Abstract
BACKGROUND
Prostate specific antigen (PSA) is a well known biomarker for early diagnosis and management of prostate cancer. Furthermore, PSA has been documented to have anti-angiogenic and anti-tumorigenic activities in both in vitro and in vivo studies. However, little is known about the molecular mechanism(s) involved in regulation of these processes, in particular the role of the serine-protease enzymatic activity of PSA.
METHODS
Enzymatic activity of PSA isolated directly from seminal plasma was inhibited specifically (>95%) by incubation with zinc2+. Human umbilical vein endothelial cells (HUVEC) were utilized to compare/contrast the physiological effects of enzymatically active versus inactive PSA.
RESULTS
Equimolar concentrations of enzymatically active PSA and PSA enzymatically inactivated by incubation with Zn2+ had similar physiological effects on HUVEC, including inhibiting the gene expression of pro-angiogenic growth factors, like VEGF and bFGF, and up-regulation of expression of the anti-angiogenic growth factor IFN-γ; suppression of mRNA expression for markers of blood vessel development, like FAK, FLT, KDR, TWIST-1; P-38; inhibition of endothelial tube formation in the in vitro Matrigel Tube Formation Assay; and inhibition of endothelial cell invasion and migration properties.
DISCUSSION
Our data provides compelling evidence that the transcriptional regulatory and the anti-angiogenic activities of human PSA are independent of the innate enzymatic activity
Keywords: angiogenesis, antiangiogenic activity, human umbilical vein endothelial cells, in vitro angiogenesis assay, matrigel invasion assay, wound healing assay, prostate cancer
INTRODUCTION
Prostate cancer (CaP) is the leading cause of cancer mortality in male in the US. CaP also is the most commonly diagnosed cancer in men, primarily due to use of the serum-based prostate-specific antigen (PSA) test. PSA is an androgen-regulated serine protease of the tissue kallikrein family with chymotrypsin like activity [1]. PSA is neither disease, nor tissue specific, with the major known function to liquefy the seminal coagulum upon discharge. PSA protein and/or gene expression has been detected to a varying degree in endometrium [2], normal breast tissue [3], breast cancer [4, 5], breast milk [6], female serum [7], adrenal neoplasm [8], renal cell carcinoma [9], and ovarian cancer [10]. In normal healthy post-pubertal males, PSA present in circulation (s-PSA) is less than 4 ng/ml, and more than 90% of it is complexed with serine protease inhibitors and, thus, is enzymatically inactive [11]. In contrast, PSA is secreted into seminal plasma (sp-PSA) by prostate epithelial cells at concentrations of 0.2–3.0 mg/ml [12]. More than 90% of sp-PSA is not complexed with chaparones, and is enzymatically active [13]. A less appreciated pool of PSA, however, is PSA sequestered within prostate tissue (t-PSA), which is present in microgram quantities, and is enzymatically active [14]. Most importantly, of the three pools, it is the t-PSA level that correlate with the physiological state of the prostate gland [15]. t-PSA levels are highest in benign prostate tissue, and decrease progressively with increasing grade and stage of CaP [16]. Furthermore, down-regulation of PSA expression in both CaP [17] and breast cancer tissue [3] has been correlated with more aggressive cancers and poorer prognosis. These correlations are in stark contrast to the relationship of s-PSA levels with CaP progression, where s-PSA level increases with progression of CaP, even though the CaP cells produce significantly lower levels of PSA than benign prostate epithelial cells [16–18]. The enhanced level of s-PSA in CaP patients that correlates with disease free survival and distant metastasis-free survival rate is hypothesized to be due to leakage of PSA into the serum, and is of unknown biological significance for disease progression [19].
Angiogenesis is a complex process that provides for development of new blood vessels from existing vasculature [20,21]. Under normal physiologic conditions, this tightly regulated process occurs only during embryonic development, the female reproductive cycle and wound healing [22]. In contrast, in pathological conditions such as cancer, rheumatoid arthritis and diabetic retinopathy, angiogenesis is persistent, apparently due to an imbalance between positive and negative regulatory signals [23]. Analysis of the mechanism(s) of “pathological angiogenesis” has indicated the role of growth factors, growth factor receptors, cell adhesion molecules, tissue hypoxia and locally active enzymes, such as metalloproteinases. Specific growth promoting factors known to induce angiogenesis include VEGF, bFGF, TGF-β, and IL-8 [24,25]. To balance these ubiquitous pro-angiogenic stimuli, multiple endogenous factors, such as IFNs, IL-12, matrix-glycoproteins, and thrombospondin negatively regulate angiogenesis.
PSA has been demonstrated to have both anti-tumorigenic and anti-angiogenic activity in an in vitro assay [26]. Transfection of PC-3 CaP cells with PSA before transplantation into nude mice prolonged doubling time, reduced tumorigenicity and reduced metastasis [27]. PSA treatment of CaP cells in vitro caused the release of anti-angiogenic fragments (angiostatin-like) by proteolytic digestion of extracellular matrix components and plasminogen [28]. Furthermore, both enzymatically active and inactive forms of PSA had anti-angiogenic activity in vitro [29]. Our group demonstrated that purified enzymatically active, and inactive free-PSA (f-PSA is un-complexed with chaperone proteins) equivalently downregulated expression in CaP cells of pro-angiogenic factors/cancer-related genes, which included VEGF, EphA2, CYR61, Bcl2, Pim-1 oncogene, and uPA, and up-regulated expression of anti-angiogenic genes, which included IFN and IFN-related genes [29]. In addition, f-PSA inhibited growth of PC-3M prostate tumor xenografts in nude mice [29]. These data suggested that enzymatic activity of f-PSA was not essential for modulation of gene expression, anti-angiogenic activity and anti-tumor activity. However, Mattsson et al. [30] reported that recombinant pro-PSA did not demonstrate anti-angiogenic activity. pro-PSA innately lacks enzymatic activity, reflecting structural differences between the un-processed pro-protein and the proteolytically activated mature PSA protein. Therefore, pro-PSA may not represent an appropriate control for definitive determination of the role of enzymatic activity in the modulation of anti-angiogenic/anti-tumorigenic effects of PSA.
This study addressed directly the role of enzymatic activity in mediation of the anti-angiogenic and anti-tumorigenic effects of PSA, and found that enzymatically active f-PSA, and f-PSA that was enzymatically inactivated by incubation with Zn2+, exhibited similar physiological activities in human umbilical vein endothelial cells (HUVEC), which included: (a) suppression of mRNA expression of pro-angiogenic growth factors, such as bFGF and VEGF, and up-regulation of mRNA for IFN-γ; (b) suppression of mRNA expression of growth factors like FAK, FLT, KDR, TWIST-1, P-38, and Cathepsin-D, that have direct roles in blood vessel development; (c) inhibition of tube formation in the in vitro Matrigel Tube Formation Assay; and (d) inhibition of endothelial cell invasion and migration. These data provide compelling evidence that the transcriptional regulatory and anti-angiogenic activities of human PSA are independent of enzymatic activity since these biological end-points are observed at Equimolar concentrations of enzymatically active and inactive f-PSA.
MATERIALS AND METHODS
Cell Culture
Human umbilical vein endothelial cells (HUVEC) were obtained from PromoCell (Heidelberg, Germany). Cells were grown in Endothelial Cell Basal Medium supplemented with Endothelial Cell Growth Medium Supplemental Pack (PromoCell) and 5 nM metribolone. Cells were maintained at 37°C in 5% CO2. Cells used were between passages 2 and 5, with populations maintaining >95% viable.
f-PSAI solation and Characterization
A two-step column chromatography procedure was used to purify f-PSA from human seminal plasma [31–33]. Briefly, seminal plasma dialyzed against buffer containing 25 mM Hepes and 1 M sodium sulfate, pH 7.0, was applied to a column packed with T-gel (Fractogel TA 650(s), Merck Darmstadt, Germany). The column was washed with equilibration buffer and bound proteins were eluted with 25 mM Hepes buffer, pH 7.0, which lacked sodium sulfate. The retained fraction of the T-gel columns contained some seminal proteins and all forms of PSA (f-PSA and complexed PSA). Fractions containing the retained protein were pooled, concentrated, and applied to a column packed with Ultrogel AcA-54, which provided gel-filtration fractionation over the range 5,000–70,000 kDa. The column was equilibrated with 10 mM sodium acetate buffer, pH 5.6, that contained 0.15 M sodium chloride. Column fractions that contained PSA were identified using Western blot analysis of column eluate containing proteins over a range of molecular weights between 25 and 40 kDa. Details of the purification of f-PSA were reported previously [31]. The f-PSA was characterized for purity using 2D-gel electrophoresis/Western blot analysis using anti-PSA and anti-PSA-complex monoclonal antibodies [31]. Several lots of f-PSA preparations subjected to amino-terminal sequencing did not reveal any protein other than PSA (W.M. Keck Foundation Biotechnology Resources Laboratory, Yale University).
Inhibition of f-PSA Enzymatic Activity by Zinc
Purified f-PSA was assayed for enzymatic activity using a PSA-specific fluorogenic substrate (Mu-His-Ser-Ser-Lys-Leu-Gln-AFC; Calbiochem, San Diego, CA) [34]. The inhibition of enzymatic activity of f-PSA (5.7 nM) by Zn2+ concentrations ranging from 0.001 to 50 mM was studied by mixing enzymatically active f-PSA with zinc chloride in the assay buffer (50 mM Tris–HCl, pH 7.9 containing 10 mM NaCl) for 10 min at 25°C before addition of the PSA-specific fluorogenic substrate (38 μM) for analysis of enzymatic activity. In the presence of zinc chloride, fluorescence readings were consistently lower, which suggested quenching of fluorescence. Therefore, zinc chloride blanks were always included. The enzymatic activity of PSA was determined from the linear increase of fluorescence over the initial 20 min, with time points taken at 200 sec intervals.
RNA Extraction
Total cellular RNA was extracted from PSA-treated and untreated HUVEC cells using an acid guanidinium thiocyanate–phenol–chloroform method [35] using TRIzol reagent (Invitrogen, Carlsbad, CA). DNA contamination was removed by treatment of the RNA preparation with DNAse (1.0 IU/mg of RNA, Promega, Inc., Madison, WI) for 30 min at 37°C, followed by Proteinase K digestion at 37°C for 15 min, extraction with phenol/chloroform and precipitation with NH4OAc/ETOH. The final pellet was dried and resuspended in diethyl-pyrocarbonate (DEPC) water. The amount of RNA was quantitated using a Nano-Drop ND-1000 spectrophotometer (Nano-Drop™ Wilmington, DE), and the RNA was stored at −80°C until used.
Reverse Transcription Real-Time Quantitative PCR (Q-PCR)
Q-PCR was used to quantitate the effect of PSA on expression of cancer-related genes in HUVEC cultures. Approximately 1×106 HUVEC cells were treated with PSA for 48 hr, cells harvested, and RNA extracted. Purified RNA was reverse transcribed to cDNA using a reverse transcriptase kit from Promega (Promega, Inc.; Cat #A3500). Relative abundance of each mRNA species was quantitated using real time quantitative PCR using specific primers and the Brilliant® SYBR® green Q-PCR master mix from Stratagene (Stratagene, Inc., La Jolla, CA; Cat #600548-51).
In Vitro Angiogenesis Assay and Quantitation
The in vitro endothelial cell tube formation assay performed in a basement membrane matrix, Matrigel (BD Biosciences, San Jose, CA), was conducted as described previously [36] with modification. A 24-well tissue culture plate was coated with 200 μl/well of Matrigel and incubated 30 min at 37°C. Approximately 7.5×104 HUVECS in 0.5 ml of media supplemented with either enzymatically active or enzymatically inactive f-PSA (1, 5, 10 μM) was applied on top of the Matrigel layer and cells incubated 16–18 hr at 37°C for endothelial cell tube formation. Control wells contained 7.5×104 HUVEC in media only. Live cell images (4× magnifications) were taken using a Nikon Eclipse TE300 inverted microscope system and analyzed using the Spot Advance software program (Diagnostic Instruments, Inc., Sterling Heights, MI). Five images were taken per well. The images were processed further using Analyze 7.0 (AnalyzeDirect, Inc., Overland Park, KS) and Angioquant v1.33 [37] to obtain the average tubule length for each image. Average tubule length of the five images is expressed in pixels. Percent inhibition is expressed in relation to tubule length in un-treated control cells.
Cell Invasion Assay
The effect of PSA on the cell invasion property of HUVEC was analyzed using the CytoSelect 24-well Cell Migration Assay [38,39]. The assay kit contains polycarbonate membrane inserts (8 μm pore size) in 24-well plates. The upper surface of the insert membrane is coated with a uniform layer of dried basement membrane matrix that serves as the barrier to discriminate invasive cells from non-invasive cells. Invasive cells degrade the matrix proteins in the layer, and ultimately pass through the pores of the polycarbonate membrane. At the conclusion of the assay, cells on the top of the membrane, and the cells that have invaded through the membrane, are removed separately, stained, and quantified. Briefly, after overnight serum-starvation, HUVEC cells were seeded in the upper chamber at 3×104 cells per well in serum-free medium, in the absence or presence of active/inactive PSA (10 μM), and the cells allowed to migrate toward FBS containing medium in the bottom chamber for 24 hr. Migratory cells were stained, the stain eluted, and quantified as absorbance at 540 nm.
Cell Migration Assay
The CytoSelect™ 24-well Wound Healing Assay Kit contains 24-well plates that contain 12 proprietary treated plastic inserts. The inserts create a wound field with a defined gap of 0.9 mm for measuring the migration and proliferation rates of cells. Migratory cells extend protrusions, and ultimately invade gap to close the wound field. Cell proliferation and migration rates can be determined using microscopic imaging. A fixing solution is provided for stopping cells at specific time points. Briefly, HUVEC cells were plated in 24 well culture plates with the proprietary treated plastic inserts and grown in endothelial cell conditioned medium with reduced FBS. The monolayers were disrupted by the plastic insert to produce a linear wound (0.9 mm width), the wells washed with PBS to remove debris, and cultures incubated with active or inactive PSA (10 μM). Wound fields were examined at different time points using phase contrast microscopy (Olympus) to measure wound size.
Statistical Analysis
All experiments were repeated a minimum of three times. Values are expressed as the mean ± SE. The significance of the difference between the control and each experimental test condition were analyzed by unpaired t-test, and a value of P < 0.05 was considered statistically significant.
RESULTS
Zinc2+ Inhibits Enzymatic Activity of Purified f-PSA
Enzymatic activity of f-PSA purified to homogeneity from human seminal plasma [31] was evaluated using a substrate highly specific for PSA protease activity (Fig. 1). f-PSA was incubated with a series of concentrations of zinc chloride, and demonstrated a dose-dependent inhibition of enzymatic activity by zinc. Inhibition of >95% was achieved at a concentration of 50 μM zinc chloride, which confirmed an earlier report [40].
Fig. 1.
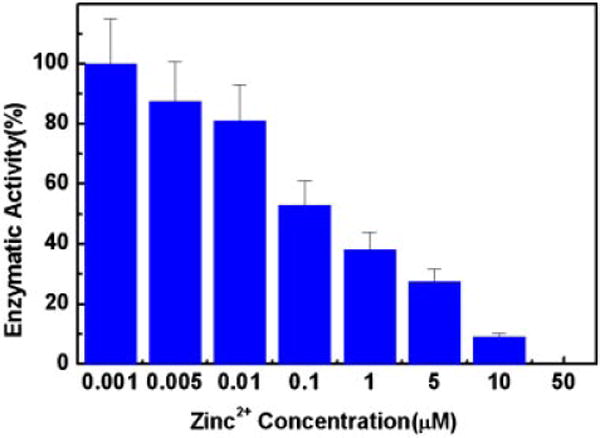
Effect of zinc2+ concentration on enzymatic activity of f-PSA. f-PSA(5.7 nM) was mixed with indicated concentration of zinc chloride, incubated for10 min at room temperature and remaining enzymatic activity was measured using PSA specific substrate. The data presented here are the mean ± SD from three independent experiments.
Enzymatic Activity of f-PSA Under Different Cell Culture Conditions
Enzymatic activity of f-PSA is routinely measured in salt buffer. Zinc2+ has been documented to inhibit enzymatic activity of PSA. It has been reported in the literature that binding of zinc to PSA is rather tight and this binding is not easily reversible [41]. In order to assess the physiological effects of enzymatically inactive f-PSA, it may be essential to document f-PSA inhibited by zinc remains “enzymatically inactive” under all cell culture conditions that includes serum containing growth medium. A series of experiments were carried out to assess the enzymatic activity of zinc2+ inhibited f-PSA in the presence of growth media and growth media containing fetal bovine serum. Assay buffer was used as control. The enzymatically inactivated f-PSA (activity inhibited with 50 μM Zinc2+) had no activity both in the presence of media or media with serum. The results are shown in Table I. It is clearly shown that f-PSA inhibited by zinc2+ remains “enzymatically inactive” under all cell culture conditions.
TABLE I.
Comparison of Enzymatic Activity of Equal Amount of PSA in Different Conditions
| Sample | Enzymatic activity (unitsa) |
|---|---|
| Active f-PSA in assay buffer | 30.44 |
| Inactive f-PSA in assay buffer | 0 |
| Active f-PSA in HUVEC media | 29.35 |
| Inactive f-PSA in HUVEC media (with serum)b | 0 |
| Inactive f-PSA in HUVEC media (without serum)b | 0 |
| PSA in seminal plasma | 35.95 |
Unit of enzymatic activity is defined as the amount of enzyme that cleaves 1 μM of substrate/min/ml.
Enzymatic activity was inhibited with 50 μM Zinc2+.
Enzymatically Inactive f-PSA Inhibits In Vitro Angiogenesis
Formation of tube-like structures in Matrigel by HUVEC is a well characterized in vitro assay for “angiogenic” activity [42–44]. Enzymatically active PSA was demonstrated previously to inhibit HUVEC tube formation in Matrigel [26]. However, the role of PSA enzymatic activity in inhibition of angiogenesis by HUVEC in Matrigel was not established. Enzymatically active f-PSA, or f-PSA inactivated by treatment with 50 μM zinc chloride (enzymatic activity inhibited > 95%), were mixed with liquid Matrigel that contained HUVEC, and the mixture plated in triplicate in 24-well tissue culture plates. Plates were incubated for 18 hr to allow formation of endothelial cell tube-like structures. Media containing 50 μM zinc chloride without PSA was used as the negative control. In the absence of f-PSA (control), HUVEC efficiently migrated, coalesced, and formed tube-like structures during the incubation (total length of tubule complexes (pixels) 11,871 ± 949; Fig. 2). In contrast, enzymatically active f-PSA inhibited significantly, in a dose-dependent manner, the migration, chemotaxis and attachment functions required for tube formation by HUVECs. The length of tubule complexes in wells treated with 1 and 10 μM PSA respectively were 5,303 ± 424 and 861 ± 69. Inactivated f-PSA was also equally effective in inhibiting the tube formation by HUVEC in Matrigel-based in vitro angiogenesis assay as enzymatically active f-PSA (length of tubule complexes for 1 and 10 μM inactive PSA were 5,305 ± 424 and 770 ± 62 respectively (Fig 2). Substituting another serine protease, Chymotrypsin, in place of f-PSA, failed to show any inhibition of HUVEC tube formation in this Matrigel assay. This suggests that inhibition of HUVEC tube formation in the Matrigel is the function associated with PSA alone and not a property of any serine protease.
Fig. 2.
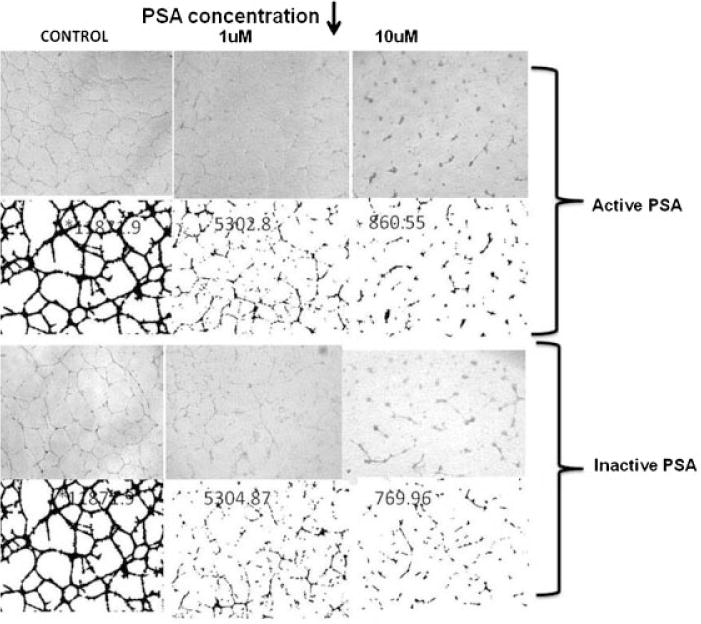
Representative figure showing the Inhibition of in vitro angiogenesis by enzymatically active and inactive f-PSA. HUVEC tube formation assay was performed in vitro in Matrigel as described in the Materials and Methods Section. The images were processed using Analyze 7.0 and Angioquant v 1.33 to obtain average tube length in pixels for each image. All experiments were performed in triplicate.
Enzymatically Inactive f-PSA Inhibits Cell Invasion
Enzymatically active or inactive f-PSA was used to evaluate the effect on the invasive property of HUVECs using the commercially available CytoSelect Cell invasion assay kit. Polycarbonate membrane inserts (8.0 mm pore size) coated with basement membrane matrix solution were seeded with HUVEC in serum-free medium in the presence of active or inactive f-PSA. Cells that invaded through the membrane were stained and quantitated (Fig. 3). In the presence of enzymatically active f-PSA, HUVEC invasion was inhibited significantly (27–35% of control). As with the effect on angiogenic activity, enzymatically inactive f-PSA inhibited the invasive potential of HUVECs comparably with Equimolar concentrations of enzymatically active PSA: the level of inhibition was 35% in the presence of enzymatically active f-PSA and 27% in the presence of enzymatically inactive f-PSA. Therefore, the enzymatic activity of f-PSA has no significant role in modulation of the physiological response to f-PSA.
Fig. 3.
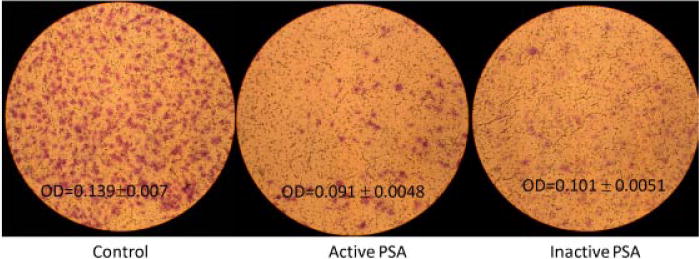
Representative figure depicting invasive potential of HUVEC in the presence/absence of enzymatically active and inactive PSA. The assay was done using commercially available CytoSelect Cell Migration assay kit as per their instructions. Control wells containing identical number of HUVEC were incubated with media only. HUVEC were allowed to migrate for 24 hr and migratory cells were stained and eluted. Stain intensity was quantitated as absorbance at 540 nm. Three independent experiments were performed.
Enzymatically Inactive f-PSA Inhibits Cell Migration
The effect on HUVEC migration of enzymatically active and inactive f-PSA was compared using the CytoSelect™ 24-well Wound Healing Assay Kit. HUVEC were plated in endothelial cell conditioned medium with reduced FBS in 24-well tissue culture dishes that contained the proprietary treated inserts. In vitro “scratch” wounds were created (0.9 mm) using treated plastic inserts in HUVEC monolayer. Wound widths were measured at 0, 24, and 48 hr. At the end of 24 hr, the average wound widths were 0.128 ± 0.01 mm for control wells; 0.89 ± 0.07 mm for wells treated with active PSA and; 0.83 ± 0.05 mm for wells treated with inactive PSA. At the end of 48 hr the wound fields in the control wells were healed completely; whereas the wound widths of the wells treated with active and inactive PSA were respectively 0.076 ± 0.06 mm and 0.64 ± 0.05 mm (Fig. 4). There were no significant differences observed between the wound healing property of HUVEC treated with active and inactive PSA. Both enzymatically active and inactive f-PSA suppressed HUVEC migration as compared to an untreated control, suggesting that enzymatic activity of PSA is not essential for this physiological response (Fig. 4).
Fig. 4.
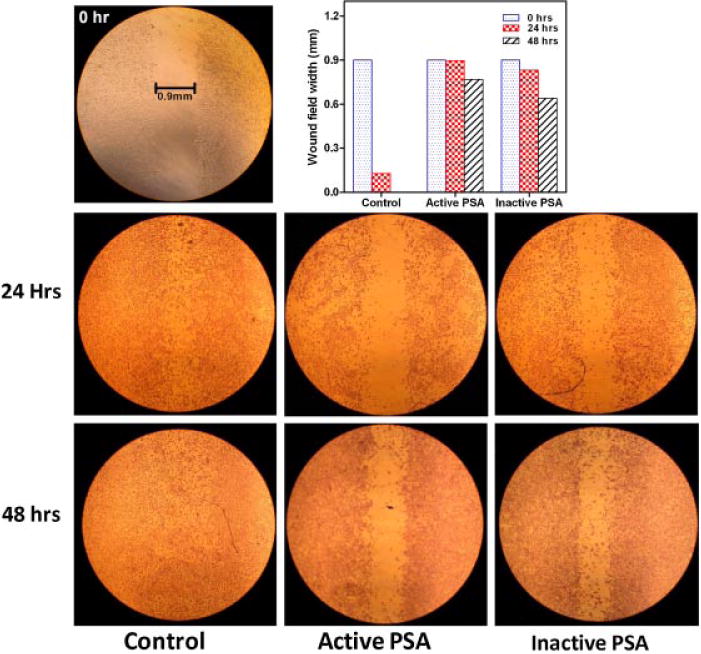
Effect of enzymatically active and inactive f-PSA on migratory property of HUVEC. Commercially available CytoSelect 24-well Wound Healing Assay kit was used. The details are described in the Materials and Methods Section. Monolayers were disrupted by the plastic insert to produce a linear wound and wounded fields were measured at 0, 24, and 48 hr using phase contrast microscopy to measure wound size.
Modulation of Gene Expression of Growth Factors and Angiogenic Markers by Enzymatically Active and Inactive f-PSA
Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (b-FGF) promote tumor growth, and interferons (IFNs) suppress tumor growth [45,46]. The VEGF/VEGF receptor axis triggers a network of signaling processes that promote endothelial cell growth, migration, and survival [47]. In addition, focal adhesion kinase (FAK), Fms-like tyrosine kinase 1 (Flt-1or VEGFR-1), vascular endothelial growth factor receptor-2 (VEGFR-2 or KDR), p-38 MAP-kinase, epithelial-mesenchymal transition-inducing factor (TWIST), and cathepsin-D also are key regulators of angiogenesis [48–51]. We reported that f-PSA suppressed VEGF gene expression, and promoted IFN-γ gene expression, in human CaP cell line PC-3M [29]. Consequently, modulation in expression of growth factors and angiogenic markers by enzymatically active or inactive f-PSA was analyzed in a biological target cell for angiogenesis, HUVEC. HUVEC were treated with enzymatically active or inactive f-PSA, RNA was extracted and message levels for the growth factors as well as angiogenic markers were determined by Real Time Q-PCR. Both enzymatically active and inactive PSA significantly modulated the expression of VEGF, bFGF and IFN-γ in HUVEC as shown in Figure 5. f-PSA inhibited VEGF and bFGF gene expression, and enhanced IFN-γ gene expression in HUVEC, and enzymatically inactive f-PSA modulated gene expression levels comparably to f-PSA (Fig. 5). Similarly, both active and inactive PSA suppressed gene expression of the angiogenic markers FAK, Flt, KDR, TWIST-1, P-38, and Cathepsin-D in HUVEC in similar fashion (Fig. 6).
Fig. 5.
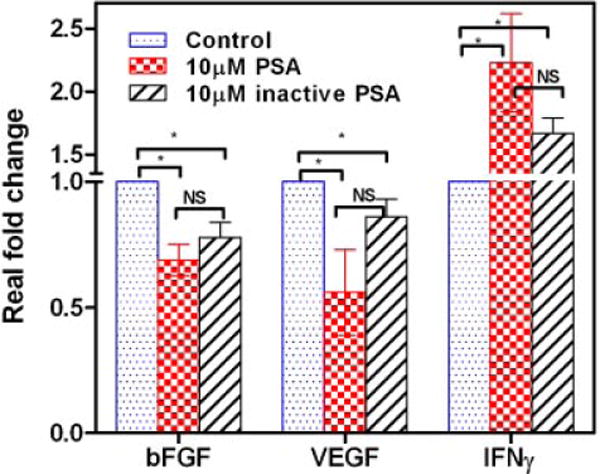
Gene expression levels of bFGF, VEGF, and IFN-γ in HUVEC treated with enzymatically active and inactive f-PSA. Nearly confluent monolayers were treated with10 μM f-PSA; RNA was extracted and reverse transcribed. The c-DNA was amplified by real-time QPCR using specific primers. The results shown are average of three experiments.
Fig. 6.
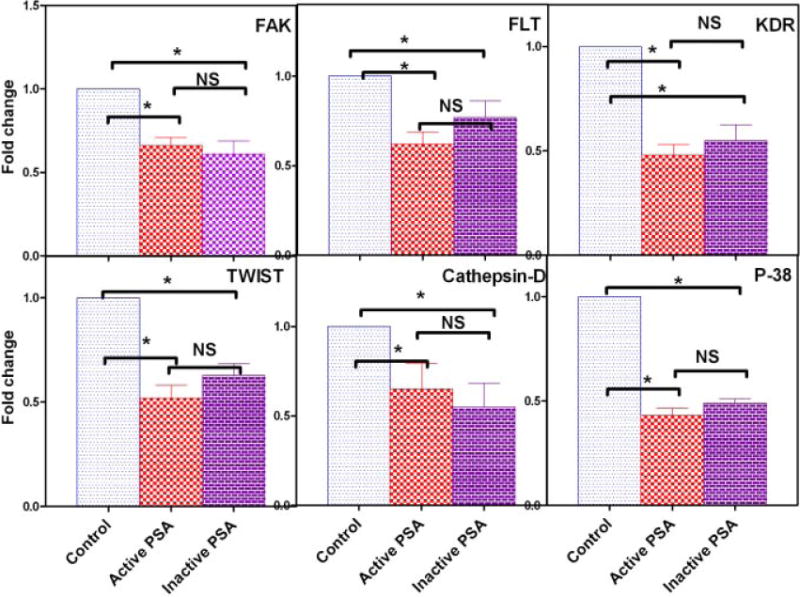
Gene expression levels of FAK, FLT, KDR, TWIST-1, Cathepsin-D, and P-38 in HUVEC treated with enzymatically active andinactive f-PSA. Nearly confluent monolayers were treated with10 μM of f-PSA; RNA was extracted and reverse transcribed. The c-DNA was amplified by real-time QPCR using specific primers. The results shown are anaverage of three experiments. Asterisk (*) denotes significant differences; NS = no significant differences.
DISCUSSION
Analysis of serum levels of prostate specific antigen (PSA), or kallikrein-related peptidase 3 (KLK3), is used extensively for the early diagnosis and management of CaP. However, the biological function of PSA in the serum is not clear. PSA is produced as an inactive pro-protein by the epithelial cells of the prostate that also produce additional proteases that enzymatically cleave PSA, activating pro-PSA into enzymatically functional f-PSA which is secreted into semen [13]. The major physiological function of enzymatically active PSA in semen is the proteolytic digestion of semenogelins, resulting in liquefaction of the seminal clot [52]. In contrast, the physiological role of serum PSA, which is complexed with protease inhibitors and enzymatically inactive, is unknown. There is an inverse correlation between the increased level of PSA in serum and decreased level of PSA production by the prostate tissue with disease progression. While serum PSA levels go up with age and with prostate disease, particularly in advanced CaP, PSA production by prostate epithelial/cancer cells goes down [18].
PSA has been shown to have anti-angiogenic activity in several angiogenesis models, including the human umbilical vein endothelial cell (HUVEC) assay, where HUVEC cells spontaneously form tubular networks in Matrigel. Fortier et al. [26] showed that enzymatically active PSA inhibited HUVEC tube formation, suggesting its anti-angiogenic activity to be linked to enzymatic function. The same group also reported that recombinant PSA reduced angiogenesis in an in vivo model where basement membrane preparations supplemented with FGF-2 were injected under the skin of mice to produce a plug. Addition of PSA to the plugs reduced blood vessel growth into the plug [53]. Consequently, these in vitro and in vivo assays suggest that PSA has a direct effect on both tumor cells, tumor vasculature and tissue compartments that would be available to PSA sequestered in the tissue microenvironment.
The mechanism by which PSA exerts its anti-angiogenic effect is not understood. However, multiple reports indicated that the anti-angiogenic effect of PSA was blocked by protease inhibitors [54]. Mattsson et al. [55] reported that enzymatic activity of PSA-isoforms is essential in inhibition of tube formation by HUVECs. It is possible that the isoforms of PSA that lack anti-angiogenic activity are “nicked,” resulting in the enzymatically “active site” being distorted, destroying the tertiary structure essential for anti-angiogenic activity. A corroborating study also hypothesized that enzymatically inactive “pro-PSA” could not inhibit endothelial cell tube formation [30]. However, processing of pro-PSA by peptidases is an essential step in converting enzymatically inactive pro-PSA into enzymatically active PSA. This post-translational processing may create a change in 3-dimensional structure that both produces the “active site,” and creates/exposes an “epitope(s)” on the surface of the PSA molecule necessary for anti-angiogenic activity. However, recombinant PSA mutated to ablate enzymatic activity for chromogenic PSA-specific substrates, maintained anti-angiogenic activity [53]. In our own studies Chymotrypsin, a serine protease that has an enzymatic activity similar to PSA, had no effect of HUVECs tube formation (unpublished data).
In this study, enzymatically active f-PSA was compared to enzymatically inactive f-PSA where enzymatic function was inhibited by incubation with Zinc. This allows a direct comparison of enzymatically active and inactive PSA that have the same three-dimensional structure and amino acid composition. The biological effects of enzymatically active and enzymatically inactive f-PSA were compared in a series of studies to evaluate the role of enzymatic activity on: (a) HUVEC tube formation; (b) HUVEC invasion; (c) HUVEC migration; (d) growth factor expression; and (e) expression of markers and regulators of angiogenesis. Zinc plays an important role in the development and normal function of the prostate. In healthy individuals, prostate tissue accumulates the highest zinc levels in the body, with the zinc concentrations in prostate tissue and prostatic fluid being approximately 9.0 mM [56]. Therefore, it is of relevance to know the effect of zinc on enzymatic activity of PSA. Zinc2+ inhibited enzymatic activity of purified f-PSA in a concentration dependent manner: zinc at 10 μM gave nearly 90% inhibition of enzymatic activity of f-PSA, and at 50 μM resulted in complete inhibition. Consequently, all studies comparing biological activity of f-PSA and enzymatically inactive f-PSA utilized 50 mM of zinc2+. Equimolar concentrations of enzymatically active and inactive f-PSA gave equivalent inhibitory activity in the Matrigel Tube Formation Assay for anti-angiogenic activity. In contrast to previous reports, Figure 2 demonstrates that inhibition of endothelial tube formation by f-PSA was not dependent on its enzymatic activity over the range of the PSA concentration studied. f-PSA significantly reduced HUVEC invasion through a polycarbonate membrane coated with basement membrane components (average pore size of 8.0 mM). Consistent with the results in the Matrigel Tube Formation Assay, there was no significant difference in invasive potential of enzymatically active and inactive f-PSA (35% vs. 27%). Similarly, enzymatically active and inactive PSA inhibit comparably the migration of HUVEC in a wound healing assay. These data suggest that enzymatic activity of PSA also does not play a significant role in modulating the inhibition of HUVEC migration, association or invasion. Several reports in the literature, including our own, suggest that PSA can modulate gene and protein expression of various growth factors and cytokines involved in angiogenesis, tumor growth, and metastasis [29,54,57]. VEGF and bFGF are known to promote tumor growth, and interferons and interferon-related genes are known to suppress tumor growth [45,46]. We demonstrated that treatment of CaP cells, PC3M, with enzymatically active f-PSA down-regulated gene/protein expression of growth factors like VEGF, EphA2, and the Pim-1 oncogene, and up-regulated the expression of anti-angiogenic markers, like interferon and related genes [29]. The present study extended earlier studies to examine the effect of PSA on the target cell for angiogenesis, the endothelial cells. Both enzymatically active and enzymatically inactive f-PSA significantly downregulated expression of pro-angiogenic growth factors like VEGF and bFGF, and up-regulated expression of anti-angiogenic growth factors like interferon γ in HUVEC (Fig. 5). In addition, f-PSA significantly down-regulated expression of focal adhesion kinase (FAK), VEGF-receptors (FLT and KDR), Wnt-inducible transcription factor TWIST-1, P-38, and Cathepsin-D, genes involved in blood vessel development and homeostasis. Importantly, as with the functional endpoints discussed above, there were no significant difference in down-regulation of gene expression between enzymatically active f-PSA and f-PSA that was enzymatically inactivated with zinc. In summary, this study addresses the effect of PSA on multiple properties of HUVEC as a prototypical endothelial cell, and suggests strongly that f-PSA sequestered in the prostate tissue microenvironment modulates the biological properties of CaP tumor epithelial cells as well as prostate epithelial cells/CaP cells, and the effect(s) are independent of enzymatic activity.
CONCLUSION
This study demonstrates directly the role of enzymatic activity in mediation of the anti-angiogenic and anti-tumorigenic effects of PSA, and found that, at Equimolar concentrations, enzymatically active f-PSA, and f-PSA that was enzymatically inactivated by incubation with Zn2+, exhibited similar physiological activities in human umbilical vein endothelial cells.
Acknowledgments
This study was supported by the Alliance Foundation, Roswell Park Cancer Institute(KCC), PO1-CA77739(GJS) and by Margaret Duffy and Robert Cameron Troup Memorial Fund for Cancer Research of Kaleida Health and the Kaleida Health Foundation(SAS). We are grateful to Dr. H Bhakoo of Infertility and IVF Associates of Western New York for kindly providing us with leftover seminal plasma for isolation of PSA. We are thankful to Jennifer Florea for her technical assistance.
References
- 1.Stenman UH, Leinonen J, Zhang WM, Finne P. Prostate-specific antigen. Semin Cancer Biol. 1999;9(2):83–93. doi: 10.1006/scbi.1998.0086. [DOI] [PubMed] [Google Scholar]
- 2.Clements J, Mukhtar A. Glandular kallikreins and prostate-specific antigen are expressed in the human endometrium. J Clin Endocrinol Metab. 1994;78(6):1536–1539. doi: 10.1210/jcem.78.6.7515392. [DOI] [PubMed] [Google Scholar]
- 3.Yu H, Diamandis EP, Levesque M, Giai M, Roagna R, Ponzone R, Sismondi P, Monne M, Croce CM. Prostate specific antigen in breast cancer, benign breast disease and normal breast tissue. Breast Cancer Res Treat. 1996;40(2):171–178. doi: 10.1007/BF01806212. [DOI] [PubMed] [Google Scholar]
- 4.Yu H, Diamandis EP, Sutherland DJ. Immunoreactive prostate-specific antigen levels in female and male breast tumors and its association with steroid hormone receptors and patient age. Clin Biochem. 1994;27(2):75–79. doi: 10.1016/0009-9120(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Levesque MA, Clark GM, Diamandis EP. Prognostic value of prostate-specific antigen for women with breast cancer: A large United States cohort study. Clin Cancer Res. 1998;4(6):1489–1497. [PubMed] [Google Scholar]
- 6.Yu H, Diamandis EP. Prostate-specific antigen in milk of lactating women. Clin Chem. 1995;41(1):54–58. [PubMed] [Google Scholar]
- 7.Yu H, Diamandis EP. Measurement of serum prostate specific antigen levels in women and in prostatectomized men with an ultrasensitive immunoassay technique. J Urol. 1995;153(3 Pt 2):1004–1008. [PubMed] [Google Scholar]
- 8.Tazawa K, Kurihara Y, Kamoshida S, Tsukada K, Tsutsumi Y. Localization of prostate-specific antigen-like immunoreactivity in human salivary gland and salivary gland tumors. Pathol Int. 1999;49(6):500–505. doi: 10.1046/j.1440-1827.1999.00900.x. [DOI] [PubMed] [Google Scholar]
- 9.Levesque M, Hu H, D’Costa M, Diamandis EP. Prostate-specific antigen expression by various tumors. J Clin Lab Anal. 1995;9(2):123–128. doi: 10.1002/jcla.1860090209. [DOI] [PubMed] [Google Scholar]
- 10.Kucera E, Kainz C, Tempfer C, Zeillinger R, Koelbl H, Sliutz G. Prostate specific antigen (PSA) in breast and ovarian cancer. Anticancer Res. 1997;17(6D):4735–4737. [PubMed] [Google Scholar]
- 11.Lilja H, Christensson A, Dahlen U, Matikainen MT, Nilsson O, Pettersson K, Lovgren T. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37(9):1618–1625. [PubMed] [Google Scholar]
- 12.Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest. 1985;76(5):1899–1903. doi: 10.1172/JCI112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malm J, Lilja H. Biochemistry of prostate specific antigen, PSA. Scand J Clin Lab Invest. 1995;55(S221):15–22. doi: 10.3109/00365519509090559. [DOI] [PubMed] [Google Scholar]
- 14.Jung K, Brux B, Lein M, Rudolph B, Kristiansen G, Hauptmann S, Schnorr D, Loening SA, Sinha P. Molecular forms of prostate-specific antigen in malignant and benign prostatic tissue: Biochemical and diagnostic implications. Clin Chem. 2000;46(1):47–54. [PubMed] [Google Scholar]
- 15.Stege RH, Tribukait B, Carlstrom KA, Grande M, Pousette AH. Tissue PSA from fine-needle biopsies of prostatic carcinoma as related to serum PSA, clinical stage, cytological grade, and DNA ploidy. Prostate. 1999;38(3):183–188. doi: 10.1002/(sici)1097-0045(19990215)38:3<183::aid-pros2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 16.Stege R, Grande M, Carlstrom K, Tribukait B, Pousette A. Prognostic significance of tissue prostate-specific antigen in endocrine-treated prostate carcinomas. Clin Cancer Res. 2000;6(1):160–165. [PubMed] [Google Scholar]
- 17.Magklara A, Scorilas A, Stephan C, Kristiansen GO, Hauptmann S, Jung K, Diamandis EP. Decreased concentrations of prostate-specific antigen and human glandular kallikrein 2 in malignant versus nonmalignant prostatic tissue. Urology. 2000;56(3):527–532. doi: 10.1016/s0090-4295(00)00621-x. [DOI] [PubMed] [Google Scholar]
- 18.Pretlow TG, Pretlow TP, Yang B, Kaetzel CS, Delmoro CM, Kamis SM, Bodner DR, Kursh E, Resnick MI, Bradley EL., Jr Tissue concentrations of prostate-specific antigen in prostatic carcinoma and benign prostatic hyperplasia. Int J Cancer. 1991;49(5):645–649. doi: 10.1002/ijc.2910490503. [DOI] [PubMed] [Google Scholar]
- 19.van Iersel MP, Thomas CM, Witjes WP, de Graaf R, de la Rosette JJ, Debruyne FM. Clinical implications of the rise and fall of prostate specific antigen after laser prostatectomy. Br J Urol. 1996;78(5):742–746. doi: 10.1046/j.1464-410x.1996.21914.x. [DOI] [PubMed] [Google Scholar]
- 20.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 21.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267(16):10931–10934. [PubMed] [Google Scholar]
- 22.Tonnesen M, Feng X, Clark R. Angiogenesis in wound healing. J Invest Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 23.Arnold F. Exploiting angiogenesis. Lancet. 1991;337(8745):865–866. doi: 10.1016/0140-6736(91)92585-p. [DOI] [PubMed] [Google Scholar]
- 24.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417(6892):954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 26.Fortier AH, Nelson BJ, Grella DK, Holaday JW. Antiangiogenic activity of prostate-specific antigen. J Natl Cancer Inst. 1999;91(19):1635–1640. doi: 10.1093/jnci/91.19.1635. [DOI] [PubMed] [Google Scholar]
- 27.Balbay MD, Juang P, Ilansa N, Williams S, McConkey D, Fidler IJ, Pettaway CA. Stable transfection of human prostate cancer cell line PC-3 with prostate-specific antigen induced apoptosis both in vivo and in vitro. Proc Am Assoc Cancer Res (Abstr) 1999;49:225–228. [Google Scholar]
- 28.Heidtmann HH, Nettelbeck DM, Mingels A, Jager R, Welker HG, Kontermann RE. Generation of angiostatin-like fragments from plasminogen by prostate-specific antigen. Br J Cancer. 1999;81(8):1269–1273. doi: 10.1038/sj.bjc.6692167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bindukumar B, Schwartz SA, Nair MP, Aalinkeel R, Kawinski E, Chadha KC. Prostate-specific antigen modulates the expression of genes involved in prostate tumor growth. Neoplasia. 2005;7(5):544. [PMC free article] [PubMed] [Google Scholar]
- 30.Mattsson JM, Valmu L, Laakkonen P, Stenman UH, Koistinen H. Structural characterization and anti-angiogenic properties of prostate-specific antigen isoforms in seminal fluid. Prostate. 2008;68(9):945–954. doi: 10.1002/pros.20751. [DOI] [PubMed] [Google Scholar]
- 31.Bindukumar B, Kawinski E, Cherrin C, Gambino LM, Nair MP, Schwartz SA, Chadha KC. Two step procedure for purification of enzymatically active prostate-specific antigen from seminal plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;813(1–2):113–120. doi: 10.1016/j.jchromb.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Chadha KC, Kawinski E, Sulkowski E. Thiophilic interaction chromatography of prostate-specific antigen. J Chromatogr B Biomed Sci Appl. 2001;754(2):521–525. doi: 10.1016/s0378-4347(00)00622-8. [DOI] [PubMed] [Google Scholar]
- 33.Chadha K, Kawinski E, Sulkowski E. US Patent # 6,379,550 B1: Method for detecting PSA and its molecular forms using thiophilic gel. USA. 2002 [Google Scholar]
- 34.Denmeade SR, Lou W, Lovgren J, Malm J, Lilja H, Isaacs JT. Specific and efficient peptide substrates for assaying the proteolytic activity of prostate-specific antigen. Cancer Res. 1997;57(21):4924–4930. [PMC free article] [PubMed] [Google Scholar]
- 35.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 36.Godoy A, Watts A, Sotomayor P, Montecinos VP, Huss WJ, Onate SA, Smith GJ. Androgen receptor is causally involved in the homeostasis of the human prostate endothelial cell. Endocrinology. 2008;149(6):2959–2969. doi: 10.1210/en.2007-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niemisto A, Dunmire V, Yli-Harja O, Zhang W, Shmulevich I. Robust quantification of in vitro angiogenesis through image analysis. Med Imag IEEE Trans. 2005;24(4):549–553. doi: 10.1109/tmi.2004.837339. [DOI] [PubMed] [Google Scholar]
- 38.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2003;302(5651):1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 39.Horwitz R, Webb D. Cell migration. Curr Biol. 2003;13(19):R756–R759. doi: 10.1016/j.cub.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Satheesh Babu AK, Vijayalakshmi MA, Smith GJ, Chadha KC. Thiophilic-interaction chromatography of enzymatically active tissue prostate-specific antigen (T-PSA) and its modulation by zinc ions. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;861(2):227–235. doi: 10.1016/j.jchromb.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malm J, Hellman J, Hogg P, Lilja H. Enzymatic action of prostate-specific antigen (PSA or hK3): Substrate specificity and regulation by Zn(2+), a tight-binding inhibitor. Prostate. 2000;45(2):132–139. doi: 10.1002/1097-0045(20001001)45:2<132::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 42.Malinda K, Nomizu M, Chung M, Delgado M, Kuratomi Y, Yamada Y, Kleinman H, Ponce M. Identification of laminin {alpha} 1 and b1 chain peptides active for endothelial cell adhesion, tube formation, and aortic sprouting. FASEB J. 1999;13(1):53. [PubMed] [Google Scholar]
- 43.Tian X, Kadaba R, You S, Liu M, Timur A, Yang L, Chen Q, Szafranski P, Rao S, Wu L. Identification of an angiogenic factor that when mutated causes susceptibility to Klippel–Trenaunay syndrome. Nature. 2004;427(6975):640–645. doi: 10.1038/nature02320.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of lami-nin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carmeliet P, Jain R. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 46.Folkman J. Successful treatment of an angiogenic disease. N Engl J Med. 1989;320(18):1211–1212. doi: 10.1056/NEJM198905043201811. [DOI] [PubMed] [Google Scholar]
- 47.Hicklin D, Ellis L. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 48.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9. [PubMed] [Google Scholar]
- 49.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15(18):2169. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 50.Ansieau S, Morel A, Hinkal G, Bastid J, Puisieux A. TWISTing an embryonic transcription factor into an oncoprotein. Oncogene. 2010;29(22):3173–3184. doi: 10.1038/onc.2010.92. [DOI] [PubMed] [Google Scholar]
- 51.Hu L, Roth J, Brooks P, Luty J, Karpatkin S. Thrombin up-regulates cathepsin D which enhances angiogenesis, growth, and metastasis. Cancer Res. 2008;68(12):4666. doi: 10.1158/0008-5472.CAN-07-6276. [DOI] [PubMed] [Google Scholar]
- 52.Robert M, Gagnon C, Semenogelin I. A coagulum forming, multifunctional seminal vesicle protein. Cell Mol Life Sci. 1999;55(6):944–9960. doi: 10.1007/s000180050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fortier AH, Holaday JW, Liang H, Dey C, Grella DK, Holland-Linn J, Vu H, Plum SM, Nelson BJ. Recombinant prostate specific antigen inhibits angiogenesis in vitro and in vivo. Prostate. 2003;56(3):212–219. doi: 10.1002/pros.10256. [DOI] [PubMed] [Google Scholar]
- 54.Mattsson J, Laakkonen P, Stenman U, Koistinen H. Antiangio-genic properties of prostate specific antigen (PSA) Scand J Clin Lab Invest. 2009;69(4):447–451. doi: 10.1080/00365510903056031. [DOI] [PubMed] [Google Scholar]
- 55.Mattsson J, Valmu L, Laakkonen P, Stenman U, Koistinen H. Structural characterization and anti-angiogenic properties of prostate-specific antigen isoforms in seminal fluid. Prostate. 2008;68(9):945–954. doi: 10.1002/pros.20751. [DOI] [PubMed] [Google Scholar]
- 56.Kavanagh J. Sodium potassium, calcium, magnesium, zinc, citrate and chloride content of human prostatic and seminal fluid. Reproduction. 1985;75(1):35. doi: 10.1530/jrf.0.0750035. [DOI] [PubMed] [Google Scholar]
- 57.Bindukumar B, Schwartz S, Aalinkeel R, Mahajan S, Lieberman A, Chadha K. Proteomic profiling of the effect of prostate-specific antigen on prostate cancer cells. Prostate. 2008;68(14):1531–1545. doi: 10.1002/pros.20811. [DOI] [PubMed] [Google Scholar]


