, median CD4 at 6 months and 5 years would have been estimated as 256 and 400 cells/mm3, respectively, had we done standard analyses that ignore LTFU, missing CD4, and death. Although we suggest using methods that properly account for these potential sources of bias, it is perhaps reassuring to note that, at least in our study, failing to account for LTFU, missing CD4, and death would have had little impact on our results.
To date, several studies have reported that the strongest predictor of long-term CD4 response is baseline CD4, even among patients with long-term HIV RNA suppression [8, 11]. In addition, studies have found that women [12] and patients initiating treatment at younger ages [13] show better CD4 response, whereas specific cART regimens have not been shown to significantly impact long-term immune response [14]. Our results corroborate these findings.
Our study has limitations worth noting. As is the case with any cohort study, as time passed, the number of individuals in active follow-up diminished due to death, LTFU, or end-of-study censoring. To address this limitation, we used inverse probability of censoring weights to account for missingness and LTFU; our weights require assuming that models have been properly specified and include all relevant common-cause variables [7]. Also, data from the clinical sites evaluated here were limited to patients from the contributing cohorts and may not be representative of all patients on cART in those countries. We were only able to incorporate HIV RNA data on a small proportion of our patients with regular measurements (35%). Finally, the inclusion criteria limited the study population to those starting treatment on or after 2000 with a baseline CD4, and therefore our findings may not be representative of the larger population of cART initiators.
CONCLUSIONS
In conclusion, we have shown that CD4 continued to increase up to 5 years after cART initiation and that higher baseline CD4 led to a more robust improved immune response. Our study population included over 12 000 treated patients followed in clinical cohorts in Latin America and the Caribbean, and thus our inferences support early start of cART in low- and middle-income countries.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Financial support. This work was funded by the National Institute of Allergy and Infectious Diseases as part of the International Epidemiologic Databases to Evaluate AIDS (grant U01 AI069923). P. M. L. and B. G. acknowledge funding from the National Council of Technological and Scientific Development and the Research Funding Agency of the State of Rio de Janeiro.
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Campbell TB, Smeaton LM, Kumarasamy N et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med 2012; 9:e1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Moing V, Thiebaut R, Chene G et al. Long-term evolution of CD4 count in patients with a plasma HIV RNA persistently <500 copies/mL during treatment with antiretroviral drugs. HIV Med 2007; 8:156–63. [DOI] [PubMed] [Google Scholar]
- 3.Luz PM, Grinsztejn B, Velasque L et al. Long-term CD4+ cell count in response to combination antiretroviral therapy. PLoS One 2014; 9:e93039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achhra AC, Phanuphak P, Amin J. Long-term immunological outcomes in treated HIV-infected individuals in high-income and low-middle income countries. Curr Opin HIV AIDS 2011; 6:258–65. [DOI] [PubMed] [Google Scholar]
- 5.McGowan CC, Cahn P, Gotuzzo E et al. Cohort profile: Caribbean, Central and South America Network for HIV Research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol 2007; 36:969–76. [DOI] [PubMed] [Google Scholar]
- 6.Lok JJ, Bosch RJ, Benson CA et al. Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection. AIDS 2010; 24:1867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008; 168:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia F, de Lazzari E, Plana M et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr 2004; 36:702–13. [DOI] [PubMed] [Google Scholar]
- 9.Nash D, Katyal M, Brinkhof MW et al. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS 2008; 22:2291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolff MJ, Cortes CP, Shepherd BE et al. Long-term outcomes of a national expanded access program to antiretroviral therapy: the Chilean AIDS cohort. J Acquir Immune Defic Syndr 2010; 55:368–74. [DOI] [PubMed] [Google Scholar]
- 11.Hunt PW, Deeks SG, Rodriguez B et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS 2003; 17:1907–15. [DOI] [PubMed] [Google Scholar]
- 12.Bastard M, Soulinphumy K, Phimmasone P et al. Women experience a better long-term immune recovery and a better survival on HAART in Lao People's Democratic Republic. BMC Infect Dis 2013; 13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright ST, Petoumenos K, Boyd M et al. Ageing and long-term CD4 cell count trends in HIV-positive patients with 5 years or more combination antiretroviral therapy experience. HIV Med 2013; 14:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanna N, Opravil M, Furrer H et al. CD4+ T cell count recovery in HIV type 1-infected patients is independent of class of antiretroviral therapy. Clin Infect Dis 2008; 47:1093–101. [DOI] [PubMed] [Google Scholar]


