Abstract
The tumor suppressor p63 is one of p53 family members and plays a vital role as a regulator of neuronal apoptosis in the development of the nervous system. However, the role of p63 in mature neuronal death has not been addressed yet. In this study, we first compared ischemia-induced effects on p63 expression in the hippocampal regions (CA1–3) between the young and adult gerbils subjected to 5 minutes of transient global cerebral ischemia. Neuronal death in the hippocampal CA1 region of young gerbils was significantly slow compared with that in the adult gerbils after transient global cerebral ischemia. p63 immunoreactivity in the hippocampal CA1 pyramidal neurons in the sham-operated young group was significantly low compared with that in the sham-operated adult group. p63 immunoreactivity was apparently changed in ischemic hippocampal CA1 pyramidal neurons in both ischemia-operated young and adult groups. In the ischemia-operated adult groups, p63 immunoreactivity in the hippocampal CA1 pyramidal neurons was significantly decreased at 4 days post-ischemia; however, p63 immunoreactivity in the ischemia-operated young group was significantly higher than that in the ischemia-operated adult group. At 7 days post-ischemia, p63 immunoreactivity was decreased in the hippocampal CA1 pyramidal neurons in both ischemia-operated young and adult groups. Change patterns of p63 level in the hippocampal CA1 region of adult and young gerbils after ischemic damage were similar to those observed in the immunohistochemical results. These findings indicate that higher and longer-term expression of p63 in the hippocampal CA1 region of the young gerbils after ischemia/reperfusion may be related to more delayed neuronal death compared to that in the adults.
Keywords: p53 tumor suppressor gene family, cerebral ischemia/reperfusion, pyramidal neurons, CA1 region, delayed neuronal death, immunohistochemistry, western blotting, neural regeneration
Introduction
Ischemic insults occur when blood flow to the brain stops or reduces by cardiac arrest or respiratory arrest, and the ischemic insults lead to irreversible neuronal damage in some regions, including the cerebral cortex, hippocampus and striatum (Petito et al., 1987; Globus et al., 1991). The CA1 region of the hippocampus is especially well known to be highly impressionable to transient global cerebral ischemia (Kirino and Sano, 1984), and a neuronal loss in the hippocampal CA1 region gradually represents 3 to 4 days after transient ischemic injury, which has been commonly termed “delayed neuronal death” (Kirino, 1982). Possible mechanisms related with the delayed neuronal death following transient global cerebral ischemia are proposed to involve excitotoxicity, oxidative stress and inflammation (Stoll et al., 1998; White et al., 2000; An et al., 2002). However, precise intrinsic mechanisms leading to delayed neuronal death are not clearly understood yet.
As a tumor suppressor, p53 is a sequence-specific DNA binding transcription factor that plays a crucial role in the regulation of neuronal death (Banasiak and Haddad, 1998; Morrison and Kinoshita, 2000). It has been reported that activation of p53 occurs in the cellular response to stress stimuli such as DNA damage and hypoxia, which can lead to cell cycle arrest and apoptosis through controlling its many target genes (Abrahamson et al., 1995; Levine, 1997; Prives and Hall, 1999). Similarly to p53, p63, as a family member of p53, regulates the genes that mediate cell cycle arrest as well as apoptosis upon DNA damage, cooperating with or without p53 (Flores et al., 2002; Candi et al., 2007). It has been reported that, in the nervous system, p63 is known to play a critical role in regulating neuronal survival (Jacobs et al., 2004, 2006; Miller and Kaplan, 2007).
Although some investigators have focused on changes of p53 in the brain following cerebral ischemia (McGahan et al., 1998; Tounai et al., 2007), there are no reports regarding changes in p63 expression in the hippocampus following transient global cerebral ischemia. In this study, therefore, we examined chronological changes in the immunoreactivity and protein level of p63 in the hippocampus following 5 minutes of transient global cerebral ischemia in gerbils, which have been well used as an animal model of transient cerebral ischemia to investigate pathophysiology of ischemia-induced neuronal death (Bertolino et al., 2013; Malek et al., 2013; Liu et al., 2014). In addition, we compared transient global cerebral ischemia-induced changes in p63 expression in the hippocampus between the young and adult gerbils, because some researchers have proposed that the condition of neuronal death following transient cerebral ischemia is different according to the age of experimental animals (Tamagaki et al., 2000; Lee et al., 2010, 2013).
Materials and Methods
Experimental animals
We used the progeny of male Mongolian gerbils (Meriones unguiculatus) obtained from the Experimental Animal Center, Kangwon National University, Chunchon, South Korea. Gerbils aged 1 month old, weighing 25–30 g, were included in the young group and those aged 6 months old, weighing 65–75 g in the adult group. The animals were maintained in pathogen-free conditions at 23°C and 60% humidity. All the experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Kangwon National University, South Korea and adhered to guidelines that are in compliance with the current international laws and policies (Guide for the Care and Use of Laboratory Animals, The National Academies Press, 8th Ed., 2011).
Induction of transient global cerebral ischemia
As previously described (Lee et al., 2013), transient global cerebral ischemia was induced as follows. A midline ventral incision was then made in the neck, and bilateral common carotid arteries were isolated, freed of nerve fibers, and occluded using non-traumatic aneurysm clips (Yasargil FE 723K, Aesculap, Tuttlingen, Germany). The complete interruption of blood flow was confirmed by observing the central artery in retinae using an ophthalmoscope (HEINE K180®, Heine Optotechnik, Herrsching, Germany). After 5 minutes of occlusion, the aneurysm clips were removed from the common carotid arteries. The restoration of blood flow (reperfusion) was observed directly using the ophthalmoscope. Body (rectal) temperature was maintained under free-regulating or normothermic (37 ± 0.5°C) conditions with a rectal temperature probe (TR-100; Fine Science Tools, Foster City, CA, USA) and a thermometric blanket before, during and after the surgery until the animals completely recovered from anesthesia. Thereafter, animals were kept in a thermal incubator (temperature 23°C; humidity 60%) (Mirae Medical Industry, Seoul, South Korea) until the animals were euthanized. The gerbils were randomized to sham and ischemia groups and the sham-operated animals were subjected to the same surgical procedures except that the common carotid arteries were not occluded.
Tissue processing for histological examination
For histological analysis, sham- and ischemia-operated adult and young gerbils (n = 7 at each time point) at the designated time points (sham, 1 day, 4 days and 7 days after reperfusion) were sacrificed. As previously described (Lee et al., 2013), the animals were deeply anesthetized with pentobarbital sodium at the designated time points and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (PB, pH 7.4). The brains were removed and postfixed in the same fixative for 6 hours. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter, frozen tissues were serially sectioned on a cryostat (Leica, Wetzler, Germany) into 30 μm thick coronal sections, and they were then collected into 6-well plates containing PBS.
Staining for neuronal nuclei (NeuN) and Fluoro-Jade B (F-J B)
To examine neuronal damage/death in the hippocampus between the young and adult animals after transient global cerebral ischemia, immunohistochemistry for NeuN, a marker for neurons and F-J B, a high affinity fluorescent marker for the localization of neuronal degeneration, was performed according to previously described methods (Schmued and Hopkins, 2000; Park et al., 2013). In brief, the sections were sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 minutes and 10% normal goat serum in 0.05 M PBS for 30 minutes. The sections were then incubated with diluted mouse anti-NeuN (diluted 1:800; Chemicon International, Temecula, CA, USA) overnight at 4°C. Thereafter, the tissues were exposed to biotinylated goat anti-mouse IgG and streptavidin peroxidase complex (1:200; Vector, Burlingame, CA, USA). They were visualized with 3,3′-diaminobenzidine (Sigma-Aldrich, St. Louis, MO, USA) in 0.1 M Tris-HCl buffer and mounted on the gelatin-coated slides. After dehydration, the sections were mounted with Canada balsam (Kanto, Tokyo, Japan).
For F-J B staining, the sections were first immersed in a solution containing 1% sodium hydroxide in 80% alcohol, and then in 70% alcohol. They were transferred to a solution of 0.06% potassium permanganate, and transferred to a 0.0004% F-J B (Histochem, Jefferson, AR, USA) staining solution. After washing, the sections were placed on a slide warmer (approximately 50°C), and then examined using an epifluorescent microscope (Carl Zeiss, Göttingen, Germany) with blue (450–490 nm) excitation light and a barrier filter.
In order to quantitatively analyze NeuN immunoreactivity and F-J B staining, as previously described (Park et al., 2014), digital images of the hippocampus were captured with an AxioM1 light microscope (Carl Zeiss) equipped with a digital camera (Axiocam, Carl Zeiss, Germany) connected to a PC monitor. NeuN immunoreactive neurons and F-J B positive cells were counted in a 250 × 250 μm2 applied approximately at the center of the CA1 region using an image analyzing system (software: Optimas 6.5, CyberMetrics, Scottsdale, AZ, USA). The studied tissue sections were selected with 120-μm interval, and cell counts were obtained by averaging the total cell numbers of six sections taken from each animal per group.
Western blot analysis for p63
To obtain the accurate data of changes in p63 protein levels in the hippocampus after transient global cerebral ischemia, the sham- and ischemia-operated young and adult animals (n = 7 at each time point) were sacrificed at designated time points (4 and 7 days after reperfusion) and used for western blotting according to our previous method (Park et al., 2013). In brief, the brains were serially and transversely cut into a thickness of 400 μm on a vibratome (Leica), and the hippocampal CA1 regions were then dissected with a surgical blade after sacrificing them and removing the hippocampus. The tissues were homogenized in 50 mM PBS (pH 7.4) containing 0.1 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (pH 8.0), 0.2% Nonidet P-40, 10 mM ethylendiamine tetraacetic acid (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and 1 mM dithiothreitol (DTT, Santa Cruz Biotechnology, Santa Cruz, CA, USA). After centrifugation, the protein level was determined in the supernatants using a Micro BCA protein assay kit with bovine serum albumin as the standard (Pierce Chemical, Rockford, IL, USA). Aliquots containing 20 μg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM dithiothreitol, 6% SDS, 0.3% bromophenol blue and 30% glycerol. The aliquots were then loaded onto a 10% polyacrylamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Corp, East Hills, NY, USA). To reduce background staining, the membranes were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 minutes, followed by incubation with rabbit anti-p63 (1:1,000; Abcam, Cambridge, UK) overnight at 4°C. The membranes were then incubated for 2 hours with horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG (Santa Cruz Biotechnology) at room temperature. An Enhanced Chemiluminescence (ECL) kit (Pierce Chemical) was used to visualize the blots.
According to our previous method (Lee et al., 2013), the result of western blot analysis was scanned, and densitometric analysis for the quantification of the bands was done using Image J 1.46 (National Institutes of Health, Bethesda, MD, USA), which was used to count relative optical density (ROD). A ratio of the ROD was calibrated as %, with sham-operated adult group designated as 100 %.
Immunohistochemistry for p63
To obtain the accurate data for immunoreactivity, the sections from sham- and ischemia-operated young and adult animals were used at designated time points (sham, 1 day, 4 days and 7 days after reperfusion) under the same conditions. According to the above-mentioned western blot analysis method of p63, immunohistochemistry for rabbit anti-p63 (1:200; Abcam, Cambridge, UK) was performed under the same incubation temperature and time. In order to establish the specificity of the immunostaining, a negative control test was carried out with only the secondary antibody without primary antibody. The negative control resulted in the absence of immunoreactivity in any structures.
Eight sections per animal were selected to quantitatively analyze p63 immunoreactivity. Digital images of the hippocampus were captured with an AxioM1 light microscope (Carl Zeiss) equipped with a digital camera (Axiocam, Carl Zeiss, Germany) connected to a PC monitor. According to the method of our previous study (Lee et al., 2014), semi-quantification of the immunostaining intensities was evaluated with digital image analysis software (MetaMorph 4.01, Universal Imaging Corp.). The level of immunoreactivity was scaled as –, ±, + or ++ representing no staining (gray scale value C200), weakly positive (gray scale value 150–199), moderate (gray scale value 100–149), or strong (gray scale value B99), respectively.
Statistical analysis
The data shown here represent the means ± SEM. Differences of the means among the groups were statistically analyzed by one-way analysis of variance (ANOVA) with a post hoc Bonferroni's multiple comparison test in order to elucidate ischemia-related differences among experimental groups. Statistical significance was considered at P < 0.05.
Results
NeuN immunoreactive neurons and F-J B positive cells
In the sham-operated adult group, NeuN immunoreactive neurons were easily observed in the striatum pyramidale of the hippocampus, and F-J B positive cells were not observed in any region of the hippocampus (Figure 1A–C). One day after ischemia/reperfusion, the distribution pattern of the NeuN immunoreactive neurons and F-J B positive cells was similar to that in the sham-operated group (data not shown). However, in the ischemia-operated adult groups, a few NeuN immunoreactive neurons and many F-J B positive cells were found in the stratum pyramidale of the hippocampal CA1 region 4 days after ischemia/reperfusion (Table 1, Figure 1G–I). Seven days after ischemia/reperfusion, the number of NeuN immunoreactive neurons and F-J B positive cells was similar to that in the ischemia-operated adult group at 4 days post-ischemia (Table 1, Figure 1M–O).
Figure 1.
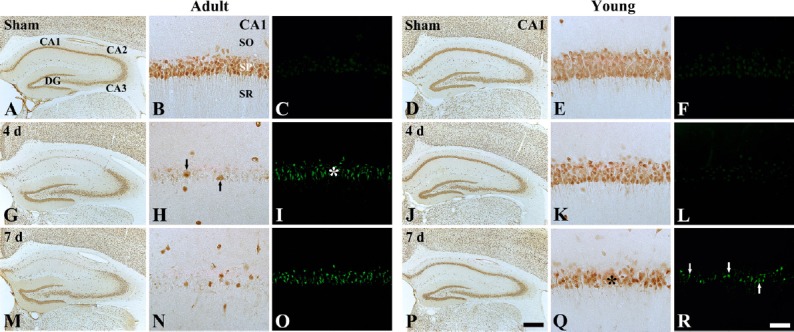
Neuronal nuclei (NeuN) immunohistochemistry and Fluoro-Jade B (F-J B) histofluorescence staining in the hippocampal CA1 region of the adult (left three columns) and young (right three columns) gerbils in the sham- (A–F) and ischemia-operated (G–R) groups.
In the ischemia-operated adult groups, a few NeuN immunoreactive neurons (black arrows) and many F-J B positive cells (white asterisk) were shown in the stratum pyramidale of the hippocampal CA1 region 4 days after ischemia/reperfusion; however, at this time point, neuronal damage is hardly found in the ischemia-operated young group. Seven days after ischemia/reperfusion, decreased NeuN immunoreactive neurons (black asterisk) and increased F-J B positive cells (white arrows) were observed in the stratum pyramidale of the hippocampal CA1 region in the ischemia-operated young group. CA: Cornus ammonis; DG: dentate gyrus; SO: stratum oriens; SR: stratum radiatum. Scale bars: 800 μm in A, D, G, J, M, P, and 50 μm in B, C, E, F, H, I, K, L, N, O, Q, R.
Table 1.
Quantification of neuronal nuclei (NeuN) immunoreactive neurons and Fluoro-Jade B (F-J B) positive cells in adult versus young gerbils
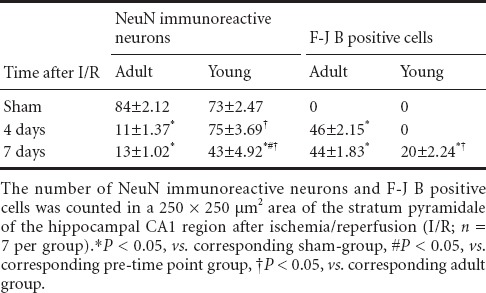
In the sham-operated young group, NeuN immunoreactive neurons in the striatum pyramidale of the hippocampus were also well observed, and no F-J B positive cells were observed in the striatum pyramidale (Figure 1D–F). In the ischemia-operated young roup at 4 days post-ischemia, the number of NeuN immunoreactive neurons in the stratum pyramidale was similar to that in the sham-operated young group, and F-J B positive cells were hardly observed in the stratum pyramidale (Table 1, Figure 1J–L). However, a significant loss of NeuN immunoreactive neurons and some F-J B positive cells was observed 7 days after ischemia/reperfusion; the number of NeuN immunoreactive neurons was significantly greater than that in the ischemia-operated adult group and the number of the F-J B positive cells was significantly less than that in the ischemia-operated adult group (Table 1, Figure 1P–R).
Changes in p63 immunoreactivity
Hippocampal CA1 region
In the sham-operated adult group, p63 immunoreactivity was strongly detected in the pyramidal neurons of the stratum pyramidale (Figure 2A). However, in the ischemia-operated adult groups, p63 immunoreactivity in the stratum pyramidale began to decrease 1 day after ischemia/reperfusion (Table 2, Figure 2C). Four days after ischemia/reperfusion, p63 immunoreactivity was hardly detected in the stratum pyramidale, and many non-pyramidale cells showed p63 immunoreaction in the stratum oriens and radiatum (Table 2, Figure 2E). Seven days after ischemia/reperfusion, the distribution pattern of p63 immunoreactivity in the ischemic hippocampal CA1 region was similar to that in the ischemia-operated adult group at 4 days post-ischemia (Table 2, Figure 2G).
Figure 2.
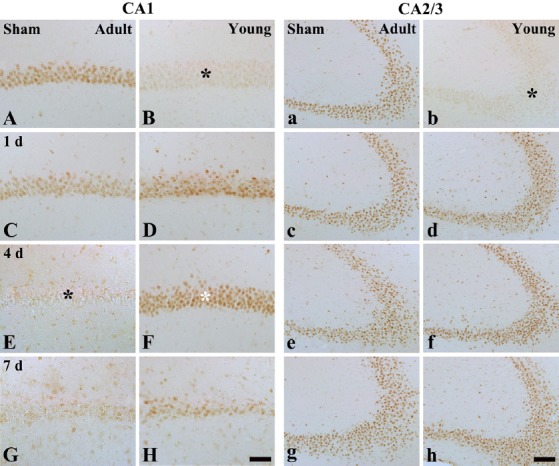
Immunohistochemistry for p63 in the hippocampal CA1 region (left two columns) and CA2/3 regions (right two columns) of the adult and young gerbils in the sham- (A, B, a and b) and ischemia-operated (C–H and c–h) groups.
In the sham-operated young group, p63 immunoreactivity in the stratum pyramidale (SP, black asterisks) was significantly lower than that in the sham-operated adult group. In the ischemia-operated adult group, p63 immunoreactivity was significantly decreased in the SP (black asterisk) of the hippocampal CA1 region 4 days after ischemia/ reperfusion. However, at this time point, p63 immunoreactivity was distinctively increased in the SP (white asterisk) of the ischemia-operated young group, and p63 immunoreactive pyramidal neurons were significantly decreased 7 days after ischemia/reperfusion. Meanwhile, in the ischemia-operated adult group, p63 immunoreactivity was not changed in the SP of the hippocampal CA2/3 regions compared with that in the sham-operated adult group. In the ischemia-operated young group, p63 immunoreactivity was similar to that in the ischemia-operated adult group. SO: Stratum oriens; SR: stratum radiatum. Scale bars: 50 μm in A–H and 100 μm in a–h.
Table 2.
Semi-quantifications of p63 immunoreactivity in the stratum pyramidale of the hippocampal CA1–3 regions of young versus adult gerbils following transient global cerebral ischemia
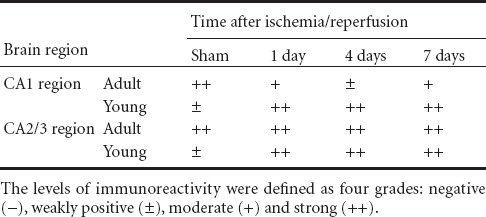
In the sham-operated young group, p63 immunoreactivity in the pyramidal neurons of the stratum pyramidale was lower than that in the sham-operated adult group (Table 2, Figure 2B). However, in the ischemia-operated young groups, p63 immunoreactivity in the stratum pyramidale was increased 1 and 4 days after ischemia/reperfusion compared with that in the sham-operated young group (Table 2, Figure 2D, F). Seven days after ischemia/reperfusion, p63 immunoreactive cells in the stratum pyramidale were decreased, and non-pyramidal cells showed p63 immunoreactivity in the stratum oriens and radiatum (Table 2, Figure 2H).
Hippocampal CA2/3 region
In the hippocampal CA2/3 region of the sham-operated adult group, p63 immunoreactivity was similar to that in the hippocampal CA1 region (Table 2, Figure 2a). In the ischemia-operated adult group, p63 immunoreactivity in the stratum pyramidale of the hippocampal CA2/3 region was not changed until 7 days post-ischemia (Table 2, Figure 2c, e, g).
In the sham-operated young group, p63 immunoreactivity in the stratum pyramidale of the hippocampal CA2/3 region was lower, like that in the hippocampal CA1 region, than that in the sham-operated adult group (Table 2, Figure 2b). However, p63 immunoreactivity was increased in the stratum pyramidale 1 day after ischemia/reperfusion and then was maintained at a stable leve until 7 days post-ischemia (Table 2, Figure 2d, f, h).
Changes in p63 protein level
The pattern of changes in p63 protein level in the adult and young hippocampal CA1 region after ischemia/reperfusion was similar to that observed in the immunohistochemical data (Figure 3).
Figure 3.
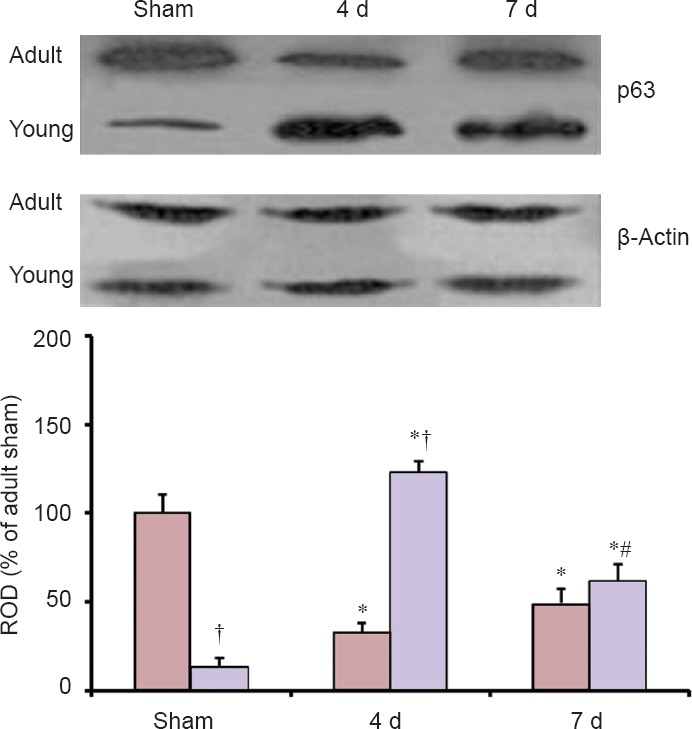
Western blot analysis of p63 protein in the hippocampal CA1 region of the adult and young gerbils in the sham- and ischemia-operated groups.
In the sham-operated young group, the protein level of p63 was significantly lower compared with that in the sham-operated adult group. Four days after ischemia/reperfusion, p63 protein level in the ischemia-operated young group was significantly higher than that in the ischemia-operated adult group. Each experiment was repeated three times, and relative optical density (ROD) was represented as % values of immunoblot band. Data were analyzed by one-way analysis of variance (ANOVA) with a post hoc Bonferroni's multiple comparison test (*P < 0.05, vs. corresponding sham-operated group, #P < 0.05, vs. corresponding pre-time point group, †P < 0.05, vs. corresponding adult group). The bars indicate the means ± SEM. d: Day(s).
p63 protein level in the sham-operated young group was significantly lower than in the sham-operated adult group. Four days after ischemia/reperfusion, p63 protein level was significantly decreased in the ischemic CA1 region of the ischemia-operated adult group; however, p63 protein level in the ischemia-operated young group was increased compared with that in the sham-operated young group. Seven days after ischemia/reperfusion, p63 protein level in the ischemia-operated adult group was increased compared with that at 4 days post-ischemia; at this time point, p63 protein level in the ischemia-operated young group was significantly decreased compared with that at 4 days post-ischemia.
Discussion
It has been well accepted that young animals have a high resistance against ischemic damage (Bertrand et al., 1996, 2000). In this study, we found that neuronal death caused by transient global cerebral ischemia in the hippocampus of young gerbil was much lower than that in the adult gerbil by NeuN immunohistochemistry and F-J B histofluorescence. This result is consistent with other and our previous studies that transient global cerebral ischemia-induced neuronal death was more delayed in the young gerbil than that in the adult gerbil (Kusumoto et al., 1995; Yan et al., 2011; Lee et al., 2013).
Flores et al. (2002) first reported that p63 acted as a pro-apoptotic protein in the developing central nervous system following gamma irradiation-induced DNA damage. In addition, Jacobs et al. (2005) showed that cultured neonatal p63–/– sympathetic neurons were highly resistant to nerve growth factor withdrawal-induced apoptosis and that embryonic p63–/– mice exhibited a noticeable deficiency in naturally occurring sympathetic neuronal death. Furthermore, it was reported that the knockdown of p63 in the embryonic telencephalon induced apoptosis of both cortical precursor cells and newly born cortical neurons (Dugani et al., 2009). As mentioned above, p63 has an important role in neuronal apoptosis during the development of the nervous system; however, the possible role of p63 in mature neuronal death has not been addressed yet. On the other hand, Bui et al. (2009) suggested that p63 is closely associated with cell survival in ischemic brain. Therefore, in this study, we first compared ischemia-induced changes of p63 expression in the hippocampal CA1/3 regions between the adult and young gerbils.
We found that p63 immunoreactivity in the pyramidal neurons of the hippocampal CA1 region, not in hippocampal CA2/3 regions, of gerbils in the ischemia-operated adult group was decreased with time and hardly found at 4 days post-ischemia. A recent study showed that the heterozygosity or acute ablation of p63 in the adult brain caused death of both neural precursor cells and adult-born neurons in the hippocampal dentate gyrus, which consequently led to hippocampal-dependent learning and memory deficits (Cancino et al., 2013). Therefore, the significant decrease of p63 expression in the adult hippocampal CA1 region after transient ischemia may be related to delayed neuronal death.
In contrast with the adult gerbils, we found that p63 immunoreactivity in the pyramidal neurons of the hippocampal CA1 region in the sham-operated young group was significantly lower than that in the sham-operated adult group. Furthermore, p63 immunoreactivity in the hippocampal CA1 pyramidal neurons of the ischemia-operated young group was significantly higher than that in the ischemia-operated adult group after ischemia/reperfusion. In addition, our present western blot result showed that change patterns in p63 protein levels in the adult and young ischemic hippocampal CA1 region were similar to those observed in the immunohistochemical data. It was reported that p63 expression was rapidly upregulated in the cerebral cortex of the young rat (postnatal day 7) after permanent focal cerebral ischemia and that the rapid induction of p63 expression initiated a transcriptional repressor Zinc finger E-box-binding homeobox 1-mediated pro-survival cascade (Bui et al., 2009). Therefore, it is likely that higher and longer-term expression of p63 in the hippocampal CA1 region of the young gerbil after ischemia/reperfusion may be related to more delayed neuronal death compared to that in the adult.
In this study, we also found that there was no change in p63 immunoreactivity in the pyramidal neurons of the ischemic hippocampal CA2/3 regions in both ischemia-operated adult and young groups. It has been well known that the hippocampal CA2/3 pyramidal neurons are relatively resistant to transient global cerebral ischemia compared with hippocampal CA1 pyramidal neurons (Kirino and Sano, 1984; Pulsinelli, 1985).
In brief, our present results show that p63 expression was apparently altered in the ischemic hippocampal CA1 region after 5 minutes of transient global cerebral ischemia and that the pattern of p63 expression in the young hippocampal CA1 region was distinctively different from that in the adult; greater and much longer-term p63 expression in the young hippocampal CA1 region was observed following transient global cerebral ischemia than in the adult hippocampal CA1 region. These findings indicate that different p63 expression levels and durations between adult and young gerbils may be associated with delayed neuronal death of the pyramidal neurons in the hippocampal CA1 region following transient global cerebral ischemia and that greater and longer-term p63 expression in the young hippocampal CA1 region after transient ischemia might be a key to explaining the difference in neuronal survival between the young and adult gerbils.
Acknowledgments
We would like to thank Mr. Sung Uk Lee for his technical help in tissue processing in this study.
Footnotes
Funding: This study was supported by 2013 Research Grant from Kangwon National University (120131480), and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A6A3A01056005).
Conflicts of Interest: None declared.
Copyedited by Yang Y, Zhai L, Zepeda A, Cavarsan C, Li CH, Song LP, Zhao M
References
- Abrahamson JL, Lee JM, Bernstein A. Regulation of p53-mediated apoptosis and cell cycle arrest by Steel factor. Mol Cell Biol. 1995;15:6953–6960. doi: 10.1128/mcb.15.12.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SJ, Kang TC, Park SK, Hwang IK, Cho SS, Chung MH, Won MH. Oxidative DNA damage and alteration of glutamate transporter expressions in the hippocampal CA1 area immediately after ischemic insult. Mol Cells. 2002;13:476–480. [PubMed] [Google Scholar]
- Banasiak KJ, Haddad GG. Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res. 1998;797:295–304. doi: 10.1016/s0006-8993(98)00286-8. [DOI] [PubMed] [Google Scholar]
- Bertolino G, De Araujo FL, Souza HC, Coimbra NC, De Araujo JE. Neuropathology and behavioral impairments after bilateral global ischemia surgery and exposure to static magnetic field: Evidence in the motor cortex, the hippocampal CA1 region and the neostriatum. Int J Radiat Biol. 2013;89:595–601. doi: 10.3109/09553002.2013.784422. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Ishii H, Spatz M. Cerebral ischemia in young and adult gerbils: effects on cholinergic metabolism. Neurochem Int. 1996;28:293–297. doi: 10.1016/0197-0186(95)00086-0. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Siren AL, Tworek D, McCarron RM, Spatz M. Differential expression of HSC73 and HSP72 mRNA and proteins between young and adult gerbils after transient cerebral ischemia: relation to neuronal vulnerability. J Cereb Blood Flow Metab. 2000;20:1056–1065. doi: 10.1097/00004647-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Bui T, Sequeira J, Wen TC, Sola A, Higashi Y, Kondoh H, Genetta T. ZEB1 links p63 and p73 in a novel neuronal survival pathway rapidly induced in response to cortical ischemia. PLoS One. 2009;4:e4373. doi: 10.1371/journal.pone.0004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancino GI, Yiu AP, Fatt MP, Dugani CB, Flores ER, Frankland PW, Josselyn SA, Miller FD, Kaplan DR. p63 Regulates adult neural precursor and newly born neuron survival to control hippocampal-dependent Behavior. J Neurosci. 2013;33:12569–12585. doi: 10.1523/JNEUROSCI.1251-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Dinsdale D, Rufini A, Salomoni P, Knight RA, Mueller M, Krammer PH, Melino G. TAp63 and DeltaNp63 in cancer and epidermal development. Cell Cycle. 2007;6:274–285. doi: 10.4161/cc.6.3.3797. [DOI] [PubMed] [Google Scholar]
- Dugani CB, Paquin A, Fujitani M, Kaplan DR, Miller FD. p63 antagonizes p53 to promote the survival of embryonic neural precursor cells. J Neurosci. 2009;29:6710–6721. doi: 10.1523/JNEUROSCI.5878-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, Jacks T. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–564. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- Globus MY, Busto R, Martinez E, Valdes I, Dietrich WD, Ginsberg MD. Comparative effect of transient global ischemia on extracellular levels of glutamate, glycine, and gamma-aminobutyric acid in vulnerable and nonvulnerable brain regions in the rat. J Neurochem. 1991;57:470–478. doi: 10.1111/j.1471-4159.1991.tb03775.x. [DOI] [PubMed] [Google Scholar]
- Jacobs WB, Govoni G, Ho D, Atwal JK, Barnabe-Heider F, Keyes WM, Mills AA, Miller FD, Kaplan DR. p63 is an essential proapoptotic protein during neural development. Neuron. 2005;48:743–756. doi: 10.1016/j.neuron.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Jacobs WB, Walsh GS, Miller FD. Neuronal survival and p73/p63/p53: a family affair. Neuroscientist. 2004;10:443–455. doi: 10.1177/1073858404263456. [DOI] [PubMed] [Google Scholar]
- Jacobs WB, Kaplan DR, Miller FD. The p53 family in nervous system development and disease. J Neurochem. 2006;97:1571–1584. doi: 10.1111/j.1471-4159.2006.03980.x. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984;62:201–208. doi: 10.1007/BF00691853. [DOI] [PubMed] [Google Scholar]
- Kusumoto M, Arai H, Mori K, Sato K. Resistance to cerebral ischemia in developing gerbils. J Cereb Blood Flow Metab. 1995;15:886–891. doi: 10.1038/jcbfm.1995.110. [DOI] [PubMed] [Google Scholar]
- Lee CH, Yoo KY, Choi JH, Park OK, Hwang IK, Kim SK, Kang IJ, Kim YM, Won MH. Neuronal damage is much delayed and microgliosis is more severe in the aged hippocampus induced by transient cerebral ischemia compared to the adult hippocampus. J Neurol Sci. 2010;294:1–6. doi: 10.1016/j.jns.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Lee CH, Park JH, Cho JH, Ahn JH, Yan BC, Lee JC, Shin MC, Cheon SH, Cho YS, Kwon YG, Lee DK, Kim YM, Won MH. Changes and expressions of Redd1 in neurons and glial cells in the gerbil hippocampus proper following transient global cerebral ischemia. J Neurol Sci. 2014;344:43–50. doi: 10.1016/j.jns.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Yan BC, Park JH, Ahn JH, Kim IH, Lee JC, Lee HY, Kim YM, Won MH, Cho JH. Differences of calcium binding proteins immunoreactivities in the young hippocampal CA1 region from the adult following transient ischemic damage. J Neurol Sci. 2013;326:40–47. doi: 10.1016/j.jns.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Liu YR, Lei RY, Wang CE, Zhang BA, Lu H, Zhu HC, Zhang GB. Effects of catalpol on ATPase and amino acids in gerbils with cerebral ischemia/reperfusion injury. Neurol Sci. 2014;35:1229–1233. doi: 10.1007/s10072-014-1687-7. [DOI] [PubMed] [Google Scholar]
- Malek M, Duszczyk M, Zyszkowski M, Ziembowicz A, Salinska E. Hyperbaric oxygen and hyperbaric air treatment result in comparable neuronal death reduction and improved behavioral outcome after transient forebrain ischemia in the gerbil. Exp Brain Res. 2013;224:1–14. doi: 10.1007/s00221-012-3283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahan L, Hakim AM, Robertson GS. Hippocampal Myc and p53 expression following transient global ischemia. Brain Res Mol Brain Res. 1998;56:133–145. doi: 10.1016/s0169-328x(98)00038-2. [DOI] [PubMed] [Google Scholar]
- Miller FD, Kaplan DR. To die or not to die: neurons and p63. Cell Cycle. 2007;6:312–317. doi: 10.4161/cc.6.3.3795. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Kinoshita Y. The role of p53 in neuronal cell death. Cell Death Differ. 2000;7:868–879. doi: 10.1038/sj.cdd.4400741. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee CH, Kim IH, Ahn JH, Cho JH, Yan BC, Lee JC, Lee TH, Seo JY, Won MH, Kang IJ. Time-course changes in immunoreactivities of glucokinase and glucokinase regulatory protein in the gerbil hippocampus following transient cerebral ischemia. Neurochem Res. 2013;38:2640–2649. doi: 10.1007/s11064-013-1182-1. [DOI] [PubMed] [Google Scholar]
- Park JH, Park O, Cho JH, Chen BH, Kim IH, Ahn JH, Lee JC, Yan BC, Yoo KY, Lee CH, Hwang IK, Kwon SH, Lee YL, Won MH, Choi JH. Anti-inflammatory effect of tanshinone I in neuroprotection against cerebral ischemia-reperfusion injury in the gerbil hippocampus. Neurochem Res. 2014;39:1300–1312. doi: 10.1007/s11064-014-1312-4. [DOI] [PubMed] [Google Scholar]
- Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112–126. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA. Selective neuronal vulnerability: morphological and molecular characteristics. Prog Brain Res. 1985;63:29–37. doi: 10.1016/S0079-6123(08)61973-1. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Tamagaki C, Murata A, Asai S, Takase K, Gonno K, Sakata T, Kinoshita T. Age-related changes of cornu ammonis 1 pyramidal neurons in gerbil transient ischemia. Neuropathology. 2000;20:221–227. doi: 10.1046/j.1440-1789.2000.00344.x. [DOI] [PubMed] [Google Scholar]
- Tounai H, Hayakawa N, Kato H, Araki T. Immunohistochemical study on distribution of NF-kappaB and p53 in gerbil hippocampus after transient cerebral ischemia: effect of pitavastatin. Meta Brain Dis. 2007;22:89–104. doi: 10.1007/s11011-006-9040-3. [DOI] [PubMed] [Google Scholar]
- White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Yan BC, Park JH, Lee CH, Yoo KY, Choi JH, Lee YJ, Cho JH, Baek YY, Kim YM, Won MH. Increases of antioxidants are related to more delayed neuronal death in the hippocampal CA1 region of the young gerbil induced by transient cerebral ischemia. Brain Res. 2011;1425:142–154. doi: 10.1016/j.brainres.2011.09.063. [DOI] [PubMed] [Google Scholar]


