Abstract
Background:
Natural compounds including flavonoids like genistein (GE) are able to inhibit cell proliferation and induce apoptosis. GE is the main representative of these groups. GE inhibits carcinogenic tumors such as colon, stomach, lung, and pancreas tumors. The aim of the present study was to analyze the apoptotic effect of GE in the hepatocellular carcinoma (HCC) PLC/PRF5 cell line.
Methods:
Cells were treated with various doses of GE (1, 5, 10, 25, 50, 75, and 100 μM/L) at different times (24, 48, and 72 h) and the MTT assay was commonly used. Furthermore, cells were treated with single dose of GE (25 μM) at different times and flow cytometry was performed.
Results:
GE inhibited the growth of liver cancer cells significantly with a time- and dose-dependent manner. The percentage of living cells in GE treatment groups with a concentration of 25 μM at different times were 53, 48 and 47%, respectively (P < 0.001). Result of flow cytometry demonstrated that GE at a 25 μM concentration induces apoptosis significantly in a time-dependent manner. The percentage of apoptotic cells at different times were 44, 56, and 60%, respectively (P < 0.001).
Conclusions:
GE can significantly inhibit the growth of HCC cells and plays a significant role in apoptosis of this cell line.
Keywords: Apoptosis, genistein, hepatocellular carcinoma, proliferation
INTRODUCTION
Hepatocellular carcinoma (HCC), malignant hepatoma, is primary cancer of hepatocytes, main cell type of liver.[1] This disease account for 85–90% of liver cancer and its survival is 6–20 months.[2] HCC is considered as the eighth most frequently diagnosed cancer worldwide, and the third most frequent cause of cancer death[3] and annually causes about 600/000 death.[4] It is clinically asymptomatic until advanced stage and has a poor prognosis, low survival and high rate of recurrence.[5] The disease has not uniformly distribution.[6] There are three geographical areas including low, moderate, and high recurrence areas.[7] More than 80% of the cases have been reported in South Africa and West Asia.[8] The incidence of the disease is moderate in France, Germany and Great Britain and low prevalence in North and South America and North Europe.[6] There are large geographical correlation between the incidence of the disease and hepatitis B. Many factors such as age and sex can affect the prevalence of the disease[9] and morbidity of the men is more than the woman.[8] HCC is the sixth most common cancer in men and the eleventh most common cancer in women.[3] Cirrhosis is the final stage of chronic diffuse liver disease[10] which in 5% of patients lead to HCC.
The main causes of cirrhosis are alcohol, hepatitis B, and hepatitis C. Infection with HBV and HCV are the major risk factors of this disease.[11] Other risk factors include alcohol, tobacco, obesity, diabetes mellitus, inherited and metabolic disease. Among all natural compounds, flavonoids with anticancer activity have pharmacological activities such as antioxidant, anti-mutagenic, anti-bacterial, anti-angiogenic and anti-inflammatory effects. These compounds are often found in cereals such as soybeans and soybean products. Genistein (GE), a hydroxyisoflavone with a heterocyclic and diphenolic structure similar to estrogen, is the main representative of these groups.[12]
Genistein inhibits carcinogenic tumors such as colon, stomach, lung, and pancreas tumors (Andres et al., 2011). Ovarian cancer, breast, and prostate cancer in Asian countries where soy foods are high is lower than in Western countries[13] and also consumption of soya foods is associated with reduced risk of HCC. It is important to note that 30–40% of cancers are because of dietary choices[14,15] which are preventable by consumption of suitable fruits, soybean, and vegetables appropriate diets.[15,16] Natural compounds including flavonoids such as quercetin,[17] resveratrol, epigallocatechin gallate, lycopene, ursolic acid, isothiocyanates, perillyl alcohol,[18] and curcumin are able to inhibit cell proliferation and induce apoptosis. Other natural compounds with anticancer effect are apigenin, GE, and daidzein.[19] Quercetin has an antiproliferative effect on colon carcinoma (HCT-116 and HT-29) and mammary adenocarcinoma (MCF-7) cell lines[20,21] and also apigenin inhibits proliferation in MCF-7 cells and HL-60 cells.[22,23] GE has a preventive effect on breast cancer. GE induces apoptosis and inhibits growth of human leukemic MOLT-4 and HL-60 cells.[24,25] Numerous studies have demonstrated that intake of high flavonoids diet decreases colon cancer and breast cancer[26,27,28] and GE induces apoptosis in human pro-myelocytic HL-60 leukemic cells, prostate cancer (PCa, LNCGP, DU-145, PC-3) cells, and H-460 nonsmall lung cancer cells in a dose-dependent manner.[29,30,31] It has been indicated that soy isoflavones (GE, genistein, daidzein, and biochanin A) inhibit growth of murine (MB-49 and MBT-2) and human (HT-1376, UM-UC-3, RT-4, J-82 and TCCSUP) bladder cancer cell lines in a dose-dependent manner. Epidemiological studies have shown that prostate cancers in Asians is lower than Americans because of consumption of soy products.[32,33,34,35] More than 100 studies have indicated that GE has an inhibitory effect on various cancers such as melanoma, lung, leukemia, lymphoma, and bladder cancer.[36]
Apoptosis, programmed cell death and vital component of various processes, is characterized by distinct morphological changes that occur during apoptosis (Hacker, 2000) including shrinkage and pyknosis (chromatin condensation). During the process of apoptosis, plasma membrane blebbing occurs followed by karyorrhexis and separation of cell fragments into apoptotic bodies during a process called “budding” which these bodies consist of cytoplasm with packed organelles with or without a nuclear fragment.[37]
However, GE compound are obtained from vegetables, fruits, spices, teas, herbs, and medicinal plants, such as flavonoids, carotenoids, phenolic compounds, and terpenoids which high intake of these natural compounds decrease risk of human malignancies, including colon, breast, lung, laryngeal, pancreatic, bladder, stomach, esophageal, and oral cancers.[38,39]
Finally, epidemiological and experimental studies demonstrated that GE has a significant role in the prevention of cancers like breast cancer, prostate cancer, colon cancer, leukemia, melanoma, etc.[40] but only limited data are available to demonstrate the effects of GE on HCC cell lines such as Bel 7402 cells, herein, a study has reported that GE significantly inhibits the growth of Bel 7402 cells and induces apoptosis in this cell line but there isn’t any report about effect of GE on PLC/PRF5 cell line, therefore, we decided to investigate apoptotic effect of GE on PLC/PRF5 cell line.[41]
METHODS
Human HCC cells (PLC/PRF5) were purchased from the National Cell Bank of Iran-Pasteur Institute. GE, Dulbecco minimal essential medium (DMEM) and MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl -2H-tetrazolium bromide) were purchased from Sigma (Sigma, St. Louis, MO, USA). All other chemicals were obtained from the best sources available.
Cell culture
The cells were cultured in DMEM with pH 7.2-7.4 (Sigma) containing 1% sodium pyruvate (sigma), 3.7 mg/ml sodium bicarbonate (Sigma), 10% fetal bovine serum (sigma) and 1% antibiotics which include 10,000 units/ml penicillin G sodium (sigma), 10,000 μg/ml streptomycin sulfate and 25 μg/ml amphotericin B (sigma) at 37°C in 5% CO2 to promote attachment. When cells became > 80% confluent, 5 × 105 cells were seeded into 24-well plates (Becton-Dickinson) for 24 h in DMEM culture medium before they were incubated with certain concentrations of GE (1, 5, 10, 25, 50, 75, and 100 μM/L), which was dissolved in dimethyl sulfoxide (DMSO); DMSO was present at 0.01-0.3% in the medium based on IC50 index, at different times (24, 48, and 72 h). The control cells were treated with DMSO only. Photography was done for cultures before and after treatment with GE at different times using inverted microscope (Nikon, TE 2000-U, Japan).
Determination of IC50 value by MTT assay
The effect of GE on cellular proliferation was assessed by MTT assay according to standard protocols. After 24, 48, and 72 h of the treatment, the IC50 value for GE in PLC/PRF5 groups were determined. The MTT assay was commonly used to assess cell proliferation and viability by measuring the reduction of yellow MTT by mitochondrial dehydrogenases in viable cells. Briefly, 5 × 105 Cells were counted and placed into each well of a 24-well microplate and were treated with various drug concentrations (1, 5, 10, 25, 50, 75, and 100 μM/L) of GE for 24, 48, and 72 h and the MTT survival assay was then carried out for the evaluation of the cell viability with different drug concentrations. The cells were measured spectrophotometrically at 570 nm. All experiments were repeated 3 times, with at least three measurements (triplicates).
Determination of cell viability by MTT assay
To determine the effect of GE, the cells were seeded in triplicate in 24-well plates and treated with GE at a concentration of 25 μM in different period times (24, 48, and 72 h). Cell viability was estimated by a colorimetric assay based on the conversion of tetrazolium dye (MTT) to a blue formazan product. The absorbance of the cell lysates in DMSO solution was read at 570 nm by a microplate reader (Bio-Rad Hercules, CA, USA).
Determination of apoptotic cells by flow cytometry assay
The cells were seeded in 24-well plates. After 24 h, the medium was changed, and medium contains GE (25 μM) was added. After 24.48 and 72 h of incubation, all the adherent cells were collected with 0.05% trypsin, washed with cold phosphate-buffered saline (PBS) and resuspended in binding buffer (×1). After addition of Annexin V-FITC and propidium iodide (PI, Becton-Dickinson, San Diego, CA, USA), analysis was carried out according to the manufacturer's protocol (BMS500F1/100CE AnnexinV-FITC, eBiscience, USA). Finally, the apoptotic cells were counted by FACScan flow cytometry (Becton Dickinson, Heidelberg, Germany). All experiments were processed independently three times. A minimum of 5 × 105 cell/ml were analyzed for each sample.
RESULTS
Result of determination of IC50 by MTT assay
Cell vitality in the human HCC cell line was analyzed using the MTT assay as described previously. The result of MTT assay indicated that GE inhibits the growth of liver cancer cells significantly in all treatment groups except the control group (P < 0.02) [Figure 1]. The IC50s value for PLC/PRF5 cells were 25 μM of GE at different time periods. According to Figure 1, the percentage of living cells in treatment groups (24, 48, and 72 h) at a concentration of 25 μM were 51, 49, and 47%, respectively (P < 0.001). The effect of GE was dose- and time-dependent. This experiment was repeated three times for each group.
Figure 1.
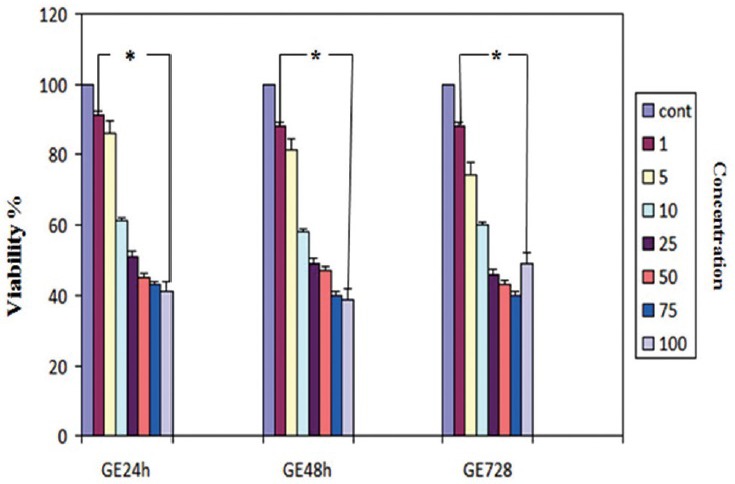
Effect of genistein (GE) on PLC/PRF5 human hepatocellular carcinoma cell proliferation. Cells were seeded in 24-well plates and allowed to attach overnight and then were treated with GE (1, 5, 10, 25, 50, 75, 100 μm/L) for 24, 48, and 72 h. Cell survival was determined by the MTT assay. Data are presented as mean ± standard error of the mean from at least three different experiments. Asterisks (*) indicate significant differences between treated cells and the control group. *P < 0.002 as compared to the control
Result of determination of cell viability by MTT assay
The cell vitality in the cells treated with GE at a concentration of 25 μM in different time periods was analyzed using the MTT assay. The amounts of reduced MTT in the all groups treated with GE were significantly lower than that of the control group (P < 0.001) and also in the 72 h treatment group than that of the other experiment groups (P < 0.001) but there isn’t any significant difference between 48 and 72 h treatment groups (P < 0.25). The percentage of living cells in treatment groups (24, 48, and 72 h) were 53%, 48%, 47%, respectively, at a concentration of 25 μM of GE. This experiment was repeated three times for each group [Figure 2].
Figure 2.
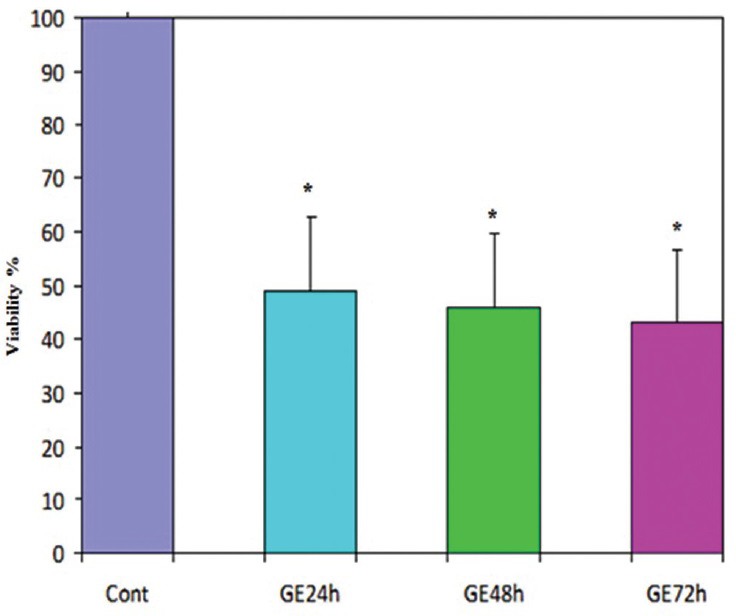
Effect of genistein (GE) at a concentration of 25 μm on cell viability of PLC/PRF5 cells. The effect of GE on the viability of PLC/PRF5 cells was determined by MTT assay at different time periods. Mean values from the three experiments ± standard error of the mean are shown. Asterisks (*) indicate significant differences between treated cells and the control group (*P, **P***P < 0.001) but there isn’t any significant difference between 48 and 72 h treatment groups (P < 0.25)
Result of determination of apoptotic cells by flow cytometry
The cells were treated with 25 μM concentration of GE for different times (24, 48 and 72 h). Flow cytometry was performed to observe the apoptotic cells which had been visualized using Annexin V-FITC and/or PI staining. Flow cytometry analysis indicated that GE at 25 μM concentration induces apoptosis in hepatocellular cancer cells in a time-dependent manner (P < 0.001). The amount of apoptotic cells was significantly increased in all three groups, but an apoptotic cell in the 72-h treatment group was more significant [Figure 3]. Percentage of apoptotic cells at different time periods (24, 48, and 72 h) were 44, 56, and 60%, respectively. Apoptotic effects were not observed in DMSO group.
Figure 3.
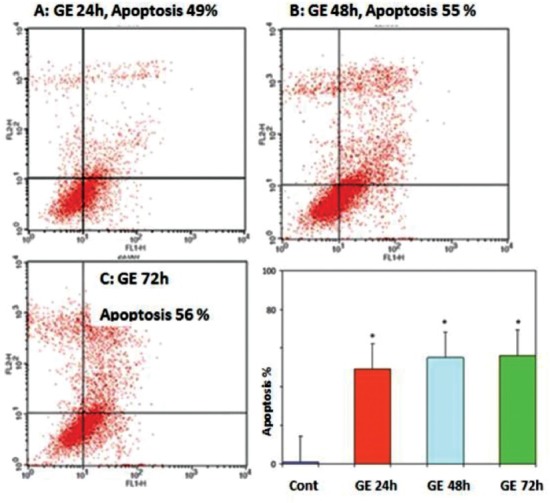
Effects of genistein (GE) on PLC/PRF5 cell apoptosis. The cells were treated with GE (25 μM) for 24, 48 and 72 h and the apoptosis-inducing effect of GE was investigated by flow cytometric analysis of PLC/PRF5 cells stained with Annexin V and propidium iodide. Results were obtained from three independent experiments and were expressed as mean ± standard error of the mean. P < 0.001, n = 3. (a) 24 h; (b) 48 h; (c) 72 h
DISCUSSION
Hepatocellular carcinoma is the most common primary malignancy of the liver and the eighth human cancer. The disease is a major complication of liver cirrhosis, and its growth rate is related to the prevalence of risk factors for chronic liver disease. In general, this disease due to a delay in diagnosis has weak pathogenesis.[1,2,3] The disease involves different molecular pathways that can affect the biological and etiologic behavior of the tumor.[4]
Genistein is a plant-derived estrogen-like compound. The precursor of this bioactive component derived from soy products, grains, nuts, and berries which becomes hormone-like compounds with estrogenic activity by intestinal bacteria.[42] The plant-derived estrogens have similar activity to beta-estradiol. It is well known that estrogen is involved in various types of cancer such as ovarian, prostate, breast, endometrial and colorectal cancers through different mechanisms that central to these mechanisms is the estrogen receptor (ER) to which estrogen bind.[43]
Our study clearly indicated that GE has a significant inhibitory effect on the growth of liver cancer cells and induces apoptosis in this cell line with a dose- and time-dependent manner.
Similarly, the same conclusion was reached by other researchers in other cancers; Chang KL et al., reported that GE inhibits proliferation and induces apoptosis in human prostate cancer[44] and also isoflavones inhibit growth of HT-29 and colo320 cell. Furthermore, other investigators reported that GE inhibits the growth of HCT 116 cells with a dose-dependent manner. This compound inhibits the growth of breast cancer cell lines ADA/MB231, MCF-7 and HBL-100 too.[45] Peterson and Barnes reported that high concentration of GE (50 or 100 μM) inhibits ER-positive breast cancer cell growth in the human (Peterson and Barnes 1991, 1996; Pagliacci et al. 1994; Chen et al. 2003). Many studies have shown that GE induces apoptosis at 50-100 μM concentrations (Pagliacci et al. 1994; 2003).
Lee et al. reported that GE can inhibit metastasis of cancer cells (Lee JY et al., 2012).[13] On the other hand, Zhang et al. and Chen et al. reported that consumption of foods rich in soy can reduce the incidence of ovarian cancer. The cancer rates are lower in the women with a high intake of GE (Zhang et al., 2004) which is agreement with the Myung's research that reported that Phytoestrogens reduce the risk of hormone-dependent tumors (Myung et al., 2009). Several other studies have demonstrated the inhibitory effect of GE on prostate cancer cells.[44,46,47] This compound with a concentration of 25 μM or higher concentration inhibits prostatic cancer cells.
These findings confirm our data obviously, but many studies have reported proliferative effects of GE that is opposite of our finding; it has been reported that GE induces apoptosis in the prostatic cancer LAPC-4 cells but has biphasic effect in the LNCap cell line. In fact, this compound has proliferative effect with physiological concentration (<10 μM) and inhibitory response with high concentration (25 or more than 25 μM).[48] Similar studies also have shown that a low dose of GE (3.7 μM) has a proliferative effect on human intestinal cells and inhibitory effect with 26-111 μM concentration. Moreover, it stimulates cell growth with <3.7 μM concentration in IEC18 cell line[39] and also stimulates cell growth in the ER-positive MCF-7 breast carcinoma cells with concentrations of 1 nM to 10 μM/L.[49]
It has demonstrated that estrogen has proliferative effect on human WRO, FRO, and ARO thyroid carcinoma cells and induces breast and uterus cancer development.[43,45] Collectively, GE induces significant growth with a low concentration (1 μM) and significant inhibition with a high concentration (25-100 μM). With regard to the estrogenic effect of GE, this compound probably acts through epigenetic mechanism although the mechanism is not fully understood.
The mechanism of estrogenic effects of GE is not fully understood although many pathways have been reported; Kim EJ et al. reported that GE inhibits proliferation and induces apoptosis in colon cancer cells (HT-29) by inhibition of insulin-like growth factor-1 receptor and the PI3k/AKT pathway,[50] also including the involvement of the MAPK pathway[51] which includes ERK-1/2 and AKT as key regulators.[52] It should be noted that isoflavones inhibit the growth of HT-29 and colo320 cell at the G2/M phase via this mechanism.[53] Other investigators reported that GE acts through ER (Setchell et al. 2005; Setchell and Clerici 2010, Barnes et al. 1996).[54] Chen et al. have shown that GE inhibits growth via ER which in response to 50-100 μM concentrations stops cell growth in the G2/M phase (Chen et al. 2003). This means that high concentrations of GE (>25 μM) inhibits ER-positive breast cancer cell growth. Similarly, Pike et al. also reported that phytoestrogens act as natural selective ER modulators, depending on the tissue and the presence of coregulator proteins (Pike et al., 1999). Besides, many studies have revealed that GE inhibits growth and proliferation of P47D breast cancer cells through signaling molecules associated with ER (ERK1/2, p90RSK, JNK, Akt, and NFκB) depending on the concentration.[55] Other investigators have reported that 90% of ovarian cancers arise from the epithelial layer which express ERα (Lee JY et al 2012).[13] In ovarian cancer, GE causes the cell cycle arrest at the G2/M phase. Some researchers have reported the relationship between GE and Bcl-2 family (Banerjee et al., 2008; Kyle et al., 1997; Spinozzi et al., 1994). This family is the most well-known apoptosis proteins which divided into apoptotic (Bcl-xL and Bcl-2) and pro-apoptotic (Bax, Bak, and Bad) groups. GE also induces apoptosis by inhibiting the NF-κB and Akt signaling pathways (Brunet et al., 1999; Cardone et al., 1998; Van Antwerp et al., 1996; Wu et al., 1996). Furthermore, tyrosine kinase inhibitor is one of the other mechanisms by which GE has an inhibitory effect on the growth of prostate cancer cells via ER.
In summary, the research about effects of GE on HCC is rare, a recent study has shown that GE (108 M) induces apoptosis in Hep3B cells were cultured in serum-free medium for 6 or 24 h but there is not any report about PLC/PRF5 cell, therefore, we selected this cell line and treated with GE (25 μM). However, based on data from many investigators, as mentioned above, GE is a plant-derived estrogen-like compound with estrogenic activity and biphasic effects that acts through ER.[56] It is important to note that GE had an apoptotic effect based on our data while other studies have reported that its effect is biphasic (inhibitory and stimulatory effects). ER acts as a tumor suppressor gene, and GE can increase expression of ER by molecular mechanisms related to apoptosis probably epigenetic pathways are involved in this process which need more researches. We did not perform enzyme activity assay related to methylation and histone modifications and also enzyme immunoassay related to protein levels, but we will perform in next researches and also further researches are needed to determine the clinical applications of GE.
CONCLUSIONS
Our findings suggest that GE may be a potent antiestrogenic compound and can effectively inhibit growth and induce apoptosis in PLC/PRF5 HCC cells. In future studies, the mechanisms and pathways of antiestrogenic effects of GE should be evaluated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Motola-Kuba D, Zamora-Valdés D, Uribe M, Méndez-Sánchez N. Hepatocellular carcinoma. An overview. Ann Hepatol. 2006;5:16–24. [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Poustchi H, Sepanlou S, Esmaili S, Mehrabi N, Ansarymoghadam A. Hepatocellular carcinoma in the world and the Middle East. Middle East J Dig Dis. 2010;2:31–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 5.Silva MF, Sherman M. Criteria for liver transplantation for HCC: What should the limits be? J Hepatol. 2011;55:1137–47. doi: 10.1016/j.jhep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Calvis D, Evert M, Dombrowski F. Review article pathogenetic and prognostic significants of interactication of RASSF proteins in human hepatocellular carcinoma. Mol Biol Int. 2012;5:1–9. doi: 10.1155/2012/849874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabibbo G, Craxi A. Epidemoilogy, risk factors and surveillance of hepatocellular carcinoma. Mol Biol Int. 2012;5:1–9. [PubMed] [Google Scholar]
- 8.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300–8. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelic S, Sotiropoulos GC, ESMO Guidelines Working Group Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v59–64. doi: 10.1093/annonc/mdq166. [DOI] [PubMed] [Google Scholar]
- 11.Franceschi S, Raza SA. Epidemiology and prevention of hepatocellular carcinoma. Cancer Lett. 2009;286:5–8. doi: 10.1016/j.canlet.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Alam S, Satpathy P, Thosar A. Plants and its parts as a source of anticancer compound: A review. Int Res J Pharm. 2014;5:244–50. [Google Scholar]
- 13.Lee JY, Kim HS, Song YS. Genistein as a potential anticancer agent against ovarian cancer. J Tradit Complement Med. 2012;2:96–104. doi: 10.1016/s2225-4110(16)30082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkar FH, Li Y, Wang Z, Padhye S. Lesson learned from nature for the development of novel anti-cancer agents: Implication of isoflavone, curcumin, and their synthetic analogs. Curr Pharm Des. 2010;16:1801–12. doi: 10.2174/138161210791208956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiseman M. The Second World Cancer Research Fund/American Institute for Cancer Research Expert Report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc Nutr Soc. 2008;67:253–6. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 16.Smith-Warner SA, Spiegelman D, Yaun SS, Albanes D, Beeson WL, van den Brandt PA, et al. Fruits, vegetables and lung cancer: A pooled analysis of cohort studies. Int J Cancer. 2003;107:1001–11. doi: 10.1002/ijc.11490. [DOI] [PubMed] [Google Scholar]
- 17.Kuno T, Tsukamoto T, Hara A, Tanaka T. Cancer chemoprevention through the induction of apoptosis by natural compounds. J Biophys Chem. 2012;3:156–73. [Google Scholar]
- 18.Pratheeshkumar P, Sreekala C, Zhang Z, Budhraja A, Ding S, Son YO, et al. Cancer prevention with promising natural products: Mechanisms of action and molecular targets. Anticancer Agents Med Chem. 2012;12:1159–84. doi: 10.2174/187152012803833035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdolmohammadi MH, Fouladdel SH, Shafiee A, Amin Gh, Ghaffari SM, Azizi E. Anticancer effects and cell cycle analysis on human breast cancer T47D cells treated with extracts of Astrodaucus persicus (Boiss) Drude in comparison to doxorubicin. DARU Journal of Pharmaceutical Sciences. 2008;16:112–9. [Google Scholar]
- 20.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007;18:427–42. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Ong CS, Tran E, Nguyen TT, Ong CK, Lee SK, Lee JJ, et al. Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in bad and hypophosphorylated retinoblastoma expressions. Oncol Rep. 2004;11:727–33. [PubMed] [Google Scholar]
- 22.Yin F, Giuliano AE, Law RE, Van Herle AJ. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001;21:413–20. [PubMed] [Google Scholar]
- 23.Wang IK, Lin-Shiau SY, Lin JK. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur J Cancer. 1999;35:1517–25. [PubMed] [Google Scholar]
- 24.Alexandrakis MG, Kyriakou DS, Kempuraj D, Huang M, Boucher W, Seretakis D, et al. The isoflavone genistein inhibits proliferation and increases histamine content in human leukemic mast cells. Allergy Asthma Proc. 2003;24:373–7. [PubMed] [Google Scholar]
- 25.Traganos F, Ardelt B, Halko N, Bruno S, Darzynkiewicz Z. Effects of genistein on the growth and cell cycle progression of normal human lymphocytes and human leukemic MOLT-4 and HL-60 cells. Cancer Res. 1992;52:6200–8. [PubMed] [Google Scholar]
- 26.Griffiths K, Hungin APS, De Meester F, Singh RB, Juneja LR. Nutrition and cancer: Dr.Douglas Wilson – Honoured. Open Nutraceuticals J. 2013;6:76–83. [Google Scholar]
- 27.Adlercreutz H, Fotsis T, Heikkinen R, Dwyer JT, Woods M, Goldin BR, et al. Excretion of the lignans enterolactone and enterodiol and of equol in omnivorous and vegetarian women and in women with breast cancer. Lancet. 1982;2:1295–9. doi: 10.1016/s0140-6736(82)91507-0. [DOI] [PubMed] [Google Scholar]
- 28.Adlercreutz H. Diet and cancer: Possible explanations for the higher risk of cancer in the poor. Gastroenterology. 1984;86:761–6. [Google Scholar]
- 29.Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein-induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer. 1998;32:123–31. doi: 10.1080/01635589809514730. [DOI] [PubMed] [Google Scholar]
- 30.Lian F, Bhuiyan M, Li YW, Wall N, Kraut M, Sarkar FH. Genistein-induced G2-M arrest, p21WAF1 upregulation, and apoptosis in a non-small-cell lung cancer cell line. Nutr Cancer. 1998;31:184–91. doi: 10.1080/01635589809514701. [DOI] [PubMed] [Google Scholar]
- 31.Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129:1628–35. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 32.Lee SA, Shu XO, Li H, Yang G, Cai H, Wen W, et al. Adolescent and adult soy food intake and breast cancer risk: Results from the Shanghai Women's Health Study. Am J Clin Nutr. 2009;89:1920–6. doi: 10.3945/ajcn.2008.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MK, Kim JH, Nam SJ, Ryu S, Kong G. Dietary intake of soy protein and tofu in association with breast cancer risk based on a case-control study. Nutr Cancer. 2008;60:568–76. doi: 10.1080/01635580801966203. [DOI] [PubMed] [Google Scholar]
- 34.Hirose K, Imaeda N, Tokudome Y, Goto C, Wakai K, Matsuo K, et al. Soybean products and reduction of breast cancer risk: A case-control study in Japan. Br J Cancer. 2005;93:15–22. doi: 10.1038/sj.bjc.6602659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonoda T, Nagata Y, Mori M, Miyanaga N, Takashima N, Okumura K, et al. A case-control study of diet and prostate cancer in Japan: Possible protective effect of traditional Japanese diet. Cancer Sci. 2004;95:238–42. doi: 10.1111/j.1349-7006.2004.tb02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suthar AC, Banavalikar MM, Biyani MK. Pharmacological activities of genistein, an isoflavone from soy (Glycine max): part I – anti-cancer activity. Indian J Exp Biol. 2001;39:511–9. [PubMed] [Google Scholar]
- 37.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishino H, Tokuda H, Satomi Y, Masuda M, Onozuka M, Yamaguchi S, et al. Cancer chemoprevention by phytochemicals and their related compounds. Asian Pac J Cancer Prev. 2000;1:49–55. [PubMed] [Google Scholar]
- 39.Nigam N, George J, Srivastava S, Roy P, Bhui K, Singh M, et al. Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B[a] P-induced mouse skin tumorigenesis. Cancer Chemother Pharmacol. 2010;65:687–96. doi: 10.1007/s00280-009-1074-x. [DOI] [PubMed] [Google Scholar]
- 40.Powis G, Hill SR, Frew TJ, Sherrill KW. Inhibitors of phospholipid intracellular signaling as antiproliferative agents. Med Res Rev. 1995;15:121–38. doi: 10.1002/med.2610150204. [DOI] [PubMed] [Google Scholar]
- 41.Gu Y, Zhu CF, Iwamoto H, Chen JS. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J Gastroenterol. 2005;11:6512–7. doi: 10.3748/wjg.v11.i41.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song RX, Santen RJ. Apoptotic action of estrogen. Apoptosis. 2003;8:55–60. doi: 10.1023/a:1021649019025. [DOI] [PubMed] [Google Scholar]
- 43.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–70. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang KL, Cheng HL, Huang LW, Hsieh BS, Hu YC, Chih TT, et al. Combined effects of terazosin and genistein on a metastatic, hormone-independent human prostate cancer cell line. Cancer Lett. 2009;276:14–20. doi: 10.1016/j.canlet.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 45.Fioravanti L, Cappelletti V, Miodini P, Ronchi E, Brivio M, Di Fronzo G. Genistein in the control of breast cancer cell growth: Insights into the mechanism of action in vitro. Cancer Lett. 1998;130:143–52. doi: 10.1016/s0304-3835(98)00130-x. [DOI] [PubMed] [Google Scholar]
- 46.Lee J, Ju J, Park S, Hong SJ, Yoon S. Inhibition of IGF-1 signaling by genistein: Modulation of E-cadherin expression and downregulation of β-catenin signaling in hormone refractory PC-3 prostate cancer cells. Nutr Cancer. 2012;64:153–62. doi: 10.1080/01635581.2012.630161. [DOI] [PubMed] [Google Scholar]
- 47.Seo YJ, Kim BS, Chun SY, Park YK, Kang KS, Kwon TG. Apoptotic effects of genistein, biochanin-A and apigenin on LNCaP and PC-3 cells by p21 through transcriptional inhibition of polo-like kinase-1. J Korean Med Sci. 2011;26:1489–94. doi: 10.3346/jkms.2011.26.11.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdalla A, Darwish AS, Elbanhawy R, Ghouraba A, Shehata S. Hepatocellular carcinoma: An overview of disease epidemiology and risk factors. Intern J Allied Med Sci Clin Res. 2014;2:205–9. [Google Scholar]
- 49.Chen AC, Donovan SM. Genistein at a concentration present in soy infant formula inhibits 50 caco-2BBe cell proliferation by causing G2/M cell cycle arrest1.Biochemical and molecular actions of nutrients. J Nutr. 2004;134:1303–8. doi: 10.1093/jn/134.6.1303. [DOI] [PubMed] [Google Scholar]
- 50.Kim EJ, Shin HK, Park JH. Genistein inhibits insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells: A possible mechanism of the growth inhibitory effect of Genistein. J Med Food. 2005;8:431–8. doi: 10.1089/jmf.2005.8.431. [DOI] [PubMed] [Google Scholar]
- 51.Kim EJ, Shin HK, Park JH. Genistein inhibits insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells: A possible mechanism of the growth inhibitory effect of Genistein. J Med Food. 2005;8:431–8. doi: 10.1089/jmf.2005.8.431. [DOI] [PubMed] [Google Scholar]
- 52.Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–39. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 53.Park JH, Oh EJ, Choi YH, Kang CD, Kang HS, Kim DK, et al. Synergistic effects of dexamethasone and genistein on the expression of Cdk inhibitor p21WAF1/CIP1 in human hepatocellular and colorectal carcinoma cells. Int J Oncol. 2001;18:997–1002. doi: 10.3892/ijo.18.5.997. [DOI] [PubMed] [Google Scholar]
- 54.Kwon Y. Effect of soy isoflavones on the growth of human breast tumors: Findings from preclinical studies. Food Sci Nutr. 2014;2:613–22. doi: 10.1002/fsn3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gwin J, Drews N, Ali S, Stamschror J, Sorenson M, Rajah TT. Effect of genistein on p90RSK phosphorylation and cell proliferation in T47D breast cancer cells. Anticancer Res. 2011;31:209–14. [PubMed] [Google Scholar]
- 56.Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, et al. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc Res. 2000;45:454–62. doi: 10.1016/s0008-6363(99)00359-4. [DOI] [PubMed] [Google Scholar]


