How do individual neurons develop and how are they integrated into neuronal circuitry? To answer this question is essential to understand how the nervous system develops and how it is maintained during the adult life. A neural stem cell must go through several stages of maturation, including proliferation, migration, differentiation, and integration, to become fully embedded to an existing neuronal circuit. The knowledge on this topic so far has come mainly from cell culture studies. Studying the development of individual neurons within intact neuronal networks in vivo is inherently difficult. Most neurons are generated form neural stem cells during embryonic and early postnatal development. In the adult brain only two major neurogenic zones persist: the subventricular zone of the lateral ventricles and the subgranular zone of the dentate gyrus (Gage, 2000). While subventricular zone-derived cells migrate to the olfactory bulb (OB) where they differentiate into at least two types of GABAergic neurons, subgranular zone-derived cells are integrated into the dentate gyrus of the hippocampus. Following damage in the adult brain, neurogenesis is upregulated in particular in the subventricular zone, showing that the adult brain attempts to repair itself. Neural precursors then migrate to the site of damage, but newly generated neurons generally fail to integrate into neuronal circuitry at the injury sites and eventually die (Christie and Turnley, 2013). The challenge of the next future will be to find ways to increase the survival rate of newborn neurons and to promote their integration into functional neuronal networks.
In addition to the subventricular and the subgranular zone, a lifelong neuronal turnover takes place also in the olfactory epithelium (OE). In order to maintain the sense of smell, new olfactory receptor neurons (ORNs) are continuously integrated into the olfactory circuit. This is necessary because of the relatively exposed position of the ORNs in the OE. Olfactory receptor neurons can be easily damaged either by exposure to toxic chemicals, infections, pollutants or trauma. Neural stem cells located close to the basal lamina of the OE, so-called basal cells, maintain the lifelong generation of new ORNs (Schwob, 2002). The first synapse in the olfactory pathway occurs between axon terminals of ORNs and dendrites of mainly mitral/tufted cells in glomeruli of the OB. These glutamatergic contacts are among the most plastic synapses in the central nervous system, with considerable changes taking place not only during development, but also throughout the whole adult life (Mori and Sakano, 2011). In addition to the normal turnover of ORNs, the OE has also the unusual capacity to recover after extensive damage (Schwob, 2002). Even after considerable lesions the peripheral olfactory system regenerates, and the olfactory map in the OB is largely restored (Cheung et al., 2014). Together, this shows that the olfactory system provides significant advantages for studying basic mechanisms that regulate stem cell renewal, neuronal development, synaptogenesis, integration of newborn neurons into neuronal circuitry, and neuronal regeneration.
In Xenopus laevis, the African clawed frog, ORN axons first reach the OB immediately after hatching of the larvae. During the larval phase the olfactory system is already fully functional, and there is a gradual increase in the number of ORNs in the OE. In this period particularly many axons grow towards the OB, where they form glomerular synapses. During metamorphosis, i.e., the transformation of the fully aquatic larva to the secondarily aquatic adult frog, the olfactory system is gradually reorganized without losing its capacity to process olfactory information. Most ORNs are replaced and a substantial rewiring of the system takes place. After metamorphosis, as in all adult vertebrates, a continuous turnover of ORNs persists also in Xenopus (Hansen et al., 1998). The cellular composition and the general organization of the olfactory system do not substantially change during the ontogeny of Xenopus. For an overview of the cellular composition and the dynamics of the peripheral olfactory system of Xenopus laevis see Figure 1. The peripheral location and the sorted cellular composition make the Xenopus OE readily accessible and easily manageable. Thereby, especially during the larval period the olfactory system is very well-suited for in vivo microscopy because of high tissue transparency. An additional substantial advantage is that in larval Xenopus ORNs can be easily activated by odorants, their natural stimuli. The successful integration of new ORNs into the olfactory circuit can therefore be easily tested by measuring odorant responses of individual ORNs, axons or glomeruli in the OB.
Figure 1.
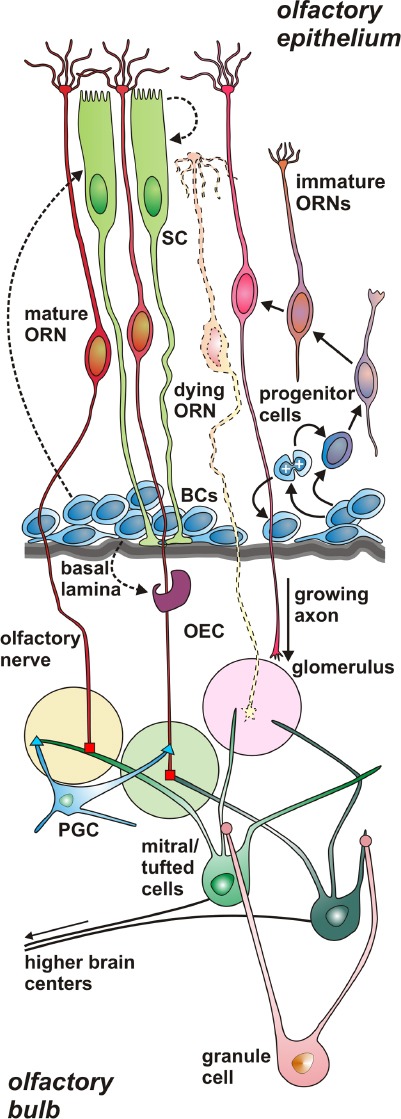
Schematic drawing of the normal peripheral olfactory system of the amphibian Xenopus laevis.
Scheme of a section through the olfactory epithelium (OE) and the olfactory bulb (OB) of larval Xenopus laevis. The three main cell types of the OE are olfactory receptor neurons (ORNs), non-neuronal sup-porting cells (SCs), and proliferative horizontal and globose basal cells (BCs), the olfactory stem cells. Throughout life, the OE contains various immature ORNs on their way to replace dying ORN. Basal cells may also give rise to SCs and olfactory ensheating cells (OEC). Axons of ORNs penetrate the basal lamina of the OE, enter the OB, and terminate in olfactory glomeruli. There they form synapses with dendrites of mitral/tufted cells, the second-order neurons of the olfactory system, and peri-glomerular cells (PGC). The axons of mitral/tufted cells merge together and convey the olfactory information to higher brain centers. The den-drites of granule cells, the most common type of interneurons of the OB, form modulatory synapses with dendrites of mitral/tufted cells.
In our laboratory, over the past few years we have been focusing on signals that control the proliferation of neural stem cells in the OE of Xenopus, and studied the development of ORN axons and their wiring properties (Hassenklöver and Manzini, 2013). We have established a method to introduce fluorescent markers into groups or individual ORNs of the OE of living larval and adult Xenopus (Hassenklöver and Manzini, 2014). By means of in vivo time lapse imaging using confocal laser-scanning or multiphoton microscopy, the animals can then be used to visualize and study the different stages of neuronal development in the living animal, from the birth of the ORN to its functional integration into neuronal circuitry. The developed technique allows to survey the early stages of neural stem/progenitor cell differentiation in the OE [see Figure 2, (i)], to observe axon development in the olfactory nerve [see Figure 2, (ii)], and to monitor the establishment of synapses in glomeruli of the OB [see Figure 2, (iii)]. An introduction of calcium sensitive dyes into developing ORNs allows to gain functional information. The possibility to introduce plasmid DNA encoding various fluorescent proteins further increases the application spectrum of this technique. A combined electroporation of dextran-coupled dyes, DNA, and/or charged morpholinos, on the other hand, allows to manipulate gene expression in individual or groups of observable cells. This allows to identify key genes that regulate the various steps of neuronal development, from the stem/progenitor cell differentiation in the OE, to axonal pathfinding and wiring, and finally the formation of synapses in the OB.
Figure 2.
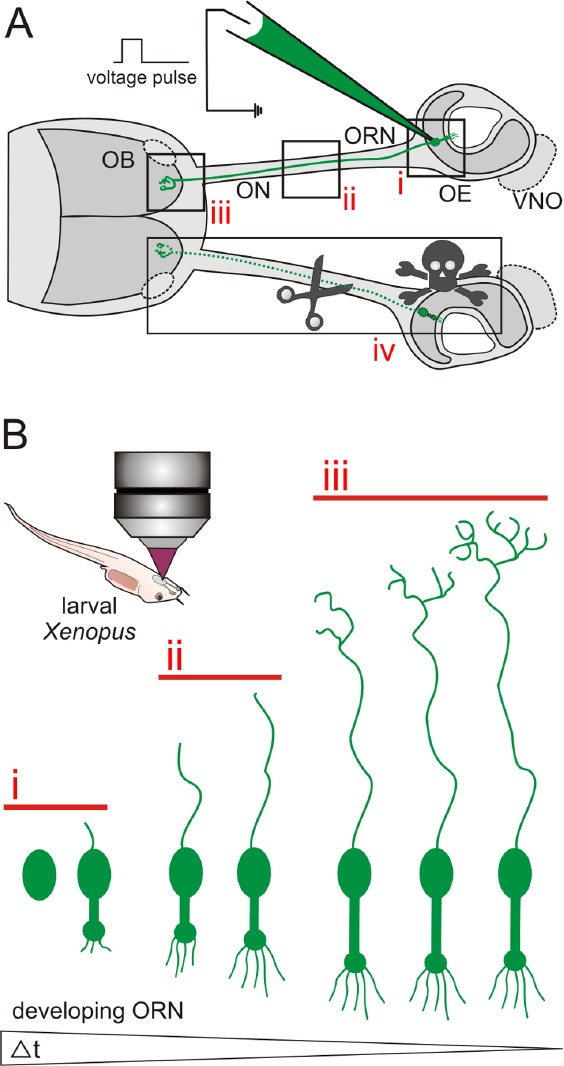
Schematic illustration of the experimental advantages of the olfactory system of larval Xenopus laevis.
(A) Schematic representation of the peripheral olfactory system of larval Xenopus laevis. Olfactory receptor neurons in the olfactory epi-thelium (OE) of anesthetized larval Xenopus can be labeled via spatially restricted electroporation of e.g., fluorescent dyes. Stained cells can be visualized in the OE (i), their axons can be followed through the olfac-tory nerve (ON, ii), and their axon terminals identified in the olfactory bulb (OB) (iii). Introduction of lesions in the OE, the ON and/or the OB is easy (iv). The vomeronasal organ (VNO) and the accessory OB are outlined by dotted lines. (B) Labeled stem/progenitor cells or im-mature ORNs can easily be investigated in anesthetized larvae using a confocal or multiphoton microscope. Using in vivo time lapse imaging, early stages of neural stem/progenitor cell differentiation can be mon-itored in the OE (i), axon development can be tracked in the ON and the OB (ii and iii), and synapse formation can be observed in glomeruli of the OB (iii). Individual cells can be followed over long time spans (up to several weeks).
The Xenopus olfactory system, particularly the larval system, is also a valuable model to study molecular and physiological mechanisms that control neuronal regeneration. It has the advantage that mechanisms involved in cellular proliferation and differentiation are particularly active. Also, the peripheral and relatively exposed position and the already mentioned high transparency of the tissue facilitate the introduction of lesions in the OE, the olfactory nerve and/or the OB [see Figure 2, (iv)]. It is possible to damage the whole OE by tissue-wide chemical induction of degeneration, or to obliterate all ORNs by cutting the olfactory nerve. Moreover, it is feasible to specifically eliminate defined cell types by targeted genetic ablation or defined subregions by introduction of focal lesions.
Malfunction of the olfactory system is a frequent affliction in humans, with up to 5% of the population suffering from anosmia and another 15% from hyposmia. Common causes for the loss of the sense of smell are chronic rhinosinusitis, polyps, neurodegenerative diseases (e.g., Alzheimer's and Parkinson's diseases), and traumatic injuries of the peripheral olfactory system. Despite the fact that the different portions of the adult human olfactory system are amongst the few regions of the brain showing neuronal regeneration, the therapeutic options for patients with olfactory loss are still limited. Currently, the most promising approach is an olfactory training paradigm which has shown to lead to increased olfactory function in patients even several years and months after the onset of the olfactory dysfunction. Still, many patients do not benefit from this exposition-based therapeutic approach and it is unclear why some do and some don’t (Hüttenbrink et al., 2013). Understanding the mechanisms that control neurogenesis, stem cell differentiation, axon outgrowth and synapse formation in the olfactory system is therefore a prerequisite to understand and eventually cure these medical conditions. The results of studies in Xenopus and other model organisms will contribute to improve our general knowledge about the basic regulation of neuronal turnover and regeneration in the olfactory system. On a long term basis, this knowledge will help clinicians to restore the human olfactory system after injury or disease. On the other hand, the Xenopus olfactory system in combination with the described electroporation method (Hassenklöver and Manzini, 2014) represents an advantageous model system to investigate general processes that regulate the development and regeneration of individual neurons, neuronal subsets or whole neuronal circuits. Basic mechanisms regulating the dynamics of neuronal development and regeneration are known to be highly conserved (Reichert, 2009). The results obtained in the Xenopus model system will therefore certainly have relevance beyond the research models and the olfactory system.
In summary, the Xenopus olfactory system with its unique strengths is a useful tool to study the different steps in the maturation and regeneration of neurons on a molecular, cellular and functional level. This will help to better understand the mechanisms that regulate olfactory neurogenesis during normal tissue maintenance and under repair conditions. The knowledge created as the result of basic research is likely to assist medical treatment and the development of new therapies targeted at replacement of lost neurons in neurodegenerative disorders or following nervous system injury.
This work was supported by DFG Schwerpunkt program 1392 (project MA 4113/2-2), cluster of Excellence and DFG Research Center Nanoscale Microscopy and Molecular Physiology of the Brain (project B1-9), and the German Ministry of Research and Education (BMBF; project 1364480).
References
- Cheung MC, Jang W, Schwob JE, Wachowiak M. Functional recovery of odor representations in regenerated sensory inputs to the olfactory bulb. Front Neural Circuits. 2014;7:207. doi: 10.3389/fncir.2013.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie KJ, Turnley AM. Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front Cell Neurosci. 2013;6:70. doi: 10.3389/fncel.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Hansen A, Reiss JO, Gentry CL, Burd GD. Ultrastructure of the olfactory organ in the clawed frog, Xenopus laevis, during larval development and metamorphosis. J Comp Neurol. 1998;398:273–288. doi: 10.1002/(sici)1096-9861(19980824)398:2<273::aid-cne8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hassenklöver T, Manzini I. Olfactory wiring logic in amphibians challenges the basic assumptions of the unbranched axon concept. J Neurosci. 2013;33:17247–17252. doi: 10.1523/JNEUROSCI.2755-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassenklöver T, Manzini I. The olfactory system as a model to study axonal growth patterns and morphology in vivo. J Vis Exp. 2014;92:e52143. doi: 10.3791/52143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttenbrink KB, Hummel T, Berg D, Gasser T, Hähner A. Olfactory dysfunction: common in later life and early warning of neurodegenerative disease. Dtsch Arztebl Int. 2013;110:1–7. doi: 10.3238/arztebl.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Sakano H. How is the olfactory map formed and interpreted in the mammalian brain? Annu Rev Neurosci. 2011;34:467–499. doi: 10.1146/annurev-neuro-112210-112917. [DOI] [PubMed] [Google Scholar]
- Reichert H. Evolutionary conservation of mechanisms for neuralregionalization, proliferation and interconnection in brain development. Biol Lett. 2009;5:112–116. doi: 10.1098/rsbl.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]


