Abstract
The morphological changes that occur in the taste buds after denervation are not well understood in rats, especially in the contralateral tongue epithelium. In this study, we investigated the time course of morphological changes in the taste buds following unilateral nerve transection. The role of the trigeminal component of the lingual nerve in maintaining the structural integrity of the taste buds was also examined. Twenty-four Sprague-Dawley rats were randomly divided into three groups: control, unilateral chorda tympani nerve transection and unilateral chorda tympani nerve transection + lingual nerve transection. Rats were allowed up to 42 days of recovery before being euthanized. The taste buds were visualized using a cytokeratin 8 antibody. Taste bud counts, volumes and taste receptor cell numbers were quantified and compared among groups. No significant difference was detected between the chorda tympani nerve transection and chorda tympani nerve transection + lingual nerve transection groups. Taste bud counts, volumes and taste receptor cell numbers on the ipsilateral side all decreased significantly compared with control. On the contralateral side, the number of taste buds remained unchanged over time, but they were larger, and taste receptor cells were more numerous postoperatively. There was no evidence for a role of the trigeminal branch of the lingual nerve in maintaining the structural integrity of the anterior taste buds.
Keywords: nerve regeneration, gustation, cytokeratin, tongue epithelium, immunohistochemistry, taste bud, trigeminal nerve disorder, NSFC grants, neural regeneration
Introduction
Taste is the main sensory modality by which we evaluate whether a potential food is good or bad. Gustatory systems detect nutritionally useful and harmful compounds in food and trigger innate behaviors leading to acceptance or rejection of potential food sources (Breslin and Huang, 2006). Taste receptor cells play an important role in transducing sapid stimuli into electrochemical signals. They constitute the multicellular rosette clusters termed “taste buds”. These sensory cells communicate via neurochemical signals with afferent nerve fibers of gustatory sensory neurons in one of several cranial nerves (VII, IX or X). These fibers project into the central nervous system at the level of the brainstem and synapse onto the nucleus of the solitary tract, where the majority of taste information is processed and integrated (Bradley and Grabauskas, 1998).
The normal function and morphological integrity of the taste buds are dependent upon innervation. Removal of neural innervation results in a dramatic loss of taste buds, which can be reversed by reinnervation (Cheal and Oakley, 1977; St. John et al., 2003; Guagliardo and Hill, 2007; Saito et al., 2011a). Although taste bud numbers may be restored following regeneration of the nerve, the cellular composition and size of the regenerated taste buds usually differ from the normal ones (Cheal and Oakley, 1977; St. John et al., 2003; Guagliardo and Hill, 2007). The epithelium of the lingual papillae also undergoes structural changes (Sollars et al., 2002; Sollars, 2005; Ohtubo and Yoshii, 2011). Although these morphological changes in taste buds after denervation have been observed in a variety of species (Cheal and Oakley, 1977; Sollars, 2005; Guagliardo and Hill, 2007), very few studies have examined the time course of the structural alterations between denervation and reinnervation. In addition, because most morphological studies have focused only on the denervated taste bud, there is very little information on the changes in taste buds on the contralateral side of the tongue.
Both the chorda tympani branch (CTN) of the cranial nerve VII and the lingual branch (LN) of the cranial nerve V innervate the anterior two-thirds of the tongue. The CTN carries taste messages from the ipsilateral taste buds of the fungiform papillae to the brain, while the LN supplies somatosensory afferents from the mucosa of the tongue (Breslin and Huang, 2006). However, the role of these nerves in maintaining the structural integrity of the taste buds is unclear (Barlow et al., 1996; Fritzsch et al., 1997; King et al., 2000; Kopka et al., 2000). Clinically, iatrogenic damage to the CTN is a well-recognized complication of middle ear surgery (Saito et al., 2011c; McManus et al., 2012). As a result, loss or change in taste perception is often a complaint from patients after middle ear surgeries (McManus et al., 2012). However, the taste disorders in half of these patients reportedly improve within 1 to 2 years, and normal fungiform papillae are seen on the ipsilateral side of the tongue, although it is not clear whether the CTN had regenerated (Saito et al., 2011b, c), although injury to the CTN does not necessarily produce symptoms. Furthermore, some patients do not experience dysgeusia, although their CTNs were transected during surgery (McManus et al., 2012). These observations prompted us to investigate the role of the lingual branch in maintaining the functional and morphological integrity of the taste buds.
Because most early studies on taste buds following denervation used hematoxylin and eosin stains (Cheal and Oakley, 1977; Sollars et al., 2002; St. John et al., 2003; Sollars, 2005), the borders of the taste buds may have been difficult to define, especially at the lateral margins. This drawback has limited the morphological analysis of taste buds. This problem can be circumvented using immunological markers for taste bud cells (Guagliardo and Hill, 2007). In the present study, we performed immunohistochemistry using a cytokeratin antibody that can easily distinguish the margins of taste buds. We investigated the time course of the morphological changes in the taste buds on both sides of the tongue following unilateral CTN transection in rats. Furthermore, we examined the role of the trigeminal component of the LN in maintaining the structural integrity of the taste buds.
Materials and Methods
Animals
Twenty-four clean adult female Sprague-Dawley rats weighing 250–300 g were used in this study. They were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. in China (license No. SCXK (Hu) 2007-0005) and housed in groups of 2–4 rats per cage at room temperature. All procedures were carried out under the approval of the Institutional Animal Care and Use Committee of Eye and ENT Hospital of Fudan University in China and in full accordance with the guidelines of the Chinese Association of Laboratory Animal Science. The rats were randomly divided into three groups: sham control (n = 4), unilateral CTN transection (CTNX, n = 10) and unilateral CTN + LN transection (CTN + LNX, n = 10).
Establishment of chorda tympani nerve transection model
All surgeries were performed using aseptic technique. Animals were anesthetized with intramuscular injection of a mixture of xylazine (15 mg/kg) and ketamine (50 mg/kg), and were placed in a supine position. An incision, 1.2–1.5 cm long, was made on the ventral surface of the neck 0.5 cm lateral (left) to the midline. The digastric and masseter muscles were bluntly dissected using micro-fine forceps. The lingual nerve was visualized and traced to the point of bifurcation with the CTN. For rats that received CTN transection (CTNX group), a cut was made distal to the bifurcation only on the CTN, leaving the lingual branch intact. For rats that received CTN + LN transection (CTN + LNX group), a cut was made proximal to the bifurcation on the lingual nerve, resulting in transection of both the CTN and LN (Figure 1). In both cases, separation of the proximal and distal stumps of the transected nerve was used to verify the completeness of the surgery, but no further step was taken to prevent nerve regeneration. Sham surgery was performed in control rats in the same manner, except that no nerve was cut. The contralateral (right) side served as the intact control (Cheal and Oakley, 1977; Sollars et al., 2002; Guagliardo and Hill, 2007). The incision was sutured, and the animals were allowed to recover on a heating pad. All the procedures were done typically in about an hour. All rats recovered well after the surgery without complication.
Figure 1.
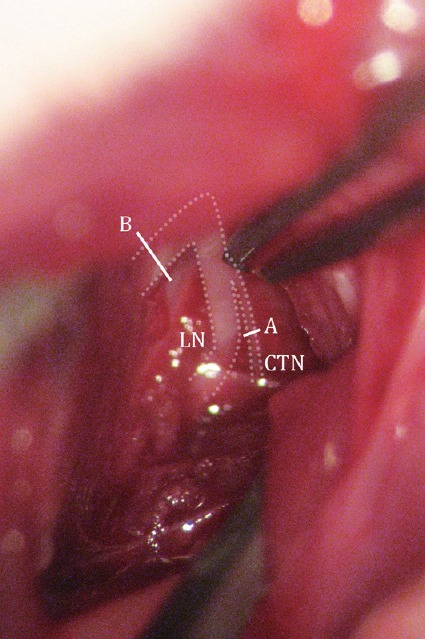
Illustration of transections performed on the nerves during surgery.
A cut was made distal to the bifurcation in CTNX rats (A) and proximal to the bifurcation in CTN + LNX rats (B). CTNX: Chorda tympani nerve transection; CTN + LNX: transection of chorda tympani nerve and lingual nerve.
Tissue preparation
Animals given unilateral CTN or CTN + LN transection were allowed 5, 10, 14, 28 or 42 days (n = 2 for each time point) to recover before being euthanized. Animals in the sham control group were sacrificed at 10 and 28 days (n = 2 for each time point) after the operation. Rats were sacrificed by cervical dislocation combined with an overdose of xylazine and ketamine. The anterior tongue was gently cut and rinsed with 0.01 M PBS solution before fixation in 4% paraformaldehyde solution overnight. After cryoprotection in 15% sucrose/PBS solution and then 30% sucrose/PBS solution overnight, the tongue sample was suspended and frozen in optimal cutting temperature compound (Sakura Finetek, Tokyo, Japan), then coronally or horizontally sectioned on a cryostat. Serial sections, 10-μm-thick, were obtained from the anterior-most 4 mm of the tongue and mounted on glass slides for immunohistochemistry.
Immunohistochemistry
Taste buds were visualized using an antibody to cytokeratin 8, which is a protein expressed in maturing or mature rat taste buds (Asano-Miyoshi et al., 2008). Slides were rinsed with 0.01 M PBS and exposed to 0.3% Triton X-100 solution before reacting with a mouse anti-rat cytokeratin 8 monoclonal antibody (1:150; Abcam, Shanghai, China) overnight at 4°C. Then, slides were rinsed with 0.01 M PBS and placed in fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG secondary antibody (1:50; Life Technologies, Grand Island, NY, USA) for 1 hour. For taste bud cell counting, slides were incubated with propidium iodide (Sigma-Aldrich, St. Louis, MO, USA; 1:1,000 in PBS solution) for 30 minutes before being air-dried and coverslipped. Images were collected with a confocal microscope (DMIRZ2; Leica Microsystems, Wetzlar, Germany) and stored in a computer for off-line analyses. The two channels were collected separately, and then merged to produce the composite image. All images were composed, measured and analyzed in Adobe Photoshop CC (Adobe Systems, Mountain View, CA, USA). To measure the taste bud volume and to quantify taste buds, images that displayed only cytokeratin 8 staining were used. A typical taste bud contained elongate cells forming a rosebud-shaped structure. An individual taste bud from an intact tongue could generally be found in 3–6 sections (i.e., 30–60 μm). In each section, the taste bud was outlined, and the area was obtained. The area from each section was multiplied by the section thickness (10 μm) and summed to estimate the total taste bud volume. Taste buds were non-repetitively counted across images of all serial slices on the same side of a tongue. To account for count difference resulting from differences in tongue sizes between animals, the total count was divided by the volume of the lingual epithelium containing the taste buds. Specifically, the lingual epithelium was outlined from the surface to the basement membrane in each section, and the area was measured with Photoshop. The area for each section was multiplied by the section thickness and summed to obtain the total volume of the lingual epithelium. In addition, taste bud counts were conducted in the sample slices approximately 1–2 mm from the tip of the tongue, because the density of taste buds was relatively high in this region, helping to obtain a strong statistical power, and these taste buds were only innervated by the ipsilateral nerves (Kinnman and Aldskogius, 1988; Hill and Phillips, 1994; Berteretche et al., 2008). To examine whether taste bud degeneration after nerve transection resulted from a loss of taste bud cells or a change in taste bud morphology, taste bud cell counts were conducted in double-labeled images. A cell was counted if its nucleus was encompassed by cytokeratin 8 staining. Images of serial slices were compared to prevent the repeated counting of a cell.
Statistical analysis
The data were expressed as the mean ± SEM. Two-way analysis of variance was used to compare differences in taste bud counts, taste bud volumes and taste cell numbers. Independent sample t-test was used to examine statistical differences in taste bud counts, taste bud volumes and taste cell numbers between individual time points or types of nerve transection. Statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, New York, USA).
Results
Changes in taste bud number after chorda tympani nerve transection
There was no significant effect of post-operative time (F = 0.03, P = 0.87) or side (F = 0.05, P = 0.83) on taste bud counts in sham control rats. The average taste bud count in sham control rats was 31.0 ± 1.8 (n = 18) per mm3 of epithelium (Figure 2, top dotted line). This value was not statistically different from taste bud counts on the contralateral side of the tongue at any post-operative time in either the CTNX group or the CTN + LNX group (Figure 2). These results indicate that the number of taste buds on the contralateral side of the tongue did not change over time regardless of the type of nerve that was transected.
Figure 2.
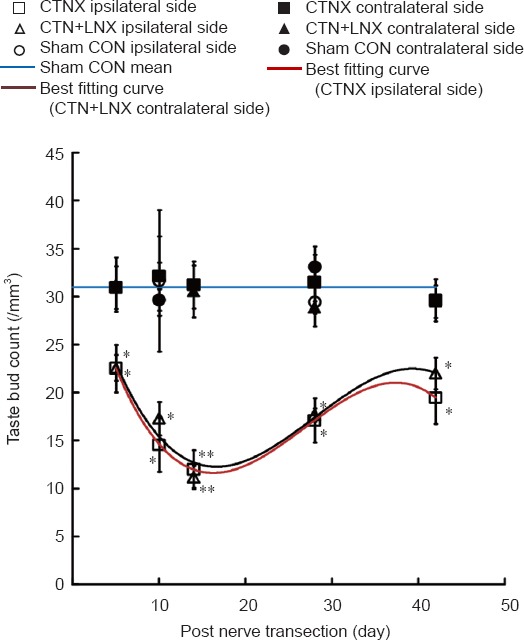
Changes in taste bud number on the tongue after chorda tympani nerve transection.
The best fitting curves for average taste bud counts on the ipsilateral side of CTNX and CTN + LNX animals were third-order polynomials. The data are expressed as the mean ± SEM. Two-way analysis of variance and independent sample t-test were used to examine statistical differences. *P < 0.05, **P < 0.01, vs. sham group. CTNX: Chorda tympani nerve transection; CTN + LNX: transection of chorda tympani nerve and lingual nerve; CON: control.
On the ipsilateral side of the tongue, the number of taste buds decreased significantly after the CTN was transected (F = 59.262, P < 0.01). Five days after CTN transection, the taste bud count was approximately 73% of the intact control value (t = 2.64, P < 0.05). The count dropped to the lowest at 14 days (t = 5.93, P < 0.01), and then increased to 66% of the intact control value 42 days after CTN transection (t = 2.51, P < 0.05).
No significant difference was found in the number of taste buds on the ipsilateral side between CTNX and CTN + LNX rats (F = 0.43, P = 0.5). The CTN + LNX and CTNX groups displayed a similar time course of changes in taste bud counts (Figure 2).
Changes in taste bud volumes after chorda tympani nerve transection
Similar to that for taste bud counts, all taste bud volumes from the sham control rats were pooled because of a non-significant effect of post-operative time (F = 0.02, P = 0.88) or side (F = 0.195, P = 0.66). The average taste bud volume in sham control rats was 4.0 ± 0.1 × 104 μm3 (Figure 3A and 4).
Figure 3.
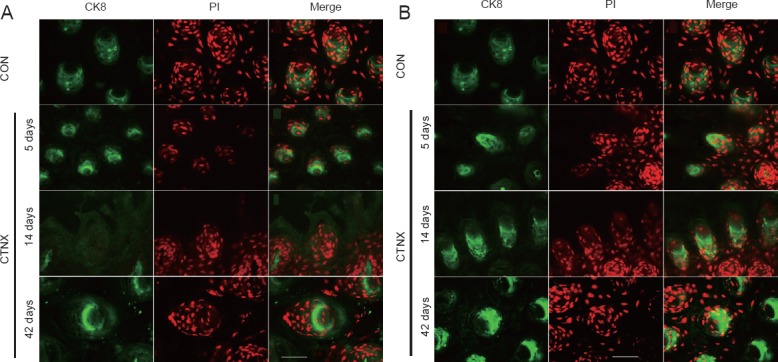
CK8 (green)/PI (red) labeling of taste buds on the ipsilateral (A) and contralateral (B) sides of the tongue at different time points after CTNX (fluorescence microscopy).
Taste buds on the ipsilateral tongue had smaller sizes and fewer cell numbers after denervation. Only fungiform papillae without CK8-positive cells were present 14 days after nerve transection. In contrast, there were increases in the size of the taste buds and in taste cell numbers on the contralateral side of the tongue postoperatively. Scale bars: 50 μm. CK8: Cytokeratin 8; PI: propidium iodide; CTNX: chorda tympani nerve transection; CON: control.
Figure 4.
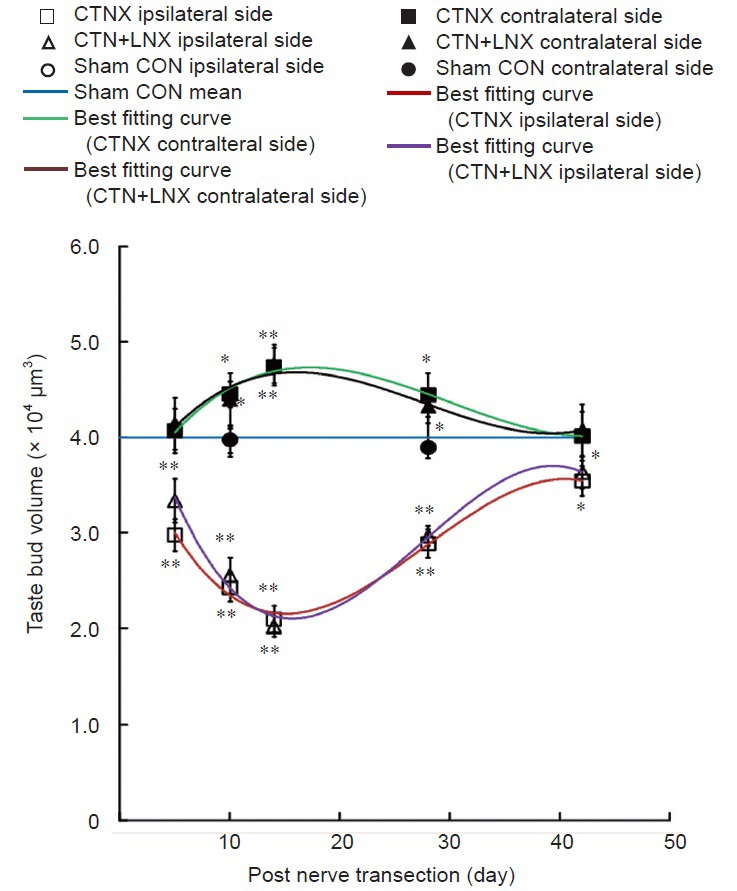
Changes in taste bud volume after CTNX.
The best fitting curves for average taste bud volumes on either side of the tongue in CTNX and CTN + LNX animals were third-order polynomials. The data are expressed as the mean ± SEM. Two-way analysis of variance and independent sample t-test were used to examine statistical differences. *P < 0.05, **P < 0.01, vs. sham group. CTNX: Chorda tympani nerve transection; CTN + LNX: transection of chorda tympani nerve and lingual nerve; CON: control.
On the ipsilateral side of the tongue, the average taste bud volume decreased significantly compared with sham control 5 days following CTN transection (t = 5.203, P < 0.01). The lowest taste bud size was observed at 14 days with a mean taste bud volume of 2.1 ± 0.1 × 104 μm3 (t = 10.297, P < 0.01). The average taste bud volume increased to 87.5% of the sham control level at 42 days (t = 2.306, P < 0.05; Figure 4).
Interestingly, the average taste bud volume on the contralateral side of the tongue also changed following CTN transection. At 10 days, there was a significant increase in average taste bud volume compared with sham control (t = 2.653, P < 0.05). The average taste bud volume on the contralateral side of the tongue peaked at 14 days (t = 3.936, P < 0.01) and remained significantly greater than sham control until 28 days following CTN transection (t = 2.308, P < 0.05; Figures 3B and 4).
No significant difference was found in mean taste bud volume between the CTNX and CTN + LNX groups at any post-operative time point on either side of the tongue (F = 0.439, P = 0.51). This indicates that the time course of changes in taste bud volume in the CTN + LNX group (Figure 4) was similar to the temporal profile of changes in the CTNX group on the same side of the tongue.
Changes in taste bud cell numbers after chorda tympani nerve transection
Neither post-operative time (F = 0.053, P = 0.82) nor side (F = 0.001, P = 0.98) had any significant effect on taste bud cell numbers in sham control rats. The average number of cells per taste bud was 76.2 ± 1.1 in sham control rats (Figures 3A and 5, n = 82).
Figure 5.
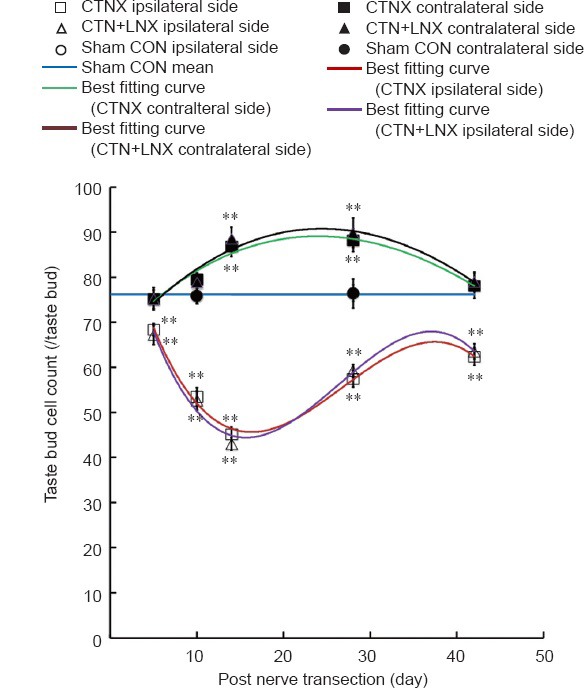
Changes in taste bud cell numbers after CTNX.
The best fitting curves for average taste bud cell numbers on either side of the tongue in CTNX and CTN + LNX rats were third-order polynomials. The data are expressed as the mean ± SEM. Two-way analysis of variance and independent sample t-test were used to examine statistical differences. **P < 0.01, vs. sham group. CTNX: Chorda tympani nerve transection; CTN + LNX: transection of chorda tympani nerve and lingual nerve; CON: control.
The average number of cells in a taste bud on the ipsilateral side of the tongue decreased to 90% of the sham control value 5 days after CTN transection (t = 3.294, P < 0.01) and continued to decline until 14 days (t = 12.896, P < 0.01). The average taste bud cell count increased to 82% of the sham control value at 42 days (t = 5.729, P < 0.01; Figures 3A and 5).
Similar to taste bud volumes, the average number of cells per taste bud on the contralateral side of the tongue increased after CTN transection, and was significantly greater than the sham control value at 14 days (t = 4.246, P < 0.01) and 28 days (t = 4.629, P < 0.01; Figures 3A and 5).
No significant difference was found in the average taste bud cell counts between the CTNX and CTN + LNX groups at any time point (F = 0.06, P = 0.81; Figure 5).
Discussion
In this study, we bilaterally examined the time course of morphological changes in the taste buds on the anterior tongue following unilateral transection of the CTN or CTN + LN. Our findings provide novel insight into the changes in taste bud structure and size in rats between denervation and reinnervation.
We did not find any evidence to support a role of the trigeminal component of the LN in maintaining the structural integrity of taste buds in rats. The trigeminal nerve and its branches contain no gustatory fibers that may be important in maintaining the number and size of the taste buds. Molecular studies have shown that neurotrophins may help determine which types of nerve fibers innervate the tongue epithelium. Specifically, brain-derived neurotrophic factor is produced by a variety of cell subpopulations in taste buds and acts as a chemoattractant molecule, while neurotrophin-4 is mainly expressed by surrounding non-gustatory epithelium and repels growing gustatory fibers from the tongue epithelium (Lopez and Krimm, 2006; Pagella et al., 2014). Brain-derived neurotrophic factor knockout results in a significant decrease in gustatory innervation, while trigeminal somatosensory innervation is less affected (Nosrat et al., 1997). A less severe loss of gustatory innervation and taste buds is observed in neurotrophin-4 knockout mice (Liebl et al., 1999).
The taste buds on the anterior two-thirds of the tongue are mainly innervated by gustatory neural fibers. Gustatory innervation regulates the differentiation of surrounding epithelial cells through the Sox2 pathway (Okubo et al., 2006; Suzuki, 2008; Pagella et al., 2014). Taste bud epithelial cells differentiate into keratinocytes instead of sensory cells after Sox2 deletion (Okubo et al., 2006). Yet, Sox2 expression is lost after denervation of taste buds (Suzuki, 2008). Taken together, these observations indicate that taste buds receive more innervation from gustatory nerve fibers than from non-gustatory ones, and only gustatory innervation is essential for the morphological integrity of the taste buds. Despite its limited role in maintaining the structure of the taste buds, the trigeminal component of the LN may still contribute in taste sensation. In patients with dental deficits, an association was found between the location of taste dysfunction and the location of deafferented teeth (Boucher et al., 2006). In addition, recovery from taste dysfunction was seen before electrogustometric threshold recovery in patients after middle ear surgeries (Berteretche et al., 2008). These findings suggest that neurophysiological convergence of the lingual somatosensory and taste pathways may occur in higher levels of the central nervous system.
The average size and number of taste buds and the number of taste bud cells all decreased significantly following ipsilateral denervation. These findings are consistent with other studies in rodents (Cheal and Oakley, 1977; Sollars et al., 2002; St. John et al., 2003; Guagliardo and Hill, 2007). These morphological parameters reached their lowest values at 14 days, and recovered to close to control levels by 42 days. Although in this report we made no attempt to examine how the taste buds regenerated, it has been shown in many studies that taste bud regeneration is dependent on regeneration of the transected gustatory nerve (Cheal and Oakley, 1977; Barry et al., 1993; Montavon et al., 1996). In support of this, we found scar tissues forming between the proximal and distal ends of the transected nerve, and there was no significant increase in taste bud count if the CTN was prevented from regenerating by cauterization (Li, unpublished observations). In addition, the regeneration of taste buds is accompanied by functional recovery as assessed behaviorally and electrophysiologically (Cheal and Oakley, 1977; Barry et al., 1993; Hill and Phillips, 1994; Montavon et al., 1996; Kopka et al., 2000; Saito et al., 2011a). In this study, the regeneration of the CTN probably started by 14 days following nerve transection.
Interestingly, the average taste bud size and the number of taste bud cells also increased on the contralateral side of the anterior tongue following unilateral CTN transection. Similar changes were reported in mice (Guagliardo and Hill, 2007). Although the underlying mechanism is unknown, it is possibly related to an inflammatory response to the nerve injury. Immunological changes in the lingual epithelium have been observed in rats following unilateral CTN transection—macrophages and neutrophils increase in numbers significantly on the intact side of the tongue (McCluskey, 2004; Wall and McCluskey, 2008; Steen et al., 2010). Recent studies suggest that this might correlate with functional plasticity of the peripheral taste system after nerve injury. Indeed, the response to sodium is extremely low on the intact side of the tongue immediately following unilateral CTN injury, but subsequently increases monotonically, resulting in supersensitivity after 1–2 days (Hill and Phillips, 1994; Wall and McCluskey, 2008; Guagliardo et al., 2009; Steen et al., 2010). Depletion of neutrophils prior to nerve sectioning restores normal sodium responses in the intact nerve, while recruiting neutrophils to the tongue produces deficits in sodium taste function (Steen et al., 2010). However, normal taste function was observed to be coupled to an increase in macrophage numbers following CTN sectioning, and could be attenuated by aldosterone (McCluskey, 2004; Guagliardo et al., 2009). Collectively, these observations suggest that the increases in taste bud size and taste cell numbers on the contralateral side of the tongue following CTN transection are likely mediated by an immunological response, which underlies the functional plasticity of gustatory sensation.
Although our study provides insight into the morphological changes in the taste buds after denervation, there are several limitations of the study design. Firstly, the time span for observation of taste bud recovery may not have been long enough. Specifically, we saw all three measures of taste bud morphology (i.e., count, volume and cell numbers) recovering to about 70–80% of baseline levels by 42 days after denervation. However, it is not known whether a full recovery of taste bud morphology can be achieved after denervation. However, based on a best fitting curve obtained from regenerating vallate papillae, the number of taste pores recovers to baseline levels in about 12 weeks following transection of the glossopharyngeal nerve in the rat (St. John et al., 2003). Thus, a better, if not full, recovery of taste bud morphology could potentially have been observed with a period of observation extending beyond 42 days. Secondly, we made no attempt to determine the source of innervation of the regenerating taste buds. The nerve terminals innervating the regenerating taste buds were likely from the original gustatory nerve (i.e., the CTN), but there could be other possibilities. Innervation may also derive from the contralateral side. Indeed, it has been reported that contralateral sprouting takes place over the midline into the denervated taste buds (Kinnman and Aldskogius, 1988). It is also possible that additional sprouts may arise from intact gustatory or non-gustatory fibers on the same side (e.g., glossopharyngeal nerve and trigeminal nerve). However, it remains unclear which nerves are the main source of reinnervation. Finally, an important question is whether the regenerated taste buds are functionally normal. Future studies using electrophysiological and behavioral tests, including training the animals to respond differentially to different taste stimuli, may help resolve this question.
Future investigations may involve comparative studies on the time course of taste bud regeneration in different species, including non-human primates. The use of neuronal tracers may help determine the source of reinnervation. In addition, molecular and genetic studies are needed to elucidate the mechanisms contributing to the regrowth of the taste buds after transection of gustatory nerves.
In summary, in the present study we found that denervation of the CTN resulted in the loss and morphological shrinkage of the ipsilateral taste buds. Enlarged taste buds and an increase in taste cell numbers were seen in the contralateral side of the tongue. There was no evidence for a role of the trigeminal component of the LN in maintaining the structural integrity of the taste buds in the anterior tongue.
Acknowledgments
We thank Cui-di Da, Jie Tian and Yunzhen Shen (Department of Research Center, Eye and ENT Hospital of Fudan University in China) for their help and instruction on microscopic imaging. We thank Wen Li (Department of Research Center, Eye and ENT Hospital of Fudan University in China) for her effort and patience in sectioning. The expertise and dedication of the staff of the Animal Care Facility in Eye and ENT Hospital of Fudan University in China were invaluable.
Footnotes
Funding: This research was supported by the Major State Basic Research Development Program of China (973 Program), No. 2011CB504500, 2011CB504506; the National Natural Science Foundation of China, No. 81271084, 81420108010, 81000413, 81370022, 81200740; the Training Program of the Excellent Young Talents of the Shanghai Municipal Health System in China, No. XYQ2013084; the Innovation Project of Shanghai Municipal Science and Technology Commission in China, No. 11411952300.
Conflicts of Interest: None declared.
Copyedited by Patel B, Pack M, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Asano-Miyoshi M, Hamamichi R, Emori Y. Cytokeratin 14 is expressed in immature cells in rat taste buds. J Mol Hist. 2008;39:193–199. doi: 10.1007/s10735-007-9151-0. [DOI] [PubMed] [Google Scholar]
- Barlow LA, Chien CB, Northcutt RG. Embryonic taste buds develop in the absence of innervation. Development. 1996;122:1103–1111. doi: 10.1242/dev.122.4.1103. [DOI] [PubMed] [Google Scholar]
- Barry MA, Larson DC, Frank ME. Loss and recovery of sodium-salt taste following bilateral chorda tympani nerve crush. Physiol Behav. 1993;53:75–80. doi: 10.1016/0031-9384(93)90013-6. [DOI] [PubMed] [Google Scholar]
- Berteretche MV, Eloit C, Dumas H, Talmain G, Herman P, Tran Ba Huy P, Faurion A. Taste deficits after middle ear surgery for otosclerosis: taste somatosensory interactions. Eur J Oral Sci. 2008;116:394–404. doi: 10.1111/j.1600-0722.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- Boucher Y, Berteretche MV, Farhang F, Arvy MP, Azérad J, Faurion A. Taste deficits related to dental deafferentation: an electrogustometric study in humans. Eur J Oral Sci. 2006;114:456–464. doi: 10.1111/j.1600-0722.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Bradley RM, Grabauskas G. Neural circuits for taste. Excitation, inhibition, and synaptic plasticity in the rostral gustatory zone of the nucleus of the solitary tract. Ann N Y Acad Sci. 1998;855:467–474. doi: 10.1111/j.1749-6632.1998.tb10607.x. [DOI] [PubMed] [Google Scholar]
- Breslin PA, Huang L. Human taste: peripheral anatomy, taste transduction, and coding. Adv Otorhinolaryngol. 2006;63:152–190. doi: 10.1159/000093760. [DOI] [PubMed] [Google Scholar]
- Cheal M, Oakley B. Regeneration of fungiform taste buds: temporal and spatial characteristics. J Comp Neurol. 1977;172:609–626. doi: 10.1002/cne.901720405. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Sarai PA, Barbacid M, Silos-Santiago I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int J Dev Neurosci. 1997;15:563–576. doi: 10.1016/s0736-5748(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Guagliardo NA, Hill DL. Fungiform taste bud degeneration in C57BL/6J mice following chorda-lingual nerve transection. J Comp Neurol. 2007;504:206–216. doi: 10.1002/cne.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagliardo NA, West KN, McCluskey LP, Hill DL. Attenuation of peripheral salt taste responses and local immune function contralateral to gustatory nerve injury: effects of aldosterone. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1103–1110. doi: 10.1152/ajpregu.00219.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DL, Phillips LM. Functional plasticity of regenerated and intact taste receptors in adult rats unmasked by dietary sodium restriction. J Neurosci. 1994;14:2904–2910. doi: 10.1523/JNEUROSCI.14-05-02904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CT, Garcea M, Spector AC. Glossopharyngeal nerve regeneration is essential for the complete recovery of quinine-stimulated oromotor rejection behaviors and central patterns of neuronal activity in the nucleus of the solitary tract in the rat. J Neurosci. 2000;20:8426–8434. doi: 10.1523/JNEUROSCI.20-22-08426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnman E, Aldskogius H. Collateral reinnervation of taste buds after chronic sensory denervation: a morphological study. J Comp Neurol. 1988;270:569–574. doi: 10.1002/cne.902700410. [DOI] [PubMed] [Google Scholar]
- Kopka SL, Geran LC, Spector AC. Functional status of the regenerated chorda tympani nerve as assessed in a salt taste discrimination task. Am J Physiol Regul Integr Comp Physiol. 2000;278:R720–731. doi: 10.1152/ajpregu.2000.278.3.R720. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Mbiene JP, Parada LF. NT4/5 mutant mice have deficiency in gustatory papillae and taste bud formation. Dev Biol. 1999;213:378–389. doi: 10.1006/dbio.1999.9385. [DOI] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Epithelial overexpression of BDNF and NT4 produces distinct gustatory axon morphologies that disrupt initial targeting. Dev Biol. 2006;292:457–468. doi: 10.1016/j.ydbio.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey LP. Up-regulation of activated macrophages in response to degeneration in the taste system: Effects of dietary sodium restriction. J Comp Neurol. 2004;479:43–55. doi: 10.1002/cne.20307. [DOI] [PubMed] [Google Scholar]
- McManus LJ, Stringer MD, Dawes PJ. Iatrogenic injury of the chorda tympani: a systematic review. J Laryngol Otol. 2012;126:8–14. doi: 10.1017/S0022215111002039. [DOI] [PubMed] [Google Scholar]
- Montavon P, Hellekant G, Farbman A. Immunohistochemical, electrophysiological, and electron microscopical study of rat fungiform taste buds after regeneration of chorda tympani through the non-gustatory lingual nerve. J Comp Neurol. 1996;367:491–502. doi: 10.1002/(SICI)1096-9861(19960415)367:4<491::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Blomlof J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- Ohtubo Y, Yoshii K. Quantitative analysis of taste bud cell numbers in fungiform and soft palate taste buds of mice. Brain Res. 2011;1367:13–21. doi: 10.1016/j.brainres.2010.10.060. [DOI] [PubMed] [Google Scholar]
- Okubo T, Pevny LH, Hogan BL. Sox2 is required for development of taste bud sensory cells. Gene Dev. 2006;20:2654–2659. doi: 10.1101/gad.1457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagella P, Jiménez-Rojo L, Mitsiadis T. Roles of innervation in developing and regenerating orofacial tissues. Cell Mol Life Sci. 2014;71:2241–2251. doi: 10.1007/s00018-013-1549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Ito T, Narita N, Yamada T, Manabe Y. Light and electron microscopic observation of regenerated fungiform taste buds in patients with recovered taste function after severing chorda tympani nerve. Ann Otol Rhinol Laryngol. 2011a;120:713–721. doi: 10.1177/000348941112001104. [DOI] [PubMed] [Google Scholar]
- Saito T, Narita N, Yamada T, Manabe Y, Ito T. Morphology of human fungiform papillae after severing chorda tympani nerve. Ann Otol Rhinol Laryngol. 2011b;120:300–306. doi: 10.1177/000348941112000504. [DOI] [PubMed] [Google Scholar]
- Saito T, Narita N, Yamada T, Ogi K, Kanno M, Manabe Y, Ito T. Length of nerve gap defects correlates with incidence of nerve regeneration but not with recovery of taste function in patients with severed chorda tympani nerve. Otol Neurotol. 2011c;32:1352–1357. doi: 10.1097/MAO.0b013e31822e96d6. [DOI] [PubMed] [Google Scholar]
- Sollars SI. Chorda tympani nerve transection at different developmental ages produces differential effects on taste bud volume and papillae morphology in the rat. J Neurobiol. 2005;64:310–320. doi: 10.1002/neu.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollars SI, Smith PC, Hill DL. Time course of morphological alterations of fungiform papillae and taste buds following chorda tympani transection in neonatal rats. J Neurobiol. 2002;51:223–236. doi: 10.1002/neu.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John SJ, Garcea M, Spector AC. The time course of taste bud regeneration after glossopharyngeal or greater superficial petrosal nerve transection in rats. Chem Senses. 2003;28:33–43. doi: 10.1093/chemse/28.1.33. [DOI] [PubMed] [Google Scholar]
- Steen PW, Shi L, He L, McCluskey LP. Neutrophil responses to injury or inflammation impair peripheral gustatory function. Neuroscience. 2010;167:894–908. doi: 10.1016/j.neuroscience.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y. Expression of Sox2 in mouse taste buds and its relation to innervation. Cell Tissue Res. 2008;332:393–401. doi: 10.1007/s00441-008-0600-1. [DOI] [PubMed] [Google Scholar]
- Wall PL, McCluskey LP. Rapid changes in gustatory function induced by contralateral nerve injury and sodium depletion. Chem Senses. 2008;33:125–135. doi: 10.1093/chemse/bjm071. [DOI] [PubMed] [Google Scholar]


