Abstract
Background
Glomus tumors are relatively uncommon subcentimeteric benign perivascular neoplasms usually located on the fingers. With their blue-red color and common subungual location, they are commonly confused for vascular or melanocytic lesions. To date there is no comprehensive review of an institutional experience with glomus tumors.
Methods
A 14-year retrospective review of all cases within University of California, Los Angeles, with either a clinical or pathological diagnosis of glomus tumor was performed. Data obtained included demographic information, tumor description, pathological diagnoses, immunohistochemical studies, radiographic and treatment information, and clinical course. Rates of concordance between clinical and pathological diagnoses and an evaluation of overlap with other entities were assessed.
Results
Clinical diagnosis of glomus tumor showed concordance with a histopathological diagnosis (45.4% of cases). The most common alternate clinical diagnoses included lipoma, cyst, or angioma. A pathological diagnosis of glomus tumor was most common in the fourth to seventh decades of life. The most common presentation was a subcentimeter lesion on the digit. Deep-seated tumors had a strikingly increased risk for malignancy (33%). Radiological studies were not relied on frequently (18.2% of cases). Immunohistochemical analysis showed diffuse αSMA and MSA expression in nearly all cases (99% and 95%, respectively), with focal to diffuse CD34 immunostaining in 32% of cases.
Discussion
Our study illustrates trends in the clinical versus pathologic diagnoses of glomus tumor, common competing diagnoses, a difference in demographics than is commonly reported (older age groups most commonly affected), and important differences in the use adjunctive diagnostic tools including radiology and immunohistochemistry.
Keywords: glomus tumor, glomangioma, glomangiomatosis, perivascular neoplasm, glomangiomyoma, glomangiosarcoma
Introduction
Glomus tumors are benign perivascular neoplasms commonly located in the distal extremities, particularly in the nail bed.1 They are hypothesized to be derived from modified smooth muscle cells of a neuromyoarterial glomus commonly termed glomus body, whose function entails temperature regulation through arteriovenous shunting of blood. Glomus tumors are neoplasms with histologic resemblance to glomus bodies and are typically small (subcentimeter), blue-red nodules associated with localized tenderness, cold sensitivity, and excruciating paroxysmal pain out of proportion to tumor size.2 Glomus tumors usually occur in areas rich in glomus bodies such as the subungual regions of digits or the deep dermis of the palm, wrist, and forearm.1 Although frequently found in the subcutis and superficial soft tissues, glomus tumors may occur in deep-seated, visceral locations throughout the body including the lung, stomach, pancreas, liver, gastrointestinal, and genitourinary tract.3 While there is a female preponderance with a subungual presentation, there is no sex predilection evident at other locations.3 Atypical or frankly malignant glomus tumor are exceedingly rare and occur more frequently as deep-seated, large tumors in the gastrointestinal system.4 Glomangioma and glomangiomyoma are classic variants of the common form of glomus tumors.5,6 Glomus tumors are typically composed of 3 components: glomus cells, smooth muscle cells, and vasculature. The classical histological features of the glomus tumor include angiocentric uniform sheets of cells with oval nuclei, forming a perivascular “collar” around vessels. The 3 different tumor variants are differentiated by their histological features. The common or solid form is found to have poor vasculature and scant smooth muscle components, while glomangiomas have a prominent vascular component, and glomangiomyomas are composed of prominent vascular and smooth muscle components.1 Immunohistochemical and electron microscopic analyses suggest glomus cells have both a smooth cell and pericyte phenotype.3,7,8 Briefly, glomus tumors are characteristically and diffusely immunoreactive for α-Smooth Muscle Actin (αSMA), Muscle Specific Actin (MSA),3,4 and h-Caldesmon.9 Although nonspecific, vimentin and collagen type IV are also expressed.1,3 Variable expression of CD34, and to a lesser extent desmin, has also been reported.4,7
Although the clinical and pathological features of glomus tumor are well published, there is to date no largescale, retrospective institutional review of all cases of glomus tumor with an emphasis on clinicopathologic correlation. Toward these purposes, a 14-year retrospective review was performed of the University of California, Los Angeles (UCLA), surgical pathology database. In total, 99 tumors were diagnosed as glomus tumor on clinical grounds. Over this same time period, 137 tumors were diagnosed as glomus tumor after histopathological examination. The cumulative clinical, radiological, histopathological, and immunohistochemical features of these lesions were compiled and compared. Overall, our study illustrates trends in the clinical versus pathologic diagnoses of glomus tumor, common competing diagnoses, and important differences in the use adjunctive diagnostic tools including radiology and immunohistochemistry.
Materials and Methods
Clinical Database Review
Computerized search of the UCLA pathology database was performed from the years 1999 to 2013. Search terms include “glomus tumor,” “glomangioma,” and “glomangiomyoma.” All pathology reports listing glomus tumor (or variants) as either a clinical or pathological diagnosis were compiled and analyzed (N = 194 patients). A clinical diagnosis was defined as the clinician writing “glomus tumor” (or variants) anywhere on the pathology requisition sheet. Of 194 patients, 99 patients were clinically diagnosed with glomus tumor (51%, 99/194). Of 194 patients, 138 cases reported glomus tumor (or variants) as a final pathologic diagnosis (69.3%, 138/194). Clinical, imaging, and pathologic findings were analyzed including age, gender, clinical description of tumor, suspected clinical diagnosis or diagnoses, radiographic imaging, tumor location, tumor size, histologic description, immunohistochemical analyses, final pathologic diagnosis, and clinical follow-up. Special attention was paid to diagnoses of glomus variants, and atypical/malignant glomus tumors. These diagnoses were made based on the criteria described by Folpe and colleagues.4 “Glomus tumor of uncertain malignant potential” (synonymous with “atypical” glomus tumors) possess high mitotic activity (>5/50 HPF) and a superficial location only, or large size only, or deep location only. Malignant tumors were classified as tumors greater than 2 cm in size while found deep in location or atypical mitotic figures, or moderate to high nuclear grade and >5 mitotic figures in 50 HPF.4,10 All clinical data were used with institutional approval under UCLA Institutional Review Board # 13-000918.
Statistical Analyses
Statistical analyses of medians were performed using a 2-tailed Wilcoxon rank sum test. Differences between mean values were evaluated by 2-tailed Student’s t test with equal variances. The dependence of a clinical, differential, or pathologic diagnosis on a tumor property such as age, location, or gender was evaluated by a χ2 test. For any of these 3 statistical analyses, *P < .05 was considered significant.
Results
Clinical Diagnosis of Glomus Tumor
Over the course of 14 years of available records, the clinical/preoperative diagnosis of glomus tumor was made in 99 cases by 46 different physicians. Most often, glomus tumor was listed on the pathology requisition as the sole diagnosis (56/99 cases, 56.6%), while in a large minority of cases a clinical differential was listed on the pathology requisition, which included glomus tumor among other entities (43/99 cases, 43.4%). The most commonly listed clinical diagnoses other than glomus tumor included “cyst” (16.3%, 7/43 cases), “lipoma” (16.3%, 7/43 cases), and “angioma” (14%, 6/43 cases). A detailed breakdown of alternative clinical diagnoses to glomus tumor can be found in Table 1. Alternative clinical diagnoses were most often darkly colored lesions (eg, nevi, melanoma, angioma, or pyogenic granuloma) or painful subcutaneous nodules (eg, neuroma, leiomyoma, or spiradenoma). Demographic information for all clinical diagnoses of glomus tumor is shown in Figure 1. Patients were most often in their fourth to sixth decades of life, with a female predilection (63/99 cases, 63.6%; Figure 1A and B). Tumors were most often located on the digits (51/99 cases, 51.5%). Other common locations included the arms (14/99 cases, 14.1%), legs (13/99 cases, 13.1%), and trunk (5/99 cases, 5.1%; Figure 2A). Clinical diagnoses of deep-seated glomus tumors were uncommon (3/99 cases, 3.03%), and included gastric, duodenal, and retroperitoneal locations. The median size of tumors clinically diagnosed as glomus tumor was 0.6 cm (range = 0.2–5.6 cm; Figure 2B). Tumors located on the digits were predominantly subcentimetric in size, with a low size variance (s2 = .15).
Table 1.
Alternative Clinical Diagnoses Included on a Differential Diagnosis With Glomus Tumora.
| Clinical Diagnoses | Cases (of 43) | Frequency (%) |
|---|---|---|
| Lipoma | 7 | 16.3 |
| Cyst | 7 | 16.3 |
| Angioma | 6 | 14.0 |
| Fibroma | 5 | 11.6 |
| Neuroma | 5 | 11.6 |
| Melanoma | 4 | 9.3 |
| Nevus | 3 | 7.0 |
| Leiomyoma | 3 | 7.0 |
| Pyogenic granuloma | 3 | 7.0 |
| Spiradenoma | 3 | 7.0 |
In 40 patient cases, the clinician/surgeon listed glomus tumor as a clinical diagnosis along with other competing diagnoses. More than 2 entities were listed in most cases (70%, 28/40).
Figure 1. Demographics of cases.
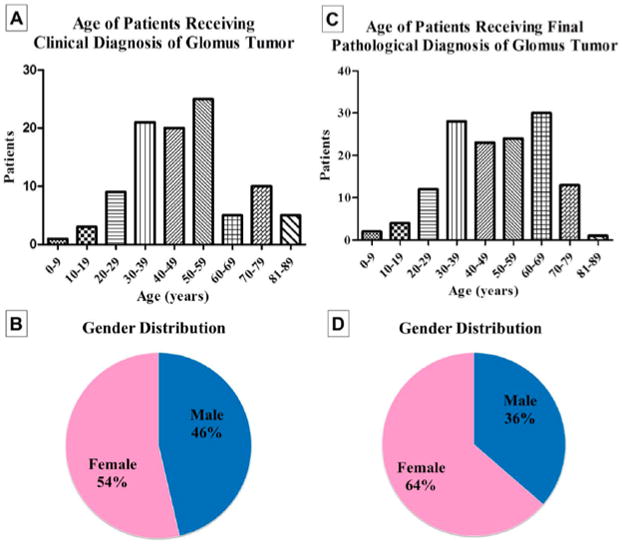
(A) Patient age with a clinical diagnosis of glomus tumor (N = 99) (B) Patient gender with a clinical diagnosis of glomus tumor (N = 99). (C) Patient age with a pathological diagnosis of glomus tumor (N = 137). (D) Patient gender with a pathological diagnosis of glomus tumor (N = 137).
Figure 2. Distribution of tumor location and size.
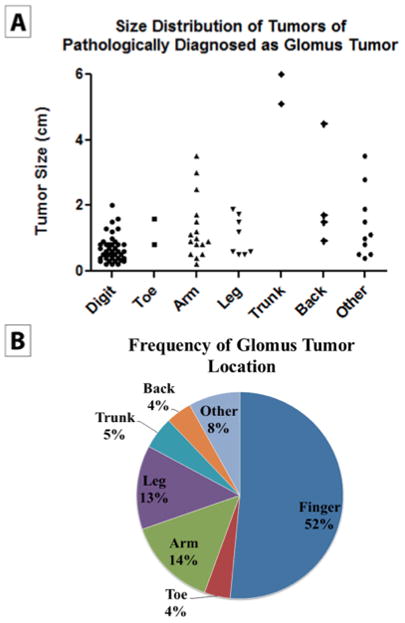
(A) Anatomic location of specimens with a pathological diagnosis of glomus tumor (N = 84). (B) Tumor size by anatomic location in specimens with a pathological diagnosis of glomus tumor (N = 84). Please note that information on tumor size was only available on a subset of patients.
Of those tumors clinically suspicious for glomus tumor, just under half were diagnosed as glomus tumor by pathological examination (45.4%, 45/99). Typical histological features of glomus tumor included well-circumscribed subcutaneous or soft tissue tumor nodules in a prominent perivascular distribution composed of small, uniform round cells with round to oval nuclei (Figure 3). Alternate pathological diagnoses for clinically diagnosed “glomus tumor” were most often vascular tumors (25.9%, 14/54 cases), other skin tumors (25.9%, 14/54 cases), or other soft tissue tumors (20.4%, 11/54 cases). A wide variety of nonneoplastic conditions were also diagnosed (20.4%, 11/54 cases). Table 2 lists specific alternative pathologic diagnoses for all lesions clinically diagnosed as glomus tumor.
Figure 3. Typical histological features of glomus tumor.
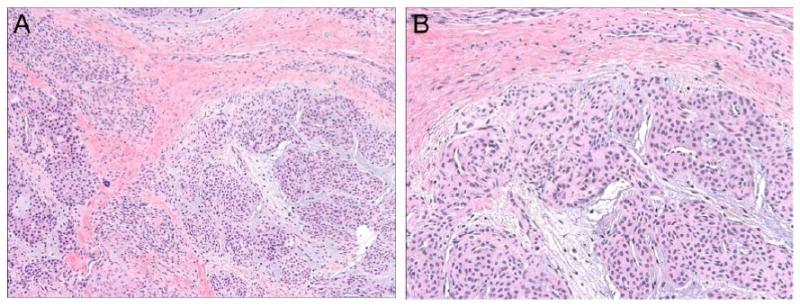
Routine hematoxylin and eosin (H&E) staining demonstrates a perivascular, proliferation of homogenous round cells with round to ovoid nuclei arranged in multicellular layers around blood vessels. Lesional cells are set in a background of myxoid matrix with stellate cells. (A) 100 × magnification; (B) 200 × magnification.
Table 2.
Alternative Pathological Diagnoses, When Glomus Tumor Was Suspected Clinicallya.
| Count | Frequency | |
|---|---|---|
| Vascular tumors | ||
| • Angioma | 11 | 20.4% |
| • Venous malformation | 3 | 5.6% |
| Total: | 14 | 25.9% |
| Other skin tumors | ||
| • Cysts | 6 | 11.1% |
| • Sweat gland neoplasms | 4 | 7.4% |
| • Salivary duct carcinoma | 1 | 1.9% |
| • Nevus | 1 | 1.9% |
| • Lentigo | 1 | 1.9% |
| • Cellular fibrous histiocytoma | 1 | 1.9% |
| Total: | 14 | 25.9% |
| Other tumors | ||
| • Epithelioid neoplasm NOS | 2 | 3.7% |
| • Mastocytosis | 1 | 1.9% |
| • Round cell neoplasm NOS | 1 | 1.9% |
| Total: | 4 | 7.4% |
| Other soft tissue tumors | ||
| • Fibroma/fibromatosis | 6 | 11.1% |
| • Leiomyoma | 2 | 3.7% |
| • Lipoma | 1 | 1.9% |
| • Histiocytic sarcoma | 1 | 1.9% |
| • Neuroma | 1 | 1.9% |
| Total: | 11 | 20.4% |
| Nontumors | 2 | 3.7% |
| • Dermal fibrosis | 3 | 5.6% |
| • Keratoma | 2 | 3.7% |
| • Hemorrhage | 1 | 1.9% |
| • Pemphigus | 1 | 1.9% |
| • Dermatitis | 1 | 1.9% |
| • Perniosis | 1 | 1.9% |
| • Fat necrosis | 1 | 1.9% |
| • Porokeratotic proliferation | 1 | 1.9% |
| Total: | 11 | 20.4% |
In 54 patient cases, the clinician/surgeon suspected a diagnosis of glomus tumor but the final pathological diagnosis differed. Diagnoses are split into categories, including tumors of vasculature (24.9%), soft tissue (20.4%), skin (25.9%), other (7.4%), and nontumors (20.4%).
As mentioned, clinicians either submitted a specimen to pathology with a sole clinical diagnosis of glomus tumor (56/99 cases, 56.6%) or with a clinical differential including glomus tumor (43/99 cases, 43.4%). Not surprisingly, a pathologic diagnosis of glomus tumor was significantly more likely when a clinician suspected glomus tumor only (67.8% likelihood vs 12.5% likelihood when a clinical differential was given, *P = 6 × 10−8). Clinicians were more apt to list glomus tumor as the sole clinical diagnosis if the lesion was present in a classic location on the fingers or fingertips (62.7% of cases, *P = .0068). In contrast, if the lesion occurred elsewhere on the arm, a clinical differential was most often listed (30% of cases, 12 of 40 cases). Other demographics such as age, gender, or tumor size did not differ between lesions clinically diagnosed as glomus tumor alone versus glomus tumor within a clinical differential.
Pathological Diagnosis of Glomus Tumor
The histopathological diagnosis of glomus tumor was made in 137 cases over a 14-year period. This included 81 cases within the UCLA health care system (59% of cases), and 56 cases referred for a second opinion (41% of cases). A clinical diagnosis of glomus tumor was listed in 32.9% of cases (45/137 cases). Other common clinical diagnoses besides glomus tumor included angioma (8 cases), lipoma (5 cases), and cyst (5 cases). Demographic information for all pathological diagnoses of glomus tumor is shown in Figure 1. Patients were most often in their fourth to seventh decades of life (Figure 1C), with a relatively equal gender distribution (54% female; (Figure 1D). Tumors were most often located on the digits (42.3%; Figure 2C). Other common locations included the hands/arms (16.1%) and legs (11.7%). Deep-seated locations were relatively uncommon (4.4%, 6 cases), included gastric, duodenal, and subglottic locations, and had an increased frequency of malignancy (33%, 2/6 cases). The median size of superficial tumors was 0.8 cm (range = 0.2–4.5 cm; Figure 2D). The median size of deep tumors was 5.1 cm (range = 3.5–6 cm). Recurrence of typical glomus tumor was rare, occurring in only 3 cases with a median follow-up period of 2 years (3/137, 2.2% of cases).
Immunohistochemical Analysis of Glomus Tumor
Immunohistochemical stains were performed in 74/137 cases (54%; Table 3). Glomus tumors were most typically positive for αSMA (99%, 68/69 samples assayed) and MSA (95%, 18/19 samples assayed) and negative for CD31, cytokeratins, and S100 in all or nearly all cases. CD34 was positive in 32% of cases (7/22 samples assayed), which ranged from focal to diffuse in distribution. Typical patterns of immunohistochemical stains are shown in Figure 4.
Table 3.
Immunohistochemical Analyses of Glomus Tumora.
| Antigen | Positive Staining (Fraction of Cases) | Frequency (%) |
|---|---|---|
| α-Smooth Muscle Actin (αSMA) | 68/69 | 99% |
| Muscle Specific Actin (MSA) | 18/19 | 95% |
| Vimentin | 12/12 | 100% |
| Calponin | 4/5 | 80% |
| CD34 | 7/22 | 32% |
| S100 | 1/44 | 2% |
| Keratins | 0/44 | 0% |
| Desmin | 0/30 | 0% |
| CD31 | 0/22 | 0% |
Results of immunohistochemical stains in specimens with a pathological diagnosis of glomus tumor, expressed as a fraction and frequency of positive staining.
Figure 4. Typical immunohistochemical profile of glomus tumor.
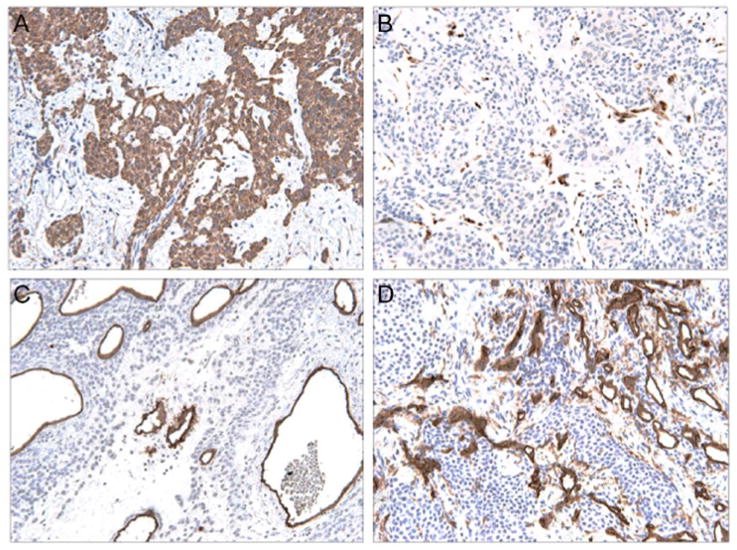
(A) αSMA immunostaining (200×), highlights diffuse staining of lesional cells. (B) S100 immunostaining, highlighting rare dendritic cells only (200×). (C) CD34 immunostaining, which predominantly highlights vessels (200×). (D) Lack of CD31 immunoreactivity, which highlights vessels only (200×).
Radiographic Analysis of Glomus Tumor
Imaging studies were infrequently employed in the workup of suspected glomus tumor. In total, 25/137 tumors were imaged (18.2%), including routine X-ray in 17 cases, magnetic resonance (MR) imaging in 5 cases, computed tomography (CT)/positron emission tomography (PET) in 2 cases, and endoscopic ultrasound (EUS) in one case. None of the imaging studies aided in the specific diagnosis of glomus tumor.
Glomus Tumor Variants
Variants of glomus tumor were infrequently observed, including tumors with features of both glomus tumor and other perivascular tumors. Four perivascular tumors with glomus tumor and myopericytoma features (glomangiopericytoma) were reported (4/137, 2.9%), 2 of which had atypical cytologic features. A single case of each of the following was described: (a) an oncocytic variant of glomus tumor, (b) a symplastic glomus tumor, (c) an otherwise typical glomus tumor that showed prominent branching vasculature in a so-called hemangiopericytoma-like pattern, and (d) a multifocal glomus tumor (each accounting for 1/137 or 0.7% of reported cases).
Malignant Glomus Tumors and Glomus Tumors of Uncertain Malignant Potential
Both malignant glomus tumor and glomus tumor of uncertain malignant potential were rarely diagnosed (4/137, 2.9%; and 5/137 3.6%, respectively). All tumors were found in adults (range = 30–61 years) with a roughly even distribution across genders (male gender in 5/9 cases, 56%). Malignant cases were found in a deep location in 75% of cases (3/4), with a size greater than 4 cm in all recorded cases (4.5 and 5.1 cm, respectively). Moderate to marked cytologic atypia was found in half of cases (2/4), and recorded mitoses were greater than 30 per 50 HPF in all cases (range = 30–125/50 HPF). In terms of glomus tumors of uncertain malignant potential, 2 cases were found in a deep location only (gastric or subglottic), 2 cases were superficial tumors measuring greater than 2 cm (2.5 and 2.8 cm, respectively), and 1 tumor show markedly increased numbers of mitotic figures without atypical forms (30/50 HPF). No significant cytologic atypia nor an infiltrative growth pattern was observed among glomus tumors of uncertain malignant potential (0/5). No known metastases were documented among malignant glomus tumors or glomus tumors of uncertain malignant potential (0/9).
Discussion
To our knowledge, this is the first institutional review that examined specimens with either a clinical or pathological diagnosis of glomus tumor. The epidemiologic and clinical findings of glomus tumor at our institution had similarities and differences from other review texts. In terms of epidemiology, a pathologic diagnosis of glomus tumor was most common in patients in the fourth to seventh decades of life. In contrast, previous reports suggest they typically occur in young adults or are evenly dispersed across all ages.11 Infrequently, glomus tumors can appear during childhood and unlike the lesions found in adults, more often are multiple.12–16 Approximately 10% of all glomus tumors are multiple, and in some instances are familial.17 A familial variant of glomangioma recently has been associated with chromosome 1p21–22, which codes for the glomulin gene.1 Truncating mutations have resulted in 17 recognized inherited variants in glomulin.18 None of the tumors in our study were documented to be familial or multiple.
Similar to other studies, an overall sex predilection was not seen, while a preponderance for females (80%, 44/55) was found in glomus tumors of the digits.4,19 The anatomic distribution of glomus tumor was relatively similar to other studies, with the vast majority in the dermis and subcutis. In particular, the digits, distal upper extremities, and lower extremities were the most common sites.3 Glomus tumors are believed to be related to the location of glomus bodies, but have been observed in extracutaneous locations not known to contain glomus cells presumably arising from perivascular smooth muscle cells that differentiate into glomoid cells. Glomus tumors have been found in a vast array of different organs including the lungs, liver, stomach, colon, and kidneys. Glomus tumors in these locations mostly are discovered incidentally or with vague symptoms, but have been found to contain nerve bundles that correlate with presenting symptoms of pain.20 Although still uncommon, deep-seated glomus tumors were more commonly represented in our study (4.4% of glomus tumors), potentially reflecting referral bias for these unusual presentations. Although the majority of tumors were less than a centimeter in greatest dimension, a wide range of reported tumor sizes was found. These relatively unusual and larger sized tumors again may represent a referral bias. Finally, given the relatively rarity of glomus tumor, the clinical to pathological concordance in diagnosis is good (45.4%)—most likely reflecting the characteristic subungual site of presentation.
Radiographic features of glomus tumor were relatively uncommonly assessed in our case series (18.2% of cases). Diverse imaging modalities were employed, including X-ray, CT/PET, ultrasound, and MRI. Importantly, none of the imaging studies used suggested a diagnosis of glomus tumor. Prior studies have emphasized the characteristic radiographic features of glomus tumor, particularly of the digits or subungual location. In contrast to its underutilization in the present study, a number of authors have reported relatively unique imaging findings of glomus tumor that aid in diagnosis. On plain films, erosive changes of the distal phalanx are present in a large minority of subungual glomus tumors.21 Marked vascularity of Doppler ultrasonographic evaluation has been described.22 MR imaging has been described as the most sensitive modality, especially for smaller tumors.22 A characteristic well-circumscribed T2 hyperintense lesion is most often found.22,23 Notably, the majority of published articles are in the form of case reports or small, retrospective case series.24–27 However, our review suggests that among all cases of glomus tumor, the supplementation of clinical impression with radiographic studies is not common practice.
The immunohistochemical profile in our study is relatively similar to other reports, with a slight higher rate of CD34 positivity (32%).4 S100 was positive in one case, in line with previous observations of rare positivity.7,28 Calponin immunohistochemical staining was underutilized in comparison other studies,4 and Type IV collagen or Caldesmon were not used.
Despite the well-documented clinical and histopathological features of glomus tumor, the exact etiopathogenesis and cellular origins of this intriguing tumor is poorly understood. Several lines of evidence suggest a modified pericytic/modified smooth muscle phenotype for glomus tumor. First, the classic appearance of glomus tumor is that of small uniform glomus cells that are seen in a perivascular arrangement.10 The immunohistochemical phenotype of glomus tumor is relatively nonspecific, but does support a pericytic/perivascular phenotype. This includes characteristic expression of αSMA and vimentin in nearly all tumors, as do pericytes.4 Some studies using electron microscopy (EM) have shown ultrastructural features of pericytic/smooth muscle differentiation. In general, EM studies have showed a scalloped nucleus, micropinocytotic vesicles, and subplasmalemmal densities, which share similarities to both pericytes and smooth muscle cells.8 Moreover, the cytoplasm of glomus cells has a variable number of actin filaments, which vary in shape from round or oval to elongated and spindle-shaped, all features suggesting that they are modified smooth muscle cells.8 Although the exact cellular etiology of glomus tumor continues to be elusive, there seems to be a difference in the genetic composition of pericytic tumors. Through RNA sequencing, an association between a novel MIR143– NOTCH fusion gene has been uncovered in greater than half of glomus tumors, regardless of malignancy and anatomic location, and has not been reported in other pericytic tumors.29 Despite this accumulating immunohistochemical, ultrastructural, and genetic data, further study is needed to determine cellular origins of glomus tumor.
References
- 1.Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448–1452. doi: 10.5858/2008-132-1448-GT. [DOI] [PubMed] [Google Scholar]
- 2.Masson P. Le glomus neuromyoarterial des regions tactiles et ses tumeurs. Lyon Chir. 1924;21:257–280. [Google Scholar]
- 3.Fletcher CDM World Health Organization, International Agency for Research on Cancer. WHO Classification of Tumours of Soft Tissue and Bone. 4. Lyon, France: IARC Press; 2013. [Google Scholar]
- 4.Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1–12. doi: 10.1097/00000478-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971–976. doi: 10.1001/archderm.140.8.971. [DOI] [PubMed] [Google Scholar]
- 6.Calduch L, Monteagudo C, Martinez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402–408. doi: 10.1046/j.1525-1470.2002.00114.x. [DOI] [PubMed] [Google Scholar]
- 7.Porter PL, Bigler SA, McNutt M, Gown AM. The immunophenotype of hemangiopericytomas and glomus tumors, with special reference to muscle protein expression: an immunohistochemical study and review of the literature. Mod Pathol. 1991;4:46–52. [PubMed] [Google Scholar]
- 8.Erlandson R. Diagnostic Transmission Electron Microscopy of Tumors. London, England: Raven Press; 1994. [Google Scholar]
- 9.Watanabe K, Kusakabe T, Hoshi N, Saito A, Suzuki T. h-Caldesmon in leiomyosarcoma and tumors with smooth muscle cell-like differentiation: its specific expression in the smooth muscle cell tumor. Hum Pathol. 1999;30:392–396. doi: 10.1016/s0046-8177(99)90113-2. [DOI] [PubMed] [Google Scholar]
- 10.Goldblum JR, Folpe AL, Weiss SW, Enzinger FM, Weiss SW. Enzinger and Weiss’s Soft Tissue Tumors. 6. Philadelphia, PA: Saunders/Elsevier; 2014. [Google Scholar]
- 11.Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996;21:257–260. doi: 10.1016/s0266-7681(96)80110-0. [DOI] [PubMed] [Google Scholar]
- 12.Wood WS, Dimmick JE. Multiple infiltrating glomus tumors in children. Cancer. 1977;40:1680–1685. doi: 10.1002/1097-0142(197710)40:4<1680::aid-cncr2820400443>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Goodman TF, Abele DC. Multiple glomus tumors. A clinical and electron microscopic study. Arch Dermatol. 1971;103:11–23. doi: 10.1001/archderm.103.1.11. [DOI] [PubMed] [Google Scholar]
- 14.Kohout E, Stout AP. The glomus tumor in children. Cancer. 1961;14:555–566. doi: 10.1002/1097-0142(199005/06)14:3<555::aid-cncr2820140316>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Charles NC. Multiple glomus tumors of the face and eyelid. Arch Ophthalmol. 1976;94:1283–1285. doi: 10.1001/archopht.1976.03910040155005. [DOI] [PubMed] [Google Scholar]
- 16.Sluiter JT, Postma C. Multiple glomus tumours of the skin. Acta Derm Venereol. 1959;39:98–107. [PubMed] [Google Scholar]
- 17.Shugart RR, Soule EH, Johnson EW., Jr Glomus tumor. Surg Gynecol Obstet. 1963;117:334–340. [PubMed] [Google Scholar]
- 18.Brouillard P, Ghassibe M, Penington A, et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): evidence for a founder effect. J Med Genet. 2005;42(2):e13. doi: 10.1136/jmg.2004.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takata H, Ikuta Y, Ishida O, Kimori K. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25–27. doi: 10.1142/s0218810401000394. [DOI] [PubMed] [Google Scholar]
- 20.Appelman HD, Helwig EB. Glomus tumors of the stomach. Cancer. 1969;23:203–213. doi: 10.1002/1097-0142(196901)23:1<203::aid-cncr2820230127>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Vandenberghe L, De Smet L. Subungual glomus tumours: a technical tip towards diagnosis on plain radiographs. Acta Orthop Belg. 2010;76:396–397. [PubMed] [Google Scholar]
- 22.Glazebrook KN, Laundre BJ, Schiefer TK, Inwards CY. Imaging features of glomus tumors. Skeletal Radiol. 2011;40:855–862. doi: 10.1007/s00256-010-1067-1. [DOI] [PubMed] [Google Scholar]
- 23.Koc O, Kivrak AS, Paksoy Y. Subungual glomus tumour: magnetic resonance imaging findings. Australas Radiol. 2007;51(Spec No):B107–B109. doi: 10.1111/j.1440-1673.2007.01797.x. [DOI] [PubMed] [Google Scholar]
- 24.de Marco L, Mamede M. Metabolic pattern of glomic tumor by F-18 FDG PET/CT. Clin Nucl Med. 2011;36:1051–1052. doi: 10.1097/RLU.0b013e31821c9aaf. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Le H, Munk P, Malfair D, Lee Ch H, Clarkson P. Glomus tumour in the forearm: a case report and review of MRI findings. JBR-BTR. 2010;93:292–295. doi: 10.5334/jbr-btr.342. [DOI] [PubMed] [Google Scholar]
- 26.Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389–393. doi: 10.1111/j.1346-8138.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu KL, Wang HP, Tseng WY, Shun CT, Chen SJ, Tsang YM. Glomus tumor of the stomach: MRI findings. AJR Am J Roentgenol. 2005;185:1190–1192. doi: 10.2214/AJR.04.1531. [DOI] [PubMed] [Google Scholar]
- 28.Nuovo M, Grimes M, Knowles D. Glomus tumors: a clinicopathologic and immunohistochemical analysis of forty cases. Surg Pathol. 1990;3:31–45. [Google Scholar]
- 29.Mosquera JM, Sboner A, Zhang L, et al. Novel MIR143-NOTCH fusions in benign and malignant glomus tumors. Genes Chromosomes Cancer. 2013;52:1075–1087. doi: 10.1002/gcc.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]


