Abstract
Cryopreservation of boar semen is still considered suboptimal due to lower fertility as compared with fresh samples when glycerol, a permeating cryoprotectant, is used. Trehalose is a non-permeable cryoprotectant and nonreducing disaccharide known to stabilize proteins and biologic membranes. The aim of this study was to evaluate the cryosurvival and in vitro penetrability of boar spermatozoa when glycerol was replaced with trehalose in a freezing extender. Ejaculated Berkshire semen samples were diluted in egg yolk-based freezing extender containing glycerol (100 mM) or trehalose (0, 50, 100, 150, 200 and 250 mM) and cryopreserved using a straw freezing procedure. Thawed samples were analyzed for motility, viability, mitochondrial membrane potential (MMP), and acrosome integrity. In experiment 2, penetrability of spermatozoa cryopreserved with 100 mM glycerol or trehalose was examined. Replacement of cryoprotectant glycerol (100 mM) with trehalose had no effect on sperm viability, but replacing it with 100 mM trehalose improved motility, MMP and acrosome integrity significantly. Sperm motility and MMP were considerably higher in 100 mM trehalose, whereas the acrosome integrity was substantially higher in 100–250 mM trehalose. The in vitro penetration rate was also significantly higher in spermatozoa cryopreserved with trehalose (61.3%) than in those cryopreserved with glycerol (43.6%). In conclusion, 100 mM non-permeable trehalose can be used to replace glycerol, a permeating cryoprotectant, for maintenance of better post-thaw quality of boar spermatozoa.
Keywords: Cryopreservation, Cryoprotectant, Pigs, Sperm
Cryopreservation of boar spermatozoa is important in the preservation of excellent genetic resources and also useful for exchange of genetic material between breeding populations across the world. However, commercial use of cryopreserved boar spermatozoa is still not extended due to their disappointingly low cryo-survivability and the high level of cryoinjuries in the biological membranes, which results in low conception rates and smaller litters after artificial insemination [1,2,3]. Cryoinjuries also lead to cryo-capacitation after thawing, thus shortening the life span of spermatozoa [4,5,6]. Therefore, further improvements in the fertility of frozen-thawed boar spermatozoa will make commercial use more efficient and definite.
Glycerol is the most common permeating cryoprotectant for livestock sperm, and the optimum concentration added during the cryopreservation process is 1–3% due to its potential toxicity, but boar spermatozoa react variably [7]. The increasing viscosity of glycerol may inhibits ice crystal growth before achieving the glassy matrix state [8]. The presence of glycerol also appears to ameliorate the rise in salts to a critical damaging concentration at temperatures below the ice transition [9]. However, fertility of cryopreserved boar spermatozoa has consistently been low due to osmotic shock sensitivity, oxidative stress and low-temperature cryoprotectant intoxication (reviewed by Rath et al. [10]). Glycerol molecules can be inserted into the membrane lipid bilayer, altering the stability of the membrane, thereby influencesing the ability of sperm to undergo capacitation and the acrosome reaction, or even resulting in cell death [11]. Therefore, glycerol may not be the most effective cryoprotectant for boar spermatozoa. Thus, more studies using non-permeating cryoprotectants such as carbohydrates including monosaccharides, disaccharides and polysaccharides have been performed, resulting in an improved sperm survival rate after freeze-thawing [12,13,14]. Non-permeable disaccharides, including trehalose, also have a high cryoprotectant ability and kinetically inhibit ice crystal growth due to high viscosity [8]. Trehalose is known to help in resisting dehydration or freezing in a number of plants and animals including freeze-resistant insect species [15]. Disaccharides such as trehalose and sucrose are also known to stabilize the membrane by interacting with the polar head groups of phospholipids and increasing its fluidity [16]. Trehalose also induces a decline in the membrane phase transition temperature of dry lipids to form a glass, and it can reduce the concentration of the original cryoprotective agent to achieve a glassy state at a given critical cooling rate [17, 18]. Some studies have reported combined beneficial effects of trehalose on the cryosurvival of boar spermatozoa in a glycerol-based extender [14, 19, 20].
Since the biological toxicity of highly permeable glycerol cannot be avoided when it is used as a cryoprotectant, application of non-permeable cryoprotectants like trehalose for the cryopreservation of boar spermatozoa is worth examining. In this study, we hypothesized that use of the non-permeable cryoprotectant trehalose for cryopreservation of boar spermatozoa could increase their post-thaw survival, motility and/or penetrability even in a glycerol-free extender. The objective of the current study was to evaluate the effect of trehalose as a substitute cryoprotecting agent for glycerol on the cryosurvival and in vitro penetrability of boar spermatozoa.
Materials and Methods
Chemicals and extenders
Unless specified, all the chemicals were purchased from Sigma Aldrich Japan (Tokyo, Japan). The basic diluent used in the current experiments was modified Modena solution (mMS) [21]. Egg yolk-based extender (20% (v/v) hen’s egg yolk in mMS) was used as the cooling extender. As the freezing extender, the cooling extender was supplemented with 0.25% (v/v) Equex STM™ (Nova Chemical Sales, Scituate, MA, USA) and glycerol (100 mM as the final concentration - 1.5% (v/v)) or trehalose (50, 100, 150, 200 or 250 mM as the final concentration; Hayashibara, Okayama, Japan).
The medium used for washing and manipulating the cumulus-oocyte complexes (COCs) and the frozen-thawed spermatozoa for experiment 2 (Exp. 2) was TL-HEPES-PVA solution [22]. The medium used for oocyte maturation was modified Porcine Oocyte Medium [23] containing 50 µM β-mercaptoethanol (mPOM) [24]. The basic fertilization medium used in Exp. 2 was modified Medium199 (mM 199) [25].
Semen collection
Semen samples were collected from three individual Berkshire boars (1–3 years old) with excellent fertility scores (supplied by a local AI center). At least three ejaculates were obtained from each boar, and collection was done once a week. The sperm-rich fraction from individual ejaculates was collected into a prewarmed tube by gloved-hand technique and diluted with mMS (1:4) before transportation to the laboratory. Considering the heat loss on the way and body temperature of the boar, the semen samples were kept in a styrofoam box with warm packs (39 C) and transported within 1.5 h. At the laboratory, the samples were assessed for sperm concentration (hemacytometer), viability (SYBR/propidium iodide) and motility (CASA). Sperm samples with > 70% of motility and viability were used in the current experiments.
Semen processing
Diluted semen samples were centrifuged (450 × g, 5 min, room temperature), and the concentration was adjusted to 1 × 108 cells/ml with 20% (v/v) seminal plasma in mMS. Then they were cooled to 15 C in 4 h using a thermoblock (ThermoStat plus, Eppendorf, Hamburg, Germany). After incubation at 15 C overnight, sperm samples were washed with mMS three times by centrifugation (620 × g, 5 min, 15 C) to remove the seminal plasma. Afterwards, concentrations of the sperm samples were readjusted to 1 × 109 cells/ml with mMS before cryopreservation.
Cryopreservation of spermatozoa
Sperm samples were suspended in the cooling extender (1:4) at 15 C and cooled down to 5 C over the course of 2 h (ThermoStat plus, Eppendorf, Hamburg, Germany). Then they were resuspended in the freezing extender (1:1) and loaded into precooled 0.5-ml straws (Fujihara Industry, Tokyo, Japan) while keeping them on ice. The straws were frozen by keeping them 4.5 cm above the level of a liquid nitrogen bath at approximately –160 C for 15 min after sealing the free end. Finally, the straws were plunged into liquid nitrogen (–196 C) and stored for 2–3 days until thawing.
Thawing of frozen spermatozoa
The straws containing spermatozoa were thawed in water at 39 C for 30 sec. Spermatozoa were diluted in prewarmed (39 C) mMS (1:2) and washed once by centrifugation (700 × g, 3 min, 39 C). Each pellet was resuspended in 1 ml of mMS and, after incubating at 39 C for 5 min, it was evaluated for motility, viability, acrosome integrity, MMP and in vitro penetrability.
Oocytes collection and in vitro maturation
Oocytes were obtained from prepuberal gilt ovaries from a local slaughterhouse and transported to the laboratory within 1 h in 0.9% NaCl solution supplemented with Antibiotic-Antimycotic (GIBCO, Life Technologies Japan, Tokyo, Japan). Cumulus-oocyte complexes (COC) were aspirated from follicles 3–6 mm in diameter and processed for washing and in vitro maturation according to our laboratory protocol [24]. After the sample was washed three times with TL-HEPES-PVA, groups of 50 COCs with a uniform ooplasma surrounded by a clear and compact cumulus cell mass were cultured in 500 µl of mPOM containing 10 IU/ml eCG, 10 IU/ml hCG and 1 mM dibutyryl cAMP in an atmosphere of 5% CO2 in air at 39 C for 20 h. Then the culture was continued in a fresh mPOM medium without the above supplements for another 24 h period [26].
In vitro fertilization and penetrability assessment
After in vitro maturation of the oocyte, groups of 30–50 oocytes were denuded from cumulus cells with 0.1% hyaluronidase by pipetting. Then the oocytes were processed for in vitro fertilization according to our laboratory protocol [25]. Briefly, 30–50 oocytes were co-cultured with frozen-thawed spermatozoa (5 × 105 cells/ml) in mM199 (100 µl) containing 5 mM caffeine-benzoate for 8 h at 39 C in an atmosphere of 5% CO2 in air. After culture for in vitro fertilization, the oocytes were fixed in 25% (v/v) acidic alcohol at room temperature for at least 3 days, stained with 1% (w/v) orcein in 45% (v/v) acetic acid for 3 min and mounted on a glass slide, and sperm penetration and pronuclear formation were observed under a phase-contrast microscope (× 400) [27].
Evaluation of fresh and post-thaw spermatozoa quality
Motility: The percentage of total motile spermatozoa was determined using a computer-assisted semen analysis system (CASA, with the Sperm Motility Analysis System software, Digital Image Technology, Tokyo, Japan) with 60 FPS. For each sample, three subsamples were analyzed, and 2 µl of each subsample was placed on an objective micrometer (Fujihira Industry, Tokyo, Japan) and a minimum of 300 sperms per subsample were analyzed.
Viability: Viability was evaluated according to the protocol of a LIVE/DEAD Sperm Viability Kit (Thermo Fisher Scientific, Waltham, MA, USA). One microliter of SYBR Safe DNA in DMSO (1:9), 5 µl of PI (1 mg/ml) and 2.5 µl of sperm cells were added to 491.5 µl of mMS. The mixture was kept in the dark for 1 min and 30 sec. Then, 16 µl of the mixture were placed on a Thoma glass slide (0.1 mm deep, Erma, Tokyo, Japan) and observed under a fluorescence microscope (Eclipse 80i, Nikon, Tokyo, Japan). Green cells indicated live sperms, while red cells were considered dead sperms. A total of 400 sperm cells were counted, and the percentage of live cells was calculated.
Acrosome integrity: Acrosome integrity (intactness of the acrosome membrane) was evaluated by chlortetracycline (CTC) assay as described previously [22, 25]. Briefly, the suspended sperm cells were stained with 4 µl of 20 mg/ml bisbenzimide in TL-HEPES. After keeping them in the dark for 3 min, they were transferred to 4 ml of 3% PVP-PBS solution. The suspension was centrifuged at 850 × g for 5 min. The sperm cells in the pellet were mixed thoroughly with 45 µl of freshly prepared CTC solution (1:1). The CTC solution contained 750 μM CTC in a buffer of 130 mM NaCl, 5 mM cysteine and 20 mM Tris-HCl. The solution was wrapped in foil to prevent the entry of light and stored at 4 C until use. Sperm cells were then fixed by adding 8 µl of 12.5% (w/v) paraformaldehyde in 0.5 M Tris-HCl buffer (final pH 7.4). Slides were prepared by placing 10 µl of this suspension on a clean slide. One drop of 0.22 M l,4-diazabicyclo[2.2.2]octane in glycerol was mixed to retard fading of fluorescence. A cover slip was placed, and the slide was gently but firmly compressed between tissues to remove excess fluid. Only living sperm cells (Hoechst negative) were examined for CTC staining. Two hundred live sperm cells were then examined under blue-violet illumination (excitation at 400–440 nm and emission at 470 nm) and classified according to CTC staining patterns. The three fluorescent staining patterns identified were as follows: F, uniform fluorescence over the whole sperm head (acrosome intact cells); B, a fluorescence-free band in the post-acrosome region (capacitated cells); and AR, with almost no fluorescence over the whole sperm head except for a thin band of fluorescence in the equatorial segment (acrosome-reacted cells) [25].
Mitochondrial membrane potential: Two microliters of JC-1 (153 µm, Invitrogen, Molecular Probes, 5,5’,6,6’–tetrachloro-1,1’,3,3’-tetraethyl benzimidazolyl-carbocyanine iodide) and 3 µl of PI (1 mg/ml ) were added to a 150 µl of sperm sample and incubated at 39 C for 8 min in the dark. Then, 8 µl of the mixture was placed on a glass slide and observed under a fluorescence microscope (Eclipse 80i, Nikon). A green sperm head with an orange-yellow midpiece indicated a viable spermatozoon with high mitochondrial membrane potential (HMMP). A total of 300 sperm cells was counted, and the percentage of sperm cells with HMMP was calculated.
Experimental design
Experiment 1 (Exp. 1): Sperm samples were frozen with glycerol (100 mM) or trehalose (50, 100, 150, 200 and 250 mM) with 0.25% Equex STM™ as the freezing extender. The negative control consisted of 0 mM of either glycerol or trehalose. Sperm cells were packed into 0.5-ml straws and cryopreserved. Samples were thawed at 39 C for 30 sec and tested for post-thaw sperm qualities.
Experiment 2 (Exp. 2): The in vitro penetrabilities of spermatozoa frozen with 100 mM glycerol or trehalose were compared. A total of 187 mature oocytes from four replicates were used in the experiment.
Statistical analysis
Statistical analyses of results replicated 4–10 times were used for treatment comparisons and carried out by one-way ANOVA followed by Tukey’s multiple range test (Exp. 1) or the Chi-square test (Exp. 2) using the GraphPad Prism 6 statistical software (GraphPad Software, La Jolla, CA, USA). The Interrelation of concentration and post-thaw variables were assessed by Pearson correlation. All percentage data were subjected to arcsine transformation before statistical analysis if the percentage data contained values less than 10% and/or more than 90%. All data were expressed as the mean ± SEM. Differences were considered significant at P < 0.05.
Results
Exp. 1: Effect of trehalose on boar spermatozoa post-thaw survival
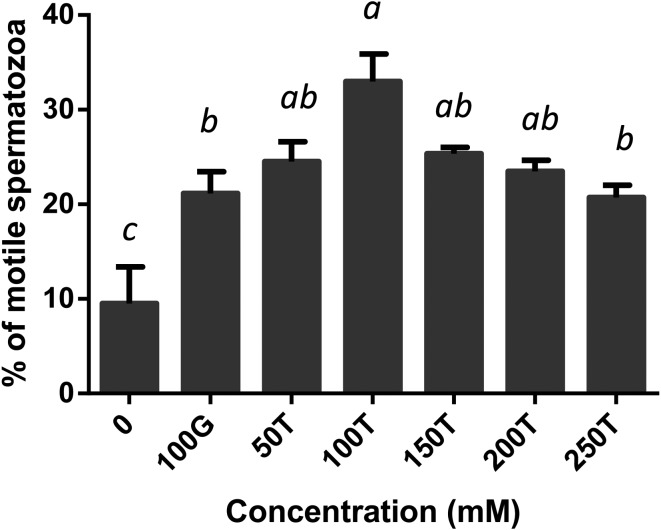
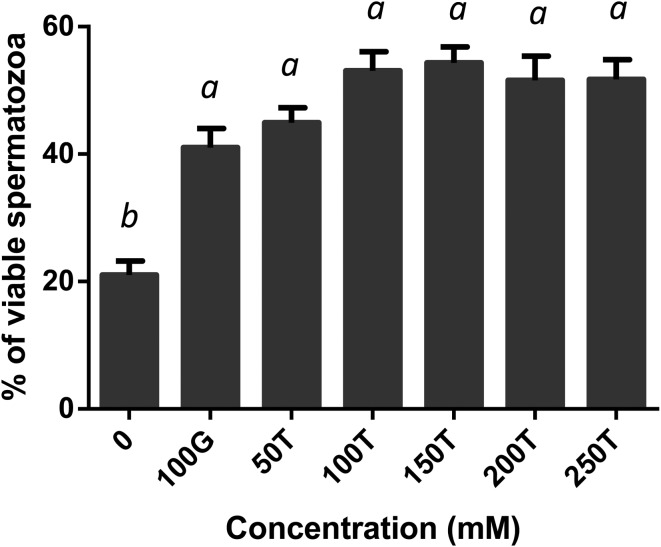
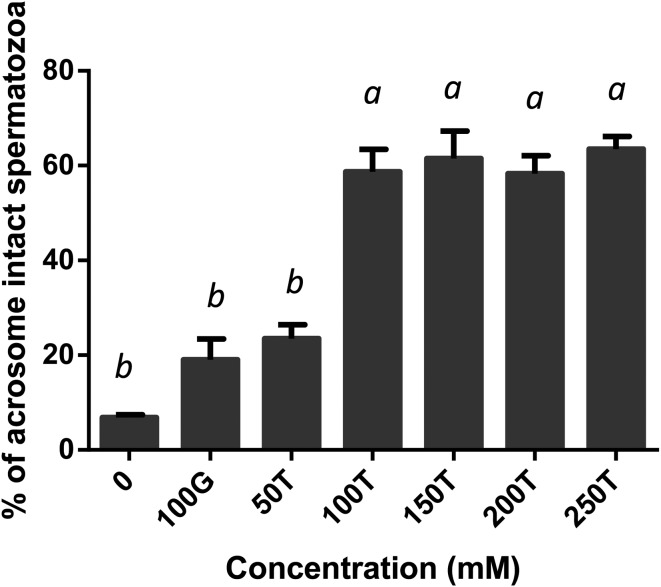
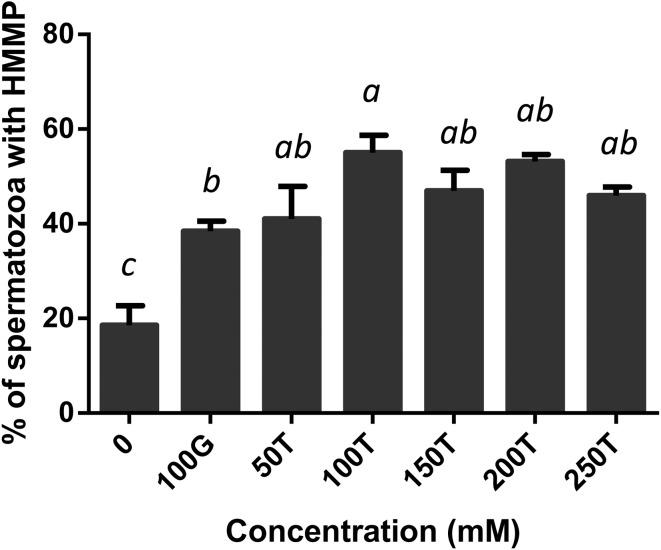
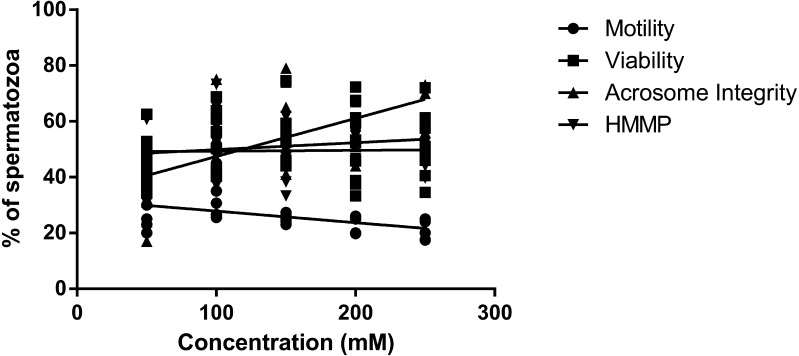
The effect of glycerol and trehalose added to the freezing extender on motility, viability, acrosome integrity and mitochondrial membrane potential of boar spermatozoa after freezing-thawing was evaluated. All the treated samples showed a significant increase in motility compared with the negative control (Fig. 1). Motility was significantly high in spermatozoa extended in 100 mM trehalose when compared with those extended in 100 mM glycerol (P < 0.05) or 250 mM trehalose (P < 0.01). It was not considerably different among the 50, 150, 200 and 250 mM trehalose extenders (P > 0.05). Post-thaw viability was significantly higher in all the treated samples when compared with the negative control (P < 0.01, Fig. 2). There were no significant differences among treatments between the 100 mM glycerol and 50–250 mM trehalose extenders. When the acrosome status was evaluated by CTC assay, spermatozoa in the extenders containing 100 mM or more of trehalose, exhibited significantly higher acrosomal integrity compared with those in the extenders containing 0 or 50 mM trehalose or 100 mM glycerol (P < 0.01, Fig. 3). Acrosome integrity was not significantly different in extenders supplemented with 100–250 mM trehalose. The percentage of viable spermatozoa with HMMP was significantly higher in all the treated extenders compared with the negative control (P < 0.01, Fig. 4). Spermatozoa extended in 100 mM trehalose exhibited a significantly high percentage of HMMP when compared with those extended in 100 mM glycerol (P < 0.05). There were no significant differences between the 50, 150, 200 and 250 mM trehalose extenders (P > 0.05). Motility and acrosome integrity showed a significant relationship with the trehalose concentration (Fig. 5). Motility was negatively correlated with the trehalose concentration (r = –0.49, P < 0.01), whereas acrosome integrity was positively correlated with it (r = 0.58, P < 0.005). Motility showed a strong negative correlation with trehalose concentrations greater than 100 mM (r = –0.95, P < 0.05). Viability and mitochondrial membrane potential were not significantly correlated with the trehalose concentration (P > 0.05).
Fig. 1.
Post-thaw motility of boar spermatozoa frozen in extenders supplemented with glycerol or trehalose. Error bars represent the SEM. a,b,c P < 0.05, n = 6.
Fig. 2.
Post-thaw viability of boar spermatozoa frozen in extenders supplemented with glycerol or trehalose. Error bars represent the SEM. a,b P < 0.05, n = 10.
Fig. 3.
Post-thaw acrosome integrity of boar spermatozoa frozen in extenders supplemented with glycerol or trehalose. Error bars represent the SEM. a,b P < 0.05, n = 6.
Fig. 4.
Percentage of boar spermatozoa frozen in extenders supplemented with glycerol or trehalose with high mitochondria membrane potential. Error bars represent the SEM. a,b,c P < 0.05, n = 6.
Fig. 5.
Correlation between the trehalose concentration and post-thaw variables.
Exp. 2: Effect of the presence of trehalose versus glycerol during cryopreservation on the in vitro penetrability of boar spermatozoa after thawing
The in vitro penetrability of frozen-thawed spermatozoa cryopreserved with glycerol or trehalose is shown in Table 1. The extenders containing trehalose considerably preserved the in vitro penetrability of spermatozoa when compared to glycerol (P < 0.01). However, the incidence of monospermic oocytes was higher when spermatozoa were frozen with glycerol (P < 0.01). The percentage of oocytes with male and female pronuclei was not significantly different between the two groups (glycerol vs trehalose), 8 h after IVF.
Table 1. Effect of glycerol and trehalose in an extender on penetrability of boar spermatozoa following freezing and thawing.
| Treatment | No. of oocytes examined | No. (% mean ± SEM) of oocytes |
||
| Penetrated 1 | Monospermy 2 | Formed male pronucleus 2 | ||
| Glycerol | 82 | 34 (43.6 ± 11.9)a | 29 (89.6 ± 7.9)a | 19 (50.0 ± 14.9) |
| Trehalose | 105 | 66 (61.3 ± 9.5)b | 47 (76.5 ± 9.1)b | 43 (59.2 ± 9.6) |
1 Percentage of the number of oocytes examined. 2 Percentage of the number of oocytes penetrated. a,b P < 0.05, n = 4.
Discussion
The results of the present study show that replacement of glycerol with trehalose as the cryoprotectant significantly improve motility, acrosome integrity, MMP and in vitro penetrability of post-thaw boar spermatozoa. The effects of trehalose on cryosurvival of spermatozoa have been reported in mammalian species other than the boar, such as the ram [28,29,30], goat [31,32,33], bull [34] and gazelle [35], for extenders containing glycerol supplemented with trehalose. Several investigators have found that the addition of trehalose to glycerol-based cryopreservation extenders protects boar spermatozoa against freeze damage. According to Gutierrez-Perez [20], almost double the sperms were alive, motile and intact in the presence of 250 mM trehalose and 1% (v/v) glycerol compared with those in 4% glycerol alone. Moreover, Malo et al. [14] stated that the addition of trehalose to the first medium before spermatozoa were extended with 3% glycerol significantly improved the freezability of boar spermatozoa, achieving higher sperm survival and fertilization rates. These results were obtained in the presence of glycerol at a relatively low concentration, and trehalose probably helped to produce a glass-forming state [17, 18]. Our findings clearly demonstrate that the beneficial effect of trehalose is observed even under glycerol-free conditions. Although a dehydration effect is expected even in the presence of trehalose alone, due to the non-permeability of trehalose, the biological toxicity in boar spermatozoa appears to be reduced compared with the presence of glycerol alone. Chemical toxicity cannot be ignored in application of glycerol to cryopreservation of boar spermatozoa despite benificial effects, since boar spermatozoa may be very sensitive to the toxicity of glycerol. However, some studies reported that supplementing an extender cotaining glycerol with trehalose had no significant effect on cryopreservation of spermatozoa [36, 37].
In the present study, in fact, the motility, HMMP and acrosome integrity were well maintained when spermatozoa were frozen with trehalose as compared with those frozen with glycerol. Similar results have been observed in previous studies in which the extenders were supplemented with 3–5% glycerol along with 100 mM trehalose [19, 29]. The beneficial effect of trehalose on biological membranes is probably active regardless of the presence of glycerol. Our results revealed that motility decreased in the presence of 150 mM or more of trehalose in a glycerol-free extender. Since the motility of frozen-thawed spermatozoa was also reduced in the presence of both trehalose (> 200 mM) and 3% glycerol [39], this reduction in motility may be due to the concentration-dependent effect of trehalose. Even though motility was negatively correlated with the trehalose concentration, MMP and viability were not affected by the high concentrations of trehalose. This fact suggests that high trehalose concentrations are not detrimental to spermatozoa membranes despite the low motility. In addition, although the osmotic effect is high, trehalose may not be involved in the organelle membrane damage that occurs due to influx and eflux of cryoprotectant during freezing and thawing, as it is non-penetrative. The viscosity of the medium is greater in the presence of a higher concentration of trehalose, making it difficult for sperm to move. Alternatively, the friction in the sperm tail is increased due to the loss of intracellular free water. This causes inhibition of sliding of the microtubule filaments or other structural elements in the flagellum [19], and consequently, motility is reduced. According to Rutllant et al. [40], trehalose broadened the boar spermatozoa osmotolerance by protecting the cell membrane and mitochondrial function but affected negatively on motility. However, a study of Bama miniature pig semen demonstrated significantly high motility in 200 mM trehalose with the combination of 9% LDL and 2% glycerol [41].
Furthermore, the results of the present work also showed that addition of 100–250 mM trehalose to a glycerol-free extender preserves the acrosome integrity and that the percentage of acrosome-intact spermatozoa was positively correlated with the trehalose concentration. According to Hu et al. [19], addition of 100–200 mM trehalose to an extender containing 3% glycerol preserves the acrosome integrity of boar spermatozoa. This result is also consistent with our current result showing that acrosome integrity was significantly maintained in the presence of 100 mM trehalose rather than 100 mM glycerol. However, higher concentrations of trehalose in the presence of glycerol have also been reported to have a detrimental effect on the acrosome integrity of frozen-thawed spermatozoa [39]. It has been suggested that the main cryoprotective effect of trehalose is preservation of the membrane structure. Lambruschini et al. [38] stated that trehalose participates in the network of hydrogen bonds between the phospholipid polar heads, thus replacing the water of hydration at the membrane-fluid interface and maintaining the head groups at their hydrated position. In addition, the osmotic effect of trehalose decreases the intracellular freezable water and hence the formation of ice crystals inside the cells [8]. Therefore, using 100 mM trehalose as a cryoprotectant in the extender for boar spermatozoa reduces the injuries in membranes and consequently improves the motility, MMP and acrosome integrity of frozen-thawed spermatozoa.
As trehalose preserves membrane fluidity during cryopreservation, it would eventually affect fertility as well. However, a limited number of studies have been carried out to evaluate the fertility of spermatozoa frozen-thawed with trehalose. The results of the current study clearly demonstrated that replacement of glycerol in an extender for cryopreservation with trehalose improved the in vitro penetrability of frozen-thawed boar spermatozoa. Malo et al. [14] achieved an in vitro penetration rate of 57.5% when boar spermatozoa were cryopreserved with trehalose in a glycerol-based extender. Supplementation of an extender containing 5% glycerol with 100 mM trehalose has been also reported to improve the fertility of frozen-thawed ram spermatozoa following artificial insemination in ewes [29]. In a recent report in mice [12], frozen-thawed spermatozoa cryopreserved with 300 mM trehalose retained significantly better fertility (79%) than those frozen in 0.3 M glycerol (11%). The presence of trehalose as a cryoprotectant in an extender improved the penetrability of frozen-thawed boar spermatozoa as compared with the combine effect of glycerol or using glycerol alone as the cryoprotectant, probably due to its favorable effects on the biological membranes.
In conclusion, trehalose, a non-permeable sugar, was capable of maintaining good levels of motility, viability, acrosome integrity, mitochondrial membrane potential and in-vitro penetrability of boar spermatozoa after cryopreservation in a glycerol-free freezing extender. We also recommend applying 100 mM trehalose as the optimum concentration to a glycerol-free freezing extender for cryopreservation of boar spermatozoa.
Acknowledgments
The authors would like to thank the Okayama Prefectural Center for Animal Husbandry & Research for providing the semen samples.
References
- 1.Johnson LA, Weitze KF, Fiser P, Maxwell WM. Storage of boar semen. Anim Reprod Sci 2000; 62: 143–172. [DOI] [PubMed] [Google Scholar]
- 2.Roca J, Rodŕiguez-Martínez H, Vázquez JM, Bolarín A, Hernández M, Saravia F, Wallgren M, Martínez EA. Strategies to improve the fertility of frozen-thawed boar semen for artificial insemination. Soc Reprod Fertil Suppl 2006; 62: 261–275. [PubMed] [Google Scholar]
- 3.Rodriguez-Martinez H, Wallgren M. Advances in boar semen cryopreservation. Vet Med Int 2011; 2011: 396181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breininger E, Beorlegui NB, O’Flaherty CM, Beconi MT. Alpha-tocopherol improves biochemical and dynamic parameters in cryopreserved boar semen. Theriogenology 2005; 63: 2126–2135. [DOI] [PubMed] [Google Scholar]
- 5.Cerolini S, Maldjian A, Surai P, Noble R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim Reprod Sci 2000; 58: 99–111. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell WMC, Johnson LA. Membrane status of boar spermatozoa after cooling or cryopreservation. Theriogenology 1997; 48: 209–219. [DOI] [PubMed] [Google Scholar]
- 7.Holt WV. Basic aspects of frozen storage of semen. Anim Reprod Sci 2000; 62: 3–22. [DOI] [PubMed] [Google Scholar]
- 8.Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Lett 2004; 25: 375–388. [PubMed] [Google Scholar]
- 9.Lovelock JE. The protective action of neutral solutes against haemolysis by freezing and thawing. Biochem J 1954; 56: 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rath D, Bathgate R, Rodriguez-Martinez H, Roca J, Strzezek J, Waberski D. Recent advances in boar semen cryopreservation. Soc Reprod Fertil Suppl 2009; 66: 51–66. [PubMed] [Google Scholar]
- 11.Leibo SP, McGrath JJ, Cravalho EG. Microscopic observation of intracellular ice formation in unfertilized mouse ova as a function of cooling rate. Cryobiology 1978; 15: 257–271. [DOI] [PubMed] [Google Scholar]
- 12.Sztein JM, Noble K, Farley JS, Mobraaten LE. Comparison of permeating and nonpermeating cryoprotectants for mouse sperm cryopreservation. Cryobiology 2001; 42: 28–39. [DOI] [PubMed] [Google Scholar]
- 13.Molinia FC, Evans G, Quintana Casares P, Maxwell W. Effect of monosaccharides and disaccharides in Tris-based diluents on motility, acrosome integrity and fertility of pellet frozen ram spermatozoa. Anim Reprod Sci 1994; 36: 113–122. [Google Scholar]
- 14.Malo C, Gil L, Gonzalez N, Cano R, de Blas I, Espinosa E. Comparing sugar type supplementation for cryopreservation of boar semen in egg yolk based extender. Cryobiology 2010; 61: 17–21. [DOI] [PubMed] [Google Scholar]
- 15.Westh P, Ramlov H. Trehalose accumulation in the Tardigrade Adorybiotus-Coronifer during anhydrobiosis. J Exp Zool 1991; 258: 303–311. [Google Scholar]
- 16.Crowe LM, Crowe JH, Rudolph A, Womersley C, Appel L. Preservation of freeze-dried liposomes by trehalose. Arch Biochem Biophys 1985; 242: 240–247. [DOI] [PubMed] [Google Scholar]
- 17.Sutton RL. Critical cooling rates for aqueous cryoprotectants in the presence of sugars and polysaccharides. Cryobiology 1992; 29: 585–598. [DOI] [PubMed] [Google Scholar]
- 18.Sutton RL. Critical cooling rates to avoid ice crystallization in solutions of cryoprotective agents. J Chem Soc, Faraday Trans 1991; 87: 101–105. [Google Scholar]
- 19.Hu JH, Li QW, Jiang ZL, Yang H, Zhang SS, Zhao HW. The cryoprotective effect of trehalose supplementation on boar spermatozoa quality. Reprod Domest Anim 2009; 44: 571–575. [DOI] [PubMed] [Google Scholar]
- 20.Gutiérrez-Pérez O, Juárez-Mosqueda ML, Carvajal SU, Ortega ME. Boar spermatozoa cryopreservation in low glycerol/trehalose enriched freezing media improves cellular integrity. Cryobiology 2009; 58: 287–292. [DOI] [PubMed] [Google Scholar]
- 21.Funahashi H, Sano T. Select antioxidants improve the function of extended boar semen stored at 10 degrees C. Theriogenology 2005; 63: 1605–1616. [DOI] [PubMed] [Google Scholar]
- 22.Funahashi H, Nagai T. Regulation of in vitro penetration of frozen-thawed boar spermatozoa by caffeine and adenosine. Mol Reprod Dev 2001; 58: 424–431. [DOI] [PubMed] [Google Scholar]
- 23.Yoshioka K, Suzuki C, Onishi A. Defined system for in vitro production of porcine embryos using a single basic medium. J Reprod Dev 2008; 54: 208–213. [DOI] [PubMed] [Google Scholar]
- 24.Akaki Y, Yoshioka K, Noguchi M, Hoshi H, Funahashi H. Successful piglet production in a chemically defined system for in-vitro production of porcine embryos: dibutyryl cyclic amp and epidermal growth factor-family peptides support in-vitro maturation of oocytes in the absence of gonadotropins. J Reprod Dev 2009; 55: 446–453. [DOI] [PubMed] [Google Scholar]
- 25.Funahashi H, Fujiwara T, Nagai T. Modulation of the function of boar spermatozoa via adenosine and fertilization promoting peptide receptors reduce the incidence of polyspermic penetration into porcine oocytes. Biol Reprod 2000; 63: 1157–1163. [DOI] [PubMed] [Google Scholar]
- 26.Funahashi H, Cantley TC, Day BN. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol Reprod 1997; 57: 49–53. [DOI] [PubMed] [Google Scholar]
- 27.Funahashi H, Day BN. Effects of the duration of exposure to hormone supplements on cytoplasmic maturation of pig oocytes in vitro. J Reprod Fertil 1993; 98: 179–185. [DOI] [PubMed] [Google Scholar]
- 28.Bucak MN, Ateşşahin A, Varişli O, Yüce A, Tekin N, Akçay A. The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen Microscopic and oxidative stress parameters after freeze-thawing process. Theriogenology 2007; 67: 1060–1067. [DOI] [PubMed] [Google Scholar]
- 29.Jafaroghli M, Khalili B, Farshad A, Zamiri MJ. The effect of supplementation of cryopreservation diluents with sugars on the post-thawing fertility of ram semen. Small Rumin Res 2011; 96: 58–63. [Google Scholar]
- 30.Tonieto RA, Goularte KL, Gastal GDA, Schiavon RS, Deschamps JC, Lucia JT. Cryoprotectant effect of trehalose and low-density lipoprotein in extenders for frozen ram semen. Small Rumin Res 2010; 93: 206–209. [Google Scholar]
- 31.Aboagla EM, Terada T. Trehalose-enhanced fluidity of the goat sperm membrane and its protection during freezing. Biol Reprod 2003; 69: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 32.Khalili B, Farshad A, Zamiri MJ, Rashidi A, Fazeli P. Effects of sucrose and trehalose on the freezability of Markhoz goat spermatozoa. Asian-australas J Anim Sci 2009; 22: 1614–1619. [Google Scholar]
- 33.Naing SW, Wahid H, Mohd Azam K, Rosnina Y, Zuki AB, Kazhal S, Bukar MM, Thein M, Kyaw T, San MM. Effect of sugars on characteristics of Boer goat semen after cryopreservation. Anim Reprod Sci 2010; 122: 23–28. [DOI] [PubMed] [Google Scholar]
- 34.Chhillar S, Singh VK, Kumar R, Atreja SK. Effects of Taurine or Trehalose supplementation on functional competence of cryopreserved Karan Fries semen. Anim Reprod Sci 2012; 135: 1–7. [DOI] [PubMed] [Google Scholar]
- 35.Garde JJ, del Olmo A, Soler AJ, Espeso G, Gomendio M, Roldan ER. Effect of egg yolk, cryoprotectant, and various sugars on semen cryopreservation in endangered Cuvier’s gazelle (Gazella cuvieri). Anim Reprod Sci 2008; 108: 384–401. [DOI] [PubMed] [Google Scholar]
- 36.Molinia FC, Evans G, Maxwell WM. In vitro evaluation of zwitterion buffers in diluents for freezing ram spermatozoa. Reprod Nutr Dev 1994; 34: 491–500. [DOI] [PubMed] [Google Scholar]
- 37.Squires EL, Keith SL, Graham JK. Evaluation of alternative cryoprotectants for preserving stallion spermatozoa. Theriogenology 2004; 62: 1056–1065. [DOI] [PubMed] [Google Scholar]
- 38.Lambruschini C, Relini A, Ridi A, Cordone L, Gliozzi A. Trehalose interacts with phospholipid polar heads in Langmuir monolayers. Langmuir 2000; 16: 5467–5470. [Google Scholar]
- 39.Aisen EG, Medina VH, Venturino A. Cryopreservation and post-thawed fertility of ram semen frozen in different trehalose concentrations. Theriogenology 2002; 57: 1801–1808. [DOI] [PubMed] [Google Scholar]
- 40.Rutllant J, Alvarado O, Fonda E. Effects of Trehalose on the osmotic stress limits of boar sperm. Biol Reprod 2008; 78: 145. [Google Scholar]
- 41.Kong D, Shang H, Guo K, Liu Y, Zhang J, Wei H. A study on optimizing the cryopreservation methods for Bama miniature pig semen. Exp Anim 2012; 61: 533–542. [DOI] [PubMed] [Google Scholar]