Abstract
Alzheimer’s disease (AD), an irreversible progressive neurodegenerative disease, causes characteristic cognitive impairment, and no curative treatments are currently available. Stem cell transplantation offers a powerful tool for the treatment of AD. We conducted a systematic review and meta-analysis of data from controlled studies to study the impact of stem cell biology and experimental design on learning and memory function following stem cell transplantation in animal models of AD. A total of 58 eligible controlled studies were included by searching PubMed, EMBASE, and Web of Science up to April 13, 2015. Meta-analysis showed that stem cell transplantation could promote both learning and memory recovery. Stratified meta-analysis was used to explore the influence of the potential factors on the estimated effect size, and meta-regression analyses were undertaken to explore the sources of heterogeneity for learning and memory function. Publication bias was assessed using funnel plots and Egger’s test. The present review reinforces the evidence supporting stem cell transplantation in experimental AD. However, it highlights areas that require well-designed and well-reported animal studies.
Alzheimer’s disease (AD), the leading cause of dementia, is a neurodegenerative disease characterized by a loss of neurons in the cerebral cortex and the hippocampus associated with the accumulation of amyloid-β plaques and neurofibrillary tangles, synaptic and neuronal loss, and cognitive decline1,2. In the United States, an estimated 5.2 million people have AD, and the number of people living with AD is expected to be 13.8 million by 20503. Worldwide, more than 35 million people have been suggested to suffer from AD today, with predictions that there could be more 125 million patients with AD by 20504. The increasing prevalence of Alzheimer’s disease represents a global challenge at personal, social, and economic levels5.Unfortunately, no effective therapeutic strategies are available today for AD to slow or stop the malfunction and death of neurons in the brain that cause AD symptoms and eventually make the disease fatal3,6,7.
Recently, with the advent of stem cell technologies and the ability to transform these cells into different types of central nervous system neurons and glial cells, the transplantation of stem cells, including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and tissue-derived stem cells such as bone marrow (BM)-and adipose-derived stem cells, has received considerable attention as a potential approach to treat various diseases, including AD8. There is now considerable preclinical literature on the possible benefits of stem cell transplantation against AD. Stem cell transplantation could lead to improvement in cognitive and memory performances and increased neuronal survival as a result of decreases in β-amyloid plaques, neurofibrillary tangles, neurodegeneration, and microglia activation in animal models of AD9,10,11,12. Moreover, neural stem cells could ameliorate the complex behavioral deficits associated with widespread AD pathology via BDNF13. In vitro and in vivo, the transplantation of bone marrow-derived mesenchymal stem cells (BM-MSCs) has been shown to ameliorate Aβ-induced neurotoxicity and cognitive decline by inhibiting apoptotic cell death and oxidative stress in the hippocampus12. It has also been demonstrated that human umbilical cord blood-derived mesenchymal stem cell (hUCB-MSCs) transplantation could rescue the impaired memory function in AD mice by reducing apoptosis and modulating oxidative stress14. Human amniotic epithelial cell (HAEC) transplantation could significantly ameliorate spatial memory deficits in transgenic mice as well as the increased acetylcholine levels and amount of hippocampal cholinergic neuritis15.
Although stem cells represent a strong candidate for treating AD in animal models, further studies are needed to determine the appropriate conditions to improve the therapeutic effects on AD, such as the ideal type of stem cell, the best source from which to harvest those stem cells for implantation, the number of cells needed, and the site of transplantation of the implanted cells. Moreover, to inform decisions regarding the design and execution of subsequent clinical trials, whether the magnitude of integrative and protective effects is large enough to be potentially clinically meaningful and whether reports of efficacy in animal models are potentially biased in favor of positive results also need to be investigated.
Therefore, we conducted a systematic review and meta-analysis of data from controlled studies to test the efficacy of stem cells as a treatment in animal models of AD. The aims of the current study were to (1) identify all animal experiments describing the efficacy of stem cell-based therapies in models of AD, (2) systematically review the literature describing the effect of stem cell-based therapies on cognitive impairment in animal models of AD, (3) perform a meta-analysis using the DerSimonian and Laird random effects model, (4) provide empirical evidence of the biological factors associated with greater efficacy, and (5) provide an assessment of the presence and impact of possible publication bias.
Methods
Search strategy
Using pre-specified inclusion and exclusion criteria (see below), we identified all publications reporting the efficacy of stem cells in an in vivo animal model of AD by searching three electronic databases (PubMed, EMBASE, and Web of Science; April 13, 2015) using the search strategy “(stem cell OR stem OR hematopoietic OR mesenchymal) AND (Alzheimer’s disease OR dementia OR senile dementia),” with various Boolean operators.
Searches of the databases using the search strategy were performed independently by two individuals. The bibliographies of relevant articles were used to identify further relevant publications. Abstracts were independently screened by two reviewers to identify those that met our inclusion criteria (see below), with differences resolved by discussion with a third reviewer.
Inclusion and Exclusion Criteria
We included studies in which learning and memory functional outcome in a group of animals with AD and treated with allogeneic or autologous stem cells was compared with learning and memory functional outcome in a control group of animals treated with placebo (saline, culture medium or similar vehicle). Labeling or transfection with markers for cellular tracing and imaging (e.g., green fluorescent protein, lacZ, bromodeoxyuridine, superparamagnetic iron oxide particles) was included.
We excluded studies using a co-treatment with another therapy or cell type. Studies that used concomitant injection with other cell types or adjuvant neuroprotective agents were also excluded.
To be included in the meta-analysis, outcomes must have been reported as a behavioral outcome, and the number of animals in each group, the mean effect size and variance must have been reported. Disagreements between investigators were resolved by consensus after discussion.
Data extraction
The following items were extracted by two investigators from each included study: reference details (publication year, and name); recipient animal (rat strain, sex); AD model; stem cells (donor species and tissue source); intervention regime (administration route and number of injections); and cognitive outcome assessments.
We extracted details of the individual study characteristics from each publication, and when a single publication reported more than 1 experiment, these data were extracted and treated as independent experiments. When neurobehavioral tests were performed serially, we only extracted data for the final time point.
In cases of missing data, we contacted the authors and requested the additional information. If data were expressed only graphically, numerical values were requested from the authors; if a response was not received, digital ruler software was used to estimate numerical values from the graphs. If the required data were not presented or obtainable, the study was excluded from the analysis.
Methodological quality of studies
We assessed the methodological quality of the included studies using the criteria based on a checklist as previously described with minor modifications16. The checklist was comprised of the following 17 items, (1) Was the research question specified and clear? (2) Were the outcome measures relevant for AD research? (3) Are the characteristics (species, background/generation, sex [and distribution], and age) of the study population clear? (4) Was the correct control group present? (5) Were the groups similar at baseline (if not randomized, were they similar with respect to characteristics such as weight and sex)? (6) Was the experiment randomized? (7) Was the type of stem cell(s) mentioned? (8) What was the age at which stem cell transplantation was started? (9) Was the duration of stem cell transplantation clear and specified? (10) Was the number of stem cells mentioned? (11) Was the administration route specified? (12) Were the methods used for outcome assessment the same in both groups? (13) Were the dropouts described for each group separately? (14) Was the outcome assessment blinded? (15) Was the outcome assessment randomized across the groups? (16) Was the total number of animals included in the statistical analyses clear? (17) Was the age at which the animals were sacrificed mentioned?
The quality of all studies was assessed independently by two reviewers. It should be noted that the quality assessment mainly assessed the quality of the reporting. Negative judgment did not necessarily indicate that the experiment was performed insufficiently; it indicated that there was inadequate information to assess the quality. (Table 3)
Statistical analysis
According to the Cochrane Handbook for Systematic Reviews of Interventions, the global estimated effect of statin treatment on cognitive outcome was determined by calculating the standardized mean difference (SMD) and 95% confidence intervals (CI) using a random effects model to avoid heterogeneity17. SMD is used as a summary statistic in meta-analyses when studies assess the same outcome but measure the outcome in a variety of ways (e.g., multiple studies measuring depression but using different psychometric scales). Within- and between-study variation or heterogeneity was assessed using Cochran’s Q-statistic18,19, with a significant Q-statistic (P < 0.10) indicating heterogeneity among studies. Heterogeneity was also assessed using the I2 statistic. Values of 25, 50 and 75% were considered to represent low, moderate and considerable heterogeneity, respectively20. For studies comparing different doses and/or times of drug administration with a single control group, we compared control group data with pooled data from all experimental groups.
Stratified meta-analysis was used to explore the influence of the potential factors on the estimated effect size21. Differences in mean effect sizes were assessed, partitioning heterogeneity using the χ2 distribution with n-1 degrees of freedom (df), and the significance level was set at P < 0.05. Meta-regression analyses were conducted to reveal potential sources of heterogeneity, as described in a previous study22. The meta-regression was univariate rather than multivariate, and we calculated adjusted R2 values to explain the proportion of the observed variability in the observed effect size for a group of experiments explained by variation in the independent variable in question16.
The presence of small effect sizes was investigated using funnel plots and Egger’s test. For Egger’s test, a P-value of < 0.10 was considered to indicate the presence of a small effect size18.
All statistical analyses were performed using Review Manager (version 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and Stata software (version 13.0, StataCorp, College Station, TX, USA).
Results
Study Selection
Our review identified 3710 publications; 58 met our pre-specified inclusion criteria. Of these, 12 studies were excluded due to inadequate reporting of the data necessary to calculate the summary effect measure outcome. Therefore, our meta-analysis was based on 46 publications, which included 52 comparisons of learning functions and 73 comparisons of memory functions (Fig. 1).
Figure 1. Flow diagram of the search process.
Description of Studies
Table S1 shows the characteristics of the included studies. A large variation existed in most study characteristics. Twenty-three studies were performed with transgenic mice, sixteen used Aβ-infused rats, four used age-induced Alzheimer’s model rats, twelve used chemically induced Alzheimer’s model rats, and four used surgery-induced AD models, including fimbria-fornix transaction23 and olfactory bulbectomy24. Babaei et al. employed two methods to establish their AD model25. Thirty-eight studies used only males, three studies used only females, five studies used both genders, and twelve studies did not mention which gender was used.
Mesenchymal stem cells (MSCs) (24 studies) derived from human umbilical cord blood, bone marrow, human placenta amniotic membrane, and human placenta were the most frequently investigated, followed by neural stem cells (NSCs, 23 studies). Stereotaxic transplantation (50 studies) was the most frequently reported route of administration. In addition, the water maze and Y-maze tests were the most frequently used measures of cognitive function. The number of cells injected ranged from 2 × 106 to (2–3) × 102.
Methodological quality of studies
The median quality score was 13 items of 20 (range 10–18). Although randomization, blinding, and description of the number of dropouts are key quality measures in the quality assessment of clinical trials, only 26 included studies randomly allocated the experimental units across the treatment groups. Eight studies described the number of dropouts per group. Moreover, the majority of included studies failed to clearly report the items, such as the age at which stem cell transplantation was started, the duration of stem cell transplantation, and the age at which the animals were sacrificed (Table S2).
Meta-analysis
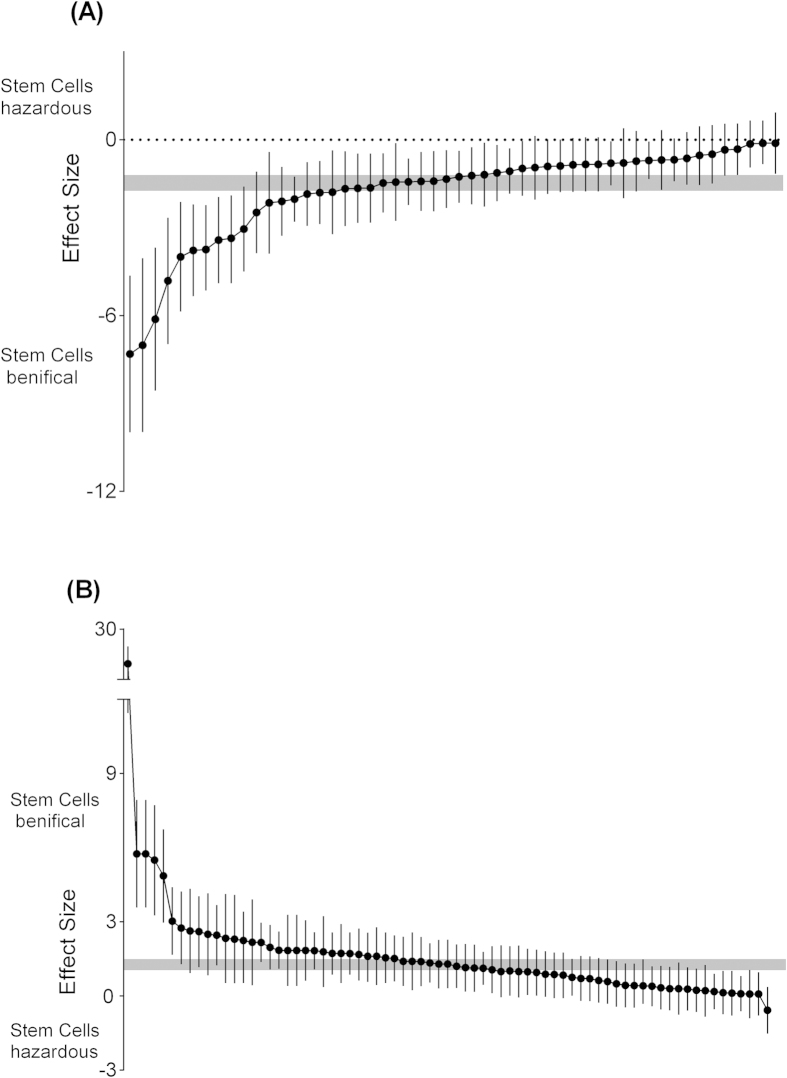
Fifty-two comparisons of 37 included studies10,12,14,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58 involving 1045 animals examined the effect of stem cell transplantation on the impaired learning function in animal models for AD using the Morris water maze test, Y-maze and the radial arm maze. The pooled analysis indicated that animals in the treatment group significantly improved in learning recovery more than animals in the control group (SMD = −1.47, 95% CI −1.76 to −1.20, P < 0.0001). There was evidence of moderate heterogeneity among studies (Chi2 = 175.5, df = 51 [P < 0.00001]; I2 = 71%) (Fig. 2A). Seventy-three comparisons of 36 included studies10,26,27,29,30,31,32,33,34,36,37,38,39,40,41,43,44,45,46,48,49,50,51,52,53,54,55,58,59,60,61,62,63,64,65,66 involving 1495 animals studied the effect of stem cell transplantation on the impaired memory function in animal models for AD using the Morris water maze test, Y-maze, novel object recognition task and single-trial passive avoidance test. The pooled analysis indicated that animals in the treatment group significantly improved in memory recovery relative to animals in the control group (SMD = 1.27, 95% CI 1.04 to 1.49, P < 0.0001). There was evidence of moderate heterogeneity among the studies (χ2 = 244.51, df = 72 [P < 0.00001]; I2 = 71%) (Fig. 2B).
Figure 2.
Summary of the data included in the meta-analysis of the use of stem cells to treat AD with individual comparisons ranked according to their effect on learning (A) and memory (B) function. The shaded gray bar represents the 95% confidence limits of the global estimate. The vertical error bars represent the 95% CIs for the individual estimates.
Stratified meta-analysis
In the stratified meta-analysis, the impact of study characteristics on the effect sizes was examined. For learning function, the stratified analysis showed that significant differences in effect sizes were observed relative to recipient species (P = 0.02), recipient sex (P = 0.0008), number of cells injected (P = 0.03), and quality scores (P < 0.0001). No significant differences in effect sizes were observed relative to the type of AD model (P = 0.1), donor species (P = 0.05), type of stem cells (P = 0.38), type of manipulation of stem cells prior to implantation (P = 0.91), and route of cell delivery (P = 0.30) (Table S3.1).
For memory function, the stratified analysis showed that significant differences in effect sizes were observed relative to the type of AD model (P = 0.01), recipient species (P = 0.007), recipient sex (P = 0.003), donor species (P = 0.004), number of cells injected (P = 0.008), and quality scores (P = 0.004). No significant differences in effect sizes were observed relative to the type of stem cells (P = 0.38), type of manipulation of the stem cells prior to implantation (P = 0.36), and route of cell delivery (P = 0.8) (Table S3.2).
Meta-regression
To further explore heterogeneity among the studies, meta-regression was conducted to investigate the effect of both continuous and categorical characteristics on learning and memory function.
For learning function, we found that the recipient species (Ad R2 = 3.72%) and the recipient sex (Ad R2 = 2.66%) accounted for a significant proportion of the between-study heterogeneity in studies (Table S3.1). For memory function, the type of AD model (Ad R2 = 28.35%), recipient species (Ad R2 = 16.96%), recipient sex (Ad R2 = 15.85%), donor species (Ad R2 = 26.22%), and number of cells injected (Ad R2 = 14.63%) were significant sources of heterogeneity (Table S3.2).
Publication bias
Finally, we sought to identify the presence of small study effects, which may have contributed to publication bias. Funnel plots showed asymmetry for both learning and memory function data, indicating the evidence of small study effects (Fig. 3A,B); (Egger regression, <0.0001 and <0.0001, respectively).
Figure 3. Funnel graph for the assessment of potential publication bias of learning.
(A) and memory (B) functions.
Discussion
Systematic reviews and meta-analyses of animal experiments not only allow for a more objective appraisal of the research evidence than is allowed by the traditional narrative reviews more commonly associated with animal research but also offer a sensible and rational approach to assessing the translational potential of promising experimental interventions before decisions are made to proceed with clinical trials67. Therefore, in this study, we intended to provide a detailed systematic review and meta-analysis of the animal literature describing stem cell-based therapy for AD.
Overall, our random-effects meta-analysis results suggested a beneficial effect of stem cell transplantation in experimental AD in terms of promoting learning and memory recovery and reinforced the evidence supporting stem cell-based therapy for experimental AD. For both learning and memory outcomes, there was a broad range of experimental approaches, reflected in the observed moderate heterogeneity. This is typical for systematic reviews in animal studies67,68 and validates our choice of a random effects model, and our summary estimates should be considered as the average efficacy rather than the best estimate of a single “true” efficacy16.
Concerning the study quality, our study quality checklist assesses aspects of both internal and external validity, and we found that the quality of most included studies was poor (13, interquartile range 12–14) and that the global estimated effect may be overestimated because many failed to report blinded and randomized assessments of outcome or describe drop-outs, which are important elements that are generally required in reports of preclinical studies69. In addition, we found that higher study quality was associated with lower efficacy for learning and memory functions. This association is consistent with the preclinical data for many other disease therapies, such as stroke and traumatic brain injury, in which higher study quality has repeatedly been associated with lower efficacy70,71. This association also decreases the confidence in their therapeutic translational potential. However, the overall quality scores accounted for a significant proportion of between-study heterogeneity.
The stratified analysis results showed that studies that did not use transgenic mouse models or the Aβ infusion models were associated with significantly larger effects. AD transgenic mouse models are the most widely used animal models for AD and have yielded significant research breakthroughs and Aβ infusion models have been a useful complement to transgenic approaches to AD neuropathology72. We found that the studies that used the fimbria-fornix lesion model showed larger behavioral gains. Of course, these results should be interpreted with caution due to the small sample sizes within the subgroups.
For both learning and memory function, these correlations seem to be the influence of high effect sizes reported in studies in which the sex of the animals used was male and in which neural precursor cells (NPCs) were transplanted into the AD model. In addition, efficacy was higher in the studies in which stem cells were delivered stereotaxically to the lateral ventricle, hippocampus, basal forebrain and cerebral cortex, rather than systematically (including intravenous and intracardiac transplantation). This observation suggests that the local implantation of stem cells into the brain may directly mediate AD pathology in animal models and that the blood-brain barrier (BBB) may mask the benefit provided by stem cells delivered intravenously or intracardially73. In addition, studies that used rats as recipient species and donor species led to significantly higher estimates of effect. It should be noted that studies in the subgroup analysis used allogeneic or xenogeneic grafts.
We did not find a dose-response relationship. Subgroup analyses revealed that transplantation of the median number of cells ([1–5] × 105) appeared to have a stronger impact on learning and memory function outcomes. There may have been no dose–response effect, or the doses used in these experiments may have all been large enough to generate maximal responses. When the dose response was formally studied, the authors also found no dose-response effect52.
The genetic modification of stem cells prior to transplantation can increase cell survival and make them more effective74,75, and modified cells could be used for the delivery of factors that can ameliorate neurological disorders76. Moreover, studies that used stem cells that over-express different neurotrophic factors, such as BDNF, acetylcholine (Ach), and nerve growth factor (NGF), found that the delivery of these genetically modified stem cells to animal models of AD is safe and effective to restore learning and memory functions, thus suggesting that stem cell-based gene therapy may be a promising treatment for AD29,53,57,58,77. However, we did not observe that prior gene modification of the implanted cells was associated with larger effects. The underlying mechanism responsible for this should also be investigated in greater depth in the future.
This study has several limitations, which are also observed in previous systematic reviews of animal studies78,79,80. First, the publication bias should be considered. Our search strategy was designed to be exhaustive to identify all potentially relevant published and unpublished studies, and our analysis was only able to include the majority of published studies in this field. The funnel plots and Egger’s test suggested the possibility of publication bias or other small study biases that affected the outcomes67. Second, extracting multiple pieces of information from a single publication has the potential to introduce bias into systematic reviews because we have observed the experiments of others and have not conducted experiments of our own; thus, this observational research should be considered to be hypothesis-generating only16. Third, we present a series of univariate analyses in which meta-regression might provide more robust insights, but these techniques are not well established; additionally, this analysis might represent an oversimplification, at least for some independent variables16. Finally, we limited our analysis to neurobehavioral outcomes, and functional outcome, in combination with histopathology, may be just as important in terms of assessing potential neuroprotective drugs81. It is thus worthy of further exploration.
As mentioned in our previous systematic reviews67,82, to improve the transition from animal experiments to human clinical trials, researchers are strongly recommended to consult and follow the ARRIVE guidelines83,84 when designing studies and to report full methodological details to allow others to reproduce and validate their results and enable more accurate reviews and meta-analyses. Second, due to the risks of tumorigenesis85, further studies are needed to investigate the safety of stem cell transplantation. Third, despite the fact that the gene modification of stem cells prior to transplantation exhibits great potential for the treatment of AD, some major problems remain to be overcome. For example, the more specific and extensive the genetic modification, the longer the stem cells must remain in vitro. Finally, the stem cells themselves have therapeutic effects; however, the sustained effects of stem cells require the long-term survival of transplanted cells, and determining the appropriate conditions to improve the therapeutic effects for AD pathology is required86.
Conclusions
The present systematic review and meta-analysis would seem to suggest that stem cells have substantial beneficial effects on learning and memory recovery in animal models of AD. However, without rigorous, robust and detailed pre-clinical evaluation, the results should be interpreted in light of the known limitations in animal experimental design and methodological quality. Therefore, more well-designed and well-reported experimental animal studies are needed.
Additional Information
How to cite this article: Wang, Z. et al. Effects of stem cell transplantation on cognitive decline in animal models of Alzheimer's disease: A systematic review and meta-analysis. Sci. Rep. 5, 12134; doi: 10.1038/srep12134 (2015).
Supplementary Material
Acknowledgments
This work was financially supported by the Young Scientists Fund of the National Natural Science Foundation of China (Grant No. 81303074, 81403259), the National Natural Science Foundation of China (Grant No.8137370), Natural Science Foundation of Hunan Province (Grant No. 13JJ3030) and the special project of high-tech industrial development of Hunan Province (Grant No. 2012SK3212).
Footnotes
Author Contributions The work presented here was carried out in collaboration between all authors. W.P. and Z.W. conceived, designed of the work, analyzed the data, interpreted the results, and drafted the manuscript. C.S., Y.W. and C.Z. carried out the literatures research, and participated in the acquisition, analysis, or interpretation of data, W.H. and R.F. co-worked on associated data collection, analyzed the data and interpreted the results. All authors reviewed and approved the final manuscript.
References
- Stygelbout V. et al. Inositol trisphosphate 3-kinase B is increased in human Alzheimer brain and exacerbates mouse Alzheimer pathology. Brain 137, 537–552 (2014). [DOI] [PubMed] [Google Scholar]
- Gjoneska E. et al. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature 518, 365–369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s A. 2014 Alzheimer’s disease facts and figures. Alzheimers Dement 10, e47–92 (2014). [DOI] [PubMed] [Google Scholar]
- Wimo A., Winblad B. & Jonsson L. The worldwide societal costs of dementia: Estimates for 2009. Alzheimers Dement 6, 98–103 (2010). [DOI] [PubMed] [Google Scholar]
- Karran E. & Hardy J. Antiamyloid therapy for Alzheimer’s disease-are we on the right road? N Engl J Med 370, 377–378 (2014). [DOI] [PubMed] [Google Scholar]
- Liu Y. H. et al. Immunity and Alzheimer’s disease: immunological perspectives on the development of novel therapies. Drug Discov Today 18, 1212–1220 (2013). [DOI] [PubMed] [Google Scholar]
- Tsai L. H. & Madabhushi R. Alzheimer’s disease: A protective factor for the ageing brain. Nature 507, 439–440 (2014). [DOI] [PubMed] [Google Scholar]
- Li M., Guo K. & Ikehara S. Stem Cell Treatment for Alzheimer’s Disease. Int J Mol Sci 15, 19226–19238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., Jin H. K. & Bae J. S. Bone Marrow-Derived Mesenchymal Stem Cells Attenuate Amyloid beta-Induced Memory Impairment and Apoptosis by Inhibiting Neuronal Cell Death. Current Alzheimer Research 7, 540–548 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang W., Wang G. M., Wang P. J., Zhang Q. & Sha S. H. Effects of neural stem cells on synaptic proteins and memory in a mouse model of Alzheimer’s disease. J Neurosci Res 92, 185–194 (2014). [DOI] [PubMed] [Google Scholar]
- Malm T. M. et al. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis 18, 134–142 (2005). [DOI] [PubMed] [Google Scholar]
- Lee J. K., Jin H. K. & Bae J. S. Bone marrow-derived mesenchymal stem cells attenuate amyloid beta-induced memory impairment and apoptosis by inhibiting neuronal cell death. Curr Alzheimer Res 7, 540–548 (2010). [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M. et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA 106, 13594–13599 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J. et al. The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer’s disease. Neurosci Lett 481, 30–35 (2010). [DOI] [PubMed] [Google Scholar]
- Xue S. R. et al. Intracerebroventricular transplantation of human amniotic epithelial cells ameliorates spatial memory deficit in the doubly transgenic mice coexpressing APPswe and PS1DeltaE9-deleted genes. Chin Med J (Engl) 124, 2642–2648 (2011). [PubMed] [Google Scholar]
- Antonic A. et al. Stem cell transplantation in traumatic spinal cord injury: a systematic review and meta-analysis of animal studies. PLoS Biol 11, e1001738 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bonilla L. et al. Evidence for the efficacy of statins in animal stroke models: a meta-analysis. J Neurochem 122, 233–243 (2012). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks H. S., Berrier J., Reitman D., Ancona-Berk V. A. & Chalmers T. C. Meta-analyses of randomized controlled trials. N Engl J Med 316, 450–455 (1987). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterinen H. M. et al. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 221, 92–102 (2014). [DOI] [PubMed] [Google Scholar]
- Liu J. B. et al. Association of vitiligo with HLA-A2: a meta-analysis. J Eur Acad Dermatol Venereol 21, 205–213 (2007). [DOI] [PubMed] [Google Scholar]
- Gu H. G. et al. Effect of neural stem cells transplantation on parvalbumin-positive neurons of the basal forebrain and abilities of learning and memory in a rat model of senile dementia. CRTER 12, 2235–2239 (2008). [Google Scholar]
- Bobkova N. V., Poltavtseva R. A., Samokhin A. N. & Sukhikh G. T. Therapeutic effect of mesenchymal multipotent stromal cells on memory in animals with Alzheimer-type neurodegeneration. Bull Exp Biol Med 156, 119–121 (2013). [DOI] [PubMed] [Google Scholar]
- Babaei P., Soltani Tehrani B. & Alizadeh A. Transplanted bone marrow mesenchymal stem cells improve memory in rat models of Alzheimer’s disease. Stem Cells Int 2012, 369417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Menachem-Zidon O., Ben-Menahem Y., Ben-Hur T. & Yirmiya R. Intra-hippocampal transplantation of neural precursor cells with transgenic over-expression of IL-1 receptor antagonist rescues memory and neurogenesis impairments in an Alzheimer’s disease model. Neuropsychopharmacology 39, 401–414 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Q. et al. Neural stem cell transplantation improves spatial learning and memory via neuronal regeneration in amyloid-beta precursor protein/presenilin 1/tau triple transgenic mice. Am J Alzheimers Dis Other Demen 29, 142–149 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N. et al. Restoration of spatial memory dysfunction of human APP transgenic mice by transplantation of neuronal precursors derived from human iPS cells. Neurosci Lett (2013). [PubMed] [Google Scholar]
- Gu G., Zhang W., Li M., Ni J. & Wang P. Transplantation of NSC-derived cholinergic neuron-like cells improves cognitive function in APP/PS1 transgenic mice. Neuroscience 291, 81–92 (2015). [DOI] [PubMed] [Google Scholar]
- Gu H. G. et al. [Effect of neural stem cells transplantation on parvalbumin-positive neurons of the basal forebrain and abilities of learning and memory in a rat model of senile dementia]. CRTER 12, 2235–2239 (2008). [Google Scholar]
- Kim K. S. et al. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol Aging 34, 2408–2420 (2013). [DOI] [PubMed] [Google Scholar]
- Lee H. J. et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol Aging 33, 588–602 (2012). [DOI] [PubMed] [Google Scholar]
- Lee J. K. et al. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells 28, 329–343 (2010). [DOI] [PubMed] [Google Scholar]
- Lee J. K., Schuchman E. H., Jin H. K. & Bae J. S. Soluble CCL5 derived from bone marrow-derived mesenchymal stem cells and activated by amyloid beta ameliorates Alzheimer’s disease in mice by recruiting bone marrow-induced microglia immune responses. Stem Cells 30, 1544–1555 (2012). [DOI] [PubMed] [Google Scholar]
- Li W. Y., Jin R. L. & Hu X. Y. [Migration of PKH26-labeled mesenchymal stem cells in rats with Alzheimer’s disease]. Journal of Zhejiang University. Medical sciences 41, 659–664 (2012). [DOI] [PubMed] [Google Scholar]
- Li X. S. et al. [Effects of human amnion membrane mesenchymal stem cell transplantation on behavior and beta-amyloid protein changes in transgenic mice with Alzheimer’s desease]. CRTER 12, 10068–10072 (2008). [Google Scholar]
- Li Z. et al. Neurotransmitter phenotype differentiation and synapse formation of neural precursors engrafting in amyloid-beta(1-40) injured rat hippocampus. J Alzheimers Dis 21, 1233–1247 (2010). [DOI] [PubMed] [Google Scholar]
- Li. Z. F. et al. [Differentiation and therapeutic effects of C17.2 neural stem cells after transplanted into hippocampus of Aβ1-40-injuried rats]. Acta Academiae Medicinae Militaris Tertiae 30, 595–599 (2008). [Google Scholar]
- Ma T. et al. Intracerebral transplantation of adipose-derived mesenchymal stem cells alternatively activates microglia and ameliorates neuropathological deficits in Alzheimer’s disease mice. Cell Transplant 22 Suppl 1, S113–126 (2013). [DOI] [PubMed] [Google Scholar]
- Moghadam F. H. et al. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation 78, 59–68 (2009). [DOI] [PubMed] [Google Scholar]
- Pan X. b. et al. Influence of neural stem cell transplantation on the number of p75NGFR positive neurons of the basal forebrain and the ethology of an animal model of Alzheimer’s disease with 192-IgG-saporin. CRTER 14, 8426–8430 (2010). [Google Scholar]
- Shen X. L., Zhang S. M., Cui M. Y. & Jia L. H. Effect of neural stem cell transplantation on cognitive function and oxidative stress in dementia rats. Progress of Anatomical Sciences 17, 304–307 (2011). [Google Scholar]
- Tang J. et al. Embryonic stem cell-derived neural precursor cells improve memory dysfunction in Abeta(1-40) injured rats. Neurosci Res 62, 86–96 (2008). [DOI] [PubMed] [Google Scholar]
- Wang Z., Ren X. Q. & Wei D. X. [Hippocampus transplantation of bone marrow mesenchymal stem cells improves the memory function of Alzheimer’s disease rats]. CRTER 18, 8130–8134 (2014). [Google Scholar]
- Wu S. et al. Neural stem cells improve learning and memory in rats with Alzheimer’s disease. Pathobiology 75, 186–194 (2008). [DOI] [PubMed] [Google Scholar]
- Xuan A. G., Luo M., Ji W. D. & Long D. H. Effects of engrafted neural stem cells in Alzheimer’s disease rats. Neurosci Lett 450, 167–171 (2009). [DOI] [PubMed] [Google Scholar]
- Xue S. et al. Therapeutic effects of human amniotic epithelial cell transplantation on double-transgenic mice co-expressing APPswe and PS1DeltaE9-deleted genes. Sci China Life Sci 55, 132–140 (2012). [DOI] [PubMed] [Google Scholar]
- Yang C., Zhang Q., Wang S. C., Qiao P. & Zhang Z. [Neural stem cells transplantation improved learning and memory abilities in Alzheimer’s disease rat]. Chinese J Appl Physiol 23, 159–161 (2007). [PubMed] [Google Scholar]
- Yang H. et al. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AbetaPP/PS1 transgenic mouse model. Stem Cell Res Ther 4, 76 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Yang H., Xie Z., Wei L. & Bi J. Systemic transplantation of human umbilical cord derived mesenchymal stem cells-educated T regulatory cells improved the impaired cognition in AbetaPPswe/PS1dE9 transgenic mice. PLoS One 8, e69129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. et al. Intravenous Administration of Human Umbilical Cord Mesenchymal Stem Cells Improves Cognitive Impairments and Reduces Amyloid-Beta Deposition in an AbetaPP/PS1 Transgenic Mouse Model. Neurochem Res 38, 2474–2482 (2013). [DOI] [PubMed] [Google Scholar]
- Yun H. M. et al. Placenta-derived mesenchymal stem cells improve memory dysfunction in an Abeta1-42-infused mouse model of Alzheimer’s disease. Cell Death Dis 4, e958 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Zhao G., Kang X. & Su L. Effects of lateral ventricular transplantation of bone marrow-derived mesenchymal stem cells modified with brain-derived neurotrophic factor gene on cognition in a rat model of Alzheimer’s disease. Neural Regen Res 7, 245–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. et al. Neural stem cell transplantation enhances mitochondrial biogenesis in a transgenic mouse model of Alzheimer’s disease-like pathology. Neurobiol Aging 36, 1282–1292 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. Neural stem cell transplants improve cognitive function without altering amyloid pathology in an APP/PS1 double transgenic model of Alzheimer’s disease. Mol Neurobiol 50, 423–437 (2014). [DOI] [PubMed] [Google Scholar]
- Zhao Z., Hu H. & Feng G. [Learning and memory amelioration of transplantation of the neural stem cells modified with human brain-derived neurotrophic factor gene on Alzheimer disease model rat]. Chinese journal of reparative and reconstructive surgery 19, 331–334 (2005). [PubMed] [Google Scholar]
- Lee H. J. et al. Human neural stem cells genetically modified to express human nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplant 21, 2487–2496 (2012). [DOI] [PubMed] [Google Scholar]
- Park D. et al. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp Neurol 234, 521–526 (2012). [DOI] [PubMed] [Google Scholar]
- Esmaeilzade B. et al. Delivery of epidermal neural crest stem cells (EPI-NCSC) to hippocamp in Alzheimer’s disease rat model. Iran Biomed J 16, 1–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia K. O. et al. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front Aging Neurosci 6, 30 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Bo H., Mu X. H., Zhang L. & Li H. S. Effects of human bone marrow mesenchymal stem cell transplantation on cognitive ability and hippocampus ultrastructure in Alzheimer’s disease rats. CRTER 14, 7453–7457 (2010). [Google Scholar]
- Li H. S., Chen Z. F. & Li H. Y. Effects of bone marrow mesenchymal stem cell transplantation on ethology of rats with alzheimer’s disease. CRTER 13, 9659–9662 (2009). [Google Scholar]
- Park D. et al. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant 21, 365–371 (2012). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest 53, 61–69 (2006). [DOI] [PubMed] [Google Scholar]
- Wu Q. Y., Li J., Feng Z. T. & Wang T. H. Bone marrow stromal cells of transgenic mice can improve the cognitive ability of an Alzheimer’s disease rat model. Neurosci Lett 417, 281–285 (2007). [DOI] [PubMed] [Google Scholar]
- Yan Y. et al. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen Res 9, 798–805 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W. et al. The efficacy of erythropoietin in treating experimental traumatic brain injury: a systematic review of controlled trials in animal models. J Neurosurg 121, 653–664 (2014). [DOI] [PubMed] [Google Scholar]
- Liao Y., Zhang X. L., Li L., Shen F. M. & Zhong M. K. Stem cell therapy for bone repair: a systematic review and meta-analysis of preclinical studies with large animal models. Br J Clin Pharmacol 78, 718–726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw J. M., Warlow C. P., Sandercock P. A., Dennis M. S. & Lindley R. I. Neuroprotection disappointment yet aGAIN. Lancet 356, 597 (2000). [DOI] [PubMed] [Google Scholar]
- O’Collins V. E. et al. 1,026 experimental treatments in acute stroke. Ann Neurol 59, 467–477 (2006). [DOI] [PubMed] [Google Scholar]
- Peng W. et al. Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Res Ther 6, 47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak D. S. Animal models of Alzheimer’s disease: therapeutic implications. J Alzheimers Dis 15, 507–521 (2008). [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M. et al. Neural stem cells genetically-modified to express neprilysin reduce pathology in Alzheimer transgenic models. Stem Cell Res Ther 5, 46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. I. & Tang Y. L. Genetic modification of stem cells for transplantation. Adv Drug Deliv Rev 60, 160–172 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. I. & Tang Y. Genetic modification of stem cells for cardiac, diabetic, and hemophilia transplantation therapies. Prog Mol Biol Transl Sci 111, 285–304 (2012). [DOI] [PubMed] [Google Scholar]
- Glat M. J. & Offen D. Cell and gene therapy in Alzheimer’s disease. Stem Cells Dev 22, 1490–1496 (2013). [DOI] [PubMed] [Google Scholar]
- Park D. et al. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol Aging 34, 2639–2646 (2013). [DOI] [PubMed] [Google Scholar]
- Ker K., Perel P. & Blackhall K. Beta-2 receptor antagonists for traumatic brain injury: a systematic review of controlled trials in animal models. CNS Neurosci Ther 15, 52–64 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perel P. et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ 334, 197 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton P., Mathias J. L. & Vink R. Impact of pharmacological treatments on outcome in adult rodents after traumatic brain injury: a meta-analysis. J Psychopharmacol 25, 1581–1599 (2011). [DOI] [PubMed] [Google Scholar]
- STAIR. Recommendations for Standards Regarding Preclinical Neuroprotective and Restorative Drug Development. Stroke 30, 2752–2758 (1999). [DOI] [PubMed] [Google Scholar]
- Peng W. et al. Impact of statins on cognitive deficits in adult male rodents after traumatic brain injury: a systematic review. Biomed Res Int 2014, 261409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Davies K., Lehn P. & Mulligan R. The ARRIVE guidelines, a welcome improvement to standards for reporting animal research. J Gene Med 12, 559–560 (2010). [DOI] [PubMed] [Google Scholar]
- Kilkenny C., Browne W. J., Cuthill I. C., Emerson M. & Altman D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8, e1000412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees J. S. et al. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. Int J Stroke 7, 582–588 (2012). [DOI] [PubMed] [Google Scholar]
- Choi S. S., Lee S. R., Kim S. U. & Lee H. J. Alzheimer’s disease and stem cell therapy. Exp Neurobiol 23, 45–52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.