Abstract
Objectives
Intestinal carriage constitutes an important reservoir of antimicrobial-resistant bacteria, with some of the highest rates reported from Asia. Antibiotic resistance has been little studied in Laos, where some antibiotics are available without restriction, but others such as carbapenems are not available.
Patients and methods
We collected stools from 397 healthy children in 12 randomly selected pre-school childcare facilities in and around Vientiane. Colonization with ESBL-producing Enterobacteriaceae (ESBLE) and carbapenemase-producing Enterobacteriaceae (CPE) was detected using a disc diffusion screening test and ESBLE were characterized using WGS. Risk factor data were collected by questionnaire.
Results
Ninety-two children (23%) were colonized with ESBLE, mainly Escherichia coli carrying blaCTX-M and Klebsiella pneumoniae carrying blaSHV or blaCTX-M, which were frequently resistant to multiple antibiotic classes. Although residence in Vientiane Capital, foreign travel, higher maternal level of education, antibiotic use in the preceding 3 months and attending a childcare facility with a ‘good’ level of hygiene were all associated with ESBLE colonization on univariable analysis, a significant association remained only for antibiotic use when a stepwise approach was used with a multivariate random-effects model. WGS analysis suggested transmission in both childcare facilities and community settings.
Conclusions
The high prevalence of paediatric colonization with ESBLE in Laos, one of the highest reported in Asia, is probably the result of inappropriate antibiotic use. Paediatric colonization with CPE was not identified in this study, but it is important to continue to monitor the spread of antibiotic-resistant Enterobacteriaceae in Laos.
Keywords: resistance, carriage, ESBLE
Introduction
ESBL-producing Enterobacteriaceae (ESBLE) are increasingly common, resulting in the wider clinical use of carbapenems. High intestinal ESBLE colonization prevalence rates have been reported in Asia, with CTX-M enzymes predominating.1 Despite limited regulation of antimicrobial prescribing in Laos, the emergence of ESBLE appears to have lagged behind other countries in the region.2 Young children represent 11% of the population in Laos3 and may act as a reservoir for antimicrobial-resistant organisms.4
The aims of this study were to: (i) determine the intestinal colonization prevalence of ESBLE and carbapenemase-producing Enterobacteriaceae (CPE) in Lao children; (ii) investigate risk factors for ESBLE/CPE; and (iii) characterize the genotype and genetic relatedness of any isolates detected.
Patients and methods
Faecal samples were collected from children ≤6 years of age in six pre-school childcare facilities in Vientiane Capital (VTE) and six in Vientiane Province (VTP), central Laos, in March–June 2011. The childcare facilities, each attended by >50 children, were selected using a random number generator.
Epidemiological data for putative risk factors were assessed by questionnaire (see Supplementary section S1 for a list of variables/translated version of questionnaire; available as Supplementary data at JAC Online). A hygiene category (good/adequate/poor) was assigned to each childcare facility (WHO guidance).5
Faecal samples were diluted 1 : 10 in saline and incubated on MacConkey agar with 10 μg cefpodoxime and imipenem discs (Oxoid, Basingstoke, UK) within 24 h of collection. Any Gram-negative/oxidase-negative bacilli growing within the antibiotic zones of inhibition and representing distinct colonial morphotypes were tested for ESBL production.6 Species identification for ESBLE was performed with the API20E system (bioMérieux, Marcy-l'Étoile, France). Isolates growing within the imipenem inhibition zone were screened using a modified Hodge test6 with 10 μg imipenem and meropenem discs. Additional disc diffusion testing was undertaken for amoxicillin/clavulanate, cefotaxime, ceftriaxone, ceftazidime, co-trimoxazole, chloramphenicol, ofloxacin, gentamicin and nitrofurantoin.6
DNA was extracted from ESBLE isolates using Quickgene (Fujifilm, Japan) with an additional mechanical lysis step (FastPrep, MP Biomedicals, USA) and sequenced on the Illumina HiSeq 2000, generating 100 base paired-end reads. Sequencing data are available at the European Nucleotide Archive (http://www.ebi.ac.uk/ena/data/view/PRJEB3980).
Reference-based mapping was used to identify nucleotide-level variation [single nucleotide variants (SNVs)] for Escherichia coli and Klebsiella pneumoniae isolates (Supplementary section S2). Maximum-likelihood phylogenies for each species were created using PhyML.7
Reads were de novo assembled into contigs using optimized parameters with Velvet/VelvetOptimiser.8,9 BLASTn was used to identify the presence of resistance gene variants in the contigs for each isolate (see Table S1 for a list of variants).10 Overlapping, partial matches across several contigs representing >80% sequence homology were aligned and this alignment was re-blasted against the database to determine which variant was present.
MLST was similarly determined for E. coli and K. pneumoniae (Achtman/Pasteur schemes); a match to all reference loci was taken as confirmation of species identification.11,12 For species identification of non-E. coli/K. pneumoniae isolates, sequences for 16S rDNA (using rrsA as the reference) were extracted using BLASTn, with top hits analysed using the SeqMatch function at the Ribosomal Database Project (http://rdp.cme.msu.edu/seqmatch/seqmatch_intro.jsp).13
Demographic/risk factor data were summarized as frequency (%) or median (IQR). Exact binomial CIs (95%) were calculated for prevalences. Groups were compared using the χ2 test, Fisher's exact test or the Mann–Whitney U-test. Independent risk factors for ESBLE colonization were determined by multivariate analysis using a random-effects model stratified by childcare facility, including all variables with P < 0.20 on univariable analysis plus variables previously reported as risk factors for ESBLE colonization (age, history of hospitalization in the previous year and history of urinary tract infection in household). P values <0.05 were considered statistically significant and a stepwise approach was used to eliminate non-significant variables from the model.
Statistical calculations were performed with STATA, versions 11 and 13 (StataCorp, College Station, TX, USA).
Ethical approval
The study was approved by the National Ethics Committee of the Ministry of Health, Lao PDR and by the Oxford Tropical Research Ethics Committee. Consent for enrolment in the study was obtained on behalf of all participants from their parents/guardians.
Results
In total, 397 individuals were sampled (202 in VTE and 195 in VTP). The median age of participants was 4.2 years (IQR: 3.4–5.2 years) (epidemiological characteristics are summarized in Table S2).
Of 236 colonies growing within cefpodoxime zones, 100 (42.4%) from 92 children were ESBLE (8 children yielded two ESBLE species). Of these, 78 were E. coli, 18 K. pneumoniae, 3 Enterobacter spp. and 1 remained unidentified. No CPE were detected. High levels of concomitant resistance to other antimicrobials were observed (Table 1).
Table 1.
Antimicrobial susceptibilities of ESBLE isolated
| Antimicrobial |
E. coli, n = 78 |
K. pneumoniae, n = 18 |
Other species, n = 4 |
Total, n = 100 |
||||
|---|---|---|---|---|---|---|---|---|
| R, n (%) | I, n (%) | R, n (%) | I, n (%) | R, n (%) | I, n (%) | R, n | I, n | |
| Amoxicillin/clavulanate | 6 (8) | 35 (45) | 0 (0) | 0 (0) | 3 (75) | 1 (25) | 9 | 36 |
| Cefotaxime | 76 (97) | 1 (1) | 11 (61) | 7 (39) | 4 (100) | 0 (0) | 91 | 8 |
| Ceftriaxone | 74 (95) | 2 (3) | 8 (44) | 9 (50) | 3 (75) | 1 (25) | 85 | 12 |
| Ceftazidime | 23 (29) | 17 (22) | 2 (11) | 1 (6) | 2 (50) | 0 (0) | 27 | 18 |
| Co-trimoxazole | 58 (74) | 0 (0) | 18 (100) | 0 (0) | 4 (100) | 0 (0) | 80 | 0 |
| Chloramphenicol | 36 (46) | 42 (54) | 14 (78) | 4 (22) | 2 (50) | 2 (50) | 52 | 48 |
| Ofloxacin | 15 (19) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 16 | 0 |
| Gentamicin | 33 (42) | 1 (1) | 4 (22) | 0 (0) | 3 (75) | 0 (0) | 40 | 1 |
| Nitrofurantoin | 1 (1) | 2 (3) | 2 (11) | 4 (22) | 1 (25) | 1 (25) | 4 | 7 |
R, resistant; I, intermediate.
Overall, the ESBLE faecal colonization prevalence was 23.2% (95% CI 19.1%–27.6%): 29.7% (95% CI 23.5%–36.5%) in VTE and 16.4% (95% CI 11.5%–22.4%) in VTP (P = 0.002). Variable colonization prevalence rates were observed between childcare facilities (Figure S1).
On univariable analysis, residence in VTE, foreign travel, antibiotic use in the preceding 3 months, higher maternal level of education and being in a childcare facility with a ‘good’ level of hygiene were all significantly associated with ESBLE colonization (Table S3). A significant association remained only for antibiotic use when a stepwise approach was used with the multivariate random-effects model, increasing the odds 2-fold (OR 2.11, 95% CI 1.21–3.68; n = 347 in the final model, stratified by childcare facility).
For the 77 successfully sequenced E. coli, we identified 33 different STs, 3 of which were novel. Common STs included: ST38 (11 isolates), ST131 (6), ST10 and ST48 (5 each); only 1 isolate was ST648 (accounting for 19% of clinical isolates in Vientiane previously).2 There was no significant difference in the distribution of STs by geographical location (P = 0.40). For the 18 K. pneumoniae, we identified 11 STs, 4 of which were novel. Only one ST was observed in both settings (ST34).
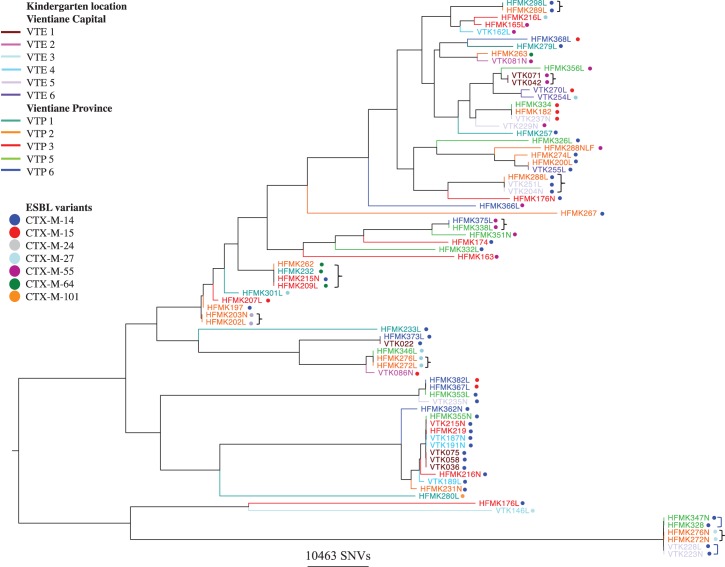
Some 141 042 variable sites were identified amongst the 77 mapped E. coli strains, with genetically identical clusters (no SNV differences) amongst isolates from children attending the same childcare facility as well as amongst those attending different childcare facilities (Figure 1). Amongst the 18 Klebsiella isolates, 269 956 variable sites were identified, with a high degree of relatedness only amongst isolates obtained from children in the same childcare facility (Figure S2).
Figure 1.
Phylogenetic relationships between ESBL-producing E. coli strains isolated from Lao children and associated ESBL gene variants. A curly, black bracket represents clusters of isolates with no SNVs between them and a square, blue bracket represents pairs of isolates with one SNV between them.
Amongst ESBL-producing E. coli, resistance was universally explained by blaCTX-M as follows: blaCTX-M-14 (36/77 isolates; 47%), blaCTX-M-15 (10/77; 13%), blaCTX-M-55 (13/77; 17%), blaCTX-M-27 (9/77; 12%), blaCTX-M-64 (5/77; 6%), blaCTX-M-24 (3/77; 4%) and blaCTX-M-101 (1/77; 1%). Amongst the K. pneumoniae isolates, 13/18 (72%) had blaSHV-2A and 5 had blaCTX-M-14 (28%). One Enterobacter sp. isolate contained blaCTX-M-63 and one blaSHV-2; in the third isolate, no class A ESBLs were detected.
Discussion
ESBLE are an emerging cause of infection in Vientiane,2 but there are no previous studies of gastrointestinal ESBLE colonization in Laos. In this study, a substantial proportion of healthy children ≤6 years (23%) were ESBLE colonized; colonization prevalence was twice as high in VTE (30%) versus VTP (16%). No CPE were identified, despite their detection in neighbouring countries.14,15
ESBL-producing E. coli and K. pneumoniae were the most common colonizers in our study (78% and 18% of isolates, respectively). CTX-M genes were the most prevalent mechanisms, with a similar distribution of variants in E. coli as found previously in local clinical samples,2 and some additional ones, including CTX-M-24, 64 and 101. By contrast, ESBL-producing K. pneumoniae contained only CTX-M-14 and SHV-2A variants. The genetic relatedness of some E. coli and K. pneumoniae strains within the same childcare facilities suggests localized transmission. Additionally, for ESBL-producing E. coli, genetically identical strains were shared between children attending different childcare facilities and in one instance between children attending childcare facilities in both VTP and VTE, suggesting a wide and fluid colonization reservoir.
Use of antibiotics in the 3 months prior to sampling was identified as the only risk factor that remained significantly associated with ESBLE colonization in the multivariate analysis, almost doubling the odds, consistent with other data.16,17 Concomitant antimicrobial resistance was common (Table 1) and may contribute to selection of ESBLE. Antibiotic selection pressures may be driven by a number of factors, including inappropriate self-medication, lack of prescribing regulations, substandard/falsified medicines containing antibiotics and agricultural use.
There are several limitations to our study. The sampling of a small number of childcare facilities may have introduced a ‘cluster effect’, given that ESBLE appear to be transmitted within these. We did, however, sample a highly divergent set of ESBL-producing E. coli/K. pneumoniae strains, as evidenced by the phylogenies. Childcare is not subsidized and the sampling may have been biased towards wealthier individuals, although 19% of participants came from households earning <65 USD/month. Our ESBLE screening method, selected for its practicability, probably lacks a degree of sensitivity for individuals colonized with small numbers of organisms and we may therefore have underestimated the colonization prevalence and strain diversity in our population. This may also have influenced our risk factor analysis, as preceding antibiotic use may be associated with an increased level of ESBLE colonization rather than ESBLE colonization per se. Finally, our WGS mapping-based approach would fail to recognize non-core genome genetic diversity and may inaccurately support a transmission hypothesis. However, similar approaches have been used to reliably assess transmission of hospital-associated pathogens18 and Mycobacterium tuberculosis in the community.19
Although there is some evidence that ESBLE have emerged relatively late in Laos compared with neighbouring countries,20 they are now well established in a healthy community reservoir, highlighting the need for ongoing surveillance and regulation of antimicrobial use. Treatment options for ESBLE infections in Laos are limited, given that carbapenems and nitrofurantoin are not currently available.
Funding
This work was supported by the Wellcome Trust of Great Britain, through funding for the Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit. N. S. is a Wellcome Trust/University of Oxford-funded doctoral research fellow. S. X. undertook part of this study as a project in fulfilment of the requirements of the Master in Tropical Medicine and International Health of IFMT. C. d. O. E. is funded by the UKCRC MMM Consortium. D. W. C. is part funded by the NIHR Oxford Biomedical Research Centre and is an NIHR Senior Investigator.
Transparency declarations
None to declare.
Supplementary data
Acknowledgements
We are grateful to Dr Sayaphet Rattanavong, all the staff of the kindergartens, the children and their parents, the Microbiology Laboratory and the Directors of Mahosot Hospital, the Minister of Health, the Directors of the Curative Department, Ministry of Health and the Ministry of Education and Sports, Government of the Lao PDR and the Agence Universitaire pour la Francophonie for their support.
We are very grateful to Dr Audrey Dubot-Pérès and Professor Xavier de Lamballerie for their help and for the advice of Professor David Livermore about the microbiological methods used in this study.
We would also like to thank the staff at the sequencing centre, Wellcome Trust Centre for Human Genetics, Oxford, UK and the Modernising Medical Microbiology software and bioinformatics team, University of Oxford, UK.
References
- 1.Woerther PL, Burdet C, Chachaty E, et al. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev 2013; 26: 744–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoesser N, Crook DW, Moore CE, et al. Characteristics of CTX-M ESBL-producing Escherichia coli isolates from the Lao People's Democratic Republic, 2004–09. J Antimicrob Chemother 2012; 67: 240–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNICEF. At a Glance: Lao People's Democratic Republic. http://www.unicef.org/infobycountry/laopdr_statistics.html.
- 4.Khennavong M, Davone V, Vongsouvath M, et al. Urine antibiotic activity in patients presenting to hospitals in Laos: implications for worsening antibiotic resistance. Am J Trop Med Hyg 2011; 85: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Water, Sanitation and Hygiene Standards for Schools in Low-cost Settings. Adams J, Bartram J, Chartier Y, et al. eds. 2009. http://www.who.int/water_sanitation_health/publications/wash_standards_school.pdf. [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-first Informational Supplement M100-S21. CLSI, Wayne, PA, USA, 2011. [Google Scholar]
- 7.Guindon S, Dufayard JF, Lefort V, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 2010; 59: 307–21. [DOI] [PubMed] [Google Scholar]
- 8.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 2008; 18: 821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gladman S, Seemann T. VelvetOptimiser. http://bioinformatics.net.au/software.velvetoptimiser.shtml. [Google Scholar]
- 10.Stoesser N, Batty EM, Eyre DW, et al. Predicting antimicrobial susceptibilities for Escherichia coli and Klebsiella pneumoniae isolates using whole genomic sequence data. J Antimicrob Chemother 2013; 68: 2234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirth T, Falush D, Lan R, et al. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 2006; 60: 1136–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diancourt L, Passet V, Verhoef J, et al. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 2005; 43: 4178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole JR, Wang Q, Cardenas E, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 2009; 37 (Database issue): D141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Zhou Z, Jiang Y, et al. Emergence of NDM-1-producing Acinetobacter baumannii in China. J Antimicrob Chemother 2011; 66: 1255–9. [DOI] [PubMed] [Google Scholar]
- 15.Rimrang B, Chanawong A, Lulitanond A, et al. Emergence of NDM-1- and IMP-14a-producing Enterobacteriaceae in Thailand. J Antimicrob Chemother 2012; 67: 2626–30. [DOI] [PubMed] [Google Scholar]
- 16.Grover SS, Sharma M, Chattopadhya D, et al. Phenotypic and genotypic detection of ESBL mediated cephalosporin resistance in Klebsiella pneumoniae: emergence of high resistance against cefepime, the fourth generation cephalosporin. J Infect 2006; 53: 279–88. [DOI] [PubMed] [Google Scholar]
- 17.Lautenbach E, Patel JB, Bilker WB, et al. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 2001; 32: 1162–71. [DOI] [PubMed] [Google Scholar]
- 18.Eyre DW, Golubchik T, Gordon NC, et al. A pilot study of rapid benchtop sequencing of Staphylococcus aureus and Clostridium difficile for outbreak detection and surveillance. BMJ Open 2012; 2: e001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker TM, Ip CL, Harrell RH, et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis 2013; 13: 137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phetsouvanh R, Phongmany S, Soukaloun D, et al. Causes of community-acquired bacteremia and patterns of antimicrobial resistance in Vientiane, Laos. Am J Trop Med Hyg 2006; 75: 978–85. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.