Abstract
The present study aimed to explore the mechanism underlying the protective effects of hydrogen sulfide against neuronal damage caused by cerebral ischemia/reperfusion. We established the middle cerebral artery occlusion model in rats via the suture method. Ten minutes after middle cerebral artery occlusion, the animals were intraperitoneally injected with hydrogen sulfide donor compound sodium hydrosulfide. Immunofluorescence revealed that the immunoreactivity of P2X7 in the cerebral cortex and hippocampal CA1 region in rats with cerebral ischemia/reperfusion injury decreased with hydrogen sulfide treatment. Furthermore, treatment of these rats with hydrogen sulfide significantly lowered mortality, the Longa neurological deficit scores, and infarct volume. These results indicate that hydrogen sulfide may be protective in rats with local cerebral ischemia/reperfusion injury by down-regulating the expression of P2X7 receptors.
Keywords: nerve regeneration; brain injury; hydrogen sulfide; cerebral ischemia/reperfusion injury; P2X7 receptor; 2,3,5-triphenyl-2H-tetrazolium chloride staining; animal model; protection; sodium hydrosulfide; immunofluorescence; middle cerebral artery occlusion; NSFC grant; neural regeneration
Introduction
Hydrogen sulfide (H2S) is a novel bioactive endogenous gaseous signal molecule among the other bioactive gaseous substances, nitrogen (NO) and carbon monoxide (CO) (Xie et al., 2013; Fu et al., 2014). A previous study has shown that H2S plays an important role in the central nervous system under physiological and pathological conditions (Wang et al., 2014). A growing body of evidence suggests that H2S exerts neuroprotective effects in animal models of Alzheimer's disease (Gong et al., 2011; Xuan et al., 2012; Giuliani et al., 2013), Parkinson's disease (Kida et al., 2011; Xie et al., 2013; Xue et al., 2015; Wang et al., 2015), cerebral ischemia (Li et al., 2011, 2012; Yin et al., 2013; Gheibi et al., 2014), and other models of neuronal damage (Xuan et al., 2012; Luo et al., 2013; Chen et al., 2014).
A preliminary study from our group has demonstrated that the H2S donor compound sodium hydrosulfide (NaHS) protects neurons against cerebral ischemia/reperfusion (I/R) injury (Ren et al., 2010). However, the neuroprotective mechanism remains poorly understood. Bai et al. (2013) have shown that the purinoceptor P2X7 plays a crucial role in the pathophysiology of cerebral ischemia injury. However, little evidence exists on the effect of H2S on the expression of the P2X7 receptor in the brain after cerebral I/R.
We speculate that H2S is protective against cerebral I/R injury via P2X7 receptors. Therefore, in this study, we investigated the effect of H2S on the immunoreactivity of the P2X7 receptor in the brains of rats with local cerebral I/R (after middle cerebral artery occlusion).
Materials and Methods
Animals
Forty-eight clean, adult male Sprague-Dawley rats weighing 200 ± 20 g were provided by the Experimental Animal Center of Henan Province (Zhengzhou, Henan Province, China; license No. SCXK (Yu) 2010-0002). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health: Eighth Edition (2010). The experimental protocol for the animals was approved by the Institutional Animal Care and Use Committee of Xinxiang Medical University in China.
Treatment groups
The 48 rats were randomly divided into three groups (n = 16 per group): sham-operated, cerebral I/R, and NaHS + I/R. If death occurred in any group, additional rats were included to meet the sample size number. In the NaHS + I/R group, rats were given an intraperitoneal (i.p.) injection of 25 μmol/kg NaHS (Sigma-Aldrich, St. Louis, MO, USA) 10 minutes after middle cerebral artery oclusion. Rats in the sham-operated and I/R groups received an i.p. injection of saline at the same time point.
Cerebral I/R model
The rat model of left cerebral ischemia-reperfusion was established according to a previously published method (Longa et al., 1989). Briefly, Sprague-Dawley rats were anesthetized with 10% chloral hydrate (3.5 mL/100 g, i.p. injection; Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China). After the skin was exposed through a midline neck incision, the left common carotid artery, external carotid artery, and internal carotid artery were exposed and isolated through a ventral midline incision. After electrocauterization of the occipital artery, the external carotid artery distal to the heart was ligated with two surgical sutures. The external carotid artery was then cut off at the ligation site and the common carotid artery and internal carotid artery were clamped. The silk sutures around the external carotid artery were lifted toward the common carotid artery ensuring that the external carotid artery and common carotid artery were placed on the same plane. A gap was made at the bifurcation of the external carotid artery adjacent to the common carotid artery, and an AA monofilament suture (Beijing Sunbio Biotech Co., Ltd., Beijing, China) was slowly inserted from the incision close to the internal carotid artery. The suture (18–19 mm deptg) was then withdrawn from the internal carotid artery until resistance was felt at the bifurcation of the external carotid artery. Furthermore, the suture tip almost reached the middle cerebral artery, thereby occluding blood flow in the left middle cerebral artery. The suture was then fixed and the wounds were sutured. The animals were subsequently returned to their cages (at 26°C). After 2 hours of ischemia, rats were re-anesthetized via ether inhalation. The suture was then lifted slightly until the resistance was felt, indicating that the suture tip had reached the external carotid artery. Blood flow in the left middle cerebral artery resumed as a result. Successful establishment of the model was confirmed by the right forelimb flexion or right side circling after the animals recovered from the anesthesia. The same protocol was applied for sham-operated rats, but only the arteries were isolated.
Mortality score and neurological deficit score
The mortality of rats in each group was calculated according to the formula: Percentage mortality = number of deaths in the group/total number of rats in the group × 100%. After the 2-hour ischemia and 24-hour reperfusion, neurological deficits of rats were evaluated according to a previously described 5-grade scale (Longa et al., 1989). The scale was graded as follows: 0 point: rats have no neurological symptoms, 1 point: minor defects (rats cannot fully extend the contralateral forepaw), 2 points: moderate defects (rats circle toward the contralateral side), 3 points: severe defects (rats fall toward the contralateral side), 4 points: rats cannot walk spontaneously or they lose consciousness. Rats with 1-3 points and no subarachnoid hemorrhaging qualified as established I/R models. Additional animals were included in each group to ensure a sample size of 16.
Measurement of infarct volume via 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) staining
The brain infarct volume of rats was measured according to a method as described previously (Hashimoto et al., 2010; Ansari et al., 2011). After 2 hours of ischemia and 24 hours of reperfusion, rats were deeply anesthetized. The brain was then harvested, and the cerebellum, brainstem, and olfactory bulb were removed. The brain tissue was stored at –20°C for 10 minutes. The brain tissue was cut into coronal slices (3 mm thickness) starting from the optic chiasma. Slices were then incubated with 20 g/L TTC (Beijing Biochemical Pharmaceutical Factory, Beijing, China) phosphate buffer (pH 7.4) at 37°C for 30 minutes in the dark and then fixed with 4% paraformaldehyde for 24 hours. Sections were photographed and Image-Pro Plus 6 (Media Cybernetics Inc., Rockville, MD, USA) was used to estimate infarct size. Infarct size was calculated according to the following formula: Percentage infarct size = the volume of the cerebral hemisphere on the normal side – the non-infarcted area on the ischemic side/the volume of the cerebral hemisphere on the normal side × 100%.
Immunofluorescence of the P2X7 receptor
After 2 hours of ischemia and 24 hours of reperfusion, rats were anesthetized and hearts were exposed. The heart was fixed through left ventricular intubation to the ascending aorta, and a small hole was cut at the right auricle for bleeding. Rats were perfused with 200 mL saline to rinse out blood from the whole body and then fixed overnight with 4% paraformaldehyde phosphate buffer (pH 7.4) at 4°C. Brains were dissected and then fixed overnight with 4% paraformaldehyde phosphate buffer (pH 7.4) at 4°C. Brains were dehydrated with gradient sucrose solution and then cut (20 μm thickness). Sections were washed (3 × 5 minutes) with 1 mL/L Triton X-100 phosphate solution. Slices were microwave repaired with sodium citrate (Beijing Zhongshan Glodern Bridge Biotechnology Co., Ltd., Beijing, China) for 15 minutes, then warmed to room temperature and rinsed (3 × 5 minutes) with PBS (pH 7.4) (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.). Sections were then blocked with goat serum for 20 minutes followed by incubation (overnight) with rabbit anti-rat P2X7 polyclonal antibody (1:150; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C. Sections incubated only with PBS served as the negative control. All sections were then incubated with Cy3-conjugated sheep anti-rabbit IgG (1:500; Beyotime Biotechnology, Haimen, Jiangsu Province, China; 1:500) at room temperature for 2 hours. After sections were rinsed (with PBS, 3 × 5 minutes), the nuclei were counterstained (3 minutes) with DAPI (Beyotime Biotechnology). Sections were rinsed (with PBS, 6 × 5 minutes) and then mounted with anti-fluorescence quenching fluid (Beyotime Biotechnology). Three sections randomly selected from each rat were photographed under fluorescence microscopy (GFM-500, Nikon, Tokyo, Japan). The number of P2X7-immunoreactive cells in each section from the cerebral cortex and hippocampal CA1 region was calculated (under × 400 magnification), and the average value was then determined.
Statistical analysis
All data are expressed as the mean ± SD and were analyzed by one-way analysis of variance followed by the least significant difference test. Significance was reached at values of P < 0.05 or P < 0.01. All data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA).
Results
Effect of NaHS on the mortality and neurological function in rats with cerebral I/R injury
Because six rats died in the NaHS + I/R group, a total of 22 rats were used. In the I/R group, a total of 28 rats were used because 12 rats died. The mortality percentage in the NaHS + I/R group (27.27%) was lower than that in the I/R group (42.86%). Neurological deficit scores in the I/R group were significantly (P < 0.01) higher compared with the sham group and significantly (P < 0.05) lower compared with the I/R group. These results indicated that exogenous H2S may be neuroprotective in rats with cerebral I/R injury (Figure 1).
Figure 1.
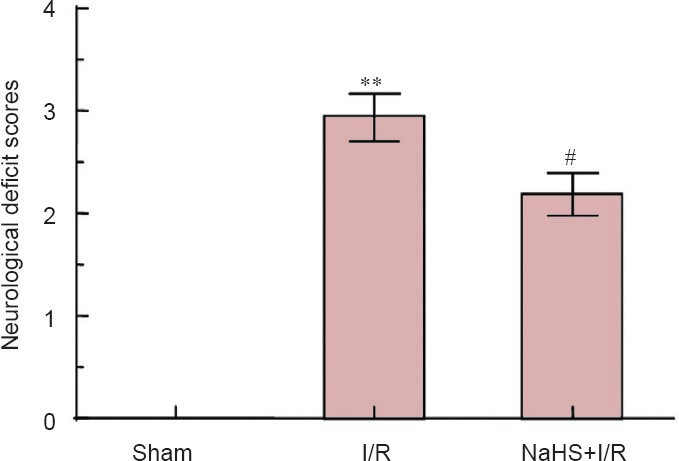
Effect of sodium hydrosulfide (NaHS) on neurological function in rats after cerebral ischemia/reperfusion (I/R).
Neurological deficit scores of rats from each group were assigned to a 5-grade scale from 0 point (indicating no neurological deficit symptoms) to 4 points (higher scores indicate more obvious symptoms of neurological deficits). **P < 0.01, vs. sham group; #P < 0.05, vs. I/R group. The data are expressed as the mean ± SD (n = 16) and analyzed by one-way analysis of variance followed by the least significant difference test. Sixteen animals per group were used.
Effect of NaHS on infarct volume in rats with cerebral I/R injury
TTC revealed red staining in the hemispheres of sham-operated rats. Red staining was also observed in normal brain tissue of I/R and NaHS + I/R rats. However, ischemic brain tissue in both groups appeared pale. The infarct volume in the NaHS + I/R group (21.88 ± 3.53%) was significantly (P < 0.01) smaller than that of the I/R group (36.71 ± 3.73%). These results indicate that exogenous H2S reduced infarct volume in rats with cerebral I/R, thereby possibly exerting neuroprotective effects (Figure 2).
Figure 2.
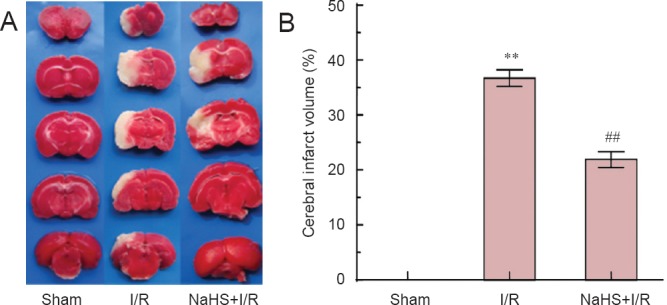
The effect of sodium hydrosulfide (NaHS) on cerebral infarct volume of rats after cerebral ischemia/reperfusion (I/R).
(A) After 2 hours of ischemia and 24 hours of reperfusion, brain slices were stained with 2,3,5-triphenyl-2H-tetrazolium chloride (TTC). TTC reveals red staining in the hemispheres of sham-operated rats. Red staining is also observed in normal brain tissue of I/R and NaHS + I/R rats. However, ischemic brain tissue in I/R and NaHS + I/R groups appears pale. (B) The cerebral infarct volume of rats. **P < 0.01, vs. sham group; ##P < 0.01, vs. I/R group. The data are expressed as the mean ± SD (n = 6) and analyzed by one-way analysis of variance followed by the least significant difference test. Six animals per group were used.
Effect of NaHS on the immunoreactivity of P2X7 receptors in the cerebral cortex and hippocampal CA1 region of rats with cerebral I/R injury
Immunofluorescence revealed that immunoreactivity of P2X7 was localized on the cell membrane and cytoplasm. Compared with the sham group, the number of P2X7-immunoreactive cells in cerebral cortex and hippocampus of rats was significantly (P < 0.01) increased in the I/R group. Compared with the I/R group, the number of P2X7-immunoreactive cells in both brain regions was significantly (P < 0.01) decreased in the NaHS + I/R group. These results indicate that exogenous H2S decreased the presence of P2X7 receptors in the brain of rats with cerebral I/R injury (Figures 3, 4).
Figure 3.
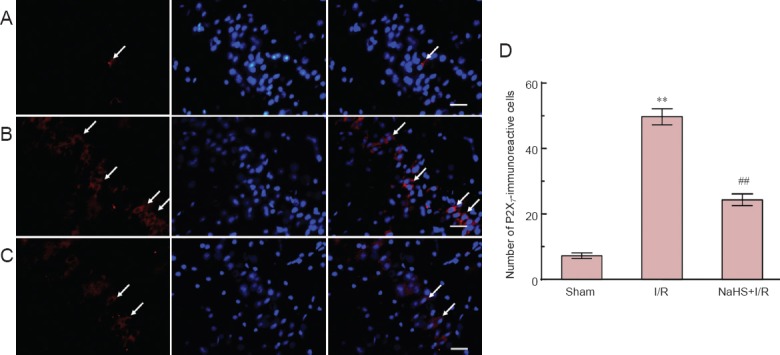
Effect of sodium hydrosulfide (NaHS) on the immunoreactivity of P2X7 receptor in the cerebral cortex of rats with cerebral ischemia/reperfusion (I/R) injury.
(A–C) Sections were incubated with the relevant primary antibody and labeled with Cy3-conjugateed secondary antibody (red) and DAPI (blue) stain. (A) Sham group, (B) I/R group, (C) NaHS + I/R group. Arrows indicate cells immunoreactive for P2X7. (D) The number of P2X7-immunoreactive cells in the cerebral cortex of rats. **P < 0.01, vs. sham group; ##P < 0.01, vs. I/R group. The data are expressed as the mean ± SD (n = 10) and analyzed by one-way analysis of variance followed by the least significant difference test. Ten animals per group were used. Scale bars: 30 μm.
Figure 4.
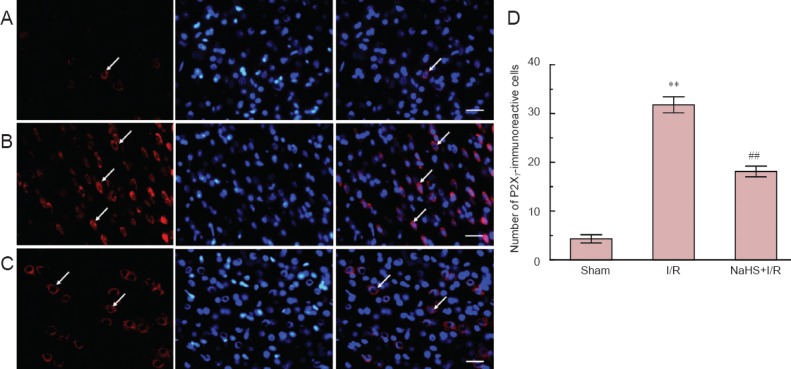
Effect of sodium hydrosulfide (NaHS) on the immunoreactivity of P2X7 receptor in the hippocampal CA1 region of rats with cerebral ischemia/reperfusion (I/R) injury.
(A–C) Sections were incubated with the relevant primary antibody and labeled with Cy3-conjugated secondary antibody (red) and DAPI (blue) stain. (A) Sham group, (B) I/R group, (C) NaHS + I/R group. Arrows indicate P2X7-immunoreactive cells. (D) The number of P2X7-immunoreactive cells in the hippocampus of rats. **P < 0.01, vs. sham group; ##P < 0.01, vs. I/R group. The data are expressed as the mean ± SD (n = 10) and analyzed by one-way analysis of variance followed by the least significant difference test. Ten animals per group were used. Scale bars: 30 μm.
Discussion
Cerebral ischemia rapidly leads to energy failure, depletion of ATP, ion imbalance, and death of neuronal cells. These events result in increased levels of extracellular ATP (Franke et al., 2004). ATP is an important neurotransmitter that exerts a variety of effects through the activation of purine energy receptors, P2X and P2Y. The P2X7 receptor is a very unique subtype of the P2X family (Sperlagh et al., 2006), with two different functions. Under physiological conditions, the concentration of extracellular ATP is within the micromolar range. At these concentrations in the brain, ATP activates the P2X7 receptors on neurons, which then selectively triggers the infux of Ca2+ and Na+, resulting in cell depolarization and excitation (Arbeloa et al., 2012). During cerebral ischemic injury, high concentrations (i.e., in the millimolar range) of ATP surround the injury site (Melani et al., 2005; Bai et al., 2013). These chronic high concentrations of ATP cause the P2X7 receptors to transform into non-selective cation channels of which their permeability is remarkably increased. This enhanced permeability allows various kinds of cations and organic matter under 900 Da to pass through the membrane (Franke et al., 2004; Pelegrin et al., 2011). Increased permeability further stimulates the production of NO, reactive oxygen species, and inflammatory molecules, and also causes calcium overload, ultimately leading to secondary brain injury and possibly apoptotic cell death (Skaper et al., 2010; Arbeloa et al., 2012).
The present study showed a significant loss of neurological function and cerebral infarction after 2 hours of ischemia and 24 hours of reperfusion. These findings are consistent with those of Melani et al. (2006). However, our findings on the expression of the P2X7 receptor contradict those of Wang et al. (2009) who have shown the down-regulation of this receptor in brains of neonatal rats with hypoxia-ischemia injury. This discrepancy may be explained by the different models used. Wang et al. (2009) used 3-day-old neonatal rats, while our group used adult rats. Therefore, neural development processes occurring in neonatal rats may influence the expression of P2X7 receptors after hypoxia-ischemia injury. Other studies have demonstrated that an increment or excessive activation of P2X7 receptors exacerbates tissue damage after MCAO in rats (Franke et al., 2004; Melani et al., 2006). Overall, our present findings indicate that increased expression of P2X7 receptors in the brains of rats with cerebral I/R injury may be involved in secondary brain injury following this insult.
A recent study has found that H2S promotes angiogenesis in rats with ischemic cerebral apoplexy (Jang et al., 2014). H2S has also been shown to suppress programmed death after cerebral I/R injury in rats, and this effect may attenuate cerebral edema (Gheibi et al., 2014). In the same rat model, H2S has been shown to be neuroprotective by inhibiting oxidative stress, inflammation, and apoptosis (Yin et al., 2013). The present study showed that administration of NaHS (25 μmol/kg) 10 minutes after cerebral ischemia significantly reduced infarct volume compared with the I/R group that did not receive the treatment. This finding is consistent with those of Gheibi et al. (2014) and Li et al. (2012). Furthermore, NaHS significantly decreased the mortality and neurological deficit scores in rats. Ren et al. (2010) have shown that when the H2S/ cystathionine β-synthase condition is down-regulated after 24 hours of cerebral I/R in rats, exogenous H2S significantly attenuates the severity of cerebral I/R injury and is neuroprotective. In the present study, we explored a possible mechanism of protection of H2S in the I/R model. Our findings demonstrate that P2X7 receptors in the cerebral cortex and hippocampus on the side of injury are significantly lower in I/R injury rats treated with H2S compared with the non-treated I/R injury rats. Various studies have shown that the P2X7 receptor antagonist is neuroprotective (Cavaliere et al., 2004; Peng et al., 2009; Friedle et al., 2010; Arbeloa et al., 2012). In summary, the present study indicates that exogenous H2S decreases the immunoreactivity of P2X7 receptors in the cerebral cortex and hippocampus on the side of injury, thereby attenuating secondary damage after cerebral I/R. This effect may be one of the mechanisms responsible for the neuroprotective effect of H2S. Further work is required to ascertain if the neuroprotective mechanism of H2S in this model is mediated by the P2X7-Ca2+ signaling pathway.
Acknowledgments
We thank all of the teachers from Department of Physiology and Neurobiology, Xinxiang Medical University, China for their help and the Laboratory of Brain Injury and Repair, Key Research Base of Henan Institutes of Higher Education of China for providing experimental room.
Footnotes
Funding: This study was financially supported by grants from the National Natural Science Foundation of China, No. 81371346, 81271376; Outstanding Postgraduate Fund of Xinxiang Medical University; and Science and Technology Key Research Project of Henan Provincial Education Department of China, No. 14A310019.
Conflicts of Interest: None declared.
Copyedited by Mark C, Raye W, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- Ansari S, Azari H, McConnell DJ, Afzal A, Mocco J. Intraluminal middle cerebral artery occlusion (MCAO) model for ischemic stroke with laser doppler flowmetry guidance in mice. J Vis Exp. 2011;8:pii:2879. doi: 10.3791/2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeloa J, Pérez-Samartín A, Gottlieb M, Matute C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis. 2012;45:954–961. doi: 10.1016/j.nbd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Bai HY, Li AP. P2X(7) receptors in cerebral ischemia. Neurosci Bull. 2013;29:390–398. doi: 10.1007/s12264-013-1338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere F, Amadio S, Sancesario G, Bernardi G, Volonté C. Synaptic P2X7 and oxygen/glucose deprivation in organotypic hippocampal cultures. J Cereb Blood Flow Metab. 2004;4:392–398. doi: 10.1097/00004647-200404000-00004. [DOI] [PubMed] [Google Scholar]
- Chen WL, Niu YY, Jiang WZ, Tang HL, Zhang C, Xia QM, Tang XQ. Neuroprotective effects of hydrogen sulfide and the underlying signaling pathways. Rev Neurosci. 2014 doi: 10.1515/revneuro-2014-0051. doi: 10.1515/revneuro-2014-0051. [DOI] [PubMed] [Google Scholar]
- Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X 7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- Friedle SA, Curet MA, Watters JJ. Recent patents on novel P2X(7) receptor antagonists and their potential for reducing central nervous system inflammation. Recent Pat CNS Drug Discov. 2010;1:35–45. doi: 10.2174/157488910789753530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZY, Liu XZ, Wu YJ, Zhu T, Jin WJ. Neuroprotective effects of hydrogen sulfide in rats with acute cauda equina syndrome. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:7914–7918. [Google Scholar]
- Gheibi S, Aboutaleb N, Khaksari M, Kalalian-Moghaddam H, Vakili A, Asadi Y, Mehrjerdi FZ, Gheibi A. Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia. J Mol Neurosci. 2014;2:264–270. doi: 10.1007/s12031-014-0284-9. [DOI] [PubMed] [Google Scholar]
- Giuliani D, Ottani A, Zaffe D, Galantucci M, Strinati F, Lodi R, Guarini S. Hydrogen sulfide slows down progression of experimental Alzheimer's disease by targeting multiple pathophysiological mechanisms. Neurobiol Learn Mem. 2013;104:82–91. doi: 10.1016/j.nlm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Gong QH, Shi XR, Hong ZY, Pan LL, Liu XH, Zhu YZ. A new hope for neurodegeneration: possible role of hydrogen sulfide. J Alzheimers Dis. 2011;2:173–182. doi: 10.3233/JAD-2011-110128. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Shibata K, Nobe K, Hasumi K, Honda K. A novel embolic model of cerebral infarction and evaluation of Stachybotrys microspora triprenyl phenol-7 (SMTP-7), a novel fungal triprenyl phenol metabolite. J Pharmacol Sci. 2010;114:41–49. doi: 10.1254/jphs.10131fp. [DOI] [PubMed] [Google Scholar]
- Jang H, Oh MY, Kim YJ, Choi IY, Yang HS, Ryu WS, Lee SH, Yoon BW. Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J Neurosci Res. 2014;92:1520–1528. doi: 10.1002/jnr.23427. [DOI] [PubMed] [Google Scholar]
- Kida K, Yamada M, Tokuda K, Marutani E, Kakinohana M, Kaneki M, Ichinose F. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson's disease. Antioxid Redox Signal. 2011;2:343–352. doi: 10.1089/ars.2010.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GF, Luo HK, Li LF, Zhang QZ, Xie LJ, Jiang H, Li LP, Hao N, Wang WW, Zhang JX. Dual effects of hydrogen sulphide on focal cerebral ischaemic injury via modulation of oxidative stress-induced apoptosis. Clin Exp Pharmacol Physiol. 2012;9:765–771. doi: 10.1111/j.1440-1681.2012.05731.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang Y, Xie Y, Yang Z, Zhang T. Protective effects of exogenous hydrogen sulfide on neurons of hippocampus in a rat model of brain ischemia. Neurochem Res. 2011;10:1840–1849. doi: 10.1007/s11064-011-0502-6. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yang X, Zhao S, Wei C, Yin Y, Liu T, Jiang S, Xie J, Wan X, Mao M, Wu J. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem Int. 2013;8:826–831. doi: 10.1016/j.neuint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volontè C, Bernardi G, Pedata F, Sancesario G. P2X 7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 2006;26:974–982. doi: 10.1038/sj.jcbfm.9600250. [DOI] [PubMed] [Google Scholar]
- Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;6:442–448. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Pelegrín P. Many ways to dilate the P2X 7 receptor pore. Br J Pharmacol. 2011;5:908–911. doi: 10.1111/j.1476-5381.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X 7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Du A, Li D, Sui J, Mayhan WG, Zhao H. Dynamic change of hydrogen sulfide during global cerebral ischemia- reperfusion and its effect in rats. Brain Res. 2010;1345:197–205. doi: 10.1016/j.brainres.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Debetto P, Giusti P. The P2X 7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24:337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- Sperlágh B, Vizi ES, Wirkner K, Illes P. P2X 7 receptors in the nervous system. Prog Neurobiol. 2006;6:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Wang JF, Li Y, Song JN, Pang HG. Role of hydrogen sulfide in secondary neuronal injury. Neurochem Int. 2014;64:37–47. doi: 10.1016/j.neuint.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Wang LY, Cai WQ, Chen PH, Deng QY, Zhao CM. Downregulation of P2X7 receptor expression in rat oligodendrocyte precursor cells after hypoxia ischemia. Glia. 2009;3:307–319. doi: 10.1002/glia.20758. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhu J, Pan Y, Dong J, Zhang L, Zhang X, Zhang L. Hydrogen sulfide functions as a neuromodulator to regulate striatal neurotransmission in a mouse model of Parkinson's disease. J Neurosci Res. 2015;3:487–494. doi: 10.1002/jnr.23504. [DOI] [PubMed] [Google Scholar]
- Wang W, Xu J, Li L, Wang P, Ji X, Ai H, Zhang L, Li L. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res Bull. 2010;83:196–201. doi: 10.1016/j.brainresbull.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Xie L, Hu LF, Teo XQ, Tiong CX, Tazzari V, Sparatore A, Del Soldato P, Dawe GS, Bian JS. Therapeutic effect of hydrogen sulfide-releasing L-Dopa derivative ACS84 on 6-OHDA-induced Parkinson's disease rat model. PLoS One. 2013;8:e60200. doi: 10.1371/journal.pone.0060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YJ, Wang AL, Guo Z, Li CS, Wang CM, Hao YY. Transplantation of bone marrow mesenchymal stem cells preconditioned with hydrogen sulfide in the treatment of rat myocardial infarction. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:8532–8538. [Google Scholar]
- Xuan A, Long D, Li J, Ji W, Zhang M, Hong L, Liu J. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in β-amyloid rat model of Alzheimer's disease. J Neuroinflammation. 2012;9:202. doi: 10.1186/1742-2094-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan A, Long D, Li J, Ji W, Zhang M, Hong L, Liu J. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol Res. 2012;84:32–44. doi: 10.1016/j.phrs.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Xue X, Bian JS. Neuroprotective effects of hydrogen sulfide in Parkinson's disease animal models: methods and protocols. Methods Enzymol. 2015;554:169–186. doi: 10.1016/bs.mie.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Yin J, Tu C, Zhao J, Ou D, Chen G, Liu Y, Xiao X. Exogenous hydrogen sulfide protects against global cerebral I/R injury via its anti-oxidative, anti-inflammatory and anti-apoptotic effects in rats. Brain Res. 2013;1491:188–196. doi: 10.1016/j.brainres.2012.10.046. [DOI] [PubMed] [Google Scholar]


