Abstract
Objective
To demonstrate the pro-osteogenic effect of burn injury on heterotopic bone formation using a novel burn ossicle in vivo model.
Background
Heterotopic ossification (HO), or the abnormal formation of bone in soft tissue, is a troubling sequela of burn and trauma injuries. The exact mechanism by which burn injury influences bone formation is unknown. The aim of this study was to develop a mouse model to study the effect of burn injury on heterotopic bone formation. We hypothesized that burn injury would enhance early vascularization and subsequent bone formation of subcutaneously implanted mesenchymal stem cells.
Methods
Mouse adipose-derived stem cells were harvested from C57/BL6 mice, transfected with a BMP-2 adenovirus, seeded on collagen scaffolds (ossicles), and implanted subcutaneously in the flank region of 8 adult mice. Burn and sham groups were created with exposure of 30% surface area on the dorsum to 60°C water or 30°C water for 18 seconds, respectively (n = 4/group). Heterotopic bone volume was analyzed in vivo by micro-computed tomography for 3 months. Histological analysis of vasculogenesis was performed with platelet endothelial cell adhesion molecule staining. Osteogenic histological analysis was performed by Safranin O, Picrosirius red, and aniline blue staining. Qualitative analysis of heterotopic bone composition was completed with ex vivo Raman spectroscopy.
Results
Subcutaneously implanted ossicles formed heterotopic bone. Ossicles from mice with burn injuries developed significantly more bone than sham control mice, analyzed by micro-computed tomography at 1, 2, and 3 months (P < 0.05), and had enhanced early and late endochondral ossification as demonstrated by Safranin O, Picrosirius red, and aniline blue staining. In addition, burn injury enhanced vascularization of the ossicles (P < 0.05). All ossicles demonstrated chemical composition characteristic of bone as demonstrated by Raman spectroscopy.
Conclusions
Burn injury increases the predilection to osteogenic differentiation of ectopically implanted ossicles. Early differences in vascularity correlated with later bone development. Understanding the role of burn injury on heterotopic bone formation is an important first step toward the development of treatment strategies aimed to prevent unwanted and detrimental heterotopic bone formation.
Keywords: adipose-derived mesenchymal cells, burn injury, heterotopic ossification, μCT, osteogenesis
Heterotopic ossification (HO) is defined as the anomalous formation of lamellar bone in extraskeletal nonosseous tissues such as muscle, fascia, or cartilage. This ectopic bone formation is thought to arise from the transformation of resident undifferentiated mesenchymal cells down an osteoblastic lineage.1,2 Clinically, HO can develop in several clinical scenarios of trauma, including blast and burn injuries. Although the overall incidence of HO in all burn patients is less than 5%, this number increases to more than 50% in major burn injuries and more than 60% of blast injuries.1,3 Thus, the size of the burn seems to influence heterotopic bone formation. Perhaps, more interesting is the fact that increased burn injury increases the amount of HO both in the region of the burn and remotely. Once this complication arises, patients experience severe pain, nerve entrapment, joint contractures, and stiffness.4,5 After HO is diagnosed by radiography, few treatment options exist and the criterion standard surgical treatments are inadequate, often leaving patients with residual contractures and a high rate of recurrence after surgical intervention.3,6,7 We set out to establish an HO model that takes into account the important component of inflammation from a burn injury, using a partial-thickness burn and ossicle implantation.
Inflammation is a well-regulated biological response to trauma and burn injuries. In orthopedic trauma, inflammation plays a crucial role in fracture repair and regeneration.8 Scientists have described in detail the essential role of inflammation for bone regeneration. Specifically, studies have identified IL-6 and TNF-α as crucial inflammatory cytokines in bone healing and mesenchymal stem cell (MSC) osteogenic differentiation.9–11 We demonstrate that the inflammation caused by a partial-thickness burn increases the angiogenesis and osteogenic differentiation of implanted MSCs, using an ossicle model.
Significant research efforts have focused on obtaining a better understanding of the pathways involved in HO. Such studies have led to the recognition of the BMPR1 (bone morphogenetic protein type I receptor), specifically coded by the ACVR1 gene as a major contributor to heterotopic bone formation.12 More specifically, studies have isolated the ALK2 receptor as the pivotal BMPR1 pathway regulating HO. Thus, previous HO models have included mice with mutations in ACVR1 (activin A receptor, type I) and MSX-2 (homeobox protein MSX-2).13,14 Researchers have targeted the BMP pathway through BMP ligand and receptor inhibitors in an effort to both better understand and potentially prevent HO.12,15
Despite advances in our understanding of the pathways involved in HO, few treatment options have resulted from these studies because these models require mutant mice that do not correlate with the true clinical development of HO from trauma. One of the limiting factors to improving treatment modalities has been the absence of animal models that mimic ectopic bone in the setting of inflammation or burn injury. Acquired HO models have focused on implantation of osteogenic compounds such as BMP-containing scaffolds or biomaterials with calcium phosphate.16 In addition, studies have reported on implanted cells with an osteogenic potential such as BMSCs.17,18 Although these previous studies have improved our mechanistic understanding of HO, they do not incorporate an inflammatory injury in a wild-type mouse, which is crucial for the development of a reproducible model. In this article, we present a new model to study HO that is directly applicable to patients with burn and blast injury by combining an established implantation model with our model of inflammation from burn injury.18,19 We believe that this model provides a good method to study the role of inflammation on heterotopic bone formation.
Methods
Animals
All experiments used 8- to 10-week-old male C57BL/6 mice (20–25 g; Harlan Laboratories, Oxford, MI). All animals were housed in standard cages with food and water available ad libitum in a specific pathogen-free facility. Animals were allowed to acclimatize for 1 week before experimentation. Experiments were performed in accordance with National Institute of Health guidelines, and prior approval was obtained from the University of Michigan Animal Care and Use Committee.
Burn Procedure
To study the effects of burn injury, we used the modified burned mouse model of Stieritz and Holder as previously described.15–18 Briefly, dorsal hair was closely clipped under isofluorane anesthesia. The mice were then placed in a custom-made, insulated mold with a rectangular opening to expose approximately 30% of the total body surface area on the dorsum of the mouse. The exposed dorsal skin surface was immersed in 60°C water for 18 seconds to produce a partial-thickness dermal burn. Sham animals were immersed in water at room temperature (30°C). The mice were immediately dried. Buprenorphine (0.01 mg/kg, Buprenex; Reckitt Benckiser Pharmaceuticals Inc, Richmond, VA) was administered by subcutaneous injection every 12 hours for the first 72 hours after burn injury. The ossicles were then harvested at either 7 days or 3 months for histology.
Isolation of Adipose-Derived Stem Cells
Six-week-old C57BL/6 littermate mice were used to isolate adipose-derived stem cells (ASCs) from the adipose compartment as previously described.20 All cells were passaged at least 4 times to ensure a near homogenous population as previously described.18
Ossicle Surgical Implantation
Simultaneously with the burn or sham injury, 1 ASC ossicle was placed in the left, dorsal flank of the mouse as previously described.18 Briefily, ASCs from C57BL/6 mice were transfected overnight with a BMP-2 adenovirus. Cells were then resuspended in 30 μL of growth medium and incorporated into gelatin sponges (Gelfoam; Pfizer, New York, NY) by capillary action. One midline longitudinal skin incision was made on the distal dorsal surface of each mouse. Two subcutaneous pockets were dissected per mouse (one over each thigh region). The ossicles were then implanted immediately in these subcutaneous pockets, and the wound was closed. A total of 4 mice received burn injury and 4 mice were nonburn control (sham) mice (n = 4/group). This number ensured statistical significance given the in vivo longitudinal imaging.
Micro–Computed Tomography
Both burn and sham groups were followed longitudinally by micro–computed tomography (μCT). μCT (GE Healthcare Bio-sciences) scans were obtained using 80 kVp, 80 mA, and 1100-ms exposure. A total of 392 projections were taken at a resolution of 45-μm voxel size. After initial calibration of known density standards, each mouse was scanned. The individual scans were reconstructed and reoriented in a 3-dimensional x, y, and z plane. Several rotations and cropping of nonbone space were undertaken to ensure uniform data analysis, with the region of interest placement corresponding with the region of heterotopic bone formation. The bone volume was assessed, calculated, and recorded using a phantom for normalization. All scans were done in vivo to allow for accurate assessment of bone formation longitudinally. Each scan took 10 minutes, with the mice under isofluorane anesthesia. No complications were observed during the scans.
Histology
Ossicles were harvested after 7 days or 3 months. The ossicles were fixed in buffered formalin for 24 hours at 4°C overnight and then decalcified for 2 weeks in 19% ethylenediaminetetraacetic acid, embedded in paraffin, and 7-μm serial sections prepared and stained with Safranin O or Picrosirius red. Every tenth slide was also stained for aniline blue as previously described.21–24 Quantification of staining was done using Adobe Photoshop magic wand tool and observer judgment.
Immunohistochemical Staining
Osteocalcin (OCN; Santa Cruz Laboratories, Santa Cruz, CA) and platelet endothelial cell adhesion molecule (PECAM; Santa Cruz Laboratories) staining was performed on 5 slides each from the same region of the ossicles in burn and nonburn mice. Slides were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide. For OCN, slides were blocked with 5% rabbit serum in phosphate-buffered saline and then incubated overnight with goat anti-OCN (1:100; Santa Cruz Laboratories) at 4°C. For PECAM, slides were blocked instead with 5% goat serum and incubated with anti-PECAM (1:80; Santa Cruz Laboratories). The appropriate biotinylated secondary antibody was used in 1:1000 dilution for PECAM, 1:200 for OCN (Vector Laboratories, Burlingame, CA). The Vectastain ABC system (Vector Laboratories) was used according to the manufacturer's instructions. Visualization was achieved with diaminobenzidine solution (Zymed Laboratories, South San Francisco, CA). Microscopy was then performed. For PECAM, vessels were quantified on 5 stained slides per defect by 3 blinded independent examiners at 20× magnification. Vessels were defined by their positive PECAM stain and their typical round or oval structure containing a lumen. Vessel surface was quantified histomorphometrically as described previously.25
Histological Sample Collection for Ex Vivo Raman Spectroscopy
The fiber-optic probe system used for these experiments has been previously described.26 Imaging was completed at 3 months after implantation. Briefly, the ossicle was placed on the probe in identical orientations. The fibers are positioned so that they just contacted the ossicle. A calibration tool (HCA, Kaiser Optical Systems) was used for white light correction and calibration of detector wavelength axis. The HCA contains a white light source for calibration of the detector wavelength response and a neon discharge lamp for calibration of the spectrograph wavelength scale. Spectra were preprocessed for removal of cosmic spikes and correction of spectrograph/detector alignment and grating-induced anamorphic magnification (curvature). Spectra were corrected for the fluorescence background by fitting background to a low-order polynomial. Overlapped bands are fitted to mixed Gaussian-Lorentzian functions. Band heights and areas are measured. Commonly, in bone, Raman spectroscopy provides the positions of mineral and matrix bands and ratios of band heights or band areas.27 The height or area of the intense PO43− v1 stretch was used as a measure of mineral content, and the width of this band was used as a measure of mineral crystallinity to identify and assess bone mineral characteristic. The height or area of the amide I band (1660 cm−1) was used as the measure of matrix content. After the emerging consensus, we used the combined proline (850 cm−1) and hydroxyproline (876 cm−1) intensity as the measure of matrix content. Standard principal components analysis–based multivariate methods were used to unmix bone and overlying tissue spectra returned by our measurements.
Statistical Analysis
Means and standard deviations were calculated from numerical data, as presented in the text, figures, and figure legends. In figures, bar graphs represent means whereas error bars represent standard deviation. Statistical analysis was performed using the 2-factor analysis of variance with replication when more than 2 groups were compared. In addition, the Welch 2-tailed t test was used when standard deviations between groups were unequal. Inequality of standard deviations was verified by employing the Levene test. P < 0.05 was considered to be significant.
Results
Burn Injury Increases TNF-α Expression
Previous studies have demonstrated TNF-α is upregulated after burn injury.28 We found a similar outcome as TNF-α expression was significantly increased in those mice with a burn injury (see Supplemental Digital Content Fig. 1, available at: http://links.lww.com/SLA/A385). Thus, our model seems to mimic the inflammatory milieu seen in humans after burn injury.
Burn Injury Increases Vascularization of Ossicles
Vasculogenesis is known to play a key role in bone formation, as studies have demonstrated overlap between the VEGF and BMP pathways.25,29,30 We first set out to examine the effect of burn injury on vessel formation, using our ossicle model. We found that 7 days after implantation, those newly forming ossicles in mice with burn injuries accompanying ASC implantation had significantly higher levels of PECAM stain and visible tubules than ossicles in nonburn mice (Fig. 1). Thus, burn injury had a positive effect on vasculogenic tendency in the implanted ossicles in this model.
Figure 1.

Burn injury increases angiogenesis in ossicles 7 days after implantation. A, PECAM quantification of ASC-seeded ossicles after burn injury or nonburn (sham) control (*, P < 0.05). B, Representative images at 20× of PECAM stain of ASC-seeded ossicles after burn injury or nonburn control (sham) 7 days after ossicle implantation. Circle indicates PECAM positive circular structure.
Burn Injury Increases Early Endochondral Ossification in Ossicles
Having noted an increase in early vascularization, we next evaluated early bone formation in the ossicle model with and without burn injury 7 days after implantation. We found, similar to our PECAM staining, more early signs of endochondral ossification by Safranin O staining in the ossicles in mice with concomitant burn injury than sham nonburn controls (Fig. 2A). Thus, a scald burn injury appears to enhance early cartilage deposition in the ossicle model. Furthermore, immunohistochemical staining for OCN at this same time point showed greater abundance and intensity in ossicles from burn mice than their sham counterparts (Fig. 2B). Consistent with Safranin O staining, it appears that the tendency for ASCs to differentiate along an osteoblastic lineage is increased after burn injury. These findings are significant, as it is likely that the early inflammatory period in burn injuries creates a more osteogenic niche for endochondral ossification to take place.
Figure 2.
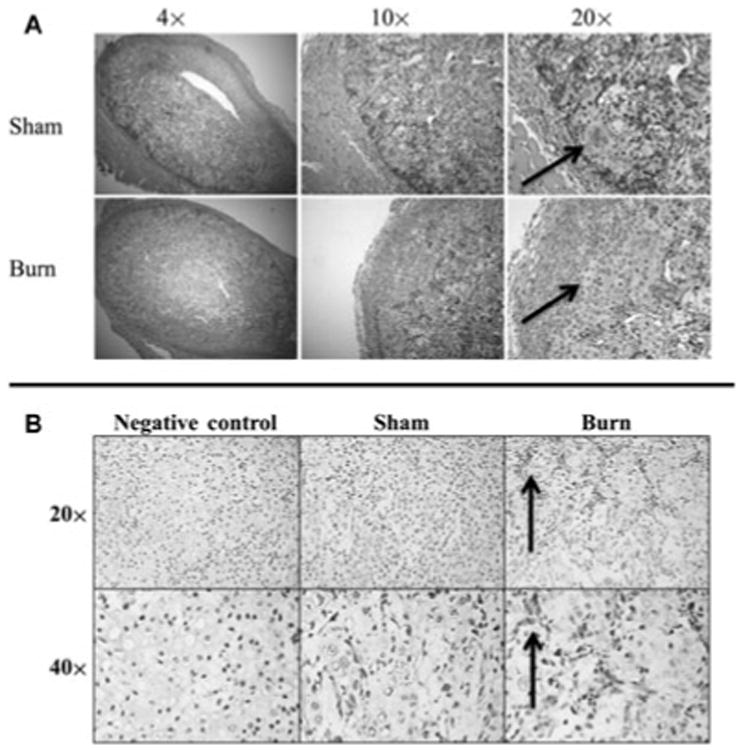
Burn injury enhances early osteogenic predilection of ASC-seeded ossicles. A, Early endochondral ossification seen in ASC-seeded ossicles. Left to Right, Images of the ASC ossicle with Safranin O staining at 4×, 10×, and 20× are shown 7 days after ossicle implantation. Black arrows point to regions of early cartilage formation with positive Safranin O staining. B, OCN immunohistochemical stain of ASC-seeded ossicles at 7 days shown at 20× and 40× magnification. Arrows indicate regions of OCN positive staining (cytoplasmic). Negative control slides were not incubated with primary OCN antibody, but otherwise similarly processed.
Burn Injury Increases Late Bone Formation Using an Ossicle Model
Having investigated early vascularization and bone formation, we next set out to follow bone formation longitudinally in ossicles after burn injury or nonburn controls using μCT. In the ossicles, we noted significantly more bone than sham controls at 1 month (Fig. 3). This trend remained and was statistically significant at 2-and 3-month time points as well. The largest difference appeared at 1 month, which corresponds to the time period within which the burn inflammatory period likely peaked then began to diminish. Thus, burn injury does seem to enhance heterotopic bone formation using an ossicle model, and μCT serves as a reasonable methodology to follow its progression.
Figure 3.
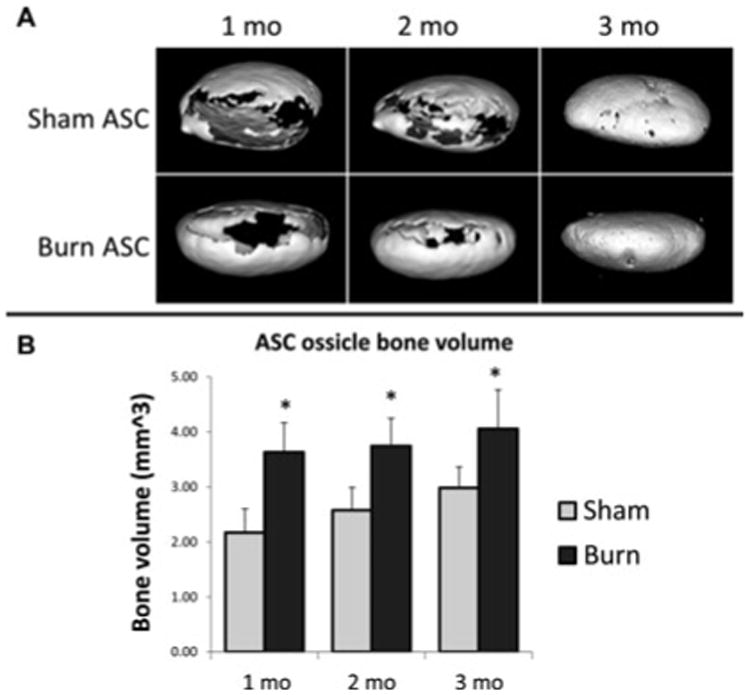
Burn injury enhances bone formation in ASC-seeded ossicles. A, 3-Dimensional reconstruction of representative ossicles from nonburn control (sham) and burn injury mice at 1, 2, and 3 months. B, Quantification of bone volume at 1, 2, and 3 months comparing burn with nonburn control (sham) (*, P < 0.05, n = 4/group).
Picrosirius Red Stain Demonstrates Increased Collagen Deposition at Early and Late Time Points After Burn Injury
Next, anionic dye Picrosirius Red staining was performed, in which mature lamellar bone appears green under polarized light.31 Similar collagen levels were noted at 7 days; however, there was significantly more lamellar bone at 3 months noted in the ASCs engrafted ossicles after burn injury (Fig. 4). Thus, consistent with the μCT data, we see that burn injury enhanced the development of early collagen deposition into bone in this heterotopic ossification model.
Figure 4.
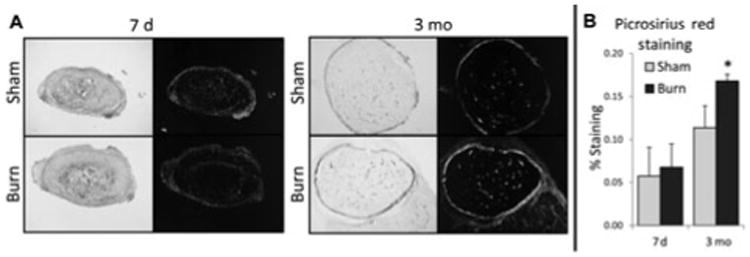
Burn injury enhances early collagen deposition and late lamellar bone deposition in ASC-seeded ossicles. Picrosirius red stain of ASC-seeded ossicles in nonpolarized (left) and polarized light (right) at 7 days and 3 months in burn and non-burn control (sham) mice. Picrosirius red quantification using Adobe Photoshop showing analysis of percentage of Picrosirius red staining under polarized light (*, P < 0.05).
Late Bone Formation in Ossicles Is Greater After Burn Injury by Histological Staining
We next sought to verify our μCT findings by direct histological staining for osteoid. Serial sections were generated through each ossicle at 7-μm thickness; approximately 50 slides were made for each ossicle. Every 10th slide was stained and examined over the entire ossicle. Results showed that the ossicle from mice with burn injuries had significantly more osteoid by aniline blue staining at 3 months (Fig. 5A). Interestingly, the greatest level of osteoid was apparent on the outside of the ossicle, which is the region most likely to receive the greatest blood flow. This additional osteoid in the burn groups was noted both around the edges of the ossicle and within the ossicle and correlated with the areas where enhanced early vascularization was observed. Next, by histomorphometric measurements, we sought to substantiate our findings. New bone regenerated within the ossicle was assessed using image analysis as previously described.21–23 Results showed a significant increase in new bone in the ASC ossicles in burn injured mice compared with nonburn control (Fig. 5B). Further confirmation of increased osteogenic activity was evidenced by OCN immunohistochemistry. Staining at this late time point yielded similar results to those obtained by OCN staining at 7 days, showing greater abundance and intensity of staining in the ossicles from burn mice than their sham counterparts (Fig. 5C). Furthermore, a similar pattern to that of aniline blue was seen, with the greatest level of OCN signal concentrated at the perimeter of the ossicle on the edges where new bone was still forming.
Figure 5.
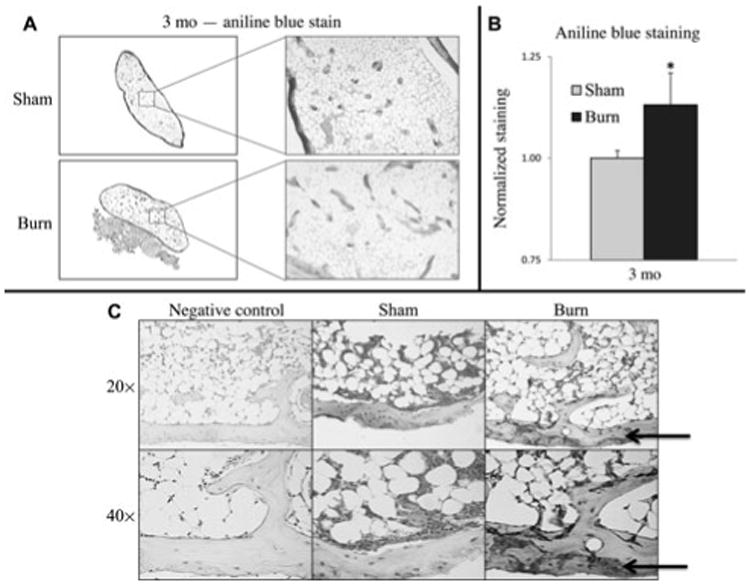
Burn injury increases osteoid formation by histology at 12 weeks. A, Representative slides of ASC seeded ossicles stained with aniline blue showing mature osteoid. B, Aniline blue quantification using Adobe Photoshop analysis of aniline blue staining (*, P < 0.05). C, OCN immunohistochemical stain of ASC-seeded ossicles at 12 weeks shown at 20× and 40× magnification. Arrows indicate regions of OCN positive staining (cytoplasmic). Negative control slides were not incubated with primary OCN antibody, but otherwise similarly processed.
Raman Spectroscopy Verifies Chemical Composition of Lamellar Bone in Ossicles
Despite the high sensitivity of μCT to detect bone, this technology depends on the density of the mineralized tissues being examined. To better understand the chemical composition of ectopic bone development, carefully explanted ossicles were imaged on a high-resolution Raman microscopy platform. We observed that the ossicles from our both burn and nonburn control mice had chemical compositions characteristic of bone tissue (Fig. 6). No differences were found, however, when comparing spectra between burn and sham mice. This suggests that although there were differences in the amount of ectopic bone formed after burn injury in this model, the bone tissue itself is similar in composition. Thus, histology, μCT, and Raman spectroscopy provided 3 different methods of analysis of the osteoid formed within the ossicle, all of which were consistent with de novo bone.
Figure 6.
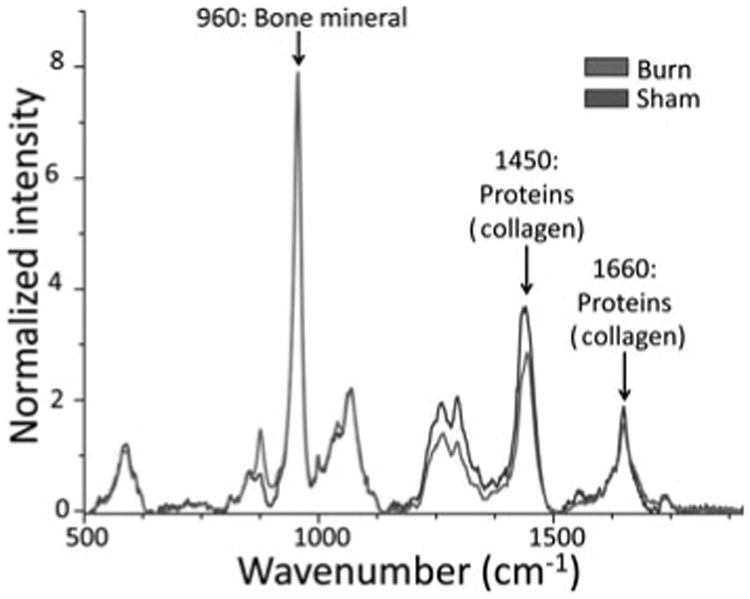
Chemical composition of bone is not different between burn and nonburn control (sham) groups as demonstrated by Raman spectroscopy of sham and burn ASC seeded ossicles. Spectra are normalized to bone mineral band (960 cm−1) and overlain to compare similarity of chemical composition signaling.
Discussion
We present a straightforward, reproducible ossicle model that introduces an inflammatory insult from a burn injury.18 Inflammation after a partial-thickness burn is known to cause the release of TNF-α, which plays a role in early osteogenic differentiation of MSCs and osteoprogenitor cells after bone fractures.8,28 Similarly, in our model, we note that mice after burn injury have increased expression of TNF-α. The ossicle model, as described by Schneider et al, uses MSCs that are transfected with a BMP-2–releasing adenovirus, placed on a collagen scaffold, and implanted subcutaneously on the dorsum of mice where bone usually does not form.18 Interestingly, earlier studies using this ossicle model did not detect bone until 3 weeks after implantation.18 In our study, we detected precursors to endochondral ossification by histology as early as 1 week after implantation in the burn injury group. Future studies will seek to investigate earlier time points and the molecular pathway that leads to the increased bone formation seen in our ossicles developed from mice with burn injuries.
Despite the promise of this model as a method of studying burn-induced HO, implantation models create an artificial system with unnaturally high concentration of osteogenic cells and osteogenic factors in one specific location that is not seen in clinically in humans. Furthermore, variations exists in the amount of bone formed in various species after implantation.6 Despite these shortcomings, we do believe that our study is internally consistent, given the use of a singular mouse strain for all ossicle implantations and similar consistency in the homogenous population of ASCs with which the ossicles were seeded. Other potential animal models to which burn injury can be introduced to further study the effect of inflammation on remote HO are the Achilles tenotomy model. Studies have shown that 60% of mice with an Achilles tenotomy lead to heterotopic ossification at 5 weeks without exogenous cytokines or cells.32 Thus, future studies will attempt to further characterize the role of burn inflammation on HO in this Achilles trauma–like model. We have recently shown that burn injury in our Achilles tenotomy model had a similar effect as in our ossicle model. Mice who received a burn injury in addition to an Achilles tenotomy demonstrated significantly more heterotopic bone formation than those mice with an Achilles tenotomy alone.33
This study extends what is already known about the ability of MSCs derived from adipose tissue to undergo osteogenic differentiation ectopically. In addition, our laboratory and others have documented the inflammatory effect of burn injury on the global transcriptome and on remote organ systems such as the heart.19,34–36 Thus, we believe that it follows, logically, that inflammation from burn injury would also have an effect on ectopically implanted MSCs. Our study sought to address 2 specific questions. First, does a burn injury affect the vasculogenic niche of the implanted ossicle? Second, does the inflammation from a burn injury lead to enhanced bone formation? We believe this second question is of specific clinical relevance, as previous treatment modalities for HO have failed to target the inflammation at the burn site. Although systemic anti-inflammatory agents have been used in clinical trials, there has only been limited success and a high complication rate.37 By demonstrating that a burn injury increases HO in this model, future studies can focus on mitigating the local inflammation, specifically at the burn site rather than systemically. Future studies will analyze the effect of TNF-α inhibition both systemically and at the site of the ossicle to establish potential pathways to target for therapeutic interventions. In addition, we are currently exploring the effect of adenosine triphosphate hydrolysis on inhibition of HO.
Thus, our model expands the armamentarium to further delve into the effects of inflammation on ectopic bone formation and the clinical maladies of HO. It appears from our study that ASCs and burn injury complement the paradigm well, as there were obvious differences seen in those with a burn injury compared with control.
Conclusions
ASCs undergo enhanced vascularization when exposed to a concomitant burn injury. In addition, this early vascularization correlated with early collagen deposition and osteoid formation. These differences were maintained over time as demonstrated by μCT and aniline blue stain.
Supplementary Material
Footnotes
Disclosure: None of the authors have a financial interest in any of the products, devices, or drugs mentioned in this article. The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal's Web site (www.annalsofsurgery.com).
References
- 1.Chen HC, Yang JY, Chuang SS, et al. Heterotopic ossification in burns: our experience and literature reviews. Burns. 2009;35:857–862. doi: 10.1016/j.burns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Shimono K, Morrison TN, Tung WE, et al. Inhibition of ectopic bone formation by a selective retinoic acid receptor alpha-agonist: a new therapy for heterotopic ossification? J Orthop Res. 2010;28:271–277. doi: 10.1002/jor.20985. [DOI] [PubMed] [Google Scholar]
- 3.Potter BK, Forsberg JA, Davis TA, et al. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am. 2010;92(suppl 2):74–89. doi: 10.2106/JBJS.J.00776. [DOI] [PubMed] [Google Scholar]
- 4.Ji Y, Christopherson GT, Kluk MW, et al. Heterotopic ossification following musculoskeletal trauma: modeling stem and progenitor cells in their microenvironment. Adv Exp Med Biol. 2011;720:39–50. doi: 10.1007/978-1-4614-0254-1_4. [DOI] [PubMed] [Google Scholar]
- 5.Tsionos I, Leclercq C, Rochet JM. Heterotopic ossification of the elbow in patients with burns. Results after early excision. J Bone Joint Surg Br. 2004;86:396–403. doi: 10.1302/0301-620x.86b3.14480. [DOI] [PubMed] [Google Scholar]
- 6.Kan L, Kessler JA. Animal models of typical heterotopic ossification. J Biomed Biotechnol. 2011;2011:309287. doi: 10.1155/2011/309287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsberg JA, Potter BK. Heterotopic ossification in wartime wounds. J Surg Orthop Adv. 2010;19:54–61. [PubMed] [Google Scholar]
- 8.Mountziaris PM, Spicer PP, Kasper FK, et al. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;17:393–402. doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Ricciardi BF, Hernandez-Soria A, et al. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928–936. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hess K, Ushmorov A, Fiedler J, et al. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45:367–376. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- 12.Yu PB, Deng DY, Lai CS, et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen Q, Little SC, Xu M, et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119:3462–3472. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YH, Kundu R, Wu L, et al. Premature suture closure and ectopic cranial bone in mice expressing Msx2 transgenes in the developing skull. Proc Natl Acad Sci U S A. 1995;92:6137–6141. doi: 10.1073/pnas.92.13.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser DL, Economides AN, Wang L, et al. In vivo somatic cell gene transfer of an engineered Noggin mutein prevents BMP4-induced heterotopic ossification. J Bone Joint Surg Am. 2003;85-A:2332–2342. doi: 10.2106/00004623-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 17.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 18.Schneider A, Taboas JM, McCauley LK, et al. Skeletal homeostasis in tissue-engineered bone. J Orthop Res. 2003;21:859–864. doi: 10.1016/S0736-0266(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 19.Ipaktchi K, Mattar A, Niederbichler AD, et al. Attenuating burn wound inflammation improves pulmonary function and survival in a burn-pneumonia model. Crit Care Med. 2007;35:2139–2144. doi: 10.1097/01.ccm.0000280568.61217.26. [DOI] [PubMed] [Google Scholar]
- 20.Levi B, Nelson ER, Brown K, et al. Differences in osteogenic differentiation of adipose-derived stromal cells from murine, canine, and human sources in vitro and in vivo. Plast Reconstr Surg. 2011;128:373–386. doi: 10.1097/PRS.0b013e31821e6e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levi B, Wan DC, Glotzbach JP, et al. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor beta1 (TGF-beta1) signaling. J Biol Chem. 2011;286:39497–39509. doi: 10.1074/jbc.M111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi B, Nelson ER, Li S, et al. Dura mater stimulates human adipose-derived stromal cells to undergo bone formation in mouse calvarial defects. Stem Cells. 2011;29:1241–1255. doi: 10.1002/stem.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi B, James AW, Nelson ER, et al. Studies in adipose-derived stromal cells: migration and participation in repair of cranial injury after systemic injection. Plast Reconstr Surg. 2011;127:1130–1140. doi: 10.1097/PRS.0b013e3182043712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behr B, Tang C, Germann G, et al. Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells. 2011;29:286–296. doi: 10.1002/stem.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okagbare PI, Morris MD. Polymer-capped fiber-optic Raman probe for non-invasive Raman spectroscopy. Analyst. 2012;137:77–81. doi: 10.1039/c1an15847c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris MD, Mandair GS. Raman assessment of bone quality. Clin Orthop Relat Res. 2011;469:2160–2169. doi: 10.1007/s11999-010-1692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finnerty CC, Jeschke MG, Herndon DN, et al. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14:553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behr B, Sorkin M, Lehnhardt M, et al. A comparative analysis of the osteogenic effects of BMP-2, FGF-2, and VEGFA in a calvarial defect model. Tissue Eng Part A. 2012;18:1079–1086. doi: 10.1089/ten.tea.2011.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levi B, Nelson ER, Hyun JS, et al. Enhancement of human adipose-derived stromal cell angiogenesis through knockdown of a BMP-2 inhibitor. Plast Reconstr Surg. 2012;129:53–66. doi: 10.1097/PRS.0b013e3182361ff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levi B, James AW, Nelson ER, et al. Acute skeletal injury is necessary for human adipose-derived stromal cell-mediated calvarial regeneration. Plast Reconstr Surg. 2011;127:1118–1129. doi: 10.1097/PRS.0b013e318205f274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClure J. The effect of diphosphonates on heterotopic ossification in regenerating Achilles tendon of the mouse. J Pathol. 1983;139:419–430. doi: 10.1002/path.1711390403. [DOI] [PubMed] [Google Scholar]
- 33.Peterson JR, Okagbare PI, De La Rosa S, et al. Early detection of burn induced heterotopic ossification using transcutaneous Raman spectroscopy. Bone. 2013;54:28–34. doi: 10.1016/j.bone.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niederbichler AD, Westfall MV, Su GL, et al. Cardiomyocyte function after burn injury and lipopolysaccharide exposure: single-cell contraction analysis and cytokine secretion profile. Shock. 2006;25:176–183. doi: 10.1097/01.shk.0000192123.91166.e1. [DOI] [PubMed] [Google Scholar]
- 35.Ipaktchi K, Mattar A, Niederbichler AD, et al. Topical p38 MAPK inhibition reduces bacterial growth in an in vivo burn wound model. Surgery. 2007;142:86–93. doi: 10.1016/j.surg.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoesel LM, Mattar AF, Arbabi S, et al. Local wound p38 MAPK inhibition attenuates burn-induced cardiac dysfunction. Surgery. 2009;146:775–785. doi: 10.1016/j.surg.2009.06.019. discussion 785–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karunakar MA, Sen A, Bosse MJ, et al. Indometacin as prophylaxis for heterotopic ossification after the operative treatment of fractures of the acetabulum. J Bone Joint Surg Br. 2006;88:1613–1617. doi: 10.1302/0301-620X.88B12.18151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


