Abstract
The Wnt/β-catenin signaling pathway plays a crucial role in neural development, axonal guidance, neuropathic pain remission and neuronal survival. In this study, we initially examined the effect of rapamycin on the Wnt/β-catenin signaling pathway after spinal cord injury, by intraperitoneally injecting spinal cord injured rats with rapamycin over 2 days. Western blot analysis and immunofluorescence staining were used to detect the expression levels of β-catenin protein, caspase-3 protein and brain-derived neurotrophic factor protein, components of the Wnt/β-catenin signaling pathway. Rapamycin increased the levels of β-catenin and brain-derived neurotrophic factor in the injured spinal cord, improved the pathological morphology at the injury site, reduced the loss of motor neurons, and promoted motor functional recovery in rats after spinal cord injury. Our experimental findings suggest that the neuroprotective effect of rapamycin intervention is mediated through activation of the Wnt/β-catenin signaling pathway after spinal cord injury.
Keywords: nerve regeneration, spinal cord injury, rapamycin, Wnt/β-catenin signaling pathway, apoptosis, caspase-3, brain-derived neurotrophic factor, neuroprotection, loss of neurons, NSFC grants, neural regeneration
Introduction
Rapamycin, an antifungal agent, is clinically utilized as an efficient immunosuppressant after solid organ transplantation (Chen et al., 2013; Li et al., 2013). Recent evidence of pleiotropic effects of rapamycin has aroused much concern; for example, rapamycin inhibits the growth of abdominal aortic aneurysms, restrains glioblastoma cancer stem cells and alleviates brain edema (Guo et al., 2014; Mendiburu-Eliçabe et al., 2014; Rouer et al., 2014). Some reports have claimed that rapamycin can induce autophagy, reduce neural tissue damage and improve the recovery of locomotor function after spinal cord injury (SCI) (Sekiguchi et al., 2012; Wang et al., 2014). However, the specific molecular mechanism of action of rapamycin after SCI has not been elucidated.
Wnts are a family of glycoproteins (Cao, 2013). The Wnt/β-catenin signaling pathway, a canonical Wnt signaling pathway, plays a significant role in neural development, axonal guidance, neuropathic pain remission and neuronal survival (Suh et al., 2011; Zhang et al., 2013). Thus, studies investigating the roles of the Wnt/β-catenin signaling pathway may provide a novel molecular mechanism and therapeutic target for treating SCI.
In the present study, we hypothesized that rapamycin would have an impact on the Wnt/β-catenin signaling pathway after SCI, and aimed to explore the expected neuroprotective mechanism of action of rapamycin in the treatment of SCI.
Materials and Methods
Animals
Seventy-two adult male Sprague-Dawley rats (of specific pathogen-free grade, aged 8–12 weeks, weighing 250–300 g) were purchased from the Laboratory Animal Centre of Liaoning Medical University (Jinzhou, Liaoning Province, China; license No. SCXK (Liao) 2003-0011). Rats were raised in the SPF Laboratory Animal Center, which maintained the environment at 23 ± 0.5°C with an alternating 12-hour light/dark cycle. The experimental procedure had full ethical approval from the Animal Ethics Committee of Liaoning Medical University in China. The rats were randomly divided into three groups: a rapamycin group (n = 24), a saline group (n = 24) and a sham group (n = 24).
Establishment of SCI model in rats
SCI models were established using the modified Allen technique (Yacoub et al., 2014). Briefly, the rats in the rapamycin and saline groups were anesthetized with 10% chloral hydrate (0.33 mL/kg) via intraperitoneal injection, the backsides of rats were disinfected and incised, and the spinal cords were clearly exposed after spinous T9/10 was removed. Then, a striker (weight: 10 g, diameter: 2 mm) was dropped from a height of 25 mm. The injury was rapidly induced and both lower extremities of rats also quickly withdrew and trembled, while gatism was also observed. Rats were not selected for the study until the SCI model was successfully established. However, the sham-operated animals (sham group) underwent laminectomy alone after being anesthetized. Then, rats were injected with antibiotics for 3 successive days and the bladder was massaged three times daily to accelerate the recovery of automatic micturition function.
Drug administration
Rapamycin (Life Technologies, Carlsbad, CA, USA) was dissolved in DMSO at a concentration of 25 mg/mL, and diluted to a final concentration of 1 mg/mL with saline before being injected. Then, rapamycin (0.5 mg/kg) and saline (0.5 mL/kg) were administered to rats in the rapamycin and saline groups via intraperitoneal injection at 1 hour after SCI and then once daily for 2 days (Chen et al., 2013).
Functional analysis
The Basso, Beattie & Bresnahan (BBB) open-field locomotor rating scale was utilized to evaluate the recovery of motor function in rats after SCI (Basso et al., 1995). Briefly, three independent examiners were blinded to assess BBB scores before operation and at 1, 3, 7, 14, 21 and 28 days after SCI. The BBB scores ranged from 0 to 21 points. The minimum score (0) indicated complete paralysis, and the maximum score implied normal function. The average scores were calculated according to the progression of locomotion recovery after SCI.
Western blot analysis
The rats were severally narcotized with 10% chloral hydrate (0.33 mL/kg) via intraperitoneal injection at 3 and 5 days post-surgery, and the T8–11 spinal cord (2 mm cephalad and caudally from the epicenter) was dissected out. The tissues were homogenized in RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% Na-deoxycholate, 1 mM EDTA). Then, the concentrations of proteins were assessed using the bicinchoninic acid kit, adjusted to 2 μg/μL, and 40 μg of protein was resolved on 12% Tris-glycine SDS-PAGE gels. Then, the proteins were transferred to polyvinylidene fluoride membranes and incubated with primary antibodies (rabbit anti-β-catenin polyclonal antibody, 1:1,000, Abcam, Cambridge, UK; rabbit anti-caspase-3 polyclonal antibody, 1:1,000, Abcam; mouse anti-β-actin polyclonal antibody, 1:1,000, Abcam) at 4°C overnight after being sealed. On the following day, the membranes were washed three times with Tris-buffered saline (TBS; 150 mM NaCl, 100 mM Tris-HCl, pH 7.4) containing 0.1% Tween-20, and then incubated with goat anti-rabbit/mouse IgG (1:3,000, Abcam) at room temperature for 2 hours. Finally, the membranes were developed using a ChemiDoc-It™ TS2 Imager (UVP, LLC, Upland, CA, USA) and relative optical density was measured using ImageJ2x software (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence analysis
At 7 days after the operation, rats were anesthetized with 10% chloral hydrate (0.33 mL/kg) and then perfused with 4% paraformaldehyde. In brief, spinal cord tissues (T8/9) 3 mm rostral to the epicenter were harvested from the rats and fixed in 4% paraformaldehyde. Tissues were immersed in 30% sucrose overnight and sliced into cross-sections at a thickness of 5 μm. Sections were placed into a box with 0.01 M citric acid for antigen retrieval. Then, the sections were blocked using blocking buffer (5% normal goat serum and 0.1% Triton-X100 in PBS) at 4°C for 1 hour and incubated with primary antibodies (rabbit anti-β-catenin polyclonal antibody,1:400, Abcam; rabbit anti-BDNF polyclonal antibody, 1:500, Novus Biologicals, Littleton, CO, USA) at 4°C overnight. Next, the tissues were incubated with FITC-goat anti-rabbit IgG (1:400, Abcam) and the nuclei were stained with DAPI (1:1,000) solution. All slides were observed under a fluorescence microscope (Leica, Heidelberger, Germany) after being mounted with Permount™ Mounting Medium (Sigma-Aldrich, St. Louis, MO, USA). The optical density of fluorescence was analyzed using ImageJ2x software.
Nissl staining
To observe the morphology and number of neurons in different groups, spinal cord tissues (T8/9, 3 mm rostral to the epicenter) were captured from the rats at 7 days after SCI. Cross-sections at 20 μm thickness were dried at room temperature and placed into miscible liquids (alcohol/chloroform = 1:1) overnight. Then, the slices were dehydrated through 100% alcohol, 95% alcohol and distilled water. The slices were stained in 0.1% cresyl violet (Sigma-Aldrich) solution at 40°C for 10 minutes and rinsed quickly in distilled water. Subsequently, the slices were differentiated in 95% ethyl alcohol for 5 minutes, dehydrated in 100% alcohol and cleared in xylene for 5 minutes. The slices were mounted and observed under a light microscope (Leica). The average quantities of Nissl bodies were counted by randomly selecting four Nissl-stained sections at the same site from each animal.
Statistical analysis
All data are expressed as the mean ± SD and were analyzed using the Graph Prism Program, Version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons between the two groups were performed using the unpaired Student's t-test; discrepancies among multiple groups were tested using one-way analysis of variance, and the BBB scores were analyzed using the Mann-Whitney U test. P-values less than 0.05 were considered statistically significant.
Results
Rapamycin treatment improved locomotion function of SCI rats
As shown in Figure 1, the BBB scores of rats were 21 points before the operation in all three groups and decreased to 0 point at 1 day after SCI. However, the tendency of BBB scores of rats showed no significant difference from 1 to 14 days after SCI in the rapamycin and saline groups (P > 0.05). At 14, 21, and 28 days post-operation, the BBB scores of rats in the rapamycin group were obviously higher than those of rats in the saline group (P < 0.05). These results indicate that the rats’ locomotion function was improved by rapamycin intervention after SCI.
Figure 1.

Rapamycin treatment improves locomotion function of rats after spinal cord injury.
The Basso, Beattie & Bresnahan (BBB) scores ranged from 0 to 21 points. The minimum number of points (0) indicated complete paralysis and the maximum number of points represented normal function. Data are expressed as the mean ± SD of five rats for each group and were analyzed by the Mann-Whitney U test. #P < 0.05, vs. saline group.
Rapamycin treatment improved pathologic morphology of spinal neurons in SCI rats
Nissl staining showed that the quantities of Nissl bodies in the spinal cord were obviously lower in the saline group than in the sham group after SCI. On the contrary, compared with the saline group, SCI was distinctly improved and the damaged tissue was also clearly reduced by rapamycin. At the same time, the quantities of motor neurons in the anterior horns were elevated (P < 0.01) and the morphology of neurons from rats in the rapamycin group was perfected compared with that of neurons from rats in the saline group (Figure 2).
Figure 2.
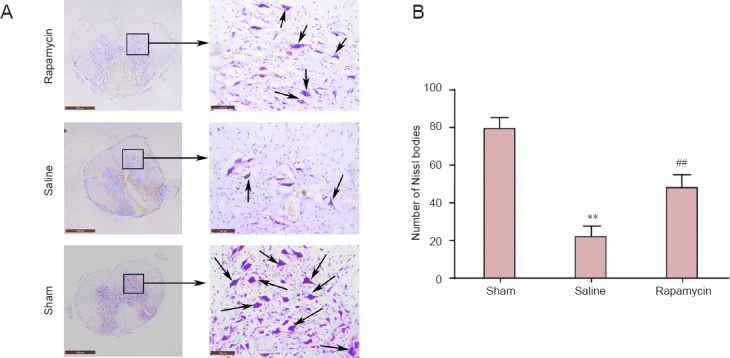
Rapamycin treatment improves morphology of spinal neurons in rats with spinal cord injury.
(A) Morphology of spinal neurons in rats (Nissl staining). Arrows indicate Nissl bodies. Scale bars: left, 500 μm; right, 100 μm. (B) Quantity of Nissl bodies in spinal neurons. Data are expressed as the mean ± SD (n = 4). Comparisons between groups were performed using the unpaired Student's t-test, and discrepancies among multiple groups were tested using one-way analysis of variance. **P < 0.01, vs. sham group; ##P < 0.01, vs. saline group.
The Wnt/β-catenin signaling pathway was activated by rapamycin in the spinal cords of SCI rats
The β-catenin protein, the hallmark protein of the canonical Wnt signaling pathway (Reya and Clevers, 2005), was detected by western blot analysis (Figure 3A) and immunofluorescence staining (Figure 4A). The expression level of β-catenin protein in the spinal cords of rats was significantly increased at 3 and 5 days after SCI in the presence of rapamycin, compared with the levels in the absence of rapamycin (saline group) (P < 0.05 or P < 0.01; Figure 3B). In addition, the intensity of fluorescence for β-catenin in the spinal cords of rats was stronger in the rapamycin group than in the other groups (P < 0.05 or P < 0.01; Figure 4B). These findings suggest that the Wnt/β-catenin signaling pathway was significantly enhanced by rapamycin in the spinal cords of SCI rats.
Figure 3.
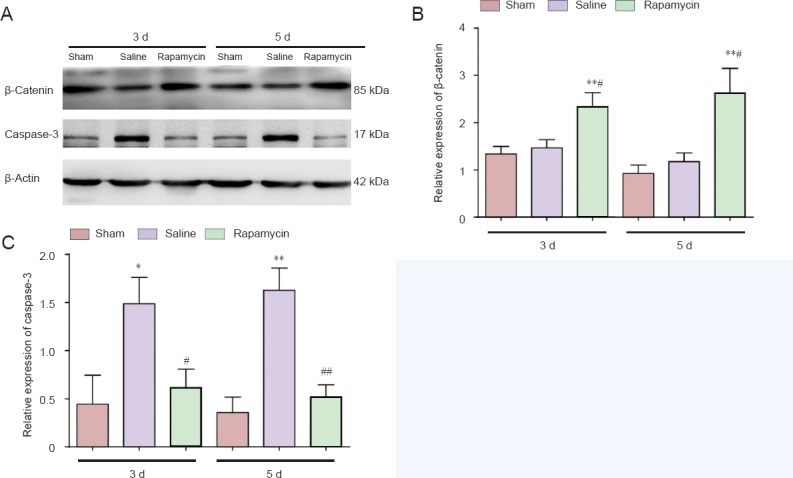
Effect of rapamycin treatment on the expression of β-catenin and caspase-3 in the spinal cord of rats with spinal cord injury.
(A) The expression levels of β-catenin protein and caspase-3 protein in the three groups were detected by western blot analysis. (B, C) Effect of rapamycin treatment on the expression of β-catenin and caspase-3 in the injured spinal cord. Relative expression levels are expressed as the optical density ratio of target proteins to β-actin. Data are expressed as the mean ± SD (n = 3). Comparisons between groups were performed using the unpaired Student's t-test and discrepancies among multiple groups were tested using one-way analysis of variance. *P < 0.05, **P < 0.01, vs. sham group; #P < 0.05, ##P < 0.01, vs. saline group. 3 d, 5 d: 3, 5 days after spinal cord injury.
Figure 4.
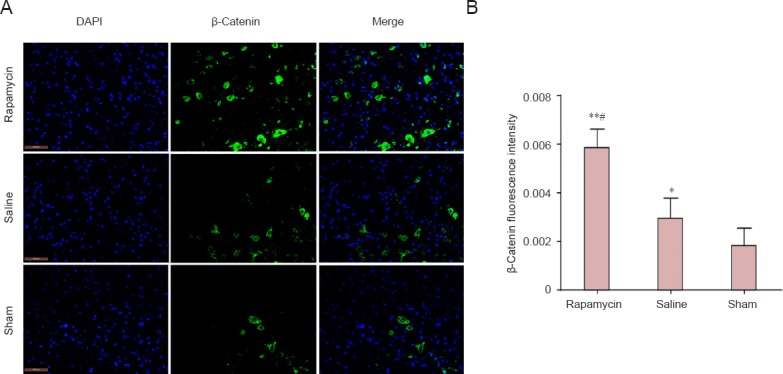
Effect of rapamycin treatment on β-catenin immunoreactivity in the spinal cord of rats after spinal cord injury.
(A) β-Catenin immunoreactivity (green) in the rat spinal cord (immunofluorescence staining). FITC was used here was a fluorescent indicator of β-catenin immunoreactivity. Scale bars: 100 μm. (B) β-Catenin fluorescence intensity in the rat spinal cord. All data are expressed as the mean ± SD (n = 3). Comparisons between groups were performed using the unpaired Student's t-test and discrepancies among multiple groups were tested using one-way analysis of variance. *P < 0.05, **P < 0.01, vs. sham group; #P < 0.05, vs. saline group.
Rapamycin inhibited apoptosis in the spinal cords of SCI rats
The expression level of caspase-3 protein in the spinal cords of SCI rats was assessed at 3 and 5 days after SCI by western blot analysis (Figure 3A). Compared with the sham group, the level of caspase-3 expression in the spinal cords of rats was increased in the saline group; compared with the saline group, the level of caspase-3 expression in the spinal cord of rats was reduced in the rapamycin group (Figure 3C, day 3: P < 0.05, day 5: P < 0.01). These results implied that rapamycin treatment inhibited apoptosis after SCI.
Rapamycin increased BDNF expression in the spinal cord of SCI rats
At day 7 after injury, immunofluorescence analysis was utilized to detect BDNF expression in the three groups of rats (Figure 5A). The relative intensity of BDNF fluorescence in the spinal cord of rats was stronger in the rapamycin group than in the other two groups (Figure 5B, P < 0.05 or P < 0.01). The level of BDNF fluorescence in the spinal cord of rats was dramatically boosted by rapamycin, which provided a beneficial environment for functional recovery after SCI.
Figure 5.
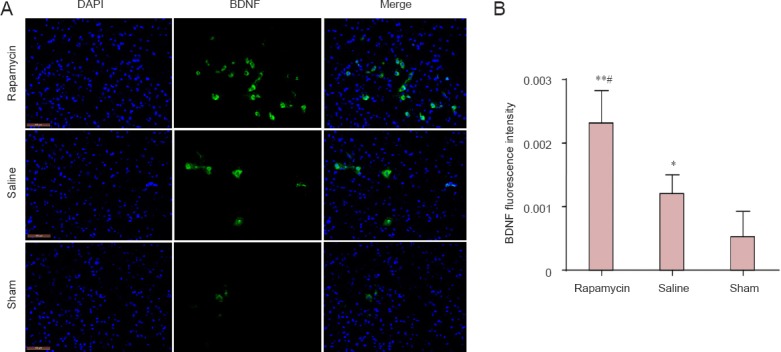
Effect of rapamycin treatment on brain-derived neurotrophic factor (BDNF) immunoreactivity in the spinal cord of rats with spinal cord injury.
(A) BDNF immunoreactivity in the rat spinal cord (immunofluorescence analysis). FITC was used here as a fluorescent indicator of BDNF immunoreactivity. Scale bars: 100 μm. (B) BDNF fluorescence intensity in the rat spinal cord. All data are expressed as the mean ± SD (n = 3). Comparisons between groups were performed using the unpaired Student's t-test and discrepancies among multiple groups were tested using one-way analysis of variance. *P < 0.05, **P < 0.01, vs. sham group; #P < 0.05, vs. saline group.
Discussion
In the present study, the expression of β-catenin protein was increased by rapamycin intervention after SCI, implying that the Wnt/β-catenin signaling pathway was activated by rapamycin after SCI. In addition, the expression level of BDNF protein was enhanced by rapamycin; on the contrary, rapamycin treatment inhibited caspase-3 expression after SCI. Furthermore, the BBB scores and Nissl staining indicated that rapamycin treatment decreased the loss of motor neurons and improved the recovery of locomotor function after SCI. Collectively, these findings suggest that the neuroprotective effect of rapamycin on SCI is mediated through activation of the Wnt/β-catenin signaling pathway.
Secondary injury plays an important part in the process of SCI, which offers a potential clinical therapeutic target for SCI (Xie et al., 2014). Secondary injury is induced by various factors: apoptosis, oxidative stress, immunodeficiency and inflammation (Hayashi et al., 2000; Profyris et al., 2004). Unfortunately, the effect of methylprednisolone, a first-line drug for treating SCI, has been questioned (Hall and Springer, 2004; Wilson and Fehlings, 2011; Fehlings et al., 2014). Hence, our study aimed to identify an alternative effective drug and illuminate the specific molecular mechanism of action of the drug.
Rapamycin is a lipophilic macrolide antibiotic produced by Streptomyces hygroscopicus, which is developed as an immunosuppressive agent in the clinic (Raught et al., 2001; Wang et al., 2014). There is growing evidence that rapamycin can inhibit tumor proliferation and has a cytostatic effect against human cancers (Vignot et al., 2005; Rouer et al., 2014; Weng et al., 2014). Interestingly, an increasing number of effects of rapamycin on SCI has attracted the attention of researchers worldwide; these include anti-inflammation, immunoregulation, autophagy promotion and neuroprotective effects (Kanno et al., 2012; Sekiguchi et al., 2012; Sontag et al., 2013). In the current study, the recovery of locomotor function was reflected in the BBB scores of rats, and the results revealed that rapamycin obviously improved locomotor neurological function at 14 days post-injury. Nissl staining indicated that rapamycin also reduced the loss of motor neurons and accelerated the recovery from injury after SCI. These results have shown that locomotor function is improved by rapamycin after SCI; however, the molecular mechanism of this effect of rapamycin on SCI has not been elucidated.
Wnts are a well-characterized family of glycoproteins, which include at least three different signaling pathways: the canonical Wnt/β-catenin pathway, the noncanonical planar cell polarity pathway, and the Wnt-Ca2+ pathway (Fernández-Martos et al., 2011; Wang et al., 2013). The Wnt/β-catenin signaling pathway plays a significant role in SCI. Briefly, β-catenin receives a signal from membrane receptors and then transmits this signal to the nucleus, where it binds Lef/Tcf transcription factors, ultimately activating target genes (Rao and Kühl, 2010). The β-catenin protein is the central player, and it promotes the regeneration of axons, inhibits apoptosis and improves functional recovery after SCI (Suh et al., 2011; Hollis ER 2nd and Zou, 2012; Sun et al., 2013; González-Fernández et al., 2014). Studies of the Wnt/β-catenin pathway may offer an original mechanism for treating SCI. Therefore, a hypothesis that the neuroprotective effect of rapamycin on SCI is mediated through influencing the Wnt/β-catenin signaling pathway was initially put forward. In the present study, the expression of β-catenin protein was detected by western blot analysis and immunofluorescence staining. Western blot analysis results suggested that the expression level of β-catenin was significantly increased by rapamycin treatment after SCI. Analogously, immunofluorescence analysis showed that β-catenin was also markedly increased in the presence of rapamycin. These phenomena clearly show that the Wnt/β-catenin signaling pathway is activated by rapamycin and suggests an underlying mechanism for the action of rapamycin following clinical application.
To further verify the neuroprotective effect of rapamycin on SCI, apoptosis and neurotrophic factors were observed in our experiment. Some researchers have reported that apoptosis is activated after SCI, which increases the loss of neurons and retards neural functional recovery. During apoptosis, the expression of Apaf-1 protein, Bcl-2 protein and caspase family members is altered; in other words, the expression of caspase-3, a vital member of the caspase family, is elevated after apoptosis (Korsmeyer, 1999; Yuan and Yankner, 2000). A decrease in the expression level of caspase-3 protein suggests that apoptosis has been inhibited, and the number of neurons should be preserved and function improved (Fernandes-Alnemri et al., 1994; Sakurai et al., 2003). In addition, recent evidence indicates that the increase in BNDF expression establishes an environment for nerves to recover, reducing the loss of nerves and apoptosis, and accelerating functional recovery after SCI (Kamei et al., 2007; Sasaki et al., 2009). In our current study, western blot analysis demonstrated that the expression level of caspase-3 was evidently lowered by rapamycin; conversely, the expression of BDNF was visibly enhanced by rapamycin in our immunofluorescence analysis.
Our findings jointly indicate that rapamycin treatment inhibited the apoptosis of neurons, increased the number of neurons, improved recovery after injury and increased the expression of BDNF, ultimately exerting a significant neuroprotective effect on SCI, via activation of the Wnt/β-catenin signaling pathway. This significant discovery demonstrates the molecular mechanism underlying the neuroprotective effect of rapamycin treatment after SCI, which could promote clinical application of rapamycin for treating SCI. Nevertheless, the effect of rapamycin has been studied only on neurons in our study, and it will be necessary to further study the effects of rapamycin on astrocytes/microglia after SCI. In addition, ours are only preliminary findings regarding the effect of rapamycin on the Wnt/β-catenin signaling pathway after SCI; further studies are needed to verify the various effects of rapamycin on the Wnt signaling pathway at different time points in rats after SCI. Simultaneously, the in-depth molecular mechanism underlying its effects requires further validation in different neural cells through both in vitro and in vivo experiments, to provide evidence supporting the clinical application of rapamycin.
Acknowledgments
We thank Jing Bi and Dong-he Han from Department of Neurobiology and Key Laboratory of Neurodegenerative Diseases of Liaoning Province, Liaoning Medical University, Jinzhou, China, for providing valuable technical assistance in this work.
Footnotes
Funding: This work was supported by grants from the National Natural Science Foundation of China, No. 81171799, 81471854 and a Special Financial Grant from the China Postdoctoral Science Foundation, No. 2013T60948.
Conflicts of Interest: None declared.
Copyedited by McGowan D, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Cao M. wnt signal pathway in neurogenesis. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:358–362. [Google Scholar]
- Chen HC, Fong TH, Hsu PW, Chiu WT. Multifaceted effects of rapamycin on functional recovery after spinal cord injury in rats through autophagy promotion, anti-inflammation, and neuroprotection. J Surg Res. 2013;179:e203–210. doi: 10.1016/j.jss.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61:36–42. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- Fernández-Martos CM, González-Fernández C, González P, Maqueda A, Arenas E, Rodríguez FJ. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One. 2011;6:e27000. doi: 10.1371/journal.pone.0027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- González-Fernández C, Fernández-Martos CM, Shields SD, Arenas E, Javier Rodríguez F. Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma. 2014;31:565–581. doi: 10.1089/neu.2013.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Feng G, Miao Y, Liu G, Xu C. Rapamycin alleviates brain edema after focal cerebral ischemia reperfusion in rats. Immunopharmacol Immunotoxicol. 2014;36:211–223. doi: 10.3109/08923973.2014.913616. [DOI] [PubMed] [Google Scholar]
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma. 2000;17:203–218. doi: 10.1089/neu.2000.17.203. [DOI] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Zou Y. Expression of the Wnt signaling system in central nervous system axon guidance and regeneration. Front Mol Neurosci. 2012;5:5. doi: 10.3389/fnmol.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei N, Tanaka N, Oishi Y, Hamasaki T, Nakanishi K, Sakai N, Ochi M. BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine. 2007;32:1272–1278. doi: 10.1097/BRS.0b013e318059afab. [DOI] [PubMed] [Google Scholar]
- Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Tateda S, Yahata K, Itoi E. The role of mTOR signaling pathway in spinal cord injury. Cell Cycle. 2012;11:3175–3179. doi: 10.4161/cc.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–1700s. [PubMed] [Google Scholar]
- Li XD, He Y, Wang DN, Hu Y, Wang WW, Zhang XZ. Rapamycin for the treatment of refractory extensive chronic graft-versus-host disease. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:3287–3294. [Google Scholar]
- Mendiburu-Eliçabe M, Gil-Ranedo J, Izquierdo M. Efficacy of rapamycin against glioblastoma cancer stem cells. Clin Transl Oncol. 2014;16:495–502. doi: 10.1007/s12094-013-1109-y. [DOI] [PubMed] [Google Scholar]
- Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Rao TP, Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci U S A. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Rouer M, Xu BH, Xuan HJ, Tanaka H, Fujimura N, Glover KJ, Furusho Y, Gerritsen M, Dalman RL. Rapamycin limits the growth of established experimental abdominal aortic aneurysms. Eur J Vasc Endovasc. 2014;47:493–500. doi: 10.1016/j.ejvs.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Nagata T, Abe K, Horinouchi T, Itoyama Y, Tabayashi K. Survival and death-promoting events after transient spinal cord ischemia in rabbits: Induction of Akt and caspase3 in motor neurons. J Thorac Cardiovasc Surg. 2003;125:370–377. doi: 10.1067/mtc.2003.112. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Radtke C, Tan AM, Zhao P, Hamada H, Houkin K, Honmou O, Kocsis JD. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting and protection of corticospinal neurons after spinal cord injury. J Neurosci. 2009;29:14932–14941. doi: 10.1523/JNEUROSCI.2769-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E. Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma. 2012;29:946–956. doi: 10.1089/neu.2011.1919. [DOI] [PubMed] [Google Scholar]
- Sontag CJ, Nguyen HX, Kamei N, Uchida N, Anderson AJ, Cummings BJ. Immunosuppressants affect human neural stem cells in vitro but not in an in vivo model of spinal cord injury. Stem Cells Transl Med. 2013;2:731–744. doi: 10.5966/sctm.2012-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HI, Min J, Choi KH, Kim SW, Kim KS, Jeon SR. Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir (Wien) 2011;153:1003–1010. doi: 10.1007/s00701-011-0945-1. [DOI] [PubMed] [Google Scholar]
- Sun L, Pan J, Peng Y, Wu Y, Li J, Liu X, Qin Y, Bauman WA, Cardozo C, Zaidi M, Qin W. Anabolic steroids reduce spinal cord injury-related bone loss in rats associated with increased Wnt signaling. J Spinal Cord Med. 2013;36:616–622. doi: 10.1179/2045772312Y.0000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang HT, Wang SH, Zhang YB. Correlation of Alzheimer's disease with Wnt signaling pathway and neural stem cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:3566–3572. [Google Scholar]
- Wang ZY, Liu WG, Muharram A, Wu ZY, Lin JH. Neuroprotective effects of autophagy induced by rapamycin in rat acute spinal cord injury model. Neuroimmunomodulation. 2014;21:257–267. doi: 10.1159/000357382. [DOI] [PubMed] [Google Scholar]
- Weng M, Gong W, Ma M, Chu B, Qin Y, Zhang M, Lun X, McFadden G, Forsyth P, Yang Y, Quan Z. Targeting gallbladder cancer: oncolytic virotherapy with myxoma virus is enhanced by rapamycin in vitro and further improved by hyaluronan in vivo. Mol Cancer. 2014;13:82. doi: 10.1186/1476-4598-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, Fehlings MG. Emerging approaches to the surgical management of acute traumatic spinal cord injury. Neurotherapeutics. 2011;8:187–194. doi: 10.1007/s13311-011-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B, Yue YS, Zhu Y, Wang JW, Cheng J. Electrical stimulation of the pudendal nerve for neurogenic bladder dysfunction after spinal cord injury: a literature research on functional reconstruction. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:7498–7502. [Google Scholar]
- Yacoub A, Hajec MC, Stanger R, Wan W, Young H, Mathern BE. Neuroprotective effects of perflurocarbon (oxycyte) after contusive spinal cord injury. J Neurotrauma. 2014;31:256–267. doi: 10.1089/neu.2013.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zhang YK, Huang ZJ, Liu S, Liu YP, Song AA, Song XJ. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest. 2013;123:2268–2286. doi: 10.1172/JCI65364. [DOI] [PMC free article] [PubMed] [Google Scholar]


