Abstract
Ovaries contain follicles at various stages of development, including primordial, primary, secondary, antral and Graafian follicles. Although the growth of these follicles is controlled to maintain regular ovulation, the mechanism through which this occurs remains unclear. In our study, we found that the growth rate of cultured secondary follicles separated from mice ovaries differed between follicles. After 4 days of culture, the size of some secondary follicles was markedly increased, while that of others had either slightly increased, remained unchanged or shrunk. We compared the expression levels of growth factors between these secondary follicles and found that the growth rate of cultured secondary follicles correlated with the expression level of insulin-like growth factor 1 (Igf1) mRNA. Igf1 mRNA expression level in secondary follicles containing theca cells was higher than that in secondary follicles without theca cells, and the granulosa cell proliferation around follicles containing theca cells was increased. Furthermore, an IGF1 inhibitor also inhibited the granulosa cell proliferation, and administration of IGF1 to secondary follicles without growth promoted granulosa cell proliferation. These results indicated that the theca cells of secondary follicles induced the expression of IGF1 and promoted the follicle growth.
Keywords: Insulin-like growth factor 1 (IGF1), Secondary follicle, Theca cell
Before the antral follicle stage, there is the pre-antral follicle stage that contains primordial, primary and secondary follicles [1]. Although it has been reported that gonadotropin, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are important for the development and survival of antral follicles, the development of pre-antral follicles is independent of gonadotropin [2]. Instead of gonadotropin, many factors control development during the pre-antral stage [3].
Previous reports have indicated that various factors induce the development of arrested primordial follicles, including bone morphogenetic protein 4 (BMP4), growth and differentiation factor 9 (GDF9), leukemia inhibitory factor (LIF), basic fibroblast growth factor (bFGF), keratinocyte growth factor (KGF) and kit ligand (KL) [4,5,6,7,8]. LIF, bFGF and KGF induce the expression of KL in granulosa cells, implying that KL may be critical to the initiation of primordial follicle development [4, 6,7,8]. These factors therefore appear to be expressed in each cell in follicles and in other parts of the ovary: BMP4 in theca and stromal cells [4], GDF9 and bFGF in oocytes, LIF and KL in granulosa cells [5,6,7] and KGF in theca cells [8]. Thus, various ovarian cells control follicle development. Furthermore, tumor necrosis factor α (TNFα), BMP7, insulin, progesterone and anti-Mullerian hormone (AMH) also control the primordial to primary follicle transition [9]. Of these factors, progesterone and AMH inhibit the transition from primordial to primary follicles.
Primordial follicle development is typically arrested as a pool of oocytes, with a decrease in number associated with ageing. Many studies have focused on the mechanism controlling the primordial to primary follicle transition. The results of these studies may enable us to utilize primordial follicles. Furthermore, to utilize primordial follicles effectively, in vitro follicle culture methods are necessary. Therefore, the understanding of the mechanism controlling follicle growth of primary and secondary follicles is important because the growth of these follicles is controlled by many factors and the detailed mechanism is not clear yet. In this study, we focused on the mechanism controlling secondary follicles. This stage is the transition period from preantral to antral follicles, and the main control factors of follicle growth change from non-gonadotropin factors to gonadotropin, FSH and LH [10].
Formation of the theca cell layer in follicles also occurs during the secondary follicle stage [2]. In this stage, the theca cells produce androgen that enhances follicle development [11,12,13,14]. Furthermore, IGF1 and KL derived from granulosa cells have been reported to increase androgen production in theca cells [15], suggesting that theca cells are important to the development of pre-antral follicles. In this study, we found that there is the relationship between the expression of Igf1 mRNA and formation of the theca cell layer and that IGF1 mainly enhances the growth of early secondary follicles. These results indicated that the expression of IGF1 induced by theca cells is important for the development of secondary follicles.
Materials and Methods
Animals
Secondary follicles were isolated from 26- to 28-day-old female ICR mice (Japan SLC, Shizuoka, Japan). All mice were housed in an environmentally controlled room at 23 ± 1 C with 12 h light and dark periods. The animal care and experiments were conducted in accordance with the Guidelines of Animal Experimentation of Bell Research Center, which were based on the guidelines published by the Science Council of Japan. The experiments in this study were approved by the Institutional Animal Care and Use Committee of Bell Research Center.
Follicle isolation and encapsulated three-dimensional alginate gel culture
Secondary follicles with diameters of 100–130 μm were isolated from 26- to 28-day-old female mice and encapsulated in 0.5% (w/v) alginate gel (Sigma-Aldrich, St Louis, MO, USA) [16]. Ovaries were placed in L15 media (Gibco, Carlsbad, CA, USA) containing 1% foetal bovine serum (FBS, Gibco), and follicles were mechanically isolated using 29 gauge needles (Terumo, Tokyo, Japan). The following criteria were used to select follicles: (1) a diameter of 100–130 μm and (2) an oocyte being round and centrally located within the follicle.
The isolated follicles were individually transferred into 3 μl 0.5% sterile sodium alginate (Sigma-Aldrich) diluted in phosphate-buffered saline (PBS), and the droplet containing the secondary follicle was then put in 50 mM CaCl2 solution (Sigma-Aldrich) for 2 min. Each alginate-encapsulated follicle was transferred into individual wells of 24-well tissue culture plates containing 500 μl minimum essential medium alpha (MEM alpha), GlutaMAX (Gibco) supplemented with 5% (v/v) FBS, 100 μIU/ml of FSH from human pituitary glands (Sigma-Aldrich) and 10 mIU/ml of LH from equine pituitary glands (Sigma-Aldrich). Encapsulated follicles were cultured at 37 C in a 5% CO2 atmosphere. On culture day 2, the follicles were treated with 100 mIU/ml of LH in medium at 37 C for 6 h to reproduce the LH surge. This treatment of LH increases the viability after the antral follicles stage around culture days 5–10. The concentrations of FSH and LH were based on a previous experiment [17].
Evaluation of the growth of cultured follicles
The condition and growth of the cultured follicles were assessed daily using an Olympus CKX41 inverted microscope and Olympus DP21 camera (Olympus, Tokyo, Japan). The follicles were concluded to be undergoing atresia if the oocyte was dark or not surrounded by a layer of granulosa cells. The diameter of each follicle was measured as the mean of the long and short axes using the ImageJ 1.44p software (National Institutes of Health, Bethesda, MD, USA).
On culture day 3, the follicles were classified into four groups according to changes in their diameter (growth rate). The high-growth group comprised follicles that increased their diameter by more than 10% of that of the initial secondary follicles; the low-growth group comprised follicles with a 2–5% increase in diameter, and the no-growth group comprised follicles that remained similar in size. The reduced group comprised follicles that continued to decrease over the 3 days. To check whether follicles contained a theca layer, we stained the cultured follicles with Hoechst 33258 (Invitrogen) and detected the layer by confocal microscopy (FV1000, Olympus). Furthermore, we confirmed whether there were apoptotic cells in each growing follicles using YO-PRO-1 (Molecular Probes, Eugene, OR, USA), which stains the nucleic acids of apoptotic cells but does not stain live cells. The follicles cultured in alginate gels were incubated in the medium containing YO-PRO-1 and Hoechst 33258 (Invitrogen) at 37 C in a 5% CO2 atmosphere for 30 min, and images were captured with a confocal microscope (FV1000; 5-μm Z-step size). The follicles were collected from two mice, and we performed the same experiment three times.
RNA extraction and quantitative reverse transcription polymerase chain reaction
Follicles were incubated with 3 IU/ml alginate lyase at 37 C for 30 min to remove the alginate gel covering the cultured follicles. Next, they were separated into groups of 10–20 follicles based on growth rate. Total RNA was isolated from follicles using NucleoSpin RNA XS (Macherey-Nagel, Duren, Germany) according to the manufacturer’s instructions. A reverse transcription with total RNA was performed with a high capacity reverse transcription kit with an RNase inhibitor (Applied Biosystems, Foster, CA, USA). Thereafter, real-time polymerase chain reaction (RT-PCR) was performed using a StepOnePlus Real-Time PCR System (Applied Biosystems) and Fast SYBR Green Master Mix (Applied Biosystems). The following primers sets were used: Igf1, 5′-TCATGTCGTCTTCACACCTCTTCT-3′ (sense) and 5′-CCACACACGAACTGAAGAGCAT-3′ (antisense); GAPDH, 5′-ATGAATACGGCTACAGCAACAGG-3′ (sense); 5′-CTGTTGCTCAGTGTCCTTGCTG-3′ (antisense). The PCR profile incubation was initiated at 95 C for 10 sec followed by 40 cycles with denaturation at 95 C for 3 sec and annealing and extension at 60 (Igf1) or 61 C (Gapdh) for 30 sec. The follicles were collected from two or three mice, and we performed the same experiment four times.
Co-culture of granulosa cells and theca cells
Secondary and antral follicles were separated from 26- to 28-day-old female ICR mice, and the granulosa cells were collected by pushing the follicles with tweezers. The isolated granulosa cells and the remaining follicle tissues containing theca cells, granulosa cells and stromal cells were incubated in the medium containing 2 mg/ml collagenase (Wako Pure Chemical Industries, Osaka, Japan,) and 30 μg/ml hyaluronidase (Sigma-Aldrich) for 60 min at 37 C in a 5% CO2 atmosphere. Next, the cells were collected by centrifuging at 800 rpm for 5 min and suspended in culture medium containing 5% FBS, 100 μIU/ml FSH and 10 mIU/ml LH. The granulosa cells were cultured in a 24-well culture dish (AGC Techno Glass, Shizuoka, Japan), and cells derived from the remaining follicle tissues were cultured in a 12 mm-culture insert (Merck Millipore, Carrigtwohill, Ireland). After 24 culture, the culture insert in which theca cells, granulosa cells and stromal cells were cultured was put into a well in which granulosa cells were cultured to ascertain whether theca cells increase the expression of Igf1 mRNA in granulosa cells. After 24 h of co-culture, granulosa cells were treated with 0.025% trypsin and 0.02% EDTA (Sigma-Aldrich) for 30 min at 37 C and collected by centrifuging at 800 rpm for 5 min. After the collection of cells, we extracted total RNA from the cells, performed a reverse transcription with total RNA and quantitated the expression of Igf1 mRNA. These procedures were the same as those above.
Effect of IGF1 on cell proliferation
To check the effect of IGF1 on granulosa cell proliferation, we treated the cultured follicles with 10 μM of 5-ethynyl-2′-deoxyuridine (EdU, Molecular Probes) and with and without 50 ng/ml of recombinant mouse IGF1 (Sigma-Aldrich) on culture day 3 for 18 h. Then, we separated the cultured follicles into growth rate groups and fixed them with 4% paraformaldehyde at room temperature for 15 min. Next, we detected EdU in the follicles with Click-iT EdU Alexa Fluor Imaging Kits (Molecular Probes) according to the manufacturer’s instruction. After staining with EdU, we transferred the follicles into 20 μl PBS (−) drops covered with 2 ml of liquid paraffin in a 35-mm glass bottom dish (IWAKI, Tokyo, Japan) before capturing images with a confocal microscope (FV1000; 5-μm Z-step size). We stained cultured follicles with Hoechst 33342 and counted the number of nuclei and EdU-positive nuclei to evaluate the cell proliferation per follicle. We picked up three images of each follicle and counted the number of total granulosa cells and Edu-positive cells. The follicles were collected from twelves mice.
Inhibition and rescue of IGF1-induced cell proliferation activity
To evaluate the effect of IGF1 on granulosa cell proliferation, we treated the cultured follicles with EdU and 10 μM AG1024 diluted with dimethyl sulfoxide, a competitive inhibitor of IGF1 (Calbiochem, San Diego, CA, US), for 18 h. After treatment, we removed the alginate gel covering the follicles and fixed and stained them as described above. In addition, we added 50 and 100 ng/ml of IGF1 with AG1024 to the culture medium to evaluate the effect AG1024. The follicles were collected from nine mice.
Statistical analysis
Statistical analyses were performed with the R software (http://www.r-project.org/). The normality of all data, including the Igf1 mRNA expression and cell proliferation levels, was evaluated by the Shapiro–Wilk test (P > 0.05). We then analyzed normally distributed data by unpaired t–test/one-way analysis of variance (one-way ANOVA, Fig. 1I, 1N , 2 , 3D , 4A , 5B and 6) and non-normally distributed data by Mann-Whitney U tests (Fig. 4B, 4C and 5A). P < 0.05 was considered statistically significant. Furthermore, multiple comparisons were sequentially carried out for statistically significant results (P < 0.05) by using the Turkey/Steel-Dawss method for normally/non-normally distributed data.
Fig. 1.
Changes in the diameter of cultured secondary follicles. (A–H) The images of cultured follicles. A and B: a high-growth follicle. C and D: low-growth follicle. E and F: no-growth follicle. G and H: reduced-growth follicle. Panels A, C, E and G are images from culture day 0, and panels B, D, F and H are images of culture day 3. The follicle diameters are as follows: A, 116 μm; B, 135 μm; C, 110 μm; D, 115 μm; E, 110 μm; F, 110 μm; G, 111 μm; and H, 106 μm. (H) On culture day 3, differences in follicle size appeared, and we separated follicles into four groups dependent on the rate of diameter increase (high-growth, low-growth, no-growth and reduced-growth groups). There were significant differences among each group except between the no-growth and reduced-growth groups (P < 0.05). (J–M) The images of follicles cultured for 4 days and stained with YO-PRO (green) and Hoechst 33258 (blue). K and M are the bright-field images of J and L, respectively. J and K are reduced-growth follicles. L and M are high-growth follicles. (N) Granulosa cell proliferation of each group was measured by an EdU labelling kit. Statistical significance is indicated by an asterisk (**P < 0.01, ***P < 0.001). Values are presented as means ± standard deviation. The number of samples per experiment is indicated under the group names. Scale bars show 100 μm.
Fig. 2.
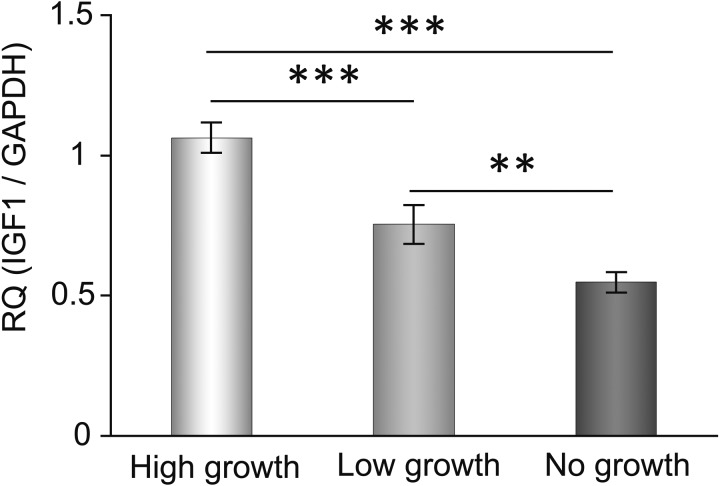
Expression of Igf1 mRNA depended on the growth rate of the cultured secondary follicles. We evaluated the expression level of Igf1 mRNA in each group on culture day 3. Statistical significance is indicated by an asterisk (**P < 0.01, *** P < 0.001). Values are presented as means ± standard deviation.
Fig. 3.
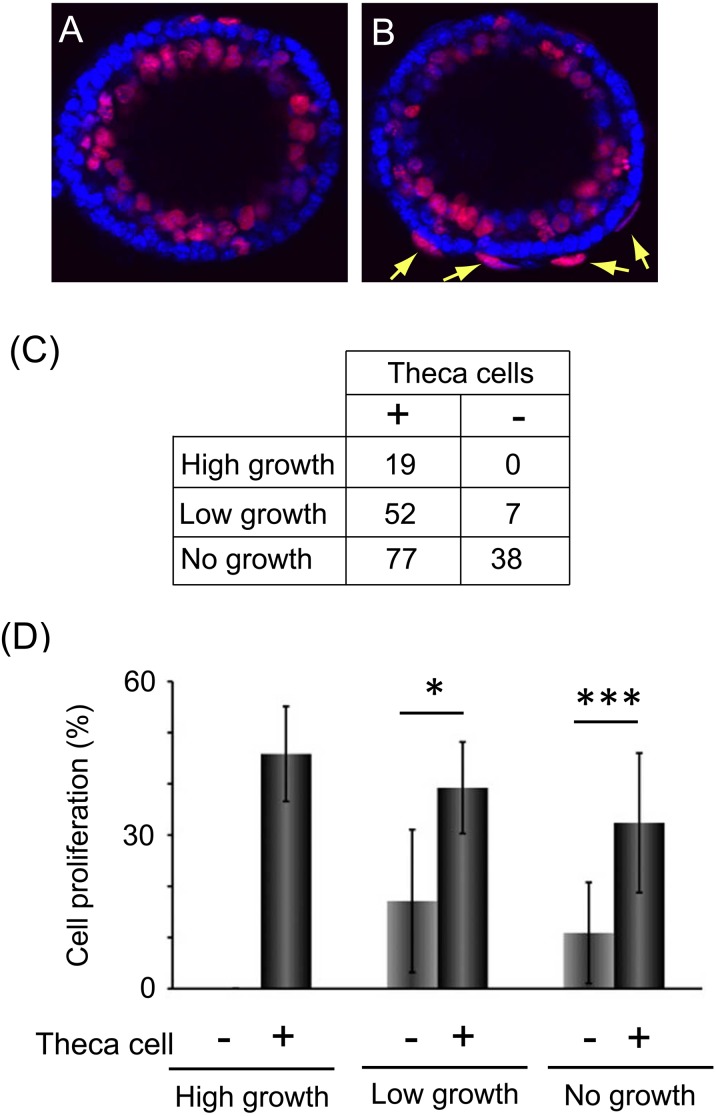
Theca cells promoted granulosa cell proliferation of secondary follicles. (A, B) The images show the granulosa cell proliferation around cultured secondary follicles stained using an EdU labelling kit (red) and Hoechst 33258 (blue). Panel A indicates follicles without theca cells, and panel B indicates follicles with theca cells (arrows). (C) The number of follicles containing theca cells (+) and no theca cells (–) in high-growth, low-growth and no-growth follicles collected from twelve mice. These follicles were used in subsequent experiments, the data for which are shown in Fig. 3D and Fig. 4A. (D) The graph indicates the rate of granulosa cell proliferation of each growing follicle. They were separated by whether they contained theca cells or not. Statistical significance is indicated by an asterisk (*P < 0.05, *** P < 0.001). Values are presented as means ± standard deviation. The numbers of samples used in this experiment were as follows: high-growth theca cell (–), 0; high-growth theca cell (+), 19; low-growth theca cell (–), 5; low growth theca cell (+), 29; no-growth theca cell (–), 19; no-growth theca cell (+), 51. These follicles were obtained from eight mice.
Fig. 4.
The addition of recombinant IGF1 and the inhibitor of IGF1, AG1024. (A) The graph indicates the granulosa cell proliferation of no-growth follicles. The follicles were separated into two groups, containing theca cells (–) or no theca cells (–), and cultured in a medium containing recombinant IGF1 (50 ng/ml). The numbers of samples used in this experiment were as follows: theca cell (–)/IGF1 (–), 19; theca cell (–)/IGF1 (+), 19; theca cell (+)/IGF1 (–), 51; theca cell (+)/IGF1 (+), 26. (B) The graph indicates the granulosa cell proliferation of no-growth follicles without theca cells. These follicles were cultured in a medium containing AG1024 or AG1024/recombinant IGF1 (50 or 100 ng/ml). The numbers of samples used in this experiment were as follows: AG1024l (–)/IGF1 (–), 12; AG1024 (+)/IGF1 (–), 22; AG1024l (+)/IGF1 (50), 16; AG1024 (+)/IGF1 (100), 13. (C) The graph indicated the granulosa cell proliferation of no-growth follicles containing theca cells. These follicles were cultured in a medium containing AG1024 or AG1024/recombinant IGF1 (50 or 100 ng/ml). The numbers of samples used in this experiment were as follows: AG1024l (–)/IGF1 (–), 47; AG1024 (+)/IGF1 (–), 45; AG1024l (+)/IGF1 (50), 28; AG1024 (+)/IGF1 (100), 31. Statistical significance is indicated by an asterisk (*P < 0.05, *** P < 0.001). Values are presented as means ± standard deviation.
Fig. 5.
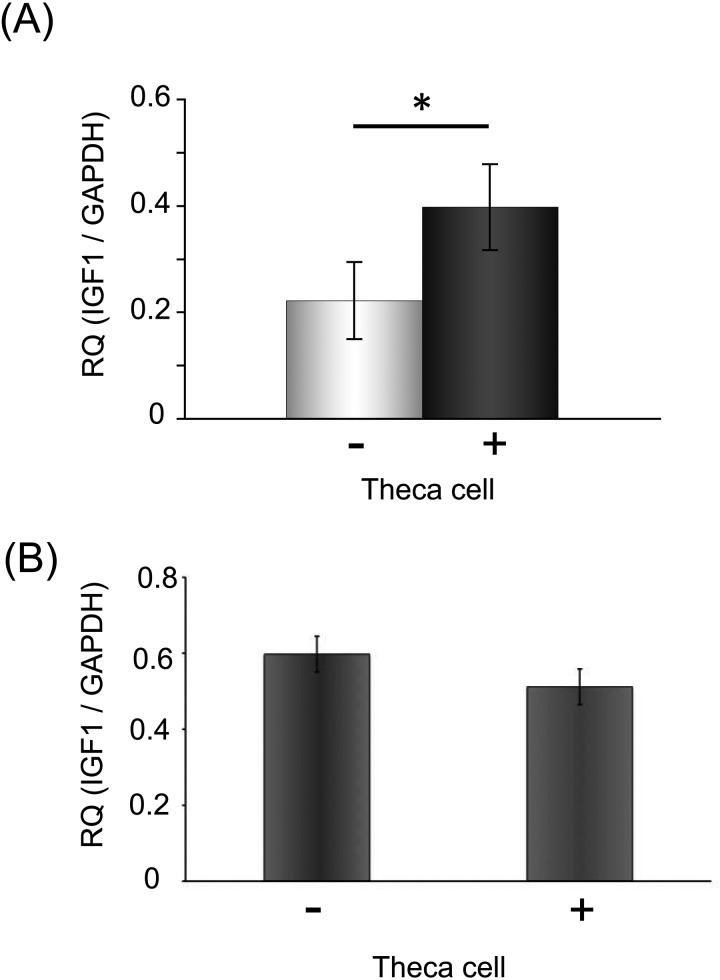
The theca cell layer increased the expression of Igf1 mRNA in the culture of secondary follicles in the no-growth group. (A) The expression level of Igf1 mRNA of cultured secondary follicles with theca cells was higher than in those without theca cells. The total mRNA of each group was extracted from ten follicles. (B) The expression level of Igf1 mRNA of culture granulosa cells. We compared the expression level of each group, cultured granulosa cells and co-cultured granulosa cells with theca cells. Statistical significance is indicated by an asterisk (*P < 0.05). Values are presented as means ± standard deviation.
Fig. 6.
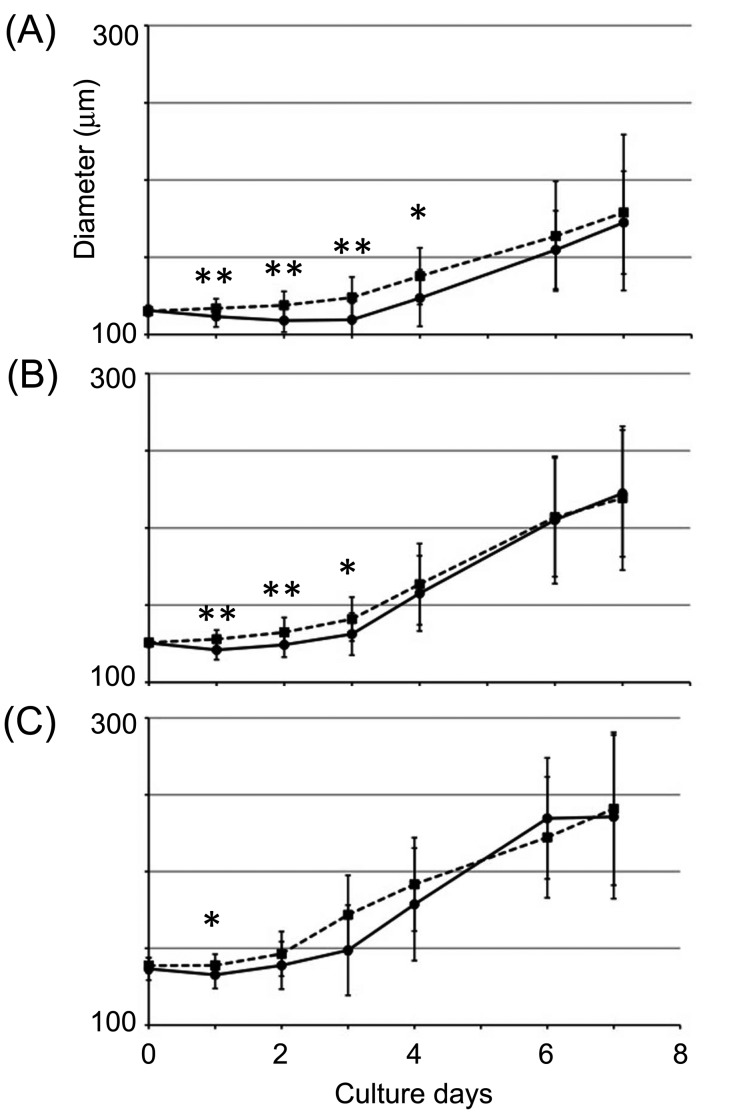
Trace of follicle growth of cultured secondary follicles for 1 week. (A) The graph indicates the result of tracing the follicle growth of follicles with a diameter of 110–120 μm. (B) The graph indicates the result of tracing the follicle growth of follicles with a diameter of 120–130 μm. (C) The graph indicates the result of tracing the follicle growth of follicles with a diameter 130 < μm. The black line indicates the follicles cultured in the normal medium (IGF1 (–)), and the dashed line indicates the follicles cultured in the medium containing IGF1 (50 ng/ml, IGF1 (+)).The numbers of samples per experiment were as follows: (A) IGF (–), 20; IGF1 (+), 29; (B) IGF1 (–), 24; IGF (+), 24; (C) IGF1 (–), 18; IGF (+), 15. Statistical significance is indicated by an asterisk (*P < 0.05, ** P < 0.01, *** P < 0.001). Values are presented as means ± standard deviation.
Results
Difference in growth rate between follicles
We compared the diameter of cultured follicles at 24-h intervals (Fig. 1A–I). All secondary follicles obtained from 26- to 28-day-old mice ovaries were 100–130 μm in diameter on culture day 0. On culture day 1, there was no difference between follicles, but the growth rate was different from culture day 2; the growth rate of the high-growth and low-growth follicles increased, while that of the no-growth and reduced-growth follicles did not change (Fig. 1I). On culture day 4, we assessed whether the growth rate differences were dependent on the proliferation of granulosa cells or the number of apoptotic cells, which revealed that the rate of granulosa cell proliferation increased in proportion to the change of follicle diameter (Fig. 1J–N). There were few apoptotic cells in follicles, and there was no difference between high-growth and reduced-growth follicles (Fig. 1J–M). On the other hand, the rates of granulosa cell proliferation were 45, 28, 16 and 5% in the high-growth, low-growth, no-growth and reduced-growth groups, respectively (Fig. 1N).
Expression of Igf1 mRNA in cultured follicles
We checked the mRNA expression levels of GDF9, BMP15 and IGF1 in each growth rate group. These growth factors have important roles in formation of the antral cavity and that promote the growth of follicles. The expression of Igf1 mRNA increased in proportion to the follicle growth rate (Fig. 2). Although other factors increased in the high-growth group, there was no difference between the low- and no-growth groups (data not shown). Furthermore, we checked the expression of FSH receptor, LH receptor and IGF-binding protein-5 (IGFBP5) mRNA, which may control the growth of late secondary follicles, but their expression levels showed no differences (data not shown).
Promotion of the growth of secondary follicles by the theca cell layer
We confirmed whether the follicles contained a theca cell layer by staining with Hoechst 33258 (Fig. 3A, B). All high-growth follicles had a theca cell layer surrounding follicles (Fig. 3B, C), while some follicles in the low- and no-growth groups contained no or few theca cells (Fig. 3A, C). The numbers of each kind of follicle used in this experiment are indicated in Fig. 3C. The number of follicles containing no theca cells in the no-growth follicles was more than in the other groups (Fig. 3C), so we focused on the no-growth group in subsequent experiments to clarify the function of theca cells.
To confirm the role of theca cells, we observed the granulosa cell proliferation around follicles (Fig. 3D), which revealed that theca cells promoted granulosa cell proliferation in the low-growth (theca cell (–), 21.8 ± 13.0%; theca cell (+), 42.1 ± 13.0%) and no-growth follicles (theca cell (−), 13.2 ± 9.5%; theca cell (+), 32.5 ± 13.3%). Furthermore, we focused on the relationship between theca cells and IGF1 (Fig. 4A). We added recombinant IGF1 (50 ng/ml) into the culture medium for no-growth follicles. As a result, recombinant IGF1 increase dthe granulosa cell proliferation of follicles without theca cells, on the other hand, the cell proliferation of follicles with theca cells was not increased (Fig. 4A; theca cell (–)/IGF1 (–),13.2 ± 9.5%; theca cell (–)/IGF1 (+), 24.2 ± 13.2%; theca cell (+)/IGF1 (–), 32.5 ± 13.3%; theca cell (+)/IGF1 (+), 32.6 ± 15.4%). Furthermore, we added the IGF1 inhibitor AG1024 to the culture medium of the no-growth follicles to elucidate whether the increase in granulosa cell proliferation was mediated by IGF1 (Fig. 4B, C). The IGF1 inhibitor had no effect on follicles without theca cells (Fig. 4B; AG1024 (–)/IGF1 (–), 10.9 ± 9.9%; AG1024 (+)/IGF1 (–), 12.2 ± 7.9%; AG1024 (+)/IGF1 (50 ng/ml), 11.8 ± 6.9%; AG1024 (+)/IGF1 (100), 12.5 ± 7.5%), while granulosa cell proliferation was decreased in follicles containing theca cells (Fig. 4C; AG1024 (–)/IGF1 (–), 32.4 ± 13.6%; AG1024 (+)/IGF1 (–), 17.7 ± 7.8%). Moreover, the effect of AG1024 was rescued by the addition of IGF1. The granulosa cell proliferation rate after the addition of AG1024 and recombinant IGF1 (100 ng/ml) was the same as that in the untreated follicles containing theca cells (Fig. 4C; AG1024 (+)/IGF1 (50 ng/ml), 25.3 ± 11.5%; AG1024 (+)/IGF1 (100), 30.1 ± 9.5%).
Enhancement of Igf1 mRNA in secondary follicles by the theca cell layer
The granulosa cell proliferation in the no-growth group was increased by the presence of theca cells and the addition of IGF1 (Fig. 3D and Fig. 4C), so we predicted that theca cells may promote IGF1 expression in secondary follicles. So, we compared Igf1 mRNA expression levels between the no-growth follicles with and without a theca cell layer, which revealed higher expression in the former group (Fig. 5A). This indicates that theca cells are important in the control of IGF1 expression in secondary follicles. Furthermore, we cultured granulosa cells isolated from secondary follicles and antral follicles with theca cells to confirm how theca cells increased the expression of Igf1 mRNA in secondary follicles (Fig. 5B). In this culture, we mechanically isolated granulosa cells from secondary and antral follicles and cultured them in a 24-well culture dish, and theca cells obtained from the residual follicle tissues that contained granulosa cells and stromal cells were cultured in the culture inserts. We put the culture inserts into the culture dish in which granulosa cells were cultured and quantitated the expression level of Igf1 mRNA after 24 h of co-culture. The results showed that the expression of Igf1 mRNA in granulosa cells did not increase after co-culture with theca cells (Fig.5B).
IGF1 enhanced only secondary follicle growth
To analyze the effect of IGF1 on follicle growth, we traced the change in the diameter of follicles cultured in normal medium or medium containing recombinant IGF1 for 1 week (Fig. 6). As a result, IGF1 promoted the follicle growth of early secondary follicles (Fig. 6). The growth of 110–120 μm diameter follicles was enhanced during culture days 1–4, but enhancement was not observed after culture day 5 (Fig. 6A). The growth of 120–130 μm diameter follicles was enhanced during culture days 1–3, but the effect of IGF1 was not observed after culture day 4 (Fig. 6B). The growth of follicles over 130 μm in diameter was enhanced during culture day 1 only (Fig. 6C). These results indicated that IGF1 enhanced the growth of only early secondary follicles with a diameter of under 150 μm.
Discussion
Previous studies reported that the secondary follicles of macaques contained fast-growth, low-growth and no-growth follicles based on growth rate [18, 19]. They selected secondary follicles without clear antral cavities, with an intact basement membrane, with attached stroma and a visible oocyte that was round and centrally located within the follicle [18]. These follicular characteristics were similar to those in our study. However, we used narrower limits for the diameter of follicles, and the secondary follicles in our study belonged to the early or middle secondary follicle stages because they contained two or three layers of granulosa cells without antral cavity (Fig. 3A and B). We found that the growth speed of each follicle was different if the follicle size was the same and that the granulosa cell proliferation correlated with the increase in the secondary follicle diameter (Fig. 1I, N). These results indicated that there are some mechanisms that control secondary follicle growth in the ovary. At first, we focused on the growth factors which are correlated with the growth of follicles, and clarified that Igf mRNA was more highly expressed in high-growth follicles than in other groups (Fig. 2). Furthermore, we revealed that the granulosa cell proliferation and expression of Igf mRNA in follicles containing theca cells were higher than in follicles without theca cells (Fig. 3D and Fig. 5A). These results indicated that theca cells enhanced the expression of IGF1 and IGF1 promoted the growth of secondary follicles. The inhibition of granulosa cell proliferation by the IGF inhibitor supported this mechanism (Fig. 4B, C).
A previous report showed that the ovaries of Igf1-knockout female mice did not contain antral follicles and were unable to ovulate [20], which indicated that IGF1 is a key factor for the transition from the pre-antral to antral stages. In our study, Igf1 mRNA expression in the high-growth follicles was higher than that in either the low-growth or the no-growth follicles (Fig. 2). Furthermore, addition of the inhibitor of IGF1 inhibited the granulosa cell proliferation of no-growth follicles containing theca cells, and this inhibition was recovered by addition of IGF1 (Fig. 4C), which revealed that IGF1 facilitates granulosa cell proliferation of follicles containing theca cells and development from the early secondary follicle stage to the antral follicle stage. To confirm whether IGF1 promoted only growth of secondary follicles, we traced the growth of culture follicles for 1 week (Fig. 6). The results revealed that the effect of IGF1 depended on the follicle size. The growth of follicles with a diameter of under 150 μm was promoted, on the other hand, the growth of follicles with a diameter of over 150 μm was not promoted by the addition of IGF1 into the medium. These results indicated that the growth of follicles over 150 μm in diameter mainly depended on FSH and LH and that the effect of IGF1 was weaker than the that for early secondary follicles.
In the secondary follicle stage,follicle growth changes from being controlled in a gonadotropin-independent manner to being controlled in a gonadotropin-dependent manner [1, 2, 21]. During this change, the function of theca cells also changes to enhance follicle growth. It was reported that formation of the theca cell layer coincided with enhanced follicle growth and increased production of androgen in theca cells [12,13,14, 22, 23]. Indeed, all high-growth follicles contained theca cells (Fig. 3C), and the rate of granulosa cell proliferation in low- and no-growth follicles with theca cells was higher than that in follicles without theca cells (Fig. 3D), so the follicles without theca cells may be in the early secondary stage. In addition, Igf1 mRNA expression in the follicles with theca cells was higher than that in the follicles without a theca cell layer (Fig. 5A). It was reported that theca cells are first observed once a follicle has two or more layers of granulosa cells [24]; therefore, we concluded that the theca cells increased not only the production of androgen but also the expression of Igf1 mRNA and facilitated the growth of early secondary follicles.
In mice and rats, Igf1 mRNA was expressed in granulosa cells of healthy growing follicles but not in theca cells, and IGF1 type I receptor mRNA was expressed in the granulosa cells from primary to preovulatory follicles [25, 26], so these reports and our results indicated that theca cells enhanced the Igf1 mRNA expression of granulosa cells in secondary follicles. To confirm how the Igf1 mRNA expression increases, we co-cultured granulosa cells and theca cells (Fig. 5B). The results revealed that the expression of Igf mRNA did not increase in granulosa cells after 24 hour co-culture with theca cells (Fig. 5B). In this co-culture system, theca cells were cultured in a culture insert, and so they did not directly contact granulosa cells. Therefore, this result indicated that the increase of Igf1 mRNA expression did not depend on the paracrine factors derived from theca cells. Direct contact between granulosa cells and theca cells may be important for enhancement of the expression of Igf1 mRNA.
Many previous reports have indicated that IGF1 is important to follicle development in various animal species [12, 27,28,29,30,31]. Furthermore, IGF1 expression has been detected in granulosa cells and in the theca cells of humans and goats [1, 32]. IGF1 was reportedly released from granulosa cells to maintain the theca layer in rats [33]. These results indicated that IGF1 has various functions in follicle development, depending on the animal species or the follicle stage. In this study, we showed that theca cells control the proliferation of granulosa cells by enhancing IGF1 expression in granulosa cells. Although it remains unclear how theca cells control IGF1 expression in granulosa cells, our results indicated that the direct interaction between theca cells and granulosa cells promoted IGF1 expression and that increased IGF1 levels facilitated the development of early secondary follicles (Fig. 7).
Fig. 7.
Proposed model for theca cell control of IGF1 expression and granulosa cell proliferation. (A) A secondary follicle containing no theca cells. (B) A follicle containing theca cells. Black circles, oocyte; gray circles, granulosa cells; gray flat circles, theca cells.
References
- 1.Silva JR, Figueiredo JR, van den Hurk R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009; 71: 1193–1208. [DOI] [PubMed] [Google Scholar]
- 2.Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res 2009; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palma GA, Argañaraz ME, Barrera AD, Rodler D, Mutto AA, Sinowatz F. Biology and biotechnology of follicle development. ScientificWorldJournal 2012; 2012: 938138. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 2003; 69: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson EE, Skinner MK. Growth and differentiation factor-9 stimulates progression of early primary but not primordial rat ovarian follicle development. Biol Reprod 2002; 67: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol 2002; 188: 65–73. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson EE, Skinner MK. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol 2004; 214: 19–25. [DOI] [PubMed] [Google Scholar]
- 8.Kezele P, Nilsson EE, Skinner MK. Keratinocyte growth factor acts as a mesenchymal factor that promotes ovarian primordial to primary follicle transition. Biol Reprod 2005; 73: 967–973. [DOI] [PubMed] [Google Scholar]
- 9.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update 2005; 11: 461–471. [DOI] [PubMed] [Google Scholar]
- 10.Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod 2010; 25: 2944–2954. [DOI] [PubMed] [Google Scholar]
- 11.Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest 1998; 101: 2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab 1999; 84: 2951–2956. [DOI] [PubMed] [Google Scholar]
- 13.Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil 1998; 113: 27–33. [DOI] [PubMed] [Google Scholar]
- 14.Spears N, Murray AA, Allison V, Boland NI, Gosden RG. Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J Reprod Fertil 1998; 113: 19–26. [DOI] [PubMed] [Google Scholar]
- 15.Huang CT, Weitsman SR, Dykes BN, Magoffin DA. Stem cell factor and insulin-like growth factor-I stimulate luteinizing hormone-independent differentiation of rat ovarian theca cells. Biol Reprod 2001; 64: 451–456. [DOI] [PubMed] [Google Scholar]
- 16.Shikanov A, Xu M, Woodruff TK, Shea LD. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J Vis Exp 2011; 49: 2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunning KR, Akison LK, Russell DL, Norman RJ, Robker RL. Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biol Reprod 2011; 85: 548–555. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction 2010; 140: 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod 2011; 26: 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellvé AR, Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol 1996; 10: 903–918. [DOI] [PubMed] [Google Scholar]
- 21.Hull KL, Harvey S. GH as a co-gonadotropin: the relevance of correlative changes in GH secretion and reproductive state. J Endocrinol 2002; 172: 1–19. [DOI] [PubMed] [Google Scholar]
- 22.Wandji SA, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod 1996; 55: 942–948. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod 2000; 62: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 24.Magoffin DA, Weitsman SR. Insulin-like growth factor-I regulation of luteinizing hormone (LH) receptor messenger ribonucleic acid expression and LH-stimulated signal transduction in rat ovarian theca-interstitial cells. Biol Reprod 1994; 51: 766–775. [DOI] [PubMed] [Google Scholar]
- 25.Adashi EY, Resnick CE, Payne DW, Rosenfeld RG, Matsumoto T, Hunter MK, Gargosky SE, Zhou J, Bondy CA. The mouse intraovarian insulin-like growth factor I system: departures from the rat paradigm. Endocrinology 1997; 138: 3881–3890. [DOI] [PubMed] [Google Scholar]
- 26.Wandji SA, Wood TL, Crawford J, Levison SW, Hammond JM. Expression of mouse ovarian insulin growth factor system components during follicular development and atresia. Endocrinology 1998; 139: 5205–5214. [DOI] [PubMed] [Google Scholar]
- 27.Iwase A, Goto M, Harata T, Takigawa S, Nakahara T, Suzuki K, Manabe S, Kikkawa F. Insulin attenuates the insulin-like growth factor-I (IGF-I)-Akt pathway, not IGF-I-extracellularly regulated kinase pathway, in luteinized granulosa cells with an increase in PTEN. J Clin Endocrinol Metab 2009; 94: 2184–2191. [DOI] [PubMed] [Google Scholar]
- 28.Gong JG, Bramley T, Webb R. The effect of recombinant bovine somatotropin on ovarian function in heifers: follicular populations and peripheral hormones. Biol Reprod 1991; 45: 941–949. [DOI] [PubMed] [Google Scholar]
- 29.Chase CC, Jr, Kirby CJ, Hammond AC, Olson TA, Lucy MC. Patterns of ovarian growth and development in cattle with a growth hormone receptor deficiency. J Anim Sci 1998; 76: 212–219. [DOI] [PubMed] [Google Scholar]
- 30.Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod 1999; 61: 353–357. [DOI] [PubMed] [Google Scholar]
- 31.Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod 1999; 14: 2328–2332. [DOI] [PubMed] [Google Scholar]
- 32.el-Roeiy A, Chen X, Roberts VJ, LeRoith D, Roberts CT, Jr, Yen SS. Expression of insulin-like growth factor-I (IGF-I) and IGF-II and the IGF-I, IGF-II, and insulin receptor genes and localization of the gene products in the human ovary. J Clin Endocrinol Metab 1993; 77: 1411–1418. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J, Taverne MA, Van Der Weijden GC, Bevers MM, Van Den Hurk R. Insulin-like growth factor-I (IGF-I) stimulates the development of cultured rat pre-antral follicles. Mol Reprod Dev 2001; 58: 287–296. [DOI] [PubMed] [Google Scholar]