Abstract
Hydrogen can relieve tissue-damaging oxidative stress, inflammation and apoptosis. Injection of hydrogen-rich saline is an effective method for transporting molecular hydrogen. We hypothesized that hydrogen-rich saline would promote the repair of spinal cord injury induced by Allen's method in rats. At 0.5, 1, 2, 4, 8, 12 and 24 hours after injury, then once daily for 2 weeks, 0.25 mL/kg hydrogen-rich saline was infused into the subarachnoid space through a catheter. Results at 24 hours, 48 hours, 1 week and 2 weeks after injury showed that hydrogen-rich saline markedly reduced cell death, inflammatory cell infiltration, serum malondialdehyde content, and caspase-3 immunoreactivity, elevated serum superoxide dismutase activity and calcitonin gene-related peptide immunoreactivity, and improved motor function in the hindlimb. The present study confirms that hydrogen-rich saline injected within 2 weeks of injury effectively contributes to the repair of spinal cord injury in the acute stage.
Keywords: nerve regeneration; spinal cord injury; hydrogen-rich saline; reactive oxygen species; physiological saline; oxidative stress; Basso, Beattie and Bresnahan score; malondialdehyde; superoxide dismutase; calcitonin gene-related peptide; caspase-3; neural regeneration
Introduction
Traumatic spinal cord injury (SCI) occurs in two phases: primary and secondary injury (Xie et al, 2014; Zhao et al., 2014). The phase in which treatment is started is often critical in determining the final outcome, and thus provides a practical target for therapeutic intervention (Cao et al., 2003; McKenna et al., 2005; Fan et al., 2013; Hao et al., 2013; Yang et al., 2014). The regulatory mechanism underlying secondary injury is complex, and various clinical treatments have been explored. For example, resveratrol has been shown to attenuate oxidative stress and inflammation in rats with SCI, an effect which may be dependent on signaling via the peroxisome proliferator-activated receptor gamma (PPARγ), Wnt/β-catenin, and insulin-like growth factor 1 (IGF-1) (Wang et al., 2013a, b). In addition, delayed administration of certain concentrations of complement component 5a (C5a) inhibits caspase-3-mediated neuronal apoptosis in SCI, and also promotes neurite outgrowth in uninjured neurons (Guo et al., 2013). Reactive oxygen species (i.e., O2− and H2O2) can damage proteins, nucleic acids and lipids, resulting in cytotoxicity. Thus, they play an important role in secondary injury, particularly in neuronal tissue because of its abundance of polyunsaturated fatty acids (Hall, 1989; Hall and Braughler, 1993; Genovese and Cuzzocrea, 2008; Chen et al., 2010). Various investigations have therefore been conducted with the aim of developing a suitable antioxidant treatment for SCI (Genovese et al., 2006; Xiong and Hall, 2009).
Hydrogen is effective against oxidative stress, inflammation, and apoptosis-induced tissue injuries, and as such is beneficial in many diseases (Xie et al., 2012a, b). The application of saline containing a therapeutic dose of hydrogen (hydrogen-rich saline) is a useful method by which to deliver molecular hydrogen (Cai et al., 2009; Chen et al., 2010; Qian et al., 2010; Zhou et al., 2013). Xu et al. (2013) showed that antioxidant hydrogen-rich saline has therapeutic potential in unilateral ureteric obstruction-induced renal injury in rats. The benefits of hydrogen-rich saline are not limited to the kidney; it also reduces acute spinal cord contusion injury, possibly by decreasing oxidative stress, inflammation and apoptosis while increasing brain-derived neurotrophic factor expression and activation of the mitochondrial adenosine triphosphate-dependent potassium (mitoKATP) channel (Chen et al., 2010; Zhou et al., 2013). However, the actions of hydrogen-rich saline in SCI have not yet been investigated in a systemic study. Neuronal apoptosis occurs when the spinal cord is injured; this is an important pathological change in secondary SCI (Emery et al., 1988) and can be observed between 6 hours and 21 days after injury (Crowe et al., 1997). Caspase-3 plays an important role in cell apoptosis. Therefore, in the present study, we compared the effects of physiological and hydrogen-rich saline administration on caspase-3 expression and other measures of spinal cord repair in rats with SCI at 6, 24 and 48 hours, and 1 and 2 weeks, after injury.
Materials and Methods
Animals
Seventy-five male adult Sprague-Dawley rats weighing 250–300 g were purchased from the Laboratory Animal Center of Central South University in China. The rats were housed in pathogen-free colonies at 20–30°C and 45–60% relative humidity under a 12-hour light/dark cycle, and were allowed free access to food and drinking water. All procedures involving animals were approved by the Animal Ethics Committee of Central South University, China.
Hydrogen-rich saline production
Hydrogen-rich saline-producing apparatus (Central South University) was used to produce hydrogen-rich saline, as described previously (Li et al., 2013). The hydrogen-saturated (0.6 mM) saline was stored at 4°C and sterilized by gamma radiation before use. Hydrogen-rich saline was freshly prepared every week and hydrogen content was measured by gas chromatography (Ohsawa et al., 2007).
Experimental groups
After 1 week of habituation, rats were randomly assigned to a sham-operated group (n = 25) and an SCI group (n = 50). Blood pressure and hindlimb performance were analyzed using the Basso, Beattie and Bresnahan (BBB) scoring system to ensure there were no differences between the two groups before the start of the experiment (Basso et al., 1995). SCI rat models were established in the SCI group according to a modification of a previously described method (Gruner, 1992). Briefly, rats were anaesthetized with 10% chloral hydrate (0.33 mL/100 g intraperitoneally). Following anesthesia, the vertebrae at T9–11 were exposed and a dorsal laminectomy was performed at the T10 level to expose the dura. The T9 and T11 vertebrae were held with stabilization clamps. A moderate contusion was created by dropping a 10 g stainless-steel weight onto the exposed cord from a height of 4 cm, and muscle and skin were sutured. Sham-operated rats only had their spinous process and vertebrae lamina removed before suturing. After surgery, the rats with SCI underwent BBB scoring again to ensure there were no differences between the rats in this group, and were equally and randomly divided into two sub-groups.
Thus, all rats were assigned to the following three groups: (1) sham-operated plus physiological saline (SP, n = 25); (2) SCI plus hydrogen-rich saline (SH, n = 25); (3) SCI plus physiological saline (SSP, n = 25). A catheter was implanted in the subarachnoid space (Wang et al., 2013a, b) to receive hydrogen-rich or physiological saline.
Animals had free access to food, water, and a heat pad. Temperature and hydration were carefully monitored for 24 hours after injury. Bladders were expressed manually three times a day until spontaneous voiding returned. All animals received preventive penicillin (2 × 105 U) twice a day for 48 hours after injury. Physiological or hydrogen-rich saline (0.25 mL/kg) was injected through the catheter at 0.5, 1, 2, 4, 8, 12 and 24 hours after injury, then once a day until sacrifice. Two investigators blinded to the experimental groups analyzed hindlimb function using the BBB scoring system at 6, 24 and 48 hours and 1 and 2 weeks postoperatively.
Tissue processing
Immediately after BBB scoring, rats were anesthetized with 10% chloral hydrate (0.33 mL/100 g intraperitoneally) and fixed on an operating table in the supine position. The skin was disinfected, the chest opened to expose the heart, and the pericardium was lifted with forceps and carefully separated. A 2 mL blood sample was taken from the right ventricle and stored at −80°C until use. The rats were then perfused with 50 mL ice cold isotonic saline followed by 150 mL paraformaldehyde (4% w/v in 0.1 M PBS; pH 6.9) through a cannula inserted in the ascending aorta. After 5 minutes, a 10 mm segment of spinal cord, centered on T10, was removed and stored in paraformaldehyde (4% w/v), and immersed in 0.02% sodium azide at 4°C until analysis.
Serum superoxide dismutase (SOD) and malondialdehyde (MDA) analysis
Serum SOD activity and MDA content were analyzed using spectrophotometric kits (Nanjing Jiancheng Biotechnology Institute, Nanjing, China) in accordance with the manufacturer's instructions and as reported previously (Ren et al., 2012b).
Morphological analyses
Spinal cord tissues were fixed with paraformaldehyde (4%, w/v) in PBS at 4°C, dehydrated through a graded ethanol series, and embedded in paraffin wax. The tissues were sectioned (5 μm thick) and mounted on slides, before being dewaxed, hydrated, and stained with hematoxylin and eosin. Under a light microscope, the integrity of neurons, axons and glial cells, nuclear staining, and infiltration of inflammatory cells were analyzed by two investigators who were blind to the experimental grouping.
Immunohistochemistry
Spinal cord tissue was fixed with paraformaldehyde (4%, w/v) for 24 hours at room temperature, and embedded in paraffin. Expression of caspase-3 and calcitonin gene-related peptide (CGRP) was measured in 3 mm paraffin-embedded sections. The sections were dewaxed in xylene, rehydrated in ethanol, and antigen retrieval was performed by microwaving (two cycles of 5 minutes each at 780 W) in ethylenediamine tetraacetic acid buffer (pH 8.0). Pure methanol and 30% hydrogen peroxide (9:1 ratio) were used to clear endogenous enzymes. Endogenous biotin and nonspecific signals were blocked by incubation with normal goat serum for 20 minutes at 20°C. For immunohistochemistry, the slides were incubated with primary antibodies (anti-CGRP and rabbit anti-caspase-3, 1:100 dilution) for 2 hours at room temperature in a humid chamber, washed in PBS, and visualized by biotinylated secondary antibodies (goat, 1:200), followed by incubation with horseradish peroxidase-conjugated streptavidin (R&D Systems, London, UK) for 30 minutes. Conditions that produced the darkest labeling of immunopositive cells combined with the lowest background staining determined the working concentrations of primary and secondary antibodies.
The chromogen was 3,3′-diaminobenzidine free base.
CGRP immunohistochemistry was performed and the optical density of CGRP-positive regions was measured in Image-Pro Plus 6.0 analysis software using the following formula: optical density = mean integrated optical density/optical density of selected positive expression area. Caspase-3-positive cells were identified by their brown nuclei under a high power lens, counted in five non-overlapping horizontal fields of view per section, and the mean value was calculated.
Statistical analysis
Data are presented as the mean ± SD and were tested to confirm normal distribution. One-way analysis of variance and the least significant difference post hoc test were used to analyze differences between groups using SPSS 16.0 software (SPSS, Chicago, IL, USA). The significance level was set at α = 0.05.
Results
Hydrogen-rich saline improved motor function in rat hindlimb after SCI
The BBB score was notably lower in the SH and SSP groups than in the SP group at 6, 24 and 48 hours and 1 and 2 weeks after surgery (P < 0.05; Table 1), confirming the success in spinal cord injury induction (Chen et al., 2010). Animals that received hydrogen-rich saline had significantly higher BBB scores than those that received physiological saline, from 24 hours after surgery until the end of the experiment (P < 0.05; Table 1).
Table 1.
Effects of hydrogen-rich saline on hindlimb motor function, SOD activity and MDA content, and spinal cord CGRP and caspase-3 immunoreactivity in rats with SCI
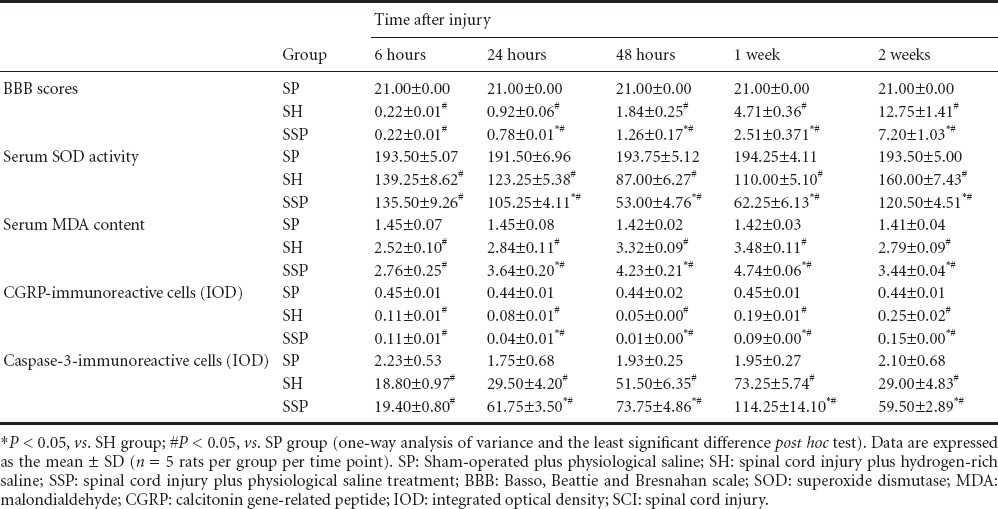
Hydrogen-rich saline reduced cell death in the injured spinal cord
SCI induced distinct cell death, hemorrhage and inflammatory cell infiltration, consistent with a previous report (Chen et al., 2010). No significant microscopic lesions were found in the SP group. However, in the SH and SPP groups, a large number of microscopic lesions, such as cell death, hemorrhage and inflammatory cell infiltration, were observed after injury (Figure 1). Lesions were less severe in the SH group than in the SPP group (Figure 1).
Figure 1.
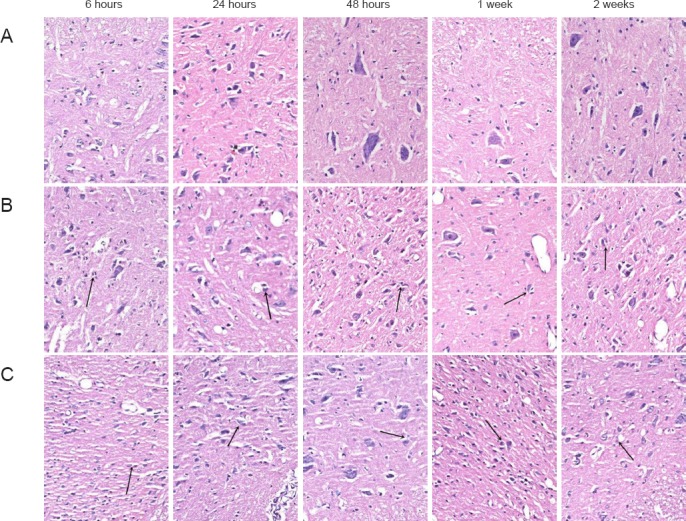
Effects of hydrogen-rich saline on cell morphology in rats with spinal cord injury (hematoxylin-eosin staining, × 200).
(A) SP group (sham-operated plus physiological saline); (B) SH group (spinal cord injury plus hydrogen-rich saline); (C) SSP group (spinal cord injury plus physiological saline). No lesion was observed on the rat spinal cord tissue in the SP group. In the SH and SSP groups, neuronal death (arrows), destruction of gray matter, and infiltration of inflammatory cells were observed. The SSP group showed more damage than the SH group.
Effects of hydrogen-rich saline on CGRP immunoreactivity in SCI rats
SCI significantly decreased CGRP expression compared to that observed in the SP group (P < 0.05), with the lowest expression occurring 48 hours after injury (Table 1; Figure 2). Hydrogen-rich saline significantly reversed this decrease in CGRP expression at 24 and 48 hours, 1 and 2 weeks after surgery (P < 0.05; Table 1; Figure 2).
Figure 2.
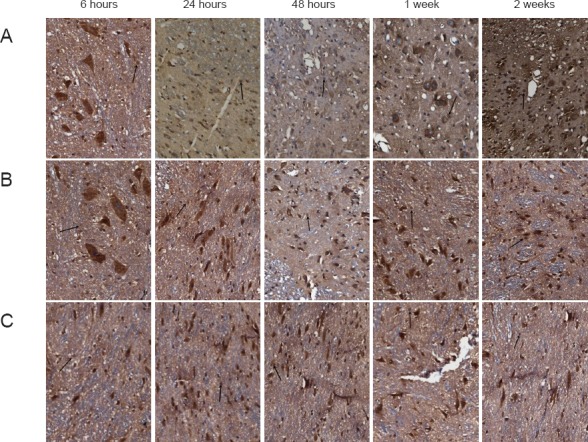
Effects of hydrogen-rich saline on calcitonin gene-related peptide (CGRP) immunoreactivity in rats with spinal cord injury (immunohistochemical staining, × 200).
(A) SP group (sham-operated plus physiological saline); (B) SH group (spinal cord injury plus hydrogen-rich saline); (C) SSP group (spinal cord injury plus physiological saline). CGRP staining (arrows) in the SH and SSP groups was lighter than in the SP group, and that in the SSP group was lighter than that of the SH group.
Hydrogen-rich saline reduced caspase-3 overexpression in SCI model rats
SCI significantly increased caspase-3 expression compared to that observed in the SP group (P < 0.05; Table 1; Figure 3), with the highest expression observed 1 week after injury. Caspase-3 protein expression was significantly lower in the SH group than in the SPP group from 24 hours until the end of the experiment (P < 0.05; Table 1; Figure 3).
Figure 3.
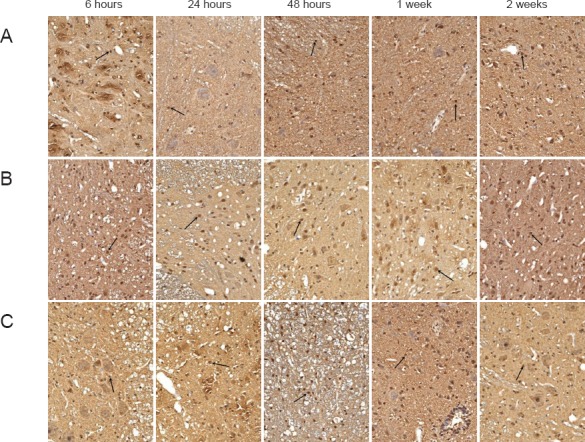
Effects of hydrogen-rich saline on caspase-3 immunoreactivity in rats with spinal cord injury (immunohistochemical staining, × 200).
(A) SP group (sham-operated plus physiological saline); (B) SH group (spinal cord injury plus hydrogen-rich saline); (C) SSP group (spinal cord injury plus physiological saline). Nuclei of caspase-3-immunoreactive apoptotic cells (arrows) were stained brown, and positive cells could be seen at each stage in the SH and SSP groups. At 24 and 48 hours and 1 and 2 weeks after the injury, caspase-3 expression was greater in the SSP group than in the SH group.
Effects of hydrogen-rich saline on serum MDA content and total SOD activity in SCI rats
SCI significantly reduced the serum total SOD activity compared to that of the SP group (P < 0.05; Table 1), with the lowest activity at 48 hours post injury. Hydrogen-rich saline significantly reversed this decrease in SOD activity at all time points after 6 hours, compared with SCI rats that received physiological saline (P < 0.05; Table 1). Conversely, SCI significantly increased serum MDA content compared with that observed in the SP group (P < 0.05; Table 1), with the highest expression occurring 1 week after injury. MDA content was significantly lower in the SH group than in the SPP group at 24 and 48 hours, 1 and 2 weeks after injury (P < 0.05; Table 1).
Discussion
We have shown that administration of hydrogen-rich saline alleviates histological and functional damage after SCI, reducing cell death and increasing hindlimb motor function, consistent with previous studies (Chen et al., 2010; Zhou et al., 2013). Indeed, the beneficial roles of hydrogen-rich saline in the brain extend beyond SCI. Li et al. (2010) and Wang et al. (2011) reported that hydrogen-rich saline prevents neuroinflammation and oxidative stress induced by amyloid beta, possibly by attenuation of c-Jun N-terminal kinase and nuclear factor-κB activity. Furthermore, hydrogen-rich saline also improves histological and functional measures in a rat model of carbon monoxide encephalopathy, showing potential as a treatment for patients with severe carbon monoxide poisoning presenting with delayed neurologic sequelae (Sun et al., 2011). In addition, hydrogen-rich saline has a notable beneficial effect in rats with transient global cerebral ischemia/reperfusion injury induced by deep hypothermic circulatory arrest (Ji et al., 2011; Shen et al., 2011), as well as protective roles in the kidney (Wang et al., 2011), intestine (He et al., 2013), lung (Xiao et al., 2013), heart (Zhang et al., 2011) and eye (Wei et al., 2012).
The mechanism underlying the roles of hydrogen-rich saline in SCI involves reducing oxidative damage by acting on MDA and SOD. MDA is a highly reactive compound, generated from reactive oxygen species. SOD catalyzes the dismutation of superoxide (O2−) into oxygen and hydrogen peroxide, thus conferring a critical antioxidant response in nearly all cells when they are exposed to oxygen. Several compelling observations have demonstrated that SCI decreases SOD activity and increases MDA levels (Ozgiray et al., 2011; Song et al., 2013; Zhou et al., 2013). In the present study, hydrogen-rich saline treatment significantly alleviated this SCI-induced change, consistent with previous findings (Chen et al., 2010; Zhou et al., 2013). Li et al. (2012) showed that hydrogen-rich saline is dose-dependently neuroprotective against permanent focal cerebral ischemia, mediated in part by a reduction in oxidative stress. Similarly, Hou et al. (2012) also observed that hydrogen-rich saline protects synaptic plasticity and cognition after mild traumatic brain injury by decreasing oxidative damage. Moreover, the antioxidant properties of hydrogen-rich saline relieve the effects of traumatic pancreatitis (Ren et al., 2012a, b). However, the biological mechanism underlying its effects on oxidative function warrants further investigation. The benefits of hydrogen-rich saline have also been implicated in immune processes, such as the immune response to viral infection (Ren et al., 2013a, b) and vaccination (Ren et al., 2013c, d, e), and innate immune activation (Ren et al., 2013d, e). It is therefore of great interest to validate the biological functions of hydrogen-rich saline in these immune processes. Mounting evidence indicates that SCI decreases CGRP expression (Jaerve et al., 2011; Kim et al., 2013). Caspase-3, one member of the cysteine-aspartic acid protease (caspase) family, plays a central role in cell apoptosis via interaction with caspase-8 and -9. Since apoptosis is also involved in the secondary phase of SCI, it is not surprising that caspase-3 is elevated in SCI (Chen et al., 2010; Lu et al., 2013). Hydrogen-rich saline treatment reverses the elevation of caspase-3 in SCI, consistent with previous evidence (Chen et al., 2010; Zhou et al., 2013).
Together, these data indicate that hydrogen-rich saline alleviates SCI via a mechanism involving the antioxidant system, CGRP, and caspase-3. To our knowledge, the present study is the first to investigate the systemic functions of hydrogen-rich saline after SCI. Our results contribute important data to the body of evidence indicating that hydrogen-rich saline is a promising potential treatment for SCI and other diseases associated with oxidative damage, inflammation and apoptosis.
Acknowledgments
We are very grateful to Wen-kai Ren from Chinese Academy of Sciences, China for his critical review of this manuscript.
Footnotes
Funding: This study was supported by a grant from Hunan Provincial Science and Technology Ministry of China, No. 2015JJ6116.
Conflicts of Interest: None declared.
Copyedited by Slone-Murphy J, Hindle A, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Cai J, Kang Z, Liu K, Liu W, Li R, Zhang JH, Luo X, Sun X. Neuroprotective effects of hydrogen saline in neonatal hypoxia-ischemia rat model. J Brain Res. 2009;1256:129–137. doi: 10.1016/j.brainres.2008.11.048. [DOI] [PubMed] [Google Scholar]
- Cao HQ, Dong ED. An update on spinal cord injury research. J Neurosci Bull. 2013;29:94–102. doi: 10.1007/s12264-012-1277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen Q, Mao Y, Xu S, Xia C, Shi X, Zhang JH, Yuan H, Sun X. Hydrogen-rich saline protects against spinal cord injury in rats. J Neurochem Res. 2010;35:1111–1118. doi: 10.1007/s11064-010-0162-y. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apotosis after traumatic human spinal cord injury. J Neurosurg. 1998;89:911–20. doi: 10.3171/jns.1998.89.6.0911. [DOI] [PubMed] [Google Scholar]
- Fan H, Liu X, Tang HB, Xiao P, Wang YZ, Ju G. Protective effects of Batroxobin on spinal cord injury in rats. J Neurosci Bull. 2013;29:501–508. doi: 10.1007/s12264-013-1354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Menegazzi M, Di Paola R, Muià C, Crisafulli C, Bramanti P, Suzuki H, Cuzzocrea S. Neuroprotection and enhanced recovery with hypericum perforatum extract after experimental spinal cord injury in mice. J Shock. 2006;25:608–617. doi: 10.1097/01.shk.0000209560.54328.69. [DOI] [PubMed] [Google Scholar]
- Genovese T, Cuzzocrea S. Role of free radicals and poly (ADP-ribose) polymerase-1 in the development of spinal cord injury: new potential therapeutic targets. J Curr Med Chem. 2008;15:477–487. doi: 10.2174/092986708783503177. [DOI] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–116. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Guo Q, Cheng J, Zhang J, Su B, Bian C, Lin S, Zhong C. Delayed post-injury administration of C5a improves regeneration and functional recovery after spinal cord injury in mice. J Clin Exp Immunol. 2013;174:318–325. doi: 10.1111/cei.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED. Free radicals and CNS injury. J Crit Care Clin. 1989;5:793–805. [PubMed] [Google Scholar]
- Hall ED, Braughler JM. Free radicals in CNS injury. J Res Publ Assoc Res Nerv Ment Dis. 1993;71:81–105. [PubMed] [Google Scholar]
- Hao HH, Wang L, Guo ZJ, Bai L, Zhang RP, Shuang WB, Jia YJ, Wang J, Li XY, Liu Q. Valproic acid reduces autophagy and promotes functional recovery after spinal cord injury in rats. J Neurosci Bull. 2013;29:484–492. doi: 10.1007/s12264-013-1355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Xiong S, Zhang J, Wang J, Sun A, Mei X, Sun X, Zhang C, Wang Q. Protective effects of hydrogen-rich saline on ulcerative colitis rat model. J Surg Res. 2013;185:174–181. doi: 10.1016/j.jss.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Hou Z, Luo W, Sun X, Hao S, Zhang Y, Xu F, Wang Z, Liu B. Hydrogen-rich saline protects against oxidative damage and cognitive deficits after mild traumatic brain injury. J Brain Res Bull. 2012;88:560–565. doi: 10.1016/j.brainresbull.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Jaerve A, Schiwy N, Schmitz C, Mueller HW. Differential effect of aging on axon sprouting and regenerative growth in spinal cord injury. J Exp Neurol. 2011;231:284–294. doi: 10.1016/j.expneurol.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Ji Q, Hui K, Zhang L, Sun X, Li W, Duan M. The effect of hydrogen-rich saline on the brain of rats with transient ischemia. J Surg Res. 2011;168:e95–101. doi: 10.1016/j.jss.2011.01.057. [DOI] [PubMed] [Google Scholar]
- Kim JY, Oh CH, Huang X, Kim MH, Yoon SH, Kim KH, Park H, Park HC, Park SR, Choi BH. Improvement in sensory function via granulocyte-macrophage colony-stimulating factor in rat spinal cord injury models. J Neurosurg Spine. 2013;18:69–75. doi: 10.3171/2012.9.SPINE1235. [DOI] [PubMed] [Google Scholar]
- Li GM, Ji MH, Sun XJ, Zeng QT, Tian M, Fan YX, Li WY, Li N, Yang JJ. Effects of hydrogen-rich saline treatment on polymicrobial sepis. J Surg Res. 2013;181:279. doi: 10.1016/j.jss.2012.06.058. [DOI] [PubMed] [Google Scholar]
- Li J, Wang C, Zhang JH, Cai JM, Cao YP, Sun XJ. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer's disease by reduction of oxidative stress. J Brain Res. 2010;1328:152–161. doi: 10.1016/j.brainres.2010.02.046. [DOI] [PubMed] [Google Scholar]
- Li J, Dong Y, Chen H, Han H, Yu Y, Wang G, Zeng Y, Xie K. Protective effects of hydrogen-rich saline in a rat model of permanent focal cerebral ischemia via reducing oxidative stress and inflammatory cytokines. J Brain Res. 2012;1486:103–111. doi: 10.1016/j.brainres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Lu PG, Feng H, Yuan SJ, Zhang RW, Li M, Hu R, Liu ZS, Yin J. Effect of preconditioning with hyperbaric oxygen on neural cell apoptosis after spinal cord injury in rats. J Neurosurg Sci. 2013;57:253–258. [PubMed] [Google Scholar]
- McKenna MT, Michaud CM, Murray CJ, Marks JS. Assessing the burden of disease in the United States using disability-adjusted life years. J Am J Prev Med. 2005;28:415–423. doi: 10.1016/j.amepre.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K, Wantanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama K, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. J Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- Ozgiray E, Serarslan Y, Oztürk OH, Altas M, Aras M, Söğüt S, Yurtseven T, Oran I, Zileli M. Protective effects of edaravone on experimental spinal cord injury in rats. J Pediatr Neurosurg. 2011;47:254–260. doi: 10.1159/000335400. [DOI] [PubMed] [Google Scholar]
- Qian L, Li B, Cai J, Gao F. The hypothesis of an effective safe and novel radioprotective agent: hydrogen-rich solution. J West Indian Med J. 2010;59:122–124. [PubMed] [Google Scholar]
- Ren J, Luo Z, Tian F, Wang Q, Li K, Wang C. Hydrogen-rich saline reduces the oxidative stress and relieves the severity of trauma-induced acute pancreatitis in rats. J Trauma Acute Care Surg. 2012a;72:1555–1561. doi: 10.1097/TA.0b013e31824a7913. [DOI] [PubMed] [Google Scholar]
- Ren W, Yin Y, Liu G, Yu X, Li Y, Yang G, Li T, Wu G. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. J Amino Acids. 2012b;42:2089–2094. doi: 10.1007/s00726-011-0942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Li Y, Yu X, Luo W, Liu G, Shao H, Yin Y. Glutamine modifies immune responses of mice infected with porcine circovirus type 2. J Br J Nutr. 2013a;110:1053–1060. doi: 10.1017/S0007114512006101. [DOI] [PubMed] [Google Scholar]
- Ren W, Luo W, Wu M, Liu G, Yu X, Fang J, Lin T, Yin Y, Wu G. Dietary L-glutamine supplementation improves pregnancy outcome in mice infected with type-2 porcine circovirus. J Amino Acids. 2013b;45:479–488. doi: 10.1007/s00726-011-1134-5. [DOI] [PubMed] [Google Scholar]
- Ren W, Yu R, Liu G, Li N, Peng Y, Wu M, Yin Y, Li Y, Fatufe AA, Li T. DNA vaccine encoding the major virulence factors of Shiga toxin type 2e (Stx2e)-expressing Escherichia coli induces protection in mice. J Vaccine. 2013c;31:367–372. doi: 10.1016/j.vaccine.2012.10.107. [DOI] [PubMed] [Google Scholar]
- Ren W, Zou L, Li N, Wang Y, Liu G, Peng Y, Ding J, Cai L, Yin Y, Wu G. Dietary arginine supplementation enhances immune responses to inactivated Pasteurella multocida vaccination in mice. J Br J Nutr. 2013d;109:867–872. doi: 10.1017/S0007114512002681. [DOI] [PubMed] [Google Scholar]
- Ren W, Zou L, Ruan Z, Li N, Wang Y, Peng Y, Liu G, Yin Y, Li T, Hou Y, Wu G. Dietary L-proline supplementation confers immunostimulatory effects on inactivated Pasteurella multocida vaccine immunized mice. J Amino Acids. 2013e;45:555–561. doi: 10.1007/s00726-013-1490-4. [DOI] [PubMed] [Google Scholar]
- Shen L, Wang J, Liu K, Wang C, Wang C, Wu H, Sun Q, Sun X, Jing H. Hydrogen-rich saline is cerebroprotective in a rat model of deep hypothermic circulatory arrest. J Neurochem Res. 2011;36:1501–1511. doi: 10.1007/s11064-011-0476-4. [DOI] [PubMed] [Google Scholar]
- Song Y, Liu J, Zhang F, Zhang J, Shi T, Zeng Z. Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. J Life Sci. 2013;92:1215–1221. doi: 10.1016/j.lfs.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Sun Q, Cai J, Zhou J, Tao H, Zhang JH, Zhang W, Sun XJ. Hydrogen-rich saline reduces delayed neurologic sequelae in experimental carbon monoxide toxicity. J Crit Care Med. 2011;39:765–769. doi: 10.1097/CCM.0b013e318206bf44. [DOI] [PubMed] [Google Scholar]
- Wang C, Li J, Liu Q, Yang R, Zhang JH, Cao YP, Sun XJ. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-kappaB activation in a rat model of amyloid-beta-induced Alzheimer's disease. J Neurosci Lett. 2010;491:127–132. doi: 10.1016/j.neulet.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Wang F, Yu G, Liu SY, Li JB, Wang JF, Bo LL, Qian LR, Sun XJ, Deng XM. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J Surg Res. 2011;167:e339–344. doi: 10.1016/j.jss.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Wang HD, Shi YM, Li L, Guo JD, Zhang YP, Hou SX. Treatment with resveratrol attenuates sublesional bone loss in spinal-cord-injured rats. J Br J Pharmacol. 2013a;170:796–806. doi: 10.1111/bph.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Sun JF, Tang YX, Guo GW, Zhou XZ, Chen YL, Shen MR. Basic fibroblast growth factor attenuates the degeneration of injuried spinal cord motor endplates. Neural Rege Res. 2013b;8:2213–2224. doi: 10.3969/j.issn.1673-5374.2013.24.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Ge L, Qin S, Shi Y, Du C, Du H, Liu L, Yu Y, Sun X. Hydrogen-rich saline protects retina against glutamate-induced excitotoxic injury in guinea pig. J Exp Eye Res. 2012;94:117–127. doi: 10.1016/j.exer.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Xiao M, Zhu T, Wang T, Wen FQ. Hydrogen-rich saline reduces airway remodeling via inactivation of NF-kappaB in a murine model of asthma. J Eur Rev Med Pharmacol Sci. 2013;17:1033–1043. [PubMed] [Google Scholar]
- Xie B, Yue YS, Zhu Y, Wang JW, Cheng J. Electrical stimulation of the pudendal nerve for neurogenic bladder dysfunction after spinal cord injury: a literature research on functional reconstruction. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:7498–7502. [Google Scholar]
- Xie K, Fu W, Xing W, Li A, Chen H, Han H, Yu Y, Wang G. Combination therapy with molecular hydrogen and hyperoxia in a murine model of polymicrobial sepsis. J Shock. 2012a;38:656–663. doi: 10.1097/SHK.0b013e3182758646. [DOI] [PubMed] [Google Scholar]
- Xie K, Yu Y, Huang Y, Zheng L, Li J, Chen H, Han H, Hou L, Gong G, Wang G. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. J Shock. 2012b;37:548–555. doi: 10.1097/SHK.0b013e31824ddc81. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hall ED. Pharmacological evidence for a role of peroxynitrite in the pathophysiology of spinal cord injury. J Exp Neurol. 2009;216:105–114. doi: 10.1016/j.expneurol.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zhang YB, Li ZZ, Yang MW, Wang S, Jiang DP. Hydrogen-rich saline ameliorates renal injury induced by unilateral ureteral obstruction in rats. J Int Immunopharmacol. 2013;17:447–452. doi: 10.1016/j.intimp.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Yang BB, Chen JT, Song XH. Proteome changes in a rat model of spinal cord injury after intrathecal injection of methylprednisolone. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:7897–7902. [Google Scholar]
- Zhang Y, Sun Q, He B, Xiao J, Wang Z, Sun X. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. J Int J Cardiol. 2011;148:91–95. doi: 10.1016/j.ijcard.2010.08.058. [DOI] [PubMed] [Google Scholar]
- Zhao B, Chen B, Zhao HY, Wang SK. Expression of sonic hedgehog in adult rat models of acute compressive spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:7903–7907. [Google Scholar]
- Zhou L, Wang X, Xue W, Xie K, Huang Y, Chen H, Gong G, Zeng Y. Beneficial effects of hydrogen-rich saline against spinal cord ischemia-reperfusion injury in rabbits. J Brain Res. 2013;1517:150–160. doi: 10.1016/j.brainres.2013.04.007. [DOI] [PubMed] [Google Scholar]


