Abstract
The transplantation of polylactic glycolic acid conduits combining bone marrow mesenchymal stem cells and extracellular matrix gel for the repair of sciatic nerve injury is effective in some respects, but few data comparing the biomechanical factors related to the sciatic nerve are available. In the present study, rabbit models of 10-mm sciatic nerve defects were prepared. The rabbit models were repaired with autologous nerve, a polylactic glycolic acid conduit + bone marrow mesenchymal stem cells, or a polylactic glycolic acid conduit + bone marrow mesenchymal stem cells + extracellular matrix gel. After 24 weeks, mechanical testing was performed to determine the stress relaxation and creep parameters. Following sciatic nerve injury, the magnitudes of the stress decrease and strain increase at 7,200 seconds were largest in the polylactic glycolic acid conduit + bone marrow mesenchymal stem cells + extracellular matrix gel group, followed by the polylactic glycolic acid conduit + bone marrow mesenchymal stem cells group, and then the autologous nerve group. Hematoxylin-eosin staining demonstrated that compared with the polylactic glycolic acid conduit + bone marrow mesenchymal stem cells group and the autologous nerve group, a more complete sciatic nerve regeneration was found, including good myelination, regularly arranged nerve fibers, and a completely degraded and resorbed conduit, in the polylactic glycolic acid conduit + bone marrow mesenchymal stem cells + extracellular matrix gel group. These results indicate that bridging 10-mm sciatic nerve defects with a polylactic glycolic acid conduit + bone marrow mesenchymal stem cells + extracellular matrix gel construct increases the stress relaxation under a constant strain, reducing anastomotic tension. Large elongations under a constant physiological load can limit the anastomotic opening and shift, which is beneficial for the regeneration and functional reconstruction of sciatic nerve. Better regeneration was found with the polylactic glycolic acid conduit + bone marrow mesenchymal stem cells + extracellular matrix gel grafts than with the polylactic glycolic acid conduit + bone marrow mesenchymal stem cells grafts and the autologous nerve grafts.
Keywords: nerve regeneration, peripheral nerve injury, rabbits, sciatic nerve injury, autologous nerve repair, polylactic glycolic acid conduit, extracellular matrix gel, grafting, stress relaxation, creep, viscoelasticity, histomorphology, electrophysiology, neural regeneration
Introduction
Combinations of cells and biomaterial scaffolds have been used successfully to repair human tissues and organs (Judd, 2009; Hillel et al., 2010; Maklad et al., 2010; Luo et al., 2013; Sun et al., 2014). Numerous studies have been published using biomaterial conduits combined with cell transplantation to treat peripheral nerve injury (Park et al., 2012; Evans et al., 2013; Ji et al., 2014; Lai et al., 2014; Liu et al., 2014; Zhu et al., 2014). Polylactic glycolic acid (PLGA) is biocompatible, biodegradable, and is the most widely studied scaffold (Gilchrist et al., 2013; Xu et al., 2015). Moore et al. (2006) created a multiple-channel biodegradable scaffold using PLGA seeded with Schwann cells and implanted the scaffold into the transected spinal cord of rats. After 1 month, the regeneration of axons was apparent.
Recently, bone marrow mesenchymal stem cells (BMSCs) have been used to repair peripheral nerve injury (Zhang et al., 2014). Dezawa et al. (2001) implanted Schwann-like cells differentiated from BMSCs into the nerve stump, reporting that BMSCs could promote nerve regeneration. Mimura et al. (2004) repaired 12-mm sciatic nerve defects in rats using the same method. After 6 months, the transplanted cells had promoted myelination. Hou et al. (2004) repaired 10-mm sciatic nerve defects with Schwann-like cells differentiated from BMSCs combined with a polylactic acid-polyglycolic acid copolymer and found satisfactory repair. Most previous studies have focused on the biocompatibility and immunological response of the sciatic nerve to PLGA (Li et al., 2013a, b, c; He et al., 2013a, b; Yao et al., 2013; Wang et al., 2013). In studies where the biomechanical properties of the PLGA scaffold were assessed, they typically focused on the mechanical characteristics of the normal PLGA conduit. Little data are available on the viscoelasticity of the injured sciatic nerve after transplantation with a PLGA conduit + BMSCs + extracellular matrix gel (ECM).
The viscoelasticity of biomaterials is an important property for matching the physiological function of human tissues. Viscoelasticity is mainly characterized by stress relaxation and creep testing. The quantitative analysis of the viscoelasticity of nerve is important for achieving appropriate regeneration of injured nerve. In the present study, sciatic nerve defects in rats were repaired by transplantation of a PLGA conduit + BMSCs + ECM, and changes in the stress relaxation and creep properties, histomorphology, and electrophysiology of the injured nerve were assessed. The biomechanical effects of the PLGA conduit + BMSCs and PLGA conduit + BMSCs + ECM transplantation on the repair of sciatic nerve injury in a rat model were investigated.
Materials and Methods
Experimental animals
A total of 72 healthy male 5-month-old Japanese rabbits weighing 2.6–2.9 kg were provided by the Changchun High-tech Medical Laboratory Animal Center (license No. SCXK (Ji) 2003-0004). The rabbits were allowed free access to food and water, fed a complete feed, and housed at 22–24°C and relative humidity of 55–70% with natural light and air circulation.
Preparation of the PLGA conduit
Following the methods of Zhang et al. (2004), PLGA (polylactic acid to glycolic acid ratio of 70:30; Changchun SinoBiomaterials Co., Ltd., Changchun, Jilin Province, China) was dissolved in dichloromethane. NaCl particles of diameter 200–300 μm were added (PLGA to NaCl mass ratio of 1:9) and mixed until uniform. The mixture was infused into a prefabricated mold to make 30 conduits that were each 60 mm long with a 1.8 mm outside diameter and a 1.6 mm inside diameter. The mixture volatilized at room temperature in a fume hood for 96 hours. Next, they were demolded to obtain columnar scaffolds that were then dried in a vacuum drying oven at 37°C for 48 hours. The columnar material was immersed in deionized water in an 800-mL beaker, and the deionized water was replaced every 5 hours. After washing with deionized water for 96 hours, the columnar material was again dried in a baking oven at 37°C for 48 hours, and then sterilized with ethylene oxide at 36°C for 12 hours. A total of 46 PLGA conduits were cut to 10 mm length with a S-5 sterile scalpel with a plastic handle. Of these 46 conduits, 20 were used for the PLGA conduit + BMSCs + ECM group, 20 for the PLGA conduit + BMSCs group, and 6 for other applications.
Experimental groups and induction of sciatic nerve injury in the rabbits
The 72 rabbits were randomly assigned to the autologous nerve, PLGA conduit + BMSCs, or PLGA conduit + BMSCs + ECM groups. Twenty rabbits from each group were used to collect electrophysiologic al data. The sciatic nerves from 10 rabbits from each group on the operated side were used for stress relaxation testing, and the sciatic nerves from 10 rabbits from each group on the operated side were used for creep testing. The non-operated side was used as a normal control group. One of the remaining specimens was prepared for histology.
We created sciatic nerve injuries in all three groups. The rabbits were anesthetized with an intraperitoneal injection of 6% chloral hydrate and secured on the operation table. Following the methods of Mimura et al. (2006), a median incision was made on the posterior thigh below the left buttocks. The semitendinosus and semimembranosus muscles were separated. The right sciatic nerve was exposed and dissociated. A 7-mm section of the sciatic nerve was resected 3 mm inferior to the piriformis muscle. After nerve contraction, a 10-mm sciatic nerve defect was present.
In the autologous nerve group, the resected portion of the autologous sciatic nerve was flipped over and sutured. Using an operating microscope, the two ends of the epineurium were sutured with 9-0 noninvasive sutures (Qingdao Nesco Medical Co., Ltd., Qingdao, China), with four stitches on each side. The muscle and skin were then sutured closed.
In the PLGA conduit + BMSCs group, a 1.5-mm nerve stump was inserted into the 10-mm PLGA conduit using the operating microscope. The epineurium was stitched to the PLGA conduit with a 9-0 noninvasive suture (Qingdao Nesco Medical Co., Ltd.) with one stitch. Next, the two ends were sutured with four stitches. Rabbit BMSCs (approximately 1 × 109/L; Shanghai EYKITS Biological Technology Co., Ltd., Shanghai, China) were infused into the conduit. The muscle and skin were then sutured closed.
In the PLGA conduit + BMSCs + ECM group, the same procedure as was done for the PLGA conduit + BMSCs group was performed, and then ECM (approximately 1 × 109/L; Shanghai Xin Yu Biotech Co., Ltd., Shanghai, China) was infused into the conduit. The muscle and skin were sutured closed after the incision was washed with gentamicin. All surgical procedures were conducted under sterile conditions.
No external fixation was used for any of the groups after surgery. The rabbits were housed in individual cages after recovery and received intraperitoneal injections of penicillin 104 U/kg, twice a day for 7 days. The incision was sterilized with 75% alcohol, once a day for 7 days. The padding in the rear of the cage kept dry. Twenty-four left sciatic nerve specimens from the rabbits from each group were used as the normal control group. Photographs showing the anastomosis of the injured sciatic nerve are shown in Figure 1.
Figure 1.
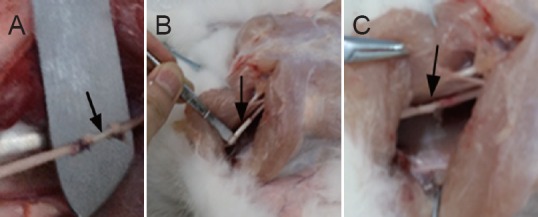
Anastomosis of the injured sciatic nerve in rabbits.
(A) Autologous nerve group, (B) PLGA conduit + BMSCs group, and (C) PLGA conduit + BMSCs + ECM group. Arrows indicate the precise anastomosis in each group. PLGA: Polylactic glycolic acid; BMSCs: bone marrow mesenchymal stem cells; ECM: extracellular matrix.
Electrophysiological measurements
Twenty-four weeks after the surgery, the rabbits were anesthetized with an intraperitoneal injection of 10% chloral hydrate (400 mg/kg). After aseptic cleaning, the rabbits were placed in a prone position, and the bilateral sciatic nerve trunk was exposed. The electrophysiological responses from the rabbits from each group were measured with an electromyograph (NIM-Neuro 2.0; Medtronic, Minneapolis, MN, USA). The recording electrode (M) was punctured into the soleus muscle. The earth wire was clamped to the edge of the wound with an alligator clip. A very strong stimulus (current 50 mA) was applied at the ischial tuberosity level (P) proximal to the stoma and at the distal sciatic nerve branch (D) to induce two motor potentials. The action potential amplitude and latency were displayed automatically by the instrument. The distance between the two stimulating electrodes was measured with a vernier caliper and input into the instrument. The instrument also automatically displayed the motor nerve conduction velocity. The left sciatic nerve was assessed using the same method, and the results were recorded as the normal control group.
After the electrophysiological measurement, the center of each anastomotic stoma was defined as the midpoint. Specimens 20-mm long on the operated side (right side) were obtained from 24 animals in each group. Twenty-four left sciatic nerve rabbit specimens were also obtained from random rabbits in each group and were used as the normal control group. All specimens were stored in physiological saline until further use.
Stress relaxation testing
An automatic control electronic universal testing machine (MODEL55100; Changchun Research Institute for Mechanical Science, Changchun, China) was used for stress relaxation testing. The testing machine had an incubator (range: −30 to 250°C), and the temperature was controlled as needed. The length and diameter of the specimens in each group were measured on a reading microscope (CGA-5; Changchun Third Optical Instrument Factory, Changchun, China). In each group, the rabbit sciatic nerve samples were 20 mm long and 1.50–1.56 mm in diameter. Following previously reported methods (Zhang et al., 2004; Liu et al., 2011; Peng et al., 2012), a presetting process was performed on each specimen. The experiment was conducted at 36.5 ± 1.0°C. Each specimen was placed in the chuck of the tester. Strain was applied to the specimen at a rate of 50%/min. In each group, the strain was kept constant when the stress reached 0.501 MPa. The strain was 12.21% in the normal control group, 12.12% in the autologous nerve group, 12.16% in the PLGA conduit + BMSCs group, and 12.18% in the PLGA conduit + BMSCs + ECM group at 0.501 MPa. The relaxation experiment was continued for 7,200 seconds. To maintain the humidity of the specimens, they were continuously wetted with physiological saline. The stress relaxation data were automatically output to a computer after each experiment.
Creep testing
The equipment, clamping method, temperature, data acquisition, and presetting process for the creep test were identical to those used for the stress relaxation test. The stress was increased at 0.5 GPa/min in each group. The stress was kept constant after reaching 0.501 MPa in each group. The strain was 12.21% in the normal control group, 12.12% in the autologous nerve group, 12.16% in the PLGA conduit + BMSCs group, and 12.18% in the PLGA conduit + BMSCs + ECM group. The experiment was continued for 7,200 seconds. The creep data were automatically output to a computer after each experiment.
Hematoxylin-eosin staining to show the sciatic nerve cross-sectional microstructure
One sciatic nerve from each group in the middle segment at the anastomotic stoma was cut into 3-mm frozen sections. The sections were fixed in 4% paraformaldehyde for 5 minutes, washed with running water, stained with hematoxylin for 2–5 minutes, washed with running water, treated with hydrochloric acid in ethanol, washed with running water, treated with lye, washed with running water, treated with eosin for 20 seconds to 3 minutes, washed with running water, dehydrated through a graded ethanol series, permeabilized with xylene I for 10 minutes and xylene II for 10 minutes, and mounted with a neutral resin. The nerve cross-sections, nerve cells, myelin sheath, axons, and basal membrane were observed using a light microscope (Olympus, Tokyo, Japan).
Statistical analysis
The data are expressed as the mean ± SD and were analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Differences among groups were assessed using one-way analysis of variance and Scheffe's method. P-values less than 0.05 were considered statistically significant.
Results
Electrophysiological function of the sciatic nerves
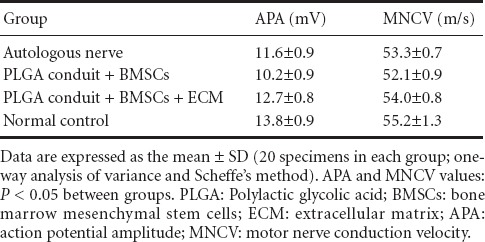
The electrophysiological testing showed that the action potential amplitudes and motor nerve conduction velocities were largest in the normal control group, followed by those in the PLGA conduit + BMSCs + ECM group, the autologous nerve group, and then the PLGA conduit + BMSCs group (P < 0.05; Table 1).
Table 1.
Results of electrophysiological testing on rabbit sciatic nerves from each group
Stress relaxation of the sciatic nerves
The stress decreased by 0.135 in the normal control group, 0.120 in the autologous nerve group, 0.125 in the PLGA conduit + BMSCs group, and 0.130 MPa in the PLGA conduit + BMSCs + ECM group at 7,200 seconds. The decreased magnitudes of stress and the normalized stress relaxation functions at 7,200 seconds were largest in the normal control group, followed by those in the PLGA conduit + BMSCs + ECM group, the PLGA conduit + BMSCs group, and finally the autologous nerve group (P < 0.05; Figure 2).
Figure 2.
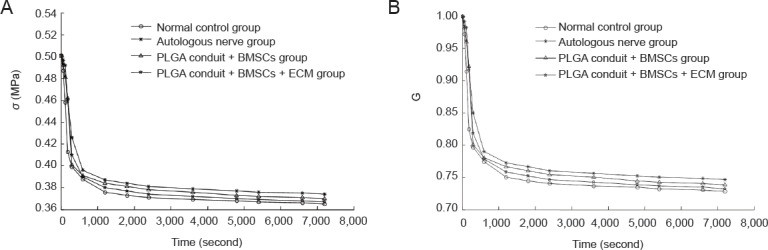
Stress relaxation curves (A) and the normalized stress relaxation functions (B) for the rabbit sciatic nerves from each group.
The stress relaxation curves and normalized stress relaxation functions gradually decreased with time in each group, becoming nearly horizontal at 7,200 seconds. Both of the two above types of curves were logarithmic in each group. Data are expressed as the mean ± SD (10 specimens in each group; one-way analysis of variance and Scheffe's method). The decreased values of stress and the normalized stress relaxation functions at 7,200 seconds were different (P < 0.05) among the groups. PLGA: Polylactic glycolic acid; BMSCs: bone marrow mesenchymal stem cells; ECM: extracellular matrix.
Normalizing the stress relaxation functions in sciatic nerve specimens
As shown in Figure 2, the stress relaxation curve functions were logarithmic:
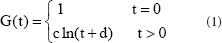
where c and d are undetermined coefficients.
If 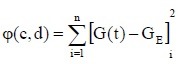
then 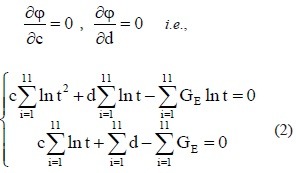
The experimental data were used in equation (2) to calculate the values of c and d for the sciatic nerves in each group. The c and d values were then used in equation (1) to obtain the following normalized stress relaxation function equations for each group:
Normal control group:
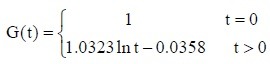
Autologous nerve group:
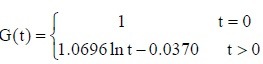
PLGA conduit + BMSCs group:
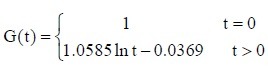
PLGA conduit + BMSCs + ECM group:
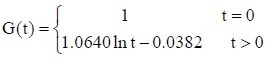
Creep curves and normalized creep functions for the sciatic nerves in each group
The strain increased to 4.767% in the normal control group, 3.144% in the autologous nerve group, 3.596% in the PLGA conduit + BMSCs group, and 4.036% in the PLGA conduit + BMSCs + ECM group. The increased amount of strain and normalized creep function at 7,200 seconds were largest in the normal control group, followed by those in the PLGA conduit + BMSCs + ECM group, the PLGA conduit + BMSCs group, and finally the autologous nerve group (P < 0.05; Figure 3).
Figure 3.
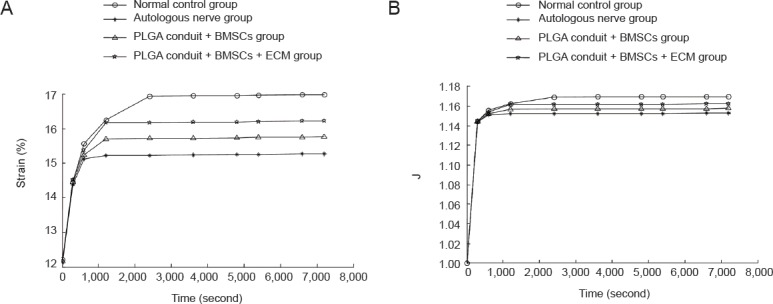
Creep curves and the normalized creep functions for the sciatic nerves from each group.
(A) Creep curves: the strain increased quickly during the initial 600 seconds before gradually slowing down. The curves were horizontal by 7,200 seconds. (B) Normalized creep functions. Y-axis: Normalized creep; X-axis: time. The normalized creep functions were horizontal by 7,200 seconds. The creep curves in each group were exponential. PLGA: Polylactic glycolic acid; BMSCs: bone marrow mesenchymal stem cells; ECM: extracellular matrix.
Normalizing the creep function equations for the sciatic nerves in each group
As shown in Figure 3, the creep curves for each group were exponential. Following previously published methods (Peng et al., 2012), we defined:
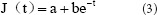
and 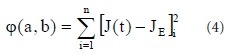
with the canonical equation:
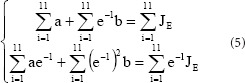
The experimental data in each group were used in equation (5) to calculate the values of a and b for the sciatic nerves in each group. The a and b values were used in equation (3) to obtain the following normalized creep function equations:
Normal control group:
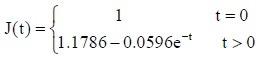
Autologous nerve group:
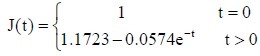
PLGA conduit + BMSCs group:
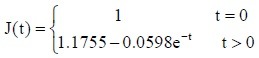
PLGA conduit + BMSCs + ECM group:
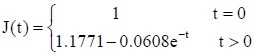
Histomorphological changes in the sciatic nerve cross-section
Light microscopy revealed that in the normal control group, the sciatic nerves were arranged regularly, and the axons were surrounded by myelin sheaths. An endoneurium formed by connective tissue was present on the surface of nerve fibers wrapped with myelin sheath and axons (Figure 4A). In the autologous nerve group, myelination was visible on the distal end of the sciatic nerves. Fibers and axons were located on one side of the elliptic Schwann cells (Figure 4B). In the PLGA conduit + BMSCs + ECM group, the sciatic nerves were completely regenerated and showed good myelination and regularly arranged nerve fibers. The conduit was completely degraded and had been resorbed (Figure 4C). In the PLGA conduit + BMSCs group, the sciatic nerves were also completely regenerated and showed good myelination and the nerve fibers were regularly arranged. The conduit was mostly degraded and resorbed (Figure 4D).
Figure 4.
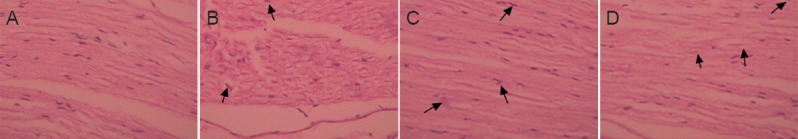
Histomorphology of the rabbit sciatic nerves at 24 weeks after transplantation (hematoxylin-eosin staining, light microscope, × 100).
(A) In the normal control group, the sciatic nerves were regularly arranged. (B) In the autologous nerve group, myelination occurred at the distal end, and axons (arrows) were located on one side of the elliptic Schwann cells. (C) In the PLGA conduit + BMSCs + ECM group, the sciatic nerve had completely regenerated with good myelination. The nerve fibers (arrows) were regularly arranged. (D) In the PLGA conduit + BMSCs group, the sciatic nerve was also completely regenerated with good myelination, and the nerve fibers (arrows) were regularly arranged. PLGA: Polylactic glycolic acid; BMSCs: bone marrow mesenchymal stem cells; ECM: extracellular matrix.
Discussion
The mechanical testing results demonstrated that the stress relaxation and creep properties were restored at 24 weeks after repair with the PLGA conduit + BMSCs, PLGA conduit + BMSCs and ECM. Under constant strain, the restoration and increases in stress relaxation and creep likely reduced the tension at the anastomotic stoma after the PLGA conduit transplantation. After repair, the increased elongation limits the opening and movement of the anastomotic stoma under constant physiological loading. The combination of a PLGA conduit with BMSCs or BMSCs + ECM showed appropriate viscoelasticity for nerve tissue.
The histomorphological results showed that at 24 weeks after repair with the PLGA conduit + BMSCs + ECM, the sciatic nerves were completely regenerated with good myelination, the nerve fibers were arranged regularly, and the conduit had been completely degraded and resorbed. These findings suggest that the combination of a PLGA conduit + BMSCs + ECM implanted in injured sciatic nerve contributes to the restoration of the sciatic nerve fibers.
The data shown here indicate that the stress relaxation and the amount of creep under constant stress and constant strain were associated with the electrophysiological index and histomorphology of the sciatic nerves after transplantation because better restoration of the electrophysiological index and histomorphology was found with better restoration of the stress relaxation and creep properties. These results suggest that stress relaxation and creep properties are useful methods for determining the extent of sciatic nerve injury repair.
Dezawa et al. (2001) reported that BMSCs could induce nerve regeneration. Liao et al. (2007) and Donzelli et al. (2006) showed that ECM contained various extracellular matrix molecules such as laminin, fibronectin, and type IV collagen and could also promote nerve regeneration. In the present study, the electrophysiological index, histomorphology, stress relaxation, and creep properties of the sciatic nerves had recovered or improved at 24 weeks after repair in the PLGA conduit + BMSCs and PLGA conduit + BMSCs + ECM groups. The PLGA conduit combined with BMSCs and the combination of PLGA conduit + BMSCs + ECM apparently promoted the regeneration of sciatic nerve after injury, as was expected. When the PLGA conduit + BMSCs + ECM was used to repair 10-mm sciatic nerve defects, the large amount of stress relaxation that occurred under constant strain likely relieved the tension on the anastomotic stoma. Under constant physiological loading, the increased elongation may restrict the opening and movement of the anastomotic stoma, contributing to the regeneration and functional reconstruction of the sciatic nerves.
PLGA conduit can be fabricated with a desired pore size and porosity, which can provide a microenvironment conducive to nerve regeneration and the retention of neurotrophic factors (Yao et al., 2014). PLGA conduits can be combined with neurotrophic factors and various types of stem cells to effectively increase the regeneration capacity of the nerve fibers after peripheral nerve injury. With continued research, the methods for using PLGA conduits combined with stem cells to repair peripheral nerve injury will be gradually improved until they can be used in the clinic.
There was variability among the individual animals, leading the experimental data for the sciatic nerves in each group to be discrete. However, these data can be used as reference values for the repair of sciatic nerve injury in the clinic.
Footnotes
Funding: This study was supported by the Science and Technology Development Program of Jilin Province in China, No. 20110492.
Conflicts of Interest: None declared.
Copyedited by McCarty W, Yajima W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosei. 2001;14:1771–1776. doi: 10.1046/j.0953-816x.2001.01814.x. [DOI] [PubMed] [Google Scholar]
- Donzelli R, Maiuri F, Piscopo GA, de Notaris M, Colella A, Divitiis E. Role of extracellular matrix components in facial nerve regeneration: an experimental study. Neurol Res. 2006;28:794–801. doi: 10.1179/016164106X110427. [DOI] [PubMed] [Google Scholar]
- Evans NR, Davies EM, Dare CJ, Oreffo RO. Tissue engineering strategies in spinal arthrodesis: the clinical imperative and challenges to clinical translation. Regen Med. 2013;8:49–64. doi: 10.2217/rme.12.106. [DOI] [PubMed] [Google Scholar]
- Gilchrist SE, Lange D, Letchford K, Bach H, Fazli L, Burt HM. Fusidic acid and rifampicin co-loaded PLGA nanofibers for the prevention of orthopedic implant associated infections. J Control Release. 2013;170:64–73. doi: 10.1016/j.jconrel.2013.04.012. [DOI] [PubMed] [Google Scholar]
- He F, Wang X, Maruyama O, Kosaka R, Sogo Y, Ito A, Ye J. Improvement in endothelial cell adhesion and retention under physiological shear stress using a laminin-apatite composite layer on titanium. J R Soc Interface. 2013;10:20130014. doi: 10.1098/rsif.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He FP, Li JY, Ye JD. Improvement of cell response of the poly(lactic-co-glycolicacid)/calcium-phosphate cement-composite scaffold with unidirectional-pore structure by the immobilization via plasma treatment. Colloid Surf B: Biointerfaces. 2013;103:209–216. doi: 10.1016/j.colsurfb.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Hillel AT, Elisseeff JH. Embryonic progenitor cells in adipose tissue engineering. Facial Plast Surg. 2010;26:405–412. doi: 10.1055/s-0030-1265021. [DOI] [PubMed] [Google Scholar]
- Hou SY, Zhang HY, Quan DP, Liu XL, Zhu JK. Tissue-engineering peripheral nerve grafting by differentiated bone-marrow stromal stem cells. Neuroscience. 2006;140:101–110. doi: 10.1016/j.neuroscience.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Ji W, Hu S, Zhou J, Wang G, Wang KZ, Zhang YL. Tissue engineering is a promising method for the repair of spinal cord injuries (Review) Exp Ther Med. 2014;7:523–528. doi: 10.3892/etm.2013.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd T. The World Health Organization's evidenced-based approach to chronic diseases: primary prevention or caring for end-stage disease? Perm J. 2009;13:65–68. doi: 10.7812/tpp/09-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai BQ, Wang JM, Ling EA, Wu JL, Zeng YS. Graft of a tissue-engineered neural scaffold serves as a promising strategy to restore myelination after rat spinal cord transection. Stem Cells Dev. 2014;23:910–921. doi: 10.1089/scd.2013.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, He FP, Ye JD. Effect of the surface topographic modification on cytocompatibility of hardened calcium phosphate cement. App Surf Sci. 2013;274:237–240. [Google Scholar]
- Li M, Guo W, Zhang P, Li H, Gu X, Yao D. Signal flow and pathways in response to early Wallerian degeneration after rat sciatic nerve injury. Neurosci Lett. 2013;536:56–63. doi: 10.1016/j.neulet.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Li S, Liu Q, Wang Y, Gu Y, Liu D, Wang C, Ding G, Chen J, Liu J, Gu X. Differential gene expression profiling and biological process analysis in proximal nerve segments after sciatic nerve transection. PLoS One. 2013;8:e57000. doi: 10.1371/journal.pone.0057000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Liu SH, Zhang SQ, Li ZH, Jiao JB, Zhang YF, Zhao YQ, Wang C, Fan M. Chitosan conduits combined with autologous mesenchymal stem cells to repair 13-mm sciatic nerve defects. Zhongguo Zuzhi Gongcheng Yanjiu. 2007;11:3517–3522. [Google Scholar]
- Liu GY, Qiao Z, Yan J. Autogous nerve anastomosis versus human amniotic membrane anastomosis Arheological comparison following simulated sciatic nerve injury. Neural Regen Res. 2011;6:2424–2428. [Google Scholar]
- Liu J, Bauer AJ, Li B. Solvent vapor annealing: an efficient approach for inscribing secondary nanostructures ontoelectrospun fibers. Macromol Rapid Commun. 2014;35:1503–1508. doi: 10.1002/marc.201400274. [DOI] [PubMed] [Google Scholar]
- Luo P, Peng QL, Xiang JP, Qi J. Synthetic nerve conduit versus autogenous nerve transplantation for repair of peripheral nerve defects. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:3010–3017. [Google Scholar]
- Maklad A, Kamel S, Wong E, Fritzsch B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010;340:303–321. doi: 10.1007/s00441-010-0944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Dezawa M, Kanno H, Sawada H, Yamamoto I. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J Neurosurg. 2004;101:806–812. doi: 10.3171/jns.2004.101.5.0806. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S, Knight AM, Lu L, Currier BL, Spinner RJ, Marsh RW, Windebank AJ, Yaszemski MJ. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419–429. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- Park BH, Zhou L, Jang KY, Park HS, Lim JM, Yoon SJ, Lee SY, Kim JR. Enhancement of tibial regeneration in a rat model by adipose-derived stromal cells in a PLGA scaffold. Bone. 2012;51:313–323. doi: 10.1016/j.bone.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Peng CG, Zhang Q, Yang Q, Zhu QS. Strain and stress variations in the human amniotic membrane and fresh corpse autologous sciatic nerve anastomosis in a model of sciatic nerve injury. Neural Regen Res. 2012;7:1779–1785. doi: 10.3969/j.issn.1673-5374.2012.23.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HW, Zhang TH, You XY, Ren YF, Zhong S. Polylactic-co-glycolic acid complex with different concentrations of Schwann cells for peripheral nerve regeneration. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:7579–7584. [Google Scholar]
- Wang XP, He FP, Li X, Ito A, Sogo Y, Maruyama O, Kosaka R, Ye JD. Tissue-engineered endothelial cell layers on surface-modified Ti for inhibiting in vitro platelet adhesion. Sci Technol Adv Mater. 2013;14:035002. doi: 10.1088/1468-6996/14/3/035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Sheybani N, Ren S, Bowlin GL, Yeudall WA, Yang H. Semi-Interpenetrating Network (sIPN) Co-Electrospun gelatin/insulin fiber formulation for transbuccal insulin delivery. Pharm Res. 2015;32:275–285. doi: 10.1007/s11095-014-1461-9. [DOI] [PubMed] [Google Scholar]
- Yao B, Li KN, Nie H. In vivo biomechanical properties of biodegradable polylactic acid-glycolic acid lumbar intertransverse fusion cage. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:8426–8432. [Google Scholar]
- Yao D, Li M, Shen D, Ding F, Lu S, Zhao Q, Gu X. Expression changes and bioinformatic analysis of Wallerian degeneration after sciatic nerve injury in rat. Neurosci Bull. 2013;29:321–332. doi: 10.1007/s12264-013-1340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CS, Lv G. Tissue engineered artificial nerves repair sciatic nerve defect in rats: an evaluation using horseradish peroxidase retrograde tracer technique. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:4658–4662. [Google Scholar]
- Zhu T, Tang Q, Gao H, Shen Y, Chen L, Zhu J. Current status of cell-mediated regenerative therapies for human spinal cord injury. Neurosci Bull. 2014;30:671–682. doi: 10.1007/s12264-013-1438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


