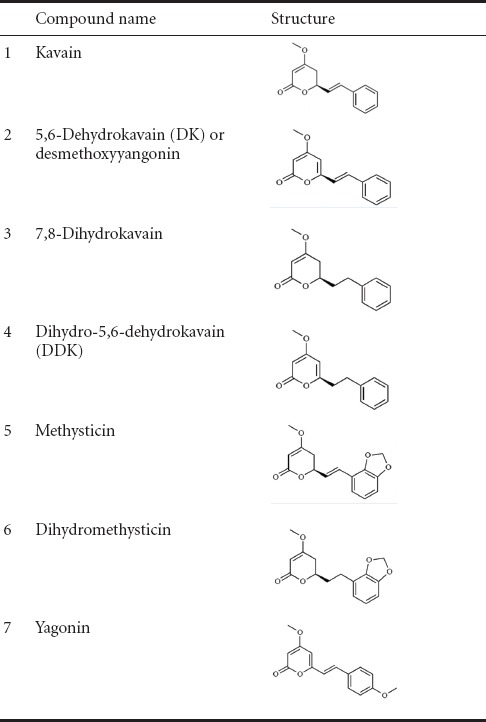
Kava or kava-kava (Piper methysticum) is a plant used for the production of a ritual drink with tranquilizing properties. The kavalactones are the main compounds of the kava drinks (see Table 1 for names and structures of the main kavalactones). There are many clinical trials on the use of kava product for anti-anxiety, and a systematic review supports the use of kava for the treatment of generalized anxiety (Sarris et al., 2009). Adverse effects on the liver have been reported, and kava is regulated in a number of countries (Teschke, 2010). Studies of kava extracts or kavalactones have been focused on elucidating the mechanism underlying its anti-anxiety and sleep-inducing ability, which has been attributed to its modulation of the γ-aminobutyric acid (GABA) receptor. Recent studies on the mechanisms of action for isolated kavalactones have revealed other neuroprotective activities not directly related to their GABAergic effects. These findings suggest that the use of kava might also be beneficial for the treatment of many degenerative diseases or nervous system conditions.
Table 1.
Names and structures of kavalactones
One of the neuroprotective effects of kavalactones is mediated by the P38/nuclear factor-kappaB (nuclear factor-κB)/cyclooxygenase 2 (COX2) signaling pathway. p38 mitogen-activated protein kinase (MAPK) is regarded as a stress-regulating kinase and plays a critical role in inflammatory responses. Downstream of the cascade of MAPKs, phosphorylation and degradation of inhibitor of kappaB (IκB) leads to translocation of NF-κB to the nucleus. Activation of cyclooxygenase enzymes, especially COX2, is one of the pro-inflammatory reactions downstream of NF-κB binding to DNA. COX converts arachidoinic acid into prostaglandins, and pharmacological inhibition of COX can provide relief from the symptoms of inflammation and pain. Elevated levels of p38 activity have been demonstrated in the amyloid beta and hyperphosphorylated tau deposits of Alzheimer's disease, Parkinson's disease, Huntington's disease, and ischemic brain models, and have been implicated in the development of these diseases (reviewed by Miloso et al., 2008). NF-κB was also regarded as a drug target for neuroprotection (Figure 1, left panel, arrows). For the signaling molecules within this pathway, we have demonstrated that dihydro-5,6-dehydrokavain (DDK) and 5,6-dehydrokavain (or desmethoxyyangonin) inhibit H2O2-induced P38 phosphorylation (Rao et al., 2014). NF-κB activity modeled by a luciferase construct in cells, was inhibited by methysticin (Shaik et al., 2009), a derivative of kavain that contains an additional 5 membered ring on top of the B ring. The inhibition activity of dihydrokawain and yangonin toward COX2 was demonstrated in a cell free system (Wu et al., 2002) (Figure 1, left panel, negative signs).
Figure 1.

Kavalactons might be neuroprotective via multiple pathways.
Kavalactone activates the p38, which in turn activates downstream kinase, and phosphorylation and degradation of IκB leads to translocation of NF-κB to the nucleus. One of the gene expression activated by NF-κB activation is COX-2. COX-2 converts arachidoinic acid into prostaglandins and up-regulation of many pro-inflamamtory cytokine follows. (2) Upon oxidative stress, the transcription factor Nrf2 was activated and released from keap1 (not shown in this diagram). ERK phosphorylation is upstream to the Nrf2 activation in neural cells treated with kavalacones. Nrf2 translocates to the nucleus and binds to the a cis-acting ARE to activate many downstream antioxidation genes, including HO-1, and protects the cells from oxidative stress and cell death.  Inhibitory activity of kavalactone single compound on signaling molecule.
Inhibitory activity of kavalactone single compound on signaling molecule.  Activation activity of kavalactone single compound on signaling molecule.
Activation activity of kavalactone single compound on signaling molecule.  Member of a signaling pathway promotes the activity of the next member.
Member of a signaling pathway promotes the activity of the next member.  Member of a signaling pathway inihibits the activity of the next member.
Member of a signaling pathway inihibits the activity of the next member.
IκB: Inhibitor of kappaB; NF-κB: nuclear factor-kappaB; COX-2: cyclooxygenase 2; Nrf2: NF-E2-related factor 2; ERK: extracellular regulated protein kinases; HO-1: heme oxygenase 1; iNOS: inducible nitric oxide synthase; NO: nitric oxide; ARE: antioxidant responsive element; DDK: dihydro-5,6-dehydrokavain; DK: dehydrokavain.
Another important neuroprotective pathway recently demonstrated is the kavalactone-mediated upregulation of antioxidation enzymes. When oxidative stress is detected by sensor molecules in the cytoplasm, possibly by modifying the cysteine residue of the keap1 protein, the transcription factor Nrf2 (NF-E2-related factor 2) is released. Nrf2 translocates to the nucleus and binds to the cis-acting antioxidant responsive element (ARE) to activate many downstream antioxidation genes and to protect the cells from oxidative stress and cell death. Activation of Nrf2 by siRNA knockout of keap1 reduces oxidative stress in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated astrocytes. Downstream to this is the heme oxygenase 1 (HO1) anti-oxidant enzyme. HO1 is responsible for catabolizing pro-oxidant heme, and the reducing product is also neuroprotective. HO1 is currently a drug target for stroke therapy (Figure 1, right panel, arrows). HO activation inhibits the level of inducible nitric oxide synthase (iNOS) and oxidative species within the cells and leads to cell survival (Figure 1, right panel, negative sign). The activation of Nrf2 and expression of HO1 were responsible for kavalctone-mediated protection against amyloid-induced neurotoxicity in neuronal and glial cell lines (Wruck et al., 2008; Terazawa et al., 2013). Methysticin, kavain, and yangonin stimulates ERK1/2 phosphorylation in PC12 cells and are responsible for the subsequent activation of Nrf2 and HO1 (Wruck et al., 2008) (Figure 1, right panel, positive signs).
Most of the neuroprotective herb extracts (for example Ginko biloba) rely on several different molecules to act on a variety of pathways. In contrast, the kavalactones’ reported neuroprotective properties were tested using purified single compounds that shared a similar structure, and a variety of drug targets were regulated by this same structure (see Table 1). The study of the relationships among the structure, conformation, and biological activities of kavalactone suggested that none of the derivatives were specific activators for intracellular signaling molecules (Rowe et al., 2011). Certain structures within the molecules are more effective than others for specific signaling pathways or cellular drug targeting. An example could be seen within the two neuroprotective pathways. The pro-inflammatory reaction and oxidative stress reaction often co-exist in a neuronal injury site, forming a positive feedback loop and either accelerating the process of cell death or extending the secondary degeneration in a paracrine or autocrine fashion. However, on the intracellular level, the two signaling pathways that were partly responsible for those reactions, i.e., the MAPK/NF-κB/COX2 and the Nrf2/ARE/antioxidation enzyme pathways, were not closely coupled. ERK phosphorylation is reported to be upstream to, and necessary for, Nrf2 activation in neural cells treated with kavalactones (Wruck et al., 2008). ERK utilized upstream kinases distinct from the P38 kinase, and hence, these two well-known MAP kinase pathways work in parallel and exhibit little cross-talks in the kinase cascade. Different conformations of the kavalactones demonstrated a preference for activated pathways. For example, compound 1 (2′,6′-dichloro-5-methoxymethyl-5,6-dehydrokawain) up-regulated Nrf2 activity without affecting COX-2 levels (Terazawa et al., 2013), and 5,6-dehydrokavain (desmethoxyyangonin) down-regulated the level of phosphorylated p38 but did not reduce the level of intracellular oxidative stress (Rao et al., 2014).
The fact that kavalactones were not specific activators of a molecular target but showed conformation-related preferences for activated pathways was further demonstrated by their interactions with other molecules. The double bond between carbon 5 and 6 in ring A and between carbon 7 and 8 in kavalactones were the most significant structural features that influence their activities. The double bond between carbon 5 and 6 in ring A rendered a locked configuration through conjugation, and this locked conformation is required for kavalactone inhibition of P-glycoprotein and monoamine oxidase B (MAO-B). On the other hand, rotational freedom around the carbon 7-8 bond (lack of double bond) is required for the inhibition of COX-1 (Rowe et al., 2011) and for the inhibition of oxidative stress (Rao et al., 2014). The double bond between carbon 7 and 8 provides a rigid configuration and is necessary for NF-κB binding and inhibition. Lastly, the inhibition activities of TNF-alpha and COX-2 were independent of the above structures (reviewed in Rowe et al., 2011). Although each drug targets has its favored conformation, it is still intriguing that the similar structures activate all of the above molecules. As they are highly philophilic molecules, one of the possible reasons that they exert such a wide spectrum of activities may be because of their ability to modulate cell membranes in the presence of certain type of receptors (this behavior is observed in their regulation of GABA receptors) or because of their direct interaction with cytoplasmic receptors (for example, with AhR, vide infra).
Another consideration for the development of kavalactones as neuroprotective agents (as either a drug or health drink) is their drug interactions and toxicity. Hepatotoxicity and carcinogenicity were reported for kava and kavalactones. Although a causal relationship has not yet been established, research in the past decade demonstrated that Kava or kavalactones may influence the concentration of ingested drugs by regulating the activities of cytochrome P450 (CYP) or P-glycoprotein (for a comprehensive review, see the introduction in Li et al., 2011). Isoforms of CYP that were modulated by individual kavalactones including CYP1A1, 1A2, 2A23, 2B1, 2C9, 2C19, 2D6, 2E1, 3A1, 3A4, and 4A9/11. Of these, CYP1A1, 1A2, 2B1, 3A1, 3A4 were increased after treatment with kavalactones (Li et al., 2011). The adverse herb-drug interactions known for kava include buprenorphine, naloxone, ethanol, leflunomide, lomitapide, mipomersen, and teriflunomide (generic names) may be closely related to CYP modulation, although a causal link has not been experimentally established.
Another aspect of the side effects of kava was reported to involve the carcinogenic CYP1A1 gene (Yamazaki et al., 2008) After binding to a ligand, aryl hydrocarbon receptor (AhR) translocates to the nucleus and binds to its nuclear translocator to form a heterodimeric transcription factor complex and regulate downstream genes, which include CYP1A1. CYP1A1 is involved in the formation of the carcinogenic metabolites of polycyclic aromatic hydrocarbons (PAHs). It is not too surprising that kavalactone's aromatic hydrocarbon structure may bind to AhR and regulate the transcription of CYP1A1. The induction of CYP1A1 by methysticin and 7,8-dihydromethysticin (Li et al., 2011) did raise concern about the long-term use of kava as a health drink.
Both conformation related pathway activities and toxicity were interesting information in terms of potential and use of a drug. If the relationship of preferred conformation and molecule acted could be established, a single kavalactone may be designed to maximally activate on different signaling pathways. The neuroprotective effects of kavalactone other than anti-anxiety suffered from lack of in vivo data, except for test on ischemic brain injury (Backhauss and Krieglstein, 1992). Further test for toxicities, additional drug target, study for the association between conformation and action on intracelluar signaling molecules, and in vivo test may lead to the conclusion that the use of purified kavalactones for an adjunct therapy in addition to other drugs for oxidative stress- and inflammation-related diseases such as Alzheimer disease and stroke.
This work was supported by the grants from the Ministry of Science and Technology, Taiwan, China (formerly National Science Council Taiwan) (NSC 99-2811-M-324-003, NSC 101-2320-B-324-001).
References
- Backhauss C, Krieglstein J. Extract of kava (Piper methysticum) and its methysticin constituents protect brain tissue against ischemic damage in rodents. Eur J Pharmacol. 1992;215:265–269. doi: 10.1016/0014-2999(92)90037-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Mei H, Wu Q, Zhang S, Fang JL, Shi L, Guo L. Methysticin and 7,8-dihydromethysticin are two major kavalactones in kava extract to induce CYP1A1. Toxicol Sci. 2011;124:388–399. doi: 10.1093/toxsci/kfr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloso M, Scuteri A, Foudah D, Tredici G. MAPKs as mediators of cell fate determination: an approach to neurodegenerative diseases. Curr Med Chem. 2008;15:538–548. doi: 10.2174/092986708783769731. [DOI] [PubMed] [Google Scholar]
- Rao YK, Shih HN, Lee YC, Cheng WT, Hung HC, Wang HC, Chen CJ, Tzeng YM, Lee MJ. Purification of kavalactones from Alpinia zerumbet and their protective actions against hydrogen peroxide-induced cytotoxicity in PC12 cells. J Biosci Bioeng. 2014;118:679–688. doi: 10.1016/j.jbiosc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Rowe A, Narlawar R, Groundwater PW, Ramzan I. Kavalactone pharmacophores for major cellular drug targets. Mini Rev Med Chem. 2011;11:79–83. doi: 10.2174/138955711793564088. [DOI] [PubMed] [Google Scholar]
- Sarris J, Kavanagh DJ, Deed G, Bone KM. St. John's wort and Kava in treating major depressive disorder with comorbid anxiety: a randomised double-blind placebo-controlled pilot trial. Hum Psychopharmacol. 2009;24:41–48. doi: 10.1002/hup.994. [DOI] [PubMed] [Google Scholar]
- Shaik AA, Hermanson DL, Xing C. Identification of methysticin as a potent and non-toxic NF-kappaB inhibitor from kava, potentially responsible for kava's chemopreventive activity. Bioorg Med Chem Lett. 2009;19:5732–5736. doi: 10.1016/j.bmcl.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terazawa R, Akimoto N, Kato T, Itoh T, Fujita Y, Hamada N, Deguchi T, Iinuma M, Noda M, Nozawa Y, Ito M. A kavalactone derivative inhibits lipopolysaccharide-stimulated iNOS induction and NO production through activation of Nrf2 signaling in BV2 microglial cells. Pharmacol Res. 2013;71:34–43. doi: 10.1016/j.phrs.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Teschke R. Kava hepatotoxicity--a clinical review. Ann Hepatol. 2010;9:251–265. [PubMed] [Google Scholar]
- Wruck CJ, Gotz ME, Herdegen T, Varoga D, Brandenburg LO, Pufe T. Kavalactones protect neural cells against amyloid beta peptide-induced neurotoxicity via extracellular signal-regulated kinase 1/2-dependent nuclear factor erythroid 2-related factor 2 activation. Mol Pharmacol. 2008;73:1785–1795. doi: 10.1124/mol.107.042499. [DOI] [PubMed] [Google Scholar]
- Wu D, Yu L, Nair MG, DeWitt DL, Ramsewak RS. Cyclooxygenase enzyme inhibitory compounds with antioxidant activities from Piper methysticum (kava kava) roots. Phytomed. 2002;9:41–47. doi: 10.1078/0944-7113-00068. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Hashida H, Arita A, Hamaguchi K, Shimura F. High dose of commercial products of kava (Piper methysticum) markedly enhanced hepatic cytochrome P450 1A1 mRNA expression with liver enlargement in rats. Food Chem Toxicol. 2008;46:3732–3738. doi: 10.1016/j.fct.2008.09.052. [DOI] [PubMed] [Google Scholar]


