Abstract
A number of medical imaging techniques are used heavily in the provision of spatially resolved information on disease and physiological status and accordingly play a critical role in clinical diagnostics and subsequent treatment. Though, for most imaging modes, contrast is potentially enhanced through the use of contrast agents or improved hardware or imaging protocols, no single methodology provides, in isolation, a detailed mapping of anatomy, disease markers or physiological status. In recent years, the concept of complementing the strengths of one imaging modality with those of another has come to the fore and been further bolstered by the development of fused instruments such as PET/CT and PET/MRI stations. Coupled with the continual development in imaging hardware has been a surge in reports of contrast agents bearing multiple functionality, potentially providing not only a powerful and highly sensitised means of co-localising physiological/disease status and anatomy, but also the tracking and delineation of multiple markers and indeed subsequent or simultaneous highly localized therapy (“theragnostics”).
Introduction
Significant advances continue to be made in medical imaging through developments in the chemistry of imaging probes and the engineering of imaging devices. One of the principal objectives of these is to seek a means of enhancing disease diagnosis and thus prognosis; within this, an ability to visualize tissue morphology, the distribution of specific cell types and even cell function/status in real time would be exceedingly powerful. Several imaging modalities can be applied in the realization of such objectives, each with associated strengths and limitations. For example, optical imaging has an associated high sensitivity and spatial resolution that is sub-micrometre in scale. The absorption and scattering characteristics of tissue components do, however, limit the penetration depth to typically less than 1 cm of tissue.1 Positron emission tomography (PET) is a highly sensitive radionuclide based imaging technique but spatially limited in resolution.1 Magnetic resonance imaging (MRI) and computed tomography (CT) are comparatively insensitive methods of imaging but present an almost irresistible marriage of convenience and high spatial resolution.
In most forms of medical imaging specific molecular probes are applied to generate, amplify, or focus, image contrast, though most, if not all, of the inherent limitations remain to some extent. Most of the currently used molecular imaging agents are rapidly cleared i.e. have a very short blood circulation time (< 30 min), due to their small size.2,3 Their application is also limited by a default non-specific biodistribution. Taken with the “no modality provides all” issue of imaging itself, it is clear that the development of high contrast targeted imaging agents, with controllable circulation/biodistribution characteristics, sensitively viewable through at least two modalities, remains important. Coupled to imaging optimization and (thus) potential impact on diagnostics remains the likely dose dependent toxicity associated with the contrast agent. There is much, then, to be gained in combining imaging techniques in a manner which, for example, can pull on the signal intensity of one imaging mode prior to making use of the spatial resolutions attainable in another in enabling the co-registration of anatomic and functional/metabolic information. This should be achieved with minimal levels of contrast agent.
By virtue of their controllable size, high surface area:volume and highly engineerable internal and external chemistries, targeted nanoparticles remain a keen focus in the development of potentially powerful multimodal imaging tools.4,5 Among these, those based on iron oxide,6–8 silica9–11 and biocompatible polymers, such as dendrimers12,13 and liposomes14–16 have been ubiquitous. For example, Reiter et al. have been using silica nanoparticles9,10,17 and polymer based frameworks18,19 to support multifunctionality, most notably MRI/optical dual modal imaging. Hyeon et al. have synthesized various iron oxide based multimodal nanoparticles with a variety of core-shell20 and core-satellite structures.21,22 Particles which promote other multifunctional modes such as MRI/SERS,23 MRI/PET,24 and MRI/CT/optical25 have also been reported. In this article, we will briefly (and necessarily selectively) discuss current developments within PET, CT and MRI imaging modes and respective strategies of enhancing image contrast. Multimodal nanoparticle magnetic resonant probes will subsequently constitute the focus. For more detailed descriptions, we would like to direct readers not only to specifically cited work herein but also recent reviews in the area.4,5,26–30
Medical imaging
Positron emission tomography (PET)
Positron emission tomography (PET) is a nuclear imaging technique that uses compounds labeled with a positron-emitting radionuclide for contrast and can be applied, for example, to the monitoring of biological cell function and failure.31,32 Radioisotopes of oxygen (14O, 15O), nitrogen (13N), carbon (11C), and fluorine (18F) are typically of choice, with an external detector collecting the emitted γ-rays and enabling the distribution and concentration of probes to be tracked in real time. Positron emitters such as isotopes of Cu, Zn, K, Br, Rb, I, P, Fe, and Ga are also used.32 An inherent complication in PET is the finite emitting lifetime of radioisotope (e.g., 18F has t1/2 = 110 min), meaning that their incorporation into vectoring or physiologically active molecules and, subsequently, the subject, must typically be rapid (though developments with 64Cu and 89Zr are enabling work over longer timescales).33 The sensitivity of PET is, though, high (in the range of 10−11–10−12mol L−1) and independent of the location depth of the reporter probe. The spatial resolution of most clinical PET scanners is 6 ~ 83 mm3, but higher resolution clinical brain scanners have been developed with a 3 mm3 resolution.31 The most commonly used positron emitter is 18F-fluorodeoxyglucose (FDG), a glucose analog,34 which provides a powerful means of imaging metabolic activity and, thus, the diagnosis, staging, and treatment of cancers. It is known35 that malignant tumor growth is, for example, associated with increased glycolysis resulting from the progressive loss of tricarboxylic acid (TCA) and the activation of the hexose monophosphate shunt, which provides the carbon backbone for the synthesis of DNA and RNA required for the high cell proliferation rates of tumors. After FGD is injected, it is taken up by cells consuming high levels of glucose in the brain, heart or tumor, where phosphorylation to 18F-FDG-6-phosphate prevents subsequent cellular release (trapping the contrast agent in the tumor).32 The distribution of FDG is then, a good indicator of the distribution of glucose activity and tumor cells.32
PET is also considered to be a reliable tool for the identification of myocardial viability, the accurate assessment of myocardial perfusion and the detection of coronary artery disease (CAD).31 There are two positron emitters, 13N-ammonia and 15O-water, which have been used in the imaging of perfusion. Due to high myocardial retention, the former provides good quality images of blood flow over a wide flow range.36 Suitable pharmacological intervention studies require repetitive measurements within short timescales, making the shorter half-life of radionuclides such as 15O-water useful.37 Cardiac neurotransmission can also be studied with PET.38 The commonly used tracer in such work, 11C-hydroxyephedrine, monitors the regional distribution of cardiac nerve terminals and labeled analogues of norepinephrine. Tracers to investigate β-receptors, alpha1-receptors, adenosine and muscarinic receptors are also under development.39,40
Single photon emission computed tomography (SPECT)
Single photon emission computed tomography (SPECT) is another radionuclide-based imaging mode. Although similar to PET in the sense that images are acquired by photon collection and subsequent mapping of radiotracer distribution, this modality is characterized by radioisotopes emitting only a single high-energy γ photon without the photon coincidence associated with PET.41 Although the sensitivity of SPECT is between one and two orders of magnitude less than PET, the radioisotopes used (133Xe, 99mTc and 123I) are more readily available and typically have longer decay times than those used in PET.42 Although the spatial resolution of a typical clinical SPECT is lower than that of PET, there are recent reports of small animal imaging where micropinhole apertures have been used to attain higher resolution (200 μm).1
SPECT imaging has been applied to the assessment of myocardial metabolism with probes including straight or branched chain fatty acid analogues, labeled with 15-p-iodophenylpentadecanoic acid (123I-IPPA) or 123I-beta-methyl-p-iodophenylpentadecanoic acid (123I-BMIPP),43–45 the latter preferentially used because of its higher myocardial retention. For example, clinical studies have demonstrated the use of 123I-BMIPP in the detection of acute and chronic ischemic events.44
The imaging of cardiac neuroreceptors is another established SPECT application with the primary clinical focus being the sympathetic nervous system.46 Changes in both presynaptic and postsynaptic cardiac function within ischemic heart disease and heart failure can, for example, be detected by SPECT using radiotracers such as 123I-meta-iodobenzylguanidine (123I-MIBG). This contrast agent is also used as a marker for norepinephrine storage where it shares the mechanism of adrenergic tissue uptake and can, therefore, provide diagnostic and prognostic information in patients with heart failure.46 In such 123IMIBG scans typically show a reduced heart-mediastinum uptake ratio, heterogeneous myocardial biodistribution, and increased washout from the heart.47
Computed tomography (CT)
Computed tomography (CT) is a medical imaging technique wherein digital geometry processing is used to generate a three-dimensional image from a larger series of constituent two-dimensional X-ray images. The intensity of a CT image, expressed as Hounsfield units, is related to the efficiency with which X-rays are attenuated as they traverse the volume element in the human body, and are represented by the picture element in the CT image. The basis of functional CT is to inject a contrast agent intravenously and then measure the increase in attenuation or enhancement of the tissue contrast after arrival of the contrast agent by CT scanning.48
There are two types of X-ray contrast agent approved for human use: barium sulfate suspensions, which are used strictly for gastrointestinal tract imaging, and water-soluble aromatic iodinated contrast agents.49 The first generation of X-ray contrast agent developed for general intravascular use were ionic triiodobenzene monomers, such as diatrizoate, though these are now suspected to be chemotoxic.49 Iohexol, a non-ionic iodinated agent, and iodixanol, a dimeric agent, are now more widely used. Iodine-based X-ray contrast agents do, though, suffer from the relatively low iodine atomic number (contrast) and typically have associated very short blood half-live (<10 min) meaning that detectable CT contrast can be minimal with current technology.50 Several other experimental X-ray contrast materials have been investigated with various degrees of success, including electron dense heavy metals.51–54 Gold nanoparticles have drawn considerable interest recently because of their well-established synthesis protocols, stability and high X-ray attenuation ability.55 Like many other nanomaterials, gold nanoparticles possess a prolonged biological half-life compared to current small molecular imaging agents such as iodine-based compounds. By way of the inherently large surface area:volume presented by these a wide range of functional groups can be conjugated. In one recent study54 1.9 nm gold nanoparticles, capable of enhancing vasculature image contrast, were intravenously injected into tumor bearing mice in order to distinguish tumor and normal tissue (the former being marked by its much denser vasculature).
Ultrasound imaging (US)
Ultrasound imaging uses a pulsed, frequency specific, sound wave focused at the anatomical region of interest. Diagnostic ultrasound normally operates at frequencies in the 2–20 MHz range, with ultrasound biomicroscopy system transducers using frequencies up to 100 MHz.56 During operation the sound waves are partially reflected back from the body (to an extent that depends on tissue density or “acoustic impedance”), received by the transducer, and subsequently processed and transformed into a digital image.57 Areas of high tissue density are generally resolved as positive contrast in the final image. Image resolution scales with the ultrasonic frequency used, reaching a current limit of ~50 microns at 60 MHz.57 Increasing spatial resolution does, however, come at the cost of decreasing tissue penetration (the image of deeper tissue/organs necessarily utilized lower, more penetrating, frequencies).
Image contrast can be greatly increased through the use of inert gas filled microbubble contrast agents from which sound waves are effectively reflected.58,59 The potential utilization of ultrasound-microbubble interaction in drug delivery (where incident high energy waves lead to controlled bubble destruction) has been considered.60 Besides microbubbles, a range of nanoparticles have been reported as ultrasound contrast agents.61,62 McPherson et al., for example, have developed a nongaseous, site (antibody) targeted acoustically reflective liposomes.61 Perfluorocarbon emulsion nanoparticles have also been used as ultrasound contrast agents. Wickline et al. have, for example, synthesized and applied ligand-targeted, lipid-encapsulated, nongaseous perfluorocarbon emulsion particles. Though these have comparatively poor inherent acoustic reflectivity compared to microbubbles, image signal:noise benefits considerably from attainable particle vectoring to specific targets.62
Optical Imaging
Biomedical optical imaging is a rapidly growing field, with a considerable and varied application across disease diagnosis and molecular biology. This modality can enable a probe of both structure and function with a remarkably high spatial resolution and temporal resolution. A range of different imaging and spectroscopic modes can be applied depending on the information required and sample physical characteristics (including absorption, emission, and scattering). A variety of clinical studies have, for example, been conducted to investigate the diagnostic potential of absorption/scattering spectroscopy in a range of organs and in the determination of hemoglobin concentration, oxygen saturation and total blood volume.63 The derived results have been used in clinical studies discriminating dysplastic from normal tissues with varying degrees of success.64,65
In fluorescence spectroscopy external light of appropriate wavelength is used to initiate the excitation of target fluorescent molecules prior to the almost simultaneous collection of emitted photons of longer-wavelength. Targets for fluorescence imaging may be endogenous molecules (such as collagen or hemoglobin), fluorescent proteins, or fluorescent contrast agents.64,65 Fiber-optic endoscopes with confocal or 2-photon laser fluorescence imaging further facilitate clinical applications of fluorescence molecular imaging with a range of targeted optical contrast agents.66 The absorption and scattering characteristics of tissue components, such as water, fat, oxyhemoglobin (HbO2), deoxyhemoglobin (Hb) and melanin, determine the penetration depth of light in tissues and, in general, this is a significant limitation of this modality. In the ultraviolet–visible range of the spectrum (<700 nm), light can penetrate tissue of a few hundred microns to a millimetre in depth. In the near infrared (NIR) spectral region (700–900 nm), however, tissues are significantly less absorbing, enabling light to propagate through several centimetres.65 In addition to the vast range of samples operating in the visible spectrum, there exists a continuously growing range of NIR probes, including Cy5.5, the Alexa dye series and indocyanine green.
In comparison to organic dyes and fluorescent proteins, quantum dots (QDs) are emerging as a new class of fluorescent label with improved brightness (quantum yields can be close to 90%),67 resistance against photobleaching68 and multicolor emission.69 The benefits of being able to excite across a broad range and obtain multicolored and highly tunable luminescence are considerable. In addition, emission is typically environmentally stable as the production of photons stems from a band gap process rather than the singlet-singlet transition typical for small molecule fluorophores. These particles typically have a 3–20 nm hydrodynamic diameter and hence are difficult to clear from general circulation by renal filtration, resulting in a potentially significant background signal. The specific targeting of quantum dots has also been investigated by, for example, their encapsulation within a tumor cell targeting ligand modified triblock copolymer.70 Multifunctional nanoparticle probes based on fluorescent quantum dots have been developed by several groups.70–72 The particles can, for example, be coated with paramagnetic chelates70 or doped with paramagnetic metal in the crystal structure71 to fabricate optical-MRI dual modal imaging agent.
Magnetic resonance imaging (MRI)
Magnetic resonance imaging is perhaps the most widely used noninvasive medical imaging technique. In the vast majority of cases image contrast is generated from the physiological variation in the nuclear magnetic resonance characteristics of water protons.73 When these are exposed to an external magnetic field, their nuclear spins will reach an equilibrium state with a (Larmor precession) frequency that is dependent on the strength of external magnetic field. On the application of an appropriate radiofrequency pulse the net magnetization vector associated with these spins will flip from a position parallel to the external field to the transverse plane. After turning off the radiofrequency pulse, the protons will relax back to the equilibrium state. There are two relaxation processes: spin–lattice or longitudinal relaxation (T1) and spin–spin or transverse relaxation (T2). The contrast in an MR image is the result of a complex interplay of numerous factors, including the relative T1 and T2 relaxation times, proton density of the imaged tissues and instrumental parameters. The MRI contrast can be further enhanced by introducing suitable MRI contrast agents. Unlike contrast agents used in CT and PET, for example, 1H MRI contrast agents are not normally directly visualized in the image; only their effects on associated (spatially or chemically) proton T1 and T2 relaxation times. The relaxivity (r1, r2) of a MRI contrast agent is defined as the change of longitudinal or transverse relaxation rate per unit concentration of the contrast agent and is a direct measure of its “effectiveness”. The relative magnitudes of r1 and r2 determine if the agent is T1-weighted agent or T2-weighted in nature. Those that predominantly reduce T1 are said to be “positive”, whereas those that largely affect T2 are “negative” and locally reduce MRI signal intensity from background. In theory, anything which locally perturbs the relaxation of magnetically excited nuclei can be applied as a contrast agent. In practice, the very strong impact of a locally fluctuating paramagnetic or superparamagnetic field on the proton relaxation is used. The effectiveness of these agents scales directly with the paramagnetic field strength and, as such, focus has been dominated by the transition metals and lanthanides. Among the latter, applications have been dominantly associated with gadolinium due to its exceptionally large paramagnetic moment (seven unpaired electrons) and unique relaxation properties.73,74 The free cation is, however, highly toxic even at low doses (0.3–0.5 mmol kg−1)2. The ionic complexes Gd-DTPA (Magnevist®) and Gd-DOTA (Dotarem®) were the first contrast agents approved for clinical practice and have become the reference points against which new agents are compared. Analogous neutral complexes, Gd-DTPA-BMA (Omniscan®) and Gd-HPDO3A (Prohance®) are now also commonly used. The high paramagnetism of divalent manganese has attracted attention.75 Manganese-dipyridoxal diphosphate (Mn-DPDP) has, for example, been approved for liver imaging, with a ratio of median lethal dose (LD50) and a dosage of 540 mmol kg−1, higher than Gd-DTPA.
In addition to 1H MRI, there is increasing interest in the development of heteronuclear imaging using other magnetic nuclei, such as 13C, 31P or 19F. MRI using these nuclei potentially avoids the need for pre-scanning and removes localization ambiguity because the resonant characteristics of the imaging agent itself are assayed directly.76 13C and 31P nuclei are of comparatively low gyromagnetic ratio and are naturally present in biological tissue, reducing their potential impact as probes. The absence of detectable 19F background in tissues has, in comparison, meant that this nucleus has attracted a good deal of positive attention over the past couple of decades. In addition to its high gyromagnetic ratio (83% of that of 1H), fluorine containing probes are typically also “biocompatible” and chemically stable.76,77 Perfluorocarbons (PFCs), fluorinated aliphatic compounds, have dominated this area thus far, and exhibit MRI characteristics that are highly dependent on chemical structure.77 Of these, perfluorooctyl bromide (PFOB), a linear PFC, was one of the earliest applied 19F MRI tracers. The PFOB 19F NMR spectrum does, though, consist of eight resonances, one for each CFn moiety; its use for cell tracking is not ideal in terms of sensitivity and requires the use of a frequency selective MRI pulse sequence to limit chemical shift artifacts.77 Further improvement in MRI sensitivity is possible with perfluorinated ethers such as perfluoro-15-crown-5 ether, PCE. PCE is a chemically and biologically inert macrocycle with 20 equivalent fluorine nuclei having a single resonance. Perfluopolyether (PFPE), a linear polymer, has a simple 19F NMR spectrum with >40 equivalent fluorines, and has recently been applied to cell tracking and quantification in vivo in a mouse diabetes model.77
Molecular gadolinium complexes contrast agents
A number of interplaying factors affect the proton relaxation afforded by a paramagnetic complex. As such a great deal of research has been, and continues to be, invested in optimizing the structure and dynamics of these complexes to maximize relaxivity at any given incident magnetic field. Solomon and Bloembergen proposed the first model in the 1950s,78,79 where the origin of paramagnetic relaxation enhancement can be divided into inner-sphere and outer-sphere contributions. Inner-sphere relaxation is associated with the paramagnetic effects on directly bound water molecules and depends intrinsically on the complex structure (namely the number of bound water molecules, the mean Gd–H separation and the mean residence time of bound water). Outer-sphere relaxation is the contribution that paramagnetic field makes to the relaxation enhancement of solvent molecules in the second coordination sphere and bulk solvent.
Current contrast agent designs focus mainly on tuning inner-sphere parameters to obtain higher longitudinal relaxivity from the protons within the first coordination sphere. Eqn (1) indicates that, if water exchange at the Gd3+ center is fast enough (τM), the relaxation rate, 1/T, is dominated by the relaxation rate of coordinated water, 1/T1m (q is the number of bound solvent molecules, and Pm is the mole fraction of water coordinated to the metal center).73 According to the Solomon–Bloembergen–Morgan (S.B.M.) theory,78,79 T1m, within a dipole–dipole (DD) relaxation mechanism scheme, is defined by eqn (2) (γ is the nuclear gyromagnetic ratio, g is the electronic g factor, μB is the Bohr magneton, r is the “electron spin-solvent nuclear spin distance”, ωS and ωH are the electron and nuclear Larmor precession frequencies, respectively).73 This equation highlights the critical role correlation time τc (defined in eqn (3) where i = 1 or 2 for T1 and T2 respectively) has in obtaining high relaxivity. In general terms, a faster water exchange rate (τM), slower molecular tumbling speed (rotational correlation time, τR) and slow electronic relaxation time (Tie) all serve to enhance imaging efficacy.73
| (1) |
| (2) |
| (3) |
The main focus of recent MRI contrast agent development has been the synthetic modification of aminocarboxylate derivatives, such as diethylenetriaminepentaacetic acid, DTPA and 1,4,7,10-tetraazacyclododecane tetraacetic acid, DOTA (Fig. 1a, 1b), for clinical use, commonly with a view to optimizing one or more of the aforementioned relaxation parameters through ligand modification.74,80 For example, Merbach et al. have reported several studies of the factors affecting water exchange rate (τM) of DTPA derivatives81 and specifically highlighted the effects of steric crowding on τM (through dissociative exchange). Relaxation rate enhancements can be achieved, for example, by varying the number of carbon atoms in the ligand scaffold, though, significantly, this is achieved at the detriment of complex thermodynamic stability. This phenomenon of losing stability upon increasing water exchange is common for aminocarboxylate ligands and must be considered prior to any projected clinical use.74 Attempts to increase the water exchange rate associated with Gd-DOTA based complexes have also been reported and, again, much of this has been based on steric crowding effects at the metal centre.82
Fig. 1.
Structures of (a) diethylenetriaminepentaacetic acid (DTPA) and (b) 1,4,7,10-tetraazacyclododecane tetraacetic acid (DOTA); (c) (d) Examples of q = 2 DTPA based complexes (q: number of bound water molecules). The increase in q is made possible by removing one carboxylate arm of the parent DTPA.83,84
As indeed predicted by eqn (1), relaxivity has been shown to be highly dependent on the number of bound water molecules (q) and numerous examples of this effect have now been noted.74,80 Examples of q = 2 complexes have been produced, for example, by combining two Gd3+ complexes to create a central core (Fig. 1c, 1d).83,84 Significantly, as with the effects of ligand steric bulk, the effects of higher q need to be balanced with those of complex thermodynamic stability. The relaxivity values of these compounds are significant higher than that of the parent complex (by virtue of both the larger q value and higher molecular weight/rotational correlation time, τR).
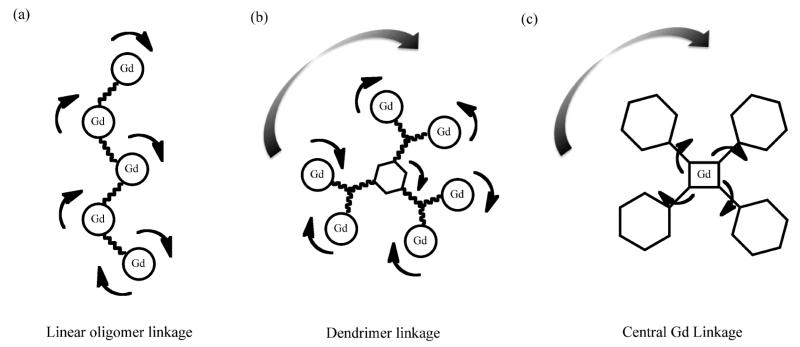
Much effort has been invested in utilizing the beneficial effects of increased rotational correlation time, τR, as afforded through oligomerisation or conjugation of paramagnetic complexes to macromolecules or nanoparticles.73,74,80 Interestingly, the linkage structure, in the sense of linker flexibility, plays a notable role85–88 (Fig. 2). Casali et al. have, for example, reported a method to link multiple gadolinium complexes with linear polymers.85 They have, specifically, reported a 52 kDa Gd-DOTA chelate modified dextran polymer with high relaxivity. Linking paramagnetic complexes in a linear fashion generates an oligomer with anisotropic rotation where rotation about the short axis of the molecule is fast and limits enhancement (Fig. 2a).85 Multiple branched dendrimers have also been used to link gadolinium complexes (Fig. 2b), where the contrasting effects of slow isotropic rotation (beneficial) and internal structural flexibility (disadvantageous) have been specifically noted.86 Another approach to optimizing the effects of motion on relaxivity has been to place the gadolinium at the center of a large molecule (Fig. 2c),87 where the lack of internal motion can result in remarkably high relaxivity. Toth et al. have proposed a related approach based on bridging multiple gadolinium complexes through (structurally rigid) ferrous iron bound bipyridyl analogues88 and have observed relaxivities remarkably better than observed with the monomeric analogues. From these select examples, it is clear not only that there exists numerous means of tuning contrast agent effectiveness (most notably through increasing τM, and decreasing τR whilst maximizing q and maintaining molecular rigidity) but also that there remains appreciable room for further improvement.
Fig. 2.
Schematic representations of Gd chelate linkage and adduct rotation/conformational fluctuation. (a) linear oligomer linkage with anisotropic rotation where rotation about the short axis of the molecule is fast and limiting in terms of relaxivity enhancement (r1 = 10.6 mM−1s−1);85 (b) dendrimer linkage with both isotropic rotation and internal motion around the core; the latter by virtue of the linkage flexibility and, again, limiting in terms of MR contrast (r1 = 16.5mM−1s−1);86 (c) Centralised Gd linkage where structure rigidification results in a remarkably high relaxivity (r1 = 39.0mM−1s−1).87
Gadolinium chelate bioconjugation
In coupling an MRI contrast agent to a biomolecule, one may be able to effectively marry the advantages afforded by increased molecular bulk with those engendered by natural biological targeting. With this in mind a range of DOTA and DTPA derivatives have been bound to peptides, monoclonal antibodies, nucleic acids or glycoproteins capable of targeting specific cell receptors.89–100 Asialoglycoprotein, a protein having high affinity for ASG receptors (specifically existing in hepatocytes), has been used for liver-specific T1 imaging,95 for example. Human amyloid-β (Aβ) peptide that is capable of selectively targeting individual amyloid plaques in the brain of Alzheimer’s disease transgenic mice. The Gd-DTPA derivative of this peptide have been successfully synthesized and enabled the in vivo MR imaging of individual amyloid plaques in the brains of Alzheimer’s disease animals or patients to enable both early diagnosis and the provision of a direct measure of the efficacy of anti-amyloid therapies being developed.98
Breast cancer cells expressing Her-2/neu receptors have been imaged in vivo with avidin-Gd-DTPA conjugates96 and antisense peptide nucleic acids (PNAs) have been DOTA tagged in generating targeted intracellular MRI contrast agents.97 The relaxivity enhancements of bioconjugation (typically 3-5 fold) have been noted in several of these studies.93,97,99 Anti-ICAM-1 antibody has also been coupled with Gd-DTPA and the specific binding of the derived complex to ICAM-1-expressing endothelial cells examined.99 A tumor angiogenesis-targeting T1 contrast agent has also been reported by the bioconjugation of Gd-DTPA with an anti-vascular endothelial growth factor receptor-2 (VEGFR2) antibody.100 A significant amount of research has been performed using Gd-DTPA derivatives of human or bovine serum albumin (HSA or BSA).101,102 Because of the long intravascular retention of the macromolecule, Gd-DTPA-BSA/HSA has been used as a blood pool agent within MR angiography. Lauffer et al. have, for example, reported a protocol for the reaction of DTPA-dianhydride with a variety of proteins.101 Again, noteworthy here is the three-fold enhancement in r1 observed with albumin-(Gd-DTPA)19 compared to the monomeric chelate.102
Antibodies have good target specificity, but their larger size limits kinetics (of both target vectoring and clearance). Though short peptides have high levels of diffusional mobility, few have been reported to have high targeting affinity.103 Antibody fragments have accordingly attracted significant interest from an in vivo imaging perspective.104,105 Affibodies represent a new class of affinity proteins based on a 58-amino acid residue protein domain, derived from one of the IgG-binding domains of staphylococcal protein.106 Being an order of magnitude smaller than antibodies a much improved penetration of solid tumors is possible. For example, a Her-2 receptor-specific affibody N-terminally modified with DOTA has been synthesized107 and applied to the in vivo 111In, 68Ga or 90Y/177Lu imaging of Her-2 expressing carcinomas.108–110
In the more general sense, the bioconjugation of contrast agents empowers the specific illumination of sites expressing cancer markers, something which has led to important advances in cancer diagnosis and treatment.111 Endocrine therapy for breast cancer is, for example, benefitting from our increasing ability to target contrast agents to estrogen receptors112 and the Her-2 receptor is playing a rapidly increasing role in tumor-specific treatment.113 These specifically vectored imaging agents enable analyses at, very often, markedly lower dose than would be required in nonspecific imaging. There do, though, remain very significant challenges. The quality of receptor-specific imaging, for example, is a very sensitive function of both the imaging modality applied and the specificity and affinity of utilized biotargeting. When binding affinities are high, one may find that even small quantities of molecular contrast agents saturate receptors,114 potentially capping signal:noise (and thus acquired diagnostic information) at levels which cannot be improved with dose. For this reason, current reports of tumor receptor molecular imaging are largely limited to (highly sensitive) PET and SPECT unless receptors are large.115 There is, then, much ground for improvement.
Multimodal molecular contrast agents
The simplest means of enabling multimodality whilst maintaining facile diffusion and tissue penetration is through molecular synthesis. The principal disadvantages of this approach (even ignoring potential synthetic demands) are the maintenance of high aqueous solubility and sufficiently long circulation times. Several constructs have been proposed to build up multimodality through the direct conjugation of metal complexes to fluorophores.92,116 Meade et al., for example, have reported a bifunctional MRI/optical contrast agent by coupling Gd-DOTA to rhodamine in a 1 : 1 ratio.116 Gd-DO3A has also been linked to fluorescein through an ethylthiourea linker.92 Very similar synthetic strategies have been applied to fuse radio and optical emission within one molecule, commonly through amide coupling of DOTA or DTPA derivatives to reactive carbocyanine dyes, with subsequent 64Cu (PET) or 177Lu (SPECT) coordination.117–120 Additional peptide conjugation can facilitate multimodal receptor specific imaging.117 A simpler method to introduce a radioisotope to a fluorophore is to iodinate the fluorophore directly using radioactive iodine.121 This approach has been employed, for example, to iodinate the photosensitizer HPPH (3-(1′-m-hexyloxyethyl)-3-devinylpyropheophorbide-R) using Iodogen beads and Na124I for potential fluorescence and PET imaging as well as photodynamic therapy.121
Nanoparticle based MRI contrast agents
Nanoparticles are potentially powerful imaging probes by virtue of their highly tunable characteristics and large surface area:volume. The latter, especially, can enable a large amount of functionality to be confined to a relatively small volume. Coupled with the comparative ease with which surface bioconjugation can be achieved, the inherently low rotational correlation time, and the possibility of multiple core-shells and one arrives at an irresistible and almost infinitely variable resonant contrast vehicle. Broadly speaking, nanoparticle based MRI agents can be categorized into those based on superparamagnetic iron oxide T2 contrast agents, gadolinium ion based T1 contrast agents and other inorganic nanoparticle T1 contrast agents.
Superparamagnetic iron oxide T2 contrast agents
Superparamagnetic iron oxide (SPIO) nanoparticles are the most widely used T2 MRI contrast agents. The relaxation induced by these can be explained by classical outer-sphere relaxation theory in considering the relaxation rates of water protons diffusing within the particle magnetization field.6,122 These agents typically exhibit strong T1 relaxation characteristics, and, by virtue of their strong and varying magnetic fields due to variable particle sizes, also produce strong T2 effects. An important consequence of outer-sphere theory is that the r2/r1 ratio increases with increasing particle size; SPIO nanoparticles were initially developed as T2 agents, producing dark (negative) contrast within images. A new generation of ultra small superparamagnetic iron oxide nanoparticles, with sizes less than 10 nm, has also been reported to have excellent r1-enhancing properties.123
Colloidal stability and biocompatibility are crucial requirements for clinical application. Generally, iron oxide nanoparticles are composed of a core of one or more magnetic crystallites embedded in a stabilizing coating, of, for example, dextran, citrate or PEG.7,8 Subsequent replacement of the organic coating with alternative surface cappings, such as those based on silanes, enables a chemically flexible/tunable surface modification. The most widely used and traditional method of preparing iron oxide nanoparticles is by the reduction and co-precipitation of a mixture of ferrous and ferric salts in aqueous media, in the presence of stabilizers such as hydrophilic polymers.124 This process has been commercially adapted in the generation of a number of MRI contrast agents based on multiple iron oxide cores encapsulated within a dextran coating, such as Feridex®, Resovist®, and Combidex®. Particles such as these are selectively up taken by Kupffer cells in the liver, spleen and bone marrow,125 a process which enables, for example, differentiation (through image contrast) between normal and abnormal liver tissue.
Generally, smaller nanoparticles have a longer plasma circulation time.126 Particles with a diameter of less than 300 nm specifically remain intravascular for a prolonged period of time and thus can serve as blood pool agents.126 Coating particles with hydrophilic PEG or dextran films has been proved to reduce phagocytic capture, leading to extended circulation and subsequent accumulation in the targeted sites.127 Weissleder et al., for example, have demonstrated the detection of lymph-node metastases in prostate cancer with dextran coated SPIO nanoparticles.128
Gadolinium ion based T1 contrast agents
The predominant T1 contrast agents are, by some margin, those based on chelated forms of gadolinium. Though there are examples of kinetically stable complexes with appended luminescent or radioimaging tags, these are limited in both range, signal intensity and by rapid renal excretion.129 In view of considerable potential, spatial resolution, ubiquitous scanners and intrinsic low signal : noise Gd agents, there is a great deal of interest in the development of improved, and particularly, targeted and non-toxic forms. In recent years, a wide range of nanoparticles (dendrimers, inorganic nanoparticles, carbon nanotubes etc), with Gd chelate conjugation have been reported with this in mind.4,5,27,28
Contrast agent doped lipid based vesicles are well established26 and present a means of incorporating a variety of agents into the internal aqueous phase or directly linked to the lipid (or indeed both). They are typically prepared by mechanical dispersion of the constituent lipids in solution and generally lie in the 80–300 nm size range.130 Through appropriate design, hybrid particles can be generated which facilitate combined dual mode imaging e.g. CT and MRI14 or MRI and optical15 or MRI and SPECT16 etc. The potential issues associated with enabling facile water exchange at the incorporated paramagnetic center have been noted and can potentially be solved by confining these centers to the outer region (lipid) of the particle. The tissue and cell penetration of these particles has been intensely studied from a gene delivery perspective.130
Dendrimers are highly branched spherical polymers of various chemical composition and structure, with those based on polyamidoamine (PAMAM) and diaminobutane (DAB) perhaps the most common.12 These particles are water soluble, size tunable and can be used as scaffolds for a potentially large number of Gd binding chelators such as derivatives of DTPA and DOTA (appropriately modified G3 PAMAM dendrimers can bind 32Gd ions per 5 nm particle; larger 15 nm G10 analogues can bind 4000 Gd centers, for example).12 Nano-sized dendrimers are generally retained in the body long enough to be used as active targeting MRI contrast agents after conjugating with vectoring materials. Wu et al. have, for example, synthesized antibody-labeled dendrimers, PAMAMAm-G2-DOTA and PAMAM-Am-G2-CHXB, and labeled these with 90Y, 111In, and Gd3+, (without loss of immunoreactivity), as potential tools for either radiotherapy or MRI.131 Wiener et al. have reported folate-conjugated dendrimer polychelates by attaching folic acid to a fourth-generation ammonia-core polyamidoamine dendrimer and observed accumulation in tumors expressing the high-affinity folate receptor (enabling targeted imaging).13
Metal–organic frameworks (MOFs) are hybrid materials consisting of metal ions bound within the well-defined coordination geometry provided by organic linkers.18,19 Reiter et al. synthesized nano-scale metal–organic nanorods with compositions of Gd(BDC)1.5(H2O)2 (where BDC is 1,4-benzanedicarboxylate) with Gd3+ coordinated in organic frameworks. The r1 MR relaxivity obtained with the suspension of 100 nm nanorods was at least an order higher than that of comparable Gd containing liposome contrast agents. The same group have also utilized (less toxic) paramagnetic manganese within these constructs while retaining good levels of in vivo r1 MR relaxivity.132
Carbon nanotubes have also been proposed as scaffolds for Gd binding. Wilson et al. for example, have loaded aqueous GdCl3 in single-walled carbon nanotubes and have measured relaxivities at some 40 times higher than observed with commercial Gd-DTPA.133 Richard et al. have, additionally, reported the non-covalent modification of carbon nanotubes with amphiphilic gadolinium chelates and, again, measured high r1 nuclear relaxivities.134
Uniform colloidal silica nanoparticles have been utilized as templates for the assembly of a number of functionalities, primarily through well-established alkoxysilane hydrolysis-condensation surface chemistry. Rieter et al., for example, have used non-porous silica nanoparticles to support Gd-DTTA or Gd-DTPA complexes and obtained one order higher r1 relaxivity than acquired from small Gd complexes.9 Besides work with non-porous silica nanoparticles reported to date, mesoporous silica nanoparticles (MSNs, Fig. 3a) have attracted a great deal of interest from a multimodal imaging perspective due to their extraordinarily high surface area. Several groups have proposed synthetic strategies enabling the incorporation of imaging functionality into these frameworks (Fig. 3b and 3c).10,11 Kim and Rieter et al. have, for example, reported the development of mesoporous silica based T1 contrast agents derived from grafted Gd-DTTA (Fig. 3b).10 Mou et al. have also directly synthesized mesoporous silica nanoparticles by the co-condensation of Gd-DTPA complexes with tetraethyl orthosilicate (TEOS) (Fig. 3c).11 With all of these thus far, r1 relaxivity is observed to be at least one order higher than that acquired with the corresponding free small molecular complexes.10,11
Fig. 3.
(a) TEM image of mesoporous silica nanoparticles; Schematic representations depicting the Gd-DTTA grafting of mesoporous silica nanoparticles (b) and Gd-DTPA modification of mesoporous silica nanorods (c). With all of these constructs, r1 relaxivity is observed to be at least one order higher than that acquired with the corresponding free small molecular complexes.10,11
Other inorganic nanoparticle T1 contrast agents
There exist a number reports on the application of nanoparticles based on transition metal or lanthanide metal oxides as T1 contrast agents. These have included, thus far, nano-sized gadolinium oxide (Gd2O3)135–137, gadolinium fluoride (GdF3),138 gadolinium phosphate (GdPO4),139 and manganese oxide (MnO).106,107 McDonald and Watkin have successfully synthesized dextran-stabilized 30 nm gadolinium oxide nanoparticles by reduction and co-precipitation reaction of GdCl3,135 though these had limited dispersion and thus lacked application without additional surface modification. Fortin et al. have reported that the reaction of Gd(NO3)·6H2O in diethylene glycol through polyol process can fabricate ultrasmall (3 nm) gadolinium oxide particles.103 The relaxivities of subsequently PEG-silane coated derivatives was reported to be twice as high as that of Gd-DTPA.103
Nanoparticles consisting of GdF3 or mixed GdF3/LaF3 have been proposed as a new class of water-soluble paramagnetic MR contrast agent. Prosser et al. have, for example, synthesized GdF3 or an 80/20 mixture of GdF3 and LaF3138 and subsequently tuned water solubility through surface modification. Dextran coated GdPO4 nanoparticles, synthesized hydrothermally, have also been developed as potential high contrast MRI agents.139
Water-dispersible and biocompatible MnO nanoparticles, synthesized by thermal decomposition of Mn-oleate complexes, have been put forward as effective T1 agents and applied to appropriately weighted brain imaging.140 These particles have also been conjugated to tumor targeting antibodies, Her2/neu, and successfully targeted to the epidermal growth factor receptors on the surface of the breast cancer cells. Hollow manganese oxide nanoparticles, prepared by an acidic etching process, have been reported as T1 MRI agents by Lee et al.141 The hollow interior of these is potentially also utilizable from a drug delivery perspective, and, to this end, the uptake and release of the anticancer drug, doxorubicin, has been demonstrated.141
Nanoparticles in multimodal MRI imaging
As mentioned in the introduction, the concept of combining imaging modes within a single administered contrast agent, and thereby achieving both high sensitivity and high spatial resolution, is a seductive and rapidly evolving one. MRI enables the high spatial resolution acquisition of physiological and anatomical images and can be powerfully combined with optical methods to provide cellular/sub-cellular information and rapid screening.10,142,143 Trimodal imaging probes have also been reported where, for example, 111In or 64Cu PET isotopes have been integrated.24 Within the highly engineerable platform presented by nanoparticles, there lie a number of distinct classes.
SPIO based multimodal imaging agents
When designing a nanoparticle based multimodal MRI imaging material, the first concern is how to incorporate each modality together without dramatically changing or reducing the functionality of either. There are various models that have been proposed to construct multimodal nanoparticles. One of these is the core-shell approach where multifunctionality is built up layer-by-layer in a synthesized particle.20,23,24,142–144 Iron oxide cores have notably been a focus in many such constructs, where the shell is a biodegradable polymer or relatively inert silica. Hyeon et al. have proposed, for example, a multifunctional polymer platform for simultaneous cancer-targeted magnetic resonance or optical imaging and magnetically guided drug delivery.142 To achieve this iron oxide nanoparticles, CdSe/ZnS quantum dots, and the anticancer drug doxorubicin were all encapsulated in biodegradable poly(d,l-lactic-co-glycolic acid) (PLGA) nanoparticles (Fig. 4a, 4b). In this work, a folate based targeting agent (poly(l-lysine)-poly(ethylene glycol)-folate) was subsequently attached to particle surfaces and T2-weighted MR imaging at 3T carried out with KB cancer cells (Fig. 4c). Hyeon et al. have also reported the synthesis of fluorescein isothiocyanate (FITC) embedded mesoporous silica nanoparticles with a single iron oxide nanoparticle core.20 Cetyltrimethylammonium bromide (CTAB) was used, not only as the stabilizer for transferring hydrophobic iron oxide to the aqueous phase, but also as the template for forming mesoporous structure and fluorescent modality (FITC, introduced via a co-condensation reaction to produce a highly luminescent porous T2 nanoparticle agent). Zhang et al. have developed iron oxide based MRI contrast agent with a similar structural concept (Fig. 5a, 5b) but with NIR emitting Nd3+ and Yb3+ chelates.143 In work by Nel et al.144 20 nm superparamagnetic iron oxide nanoparticles have been incorporated into mesoporous silica shells, the latter also luminescent by virtue of a co-condensation with a FITC silane. Surface modification with folic acid engendered the targeting of the α-folate receptor in human cancer cells line PANC-1 with particles also subsequently capable of being loaded with significant levels of the cancer drugs camptothscin or paclitaxel. Fig. 5c and 5d show the increased uptake of folate-modified nanoparticles in PANC-1 (overexpressed folate receptor). Folica acid modification can selectively increase the delivery of drugs to the cells with overexpressed folate receptor and the luminescence enables to monitor the drug treatment.
Fig. 4.
Schematic structure (a) and TEM image (b) of PLGA polymer/iron oxide hybrid nanoparticles with encapsulated 15 nm Fe3O4 nanocrystals; (c) In vitro T2-weighted MRI images of KB cancer cells. From left to right: untreated cells; cells treated with plain nanoparticles; cells treated with PEGylated nanoparticles; cells treated with targeting nanoparticles; cells treated with targeting nanoparticles under an uptake-promoting external magnetic field142 (maximum contrast).
Fig. 5.
Schematic representation (a) and TEM image (b) of multimodal mesoporous silica nanoparticles with iron oxide cores and additional NIR fluorescent modification;143 fluorescence microscopy images of PANC-1 cells (overexpressing folate receptors) treated with (c) unmodified nanoparticles and (d) folate-modified nanoparticles. Increased uptake of the folate-modified NPs was observed with the PANC-1 cells. Green: nanoparticles; Blue: cell nuclei stained with 4′-6-diamidino-2-phenylindole (DAPI); Red: cell membranes stained with wheat germ agglutinin (WGA).144
In addition to the burying of superparamagnetic cores within a modifiable silica matrix, the subsequent surface functionalization of synthesized iron oxide nanoparticles presents a popular (and potentially simpler) method of introducing additional modality.145–147 Kircher et al. have, for example, reported the decoration of aminated iron oxide nanoparticles with the near-IR fluorescence dye, Cy5.5.145 On intravenous injection of these into brain tumor bearing rats, T2 weighted MRI imaging at 4.7 T indicated particle accumulation within the tumor. Bao et al. have also developed MRI/PET dual modal contrast agent with aminated iron oxide nanoparticles (Fig. 6a).146 Dual functional PEG derivatives have been used to aminate iron oxide nanoparticles and DOTA-NHS esters then subsequently conjugated to enable Cu2+chelation. These particles, stable in serum for 24 h, produced strong MR (Fig. 6b) and PET (Fig. 6c) signals.
Fig. 6.
Schematic structure (a) of dual modal 64Cu-modified iron oxide nanoparticles; (b) T2-weighted MR image of 25 μg mL−1 (top) and 10 μg mL−1 (bottom) 64Cu-labeled magnetic nanoparticles (the scale bar corresponding to particle concentration). (c) Decay-corrected microPET image of 25 μg mL−1 (top) and 10 μg mL−1 (bottom) 64Cu-labeled magnetic nanoparticles (the scale bar corresponds to 64Cu concentration).146
One can also directly grow a luminescent shell around a generated iron oxide core. Shells of CdSe148,149 and Eu : Gd2O3150 have, for example, been generated to enable effective MRI and optical imaging, made more effective still through subsequent surface silanisation and biofunctionalisation.149
Other core-shell dual-mode probes have been developed for MRI-SERS and MRI-PET. Lee et al. have, for example, reported multifunctional silver embedded magnetic nanoparticles as surface enhanced Raman spectroscopy (SERS) probes23 by coating iron oxide nanoparticles with silica shells and then attaching silver nanoparticles, Raman probes and antibodies. Subsequent Raman analyses enabled a differentiation of cancerous cells from normal cells (although this study did not utilize the magnetic particle cores, an extrapolation of this selective cell uptake and Raman signature to an additional MRI modality is obvious). Numerous probes with MRI and PET functionality have been reported. In work by Patel et al.24 superparamagnetic iron oxide cores have been encapsulated within a porous silica shell additionally impregnated with ligands capable of chelating the positron-emitting metal, Cu2+ making the particles potential PET agents. Subsequent MRI assessments highlighted highly core dependent r2 relaxivities comparable to Feridex®. In addition to the 64Cu surface modification of generated iron oxide nanoparticles, labeling has also been carried out with 124I.151 For example, Cheon et al. have labeled serum albumin modified iron oxide nanoparticles with 124I by iodinating the ortho position of tyrosine residues on the protein.151 The resulting 32 nm nanoparticles were reported to enable highly effective MRI and PET imaging. Gold shells have also been introduced around iron or iron oxide cores as a means of introducing a CT modality (and a means of additional further surface functionalisation).152,153
Several structures other than those which are core-shell have also been utilized to engender multimodality.21,22,114 Hyeon et al. have fabricated core-satellite structural hybrid nanoparticles (Fig. 7a, 7b)21,22 with a core material of either dye-doped mesoporous silica or solid silica and satellite material comprising externally appended nanometre scale superparamagnetic iron oxide, quantum dots or gold clusters, enabling MR, luminescent or plasmon resonant characteristics. Mou et al. have also proposed nanoparticles (Fig. 7c, 7d)154 based on luminescent and magnetic mesoporous hybrid silica nanoparticles, where Fe3O4@silica nanoparticles and mesoporous silica nanoparticles are attached, as potential T2 agents with high relaxivity at 0.47T.
Fig. 7.
Schematic structure (a) and TEM micrograph (b) of hybrid silica nanoparticles with iron oxide, gold and quantum dot (QD)21 satellites; (c) (d) schematic and electron microscopy depictions of hybrid nanoparticles comprising of a Fe3O4@silica body with a fluorescent mesoporous silica nanoparticle appendage.154
Gadolinium based multimodal nanoparticle imaging agents
The paramagnetic and electronic characteristics of gadolinium remain unsurpassed and, providing kinetic stability is both high and reliably assessed, there remains a good deal of development and application possible with this metal through its incorporation into designed nanostructures. Silica nanoparticles present one of the most ideal platforms by virtue of their chemical, physiological, optical and tailorable multimodal characteristics. A number of research teams have, accordingly, reported the incorporation of Gd chelates onto silica particles which are, additionally, luminescent (Fig. 8a, 8b).9,10,17 This dual mode probe allows the co-confirmation of obtained information between MRI and optical imaging and the luminescent modality makes the optical guide of surgery possible. In most cases the determined T1 relaxivity is 5–10 times higher than that observed with the free chelate, an observation generally assigned to the reduced tumbling rate of the paramagnetic centre. Significantly, from the perspective of maximizing signal, it has been observed that relaxivities are limited by water access, most clearly when the paramagnetic payload is present in multilayers.9 Conversely, in related work, Gd-DOTA complexes have been deposited on Gd-DTTA modified silica nanoparticles via electrostatic interactions between the complexes17 (Fig. 8c, 8d). As the number of layers increased, the authors here report a proportional increase in r1 relaxivity on a per particle basis, suggesting significant water access through the hydrophilic multilayers. Mesoporous silica presents a means of greatly increasing Gd loading in a closely related platform. Lin and Reiter et al. have, for example, prepared 75 nm dye-doped mesoporous silica nanoparticles and subsequently post-functionalized the particles with Gd-DTTA.10 Significantly, the observations within this work are consistent with facile water access to the particle interior (enabling the considerable internal surface area to be utilized in a T1 manner). The same group have recently reported the intravenous injection of these particles into mice and subsequent 9.4 T T1 weighted imaging.10 Nanoparticles of this type are also associated with effective cell uptake (Fig. 9a). Santra et al. have reported a conceptually simple extension of this to a trimodal format with Ru(bpy) dye doped silica particles with paramagnetic gadolinium complexes on the particle surface.155 The presence of ruthenium enables additional application of the particles as CT contrast agents. Mou et al. have synthesized fluorescent mesoporous silica nanorods (aspect ratio 4) with Gd-DTPA co-condensed within the pore structure11 and shown that these similarly enjoy r1 relaxivities approximately an order of magnitude greater than the free complex. The group has additionally demonstrated effective particle uptake by 3T3-L1 mouse fibroblast cells and their subcellular luminescent imaging.
Fig. 8.
Schematic structure (a) and TEM image (b) of Gd complex modified fluorescent silica nanoparticles9 and (c) (d) multilayered fluorescent silica nanoparticles. As the number of layers increases, a proportional increase in r1 relaxivity on a per particle basis is observed, suggestive of significant water access through the hydrophilic multilayers.17
Fig. 9.
(a) Merged confocal fluorescent image of Hela cells treated with rhodamine modified mesoporous silica nanoparticles co-localized with various cell stains (Red: nanoparticles; Blue: nucleus; Green: lysosomes); (b) MRI and (c) CT images of mice abdomens prior to and 24 h post injection of the trimodal nanoparticles (Au nanoparticles coated with Gd and cy5.5 labeled lipids). The T1-weighted images of livers showed a 24% intensity enhancement post nanoparticle injection, while CT images demonstrated a 50% increase in X-ray attenuation;25 (d) fluorescence and MRI images of particle (Gd and cy5.5 labeled dendrimer nanoparticle) injected mouse taken from the back (arrow: injected site, mammary fat pad on the front of the mouse; arrow head: sentinel lymph node). Left: Optical image obtained with excitation light (615–665 nm) and detected with the emission filter set to 720 nm. Middle: image from left shown in false color showing particle distribution. Right: MRI image at 3T.167
Optical-MRI-CT trimodal imaging agents can be configured from an Au core based paramagnetic silica particle. Mulder et al. have, for example, fabricated such particles with r1 relaxivity of 14.0 mM−1s−125 and carried out T1 weighted liver imaging and CT imaging in mice (Fig. 9b, 9c).25 The MR liver imaging showed a 24% intensity enhancement compared to the value of the image prior to particle injected, while CT images demonstrating a striking 50% increase in X-ray attenuation. A number of other gold nanoparticle based constructs have also been proposed.156,157 For example, Gd enriched DNA-Au conjugates have been reported to enable highly efficient cell penetration and accumulation that provides an MRI contrast enhancement sufficient to enable the imaging small cell populations.156 The additional modification of these particles with organic fluorophores facilitates an optical determination of cell uptake and intracellular accumulation as well as a means of histological validation. Kim et al. have also synthesized small gold nanoparticles with a gadolinium chelate modification.157 The ability to incorporate several thousand chelated on top the surface of each particle reportedly enables both MRI imaging with very high r1 relaxivity and effective X-ray attenuation.
As mentioned previously, quantum dots have been exploited as optical indicators because of their narrow and tunable emission spectrum.72,158 Several groups have developed multifunctional nanoparticle probes based on fluorescent quantum dots. A common approach is to attach PET or MRI enabling chelates to the surface of aminated QDs. In work of Jin et al., for example, CdSeTe/CdS QDs were first surface coated with glutathione then coupled to Gd-DOTA units through standard succinimide chemistry to produce particles of high r1 relaxivity.159 In other work by Gerion et al., 10 nm CdSe/ZnS QDs were coated with a 1–2 nm thick PEGylated silica shell prior to Gd-DOTA decoration.160 The generation and application of paramagnetic quantum dots with paramagnetic PEGylated lipid coating has also been reported70 and Louie et al. have developed a series of core/shell CdSe/Zn1-xMnxS nanoparticles synthesized for use in dual-mode optical and MRI techniques.71 These particles exhibit quantum yields reaching 60% and relaxivity (r1) values in the range of 11–18 mM−1s−1.
Liposomes represent another common platform for supporting multimodality. One of the simplest approaches has been the encapsulation of more than one type of contrast agent into the aqueous liposome phase by inclusion in solution during liposome formation. This approach facilitates flexible multimodality without the requirement of multiple synthetic steps. One example, of many such approaches has been the incorporation of the CT contrast agent, iodohexol, with MRI contrast agent, gadoteridol, into unilamellar bilayer liposomes.14,161 This marriage facilitates image-guided radiotherapy where CT is used to perform radiation dose mapping and MRI contrast to identify target soft tissues. Such contrast agent encapsulation also increases agent half-life (that is they remain in circulation longer than the individual molecular probes would have). Multimodality can additionally be generated within the liposomal membrane. For example, gadolinium chelates for MRI162 and iodinated contrast agents for CT163 have both been conjugated to lipid head groups prior to liposome formation. Besides MRI/CT, a number of groups have reported the synthesis of liposomes containing a rhodamine phosphotidylethanolamine derivative and a Gd-lipid for dual-modal MRI and fluorescence imaging.164–166
Dendrimers have been fairly exhaustively investigated as potential multimodal imaging probes because their structure presents a potentially large number of modifiable groups. The representative water-soluble and biocompatible polyamidoamine (PAMAM) dendrimers have been utilised in this way.167 Talanov et al. have, for example, conjugated gadolinium chelates and NIR emissive Cy5.5 with the 256 surface amino groups presented by a G6 PAMAM dendrimer.167 These particles were subsequently injected into the mammary fat pad of mice and lymph node imaging carried out by both T1 weighted MRI and NIR imaging (Fig. 9d). The high paramagnetism of gadolinium oxide has also been utilized in the generation of Gd2O3 nanoparticle T1 agents.135–137 On encapsulating the same particles within a luminescent polysiloxane shell, one arrives at an effective size tunable bimodal agent.136 Interestingly, this study also highlighted not only the potential water accessibility of internalized Gd centers but also the chemical tunability of subsequent nanoparticle biodistribution in mice.
Conclusion and future challenges
The potential benefits of combining medical imaging modes are clear and there has been a surge in both the development of contrast agents and in associated experimental imaging methods. The vast explosion of literature in this area during the last five years does shed light on the intriguing and equally vast possibilities that remain but also continues to generate (often) as many questions as answers. Though a number of powerful platforms have been reported, none have yet progressed from the research laboratory to the clinical coalface. Nanoparticles render a range of highly tailorable properties including size, shape, surface chemistry, charge, biodistribution, emission etc., and can potentially provide a comprehensive mapping of disease status across multiple analytical platforms. Issues of biocompatibility, toxicity, in vivo targeting efficacy, and long-term stability remain, however, to be addressed. The highly beneficial collaborative effort between chemists, biologists, engineers, microscopists and clinicians is a clear and powerful one but it remains important that tools are not developed solely for the sake of being able to report the development of tools; though the concept of multimodality has driven innovation in not only contrast agent synthesis (most markedly with nanoparticles), but also in instrumental design, too often reports of new agents precede any consideration of practical application. The clinically relevant capabilities and potential toxicities of new materials remain paramount and this is where much of the next phase of development needs to be focused. This mini-review has sought to briefly summarize some of the key developments thus far.
Acknowledgements
Much of the cellular imaging reported herein was carried out in the Nikon Oxford Molecular Imaging Centre (NOMIC).
References
- 1.Massoud TF, Gambhir SS. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 2.Oksendal AN, Hals PA. J. Magn. Reson. Imaging. 1993;3:157–165. doi: 10.1002/jmri.1880030128. [DOI] [PubMed] [Google Scholar]
- 3.Yang JJ, Yang JH, Wei LX, Zurkiya O, Yang W, Li SY, Zou J, Zhou YB, Maniccia ALW, Mao H, Zhao FQ, Malchow R, Zhao SM, Johnson J, Hu XP, Krogstad E, Liu ZR. J. Am. Chem. Soc. 2008;130:9260–9267. doi: 10.1021/ja800736h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louie A. Chem. Rev. 2010;110:3146–3195. doi: 10.1021/cr9003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Piao Y, Hyeon T. Chem. Soc. Rev. 2009;38:372–390. doi: 10.1039/b709883a. [DOI] [PubMed] [Google Scholar]
- 6.Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN. Chem. Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 7.LaConte L, Nitin N, Bao G. Nano Today. 2005:32–38. [Google Scholar]
- 8.Wang YXJ, Hussain SM, Krestin GP. Eur. Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 9.Rieter WJ, Kim JS, Taylor KML, An HY, Lin WL, Tarrant T, Lin WB. Angew. Chem., Int. Ed. 2007;46:3680–3682. doi: 10.1002/anie.200604738. [DOI] [PubMed] [Google Scholar]
- 10.Taylor KML, Kim JS, Rieter WJ, An H, Lin WL, Lin WB. J. Am. Chem. Soc. 2008;130:2154–2155. doi: 10.1021/ja710193c. [DOI] [PubMed] [Google Scholar]
- 11.Tsai CP, Hung Y, Chou YH, Huang DM, Hsiao JK, Chang C, Chen YC, Mou CY. Small. 2008;4:186–191. doi: 10.1002/smll.200700457. [DOI] [PubMed] [Google Scholar]
- 12.Longmire M, Choyke PL, Kobayashi H. Curr. Top. Med. Chem. 2008;8:1180–1186. doi: 10.2174/156802608785849021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konda SD, Aref M, Brechbiel M, Wiener EC. Invest. Radiol. 2000;35:50–57. doi: 10.1097/00004424-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Zheng JZ, Liu JB, Dunne M, Jaffray DA, Allen C. Pharm. Res. 2007;24:1193–1201. doi: 10.1007/s11095-006-9220-1. [DOI] [PubMed] [Google Scholar]
- 15.Mikhaylova M, Stasinopoulos I, Kato Y, Artemov D, Bhujwalla ZM. Cancer Gene Therapy. 2009;16:217–226. doi: 10.1038/cgt.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zielhuis SW, Seppenwoolde JH, Mateus VAP, Bakker CJG, Krijger GC, Storm G, Zonnenberg BA, van het Schip AD, Koning GA, Nijsen JFW. Cancer Biother. Radiopharm. 2006;21:520–527. doi: 10.1089/cbr.2006.21.520. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Rieter WJ, Taylor KML, An H, Lin WL, Lin WB. J. Am. Chem. Soc. 2007;129:8962–8963. doi: 10.1021/ja073062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieter WJ, Taylor KML, Lin WB. J. Am. Chem. Soc. 2007;129:9852–9853. doi: 10.1021/ja073506r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieter WJ, Taylor KML, An HY, Lin WL, Lin WB. J. Am. Chem. Soc. 2006;128:9024–9025. doi: 10.1021/ja0627444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, Song IC, Moon WK, Hyeon T. Angew. Chem., Int. Ed. 2008;47:8438–8441. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Lee JE, Lee J, Jang Y, Kim SW, An K, Yu HH, Hyeon T. Angew. Chem., Int. Ed. 2006;45:4789–4793. doi: 10.1002/anie.200504107. [DOI] [PubMed] [Google Scholar]
- 22.Lee JE, Lee N, Kim H, Kim J, Choi SH, Kim JH, Kim T, Song IC, Park SP, Moon WK, Hyeon T. J. Am. Chem. Soc. 2010;132:552–557. doi: 10.1021/ja905793q. [DOI] [PubMed] [Google Scholar]
- 23.Jun BH, Noh MS, Kim J, Kim G, Kang H, Kim MS, Seo YT, Baek J, Kim JH, Park J, Kim S, Kim YK, Hyeon T, Cho MH, Jeong DH, Lee YS. Small. 2010;6:119–125. doi: 10.1002/smll.200901459. [DOI] [PubMed] [Google Scholar]
- 24.Patel D, Kell A, Simard B, Deng JX, Xiang B, Lin HY, Gruwel M, Tian GH. Biomaterials. 2010;31:2866–2873. doi: 10.1016/j.biomaterials.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 25.van Schooneveld MM, Cormode DP, Koole R, van Wijngaarden JT, Calcagno C, Skajaa T, Hilhorst J, Hart D. C. ’t., Fayad ZA, Mulder WJM, Meijerink A. Contrast Media & Molecular Imaging. 2010;5:231–236. doi: 10.1002/cmmi.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulder WJM, Strijkers GJ, Van Tilborg GAF, Cormode DP, Fayad ZA, Nicolay K. Acc. Chem. Res. 2009;42:904–914. doi: 10.1021/ar800223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Na HB, Song IC, Hyeon T. Adv. Mater. 2009;21:2133–2148. [Google Scholar]
- 28.Na HB, Hyeon T. J. Mater. Chem. 2009;19:6267–6273. [Google Scholar]
- 29.Gao JH, Gu HW, Xu B. Acc. Chem. Res. 2009;42:1097–1107. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- 30.Cheon J, Lee JH. Acc. Chem. Res. 2008;41:1630–1640. doi: 10.1021/ar800045c. [DOI] [PubMed] [Google Scholar]
- 31.Knuuti J, Bengel FM. Heart. 2008;94:360–367. doi: 10.1136/hrt.2007.118992. [DOI] [PubMed] [Google Scholar]
- 32.Phelps ME. Annual Review of Nuclear and Particle Science. 2002;52:303–338. [Google Scholar]
- 33.Dijkers EC, Munnink THO, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schroder CP, Lub-de Hooge MN, de Vries EG. Clin. Pharmacol. Ther. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 34.Zijlstra JM, Lindauer-van der Werf G, Hoekstra OS, Hooft L, Riphagen II, Huijgens PC. Haematologica-the Hematology Journal. 2006;91:522–529. [PubMed] [Google Scholar]
- 35.Briere JJ, Favier J, Gimenez-Roqueplo AP, Rustin P. Am. J. Physiol.: Cell Physiol. 2006;291:C1114–C1120. doi: 10.1152/ajpcell.00216.2006. [DOI] [PubMed] [Google Scholar]
- 36.Shah A, Schelbert HR, Schwaiger M, Henze E, Hansen H, Selin C, Huang SC. J. Am. Coll. Cardiol. 1985;5:92–100. doi: 10.1016/s0735-1097(85)80089-9. [DOI] [PubMed] [Google Scholar]
- 37.Bergmann SR, Fox KA, Rand AL, McElvany KD, Welch MJ, Markham J, Sobel BE. Circulation. 1984;70:724–733. doi: 10.1161/01.cir.70.4.724. [DOI] [PubMed] [Google Scholar]
- 38.Bengel FM, Schwaiger M. J. Nucl. Cardiol. 2004;11:603–616. doi: 10.1016/j.nuclcard.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 39.LeGuludec D, CohenSolal A, Delforge J, Delahaye N, Syrota A, Merlet P. Circulation. 1997;96:3416–3422. doi: 10.1161/01.cir.96.10.3416. [DOI] [PubMed] [Google Scholar]
- 40.Higuchi T, Schwaiger M. Curr. Cardiol. Rep. 2006;8:131–138. doi: 10.1007/s11886-006-0024-z. [DOI] [PubMed] [Google Scholar]
- 41.Cassidy PJ, Radda GK. J. R. Soc. Interface. 2005;2:133–144. doi: 10.1098/rsif.2005.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandl S, Schimmelpfennig C, Edinger M, Negrin RS, Contag CH. J. Cell. Biochem. 2002:239–248. doi: 10.1002/jcb.10454. [DOI] [PubMed] [Google Scholar]
- 43.Dilsizian V, Bateman TM, Bergmann SR, Prez RD, Magram MY, Goodbody AE, Babich JW, Udelson JE. Circulation. 2005;112:2169–2174. doi: 10.1161/CIRCULATIONAHA.104.530428. [DOI] [PubMed] [Google Scholar]
- 44.Kida K, Akashi YJ, Yoneyama K, Shimokawa M, Musha H. Ann. Nucl. Med. 2008;22:769–775. doi: 10.1007/s12149-008-0180-x. [DOI] [PubMed] [Google Scholar]
- 45.Nanasato M, Goto N, Isobe S, Unno K, Hirayama H, Sato T, Matsuoka S, Nagasaka T, Tominaga Y, Uchida K, Murohara T. Circ. J. 2009;73:1956–1960. doi: 10.1253/circj.cj-08-0415. [DOI] [PubMed] [Google Scholar]
- 46.Dobrucki LW, Sinusas AJ. Nat. Rev. Cardiol. 2009;7:38–47. doi: 10.1038/nrcardio.2009.201. [DOI] [PubMed] [Google Scholar]
- 47.Carrio I. Journal of Nuclear Medicine. 2001;42:1062–1076. [PubMed] [Google Scholar]
- 48.Lee T-Y. Trends Biotechnol. 2002;20:S3–S10. doi: 10.1016/s1471-1931(02)00197-0. [DOI] [PubMed] [Google Scholar]
- 49.Yu SB, Watson AD. Chem. Rev. 1999;99:2353–2377. doi: 10.1021/cr980441p. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Chaudhary A, Chmura SJ, Pelizzari C, Rajh T, Wietholt C, Kurtoglu M, Aydogan B. Phys. Med. Biol. 2010;55:4389–4397. doi: 10.1088/0031-9155/55/15/013. [DOI] [PubMed] [Google Scholar]
- 51.Vera DR, Mattrey RF. Acad. Radiol. 2002;9:784–792. doi: 10.1016/s1076-6332(03)80348-3. [DOI] [PubMed] [Google Scholar]
- 52.Fruman SA, Harned RK, Marcus D, Kaufman S, Swenson RB, Bernardino ME. Acad. Radiol. 1994;1:151–153. doi: 10.1016/s1076-6332(05)80834-7. [DOI] [PubMed] [Google Scholar]
- 53.Kao CY, Hoffman EA, Beck KC, Bellamkonda RV, Annapragada AV. Acad. Radiol. 2003;10:475–483. doi: 10.1016/s1076-6332(03)80055-7. [DOI] [PubMed] [Google Scholar]
- 54.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Br. J. Radiol. 2006;79:248–253. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 55.Guo R, Wang H, Peng C, Shen MW, Pan MJ, Cao XY, Zhang GX, Shi XY. J. Phys. Chem. C. 2010;114:50–56. [Google Scholar]
- 56.Foster FS, Pavlin CJ, Harasiewicz KA, Christopher DA, Turnbull DH. Ultrasound Med. Biol. 2000;26:1–27. doi: 10.1016/s0301-5629(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 57.Coatney RW. ILAR Journal. 2001;42:233–247. doi: 10.1093/ilar.42.3.233. [DOI] [PubMed] [Google Scholar]
- 58.Fowlkes JB, Kripfgans OD, Carson PL. 2004 2nd Ieee International Symposium on Biomedical Imaging: Macro to Nano, Vols 1 and 2.2004. p. 1042. [Google Scholar]
- 59.Klibanov AL, Rasche PT, Hughes MS, Wojdyla JK, Galen KP, Wible JH, Brandenburger GH. Invest. Radiol. 2004;39:187–195. doi: 10.1097/01.rli.0000115926.96796.75. [DOI] [PubMed] [Google Scholar]
- 60.Shotet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, Grayburn PA. Circulation. 2000;101:2554–2556. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 61.AlkanOnyuksel H, Demos SM, Lanza GM, Vonesh MJ, Klegerman ME, Kane BJ, Kuszak J, McPherson DD. J. Pharm. Sci. 1996;85:486–490. doi: 10.1021/js950407f. [DOI] [PubMed] [Google Scholar]
- 62.Lanza GM, Wickline SA. Curr. Probl. Cardiol. 2003;28:625–653. doi: 10.1016/j.cpcardiol.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Zonios G, Perelman LT, Backman VM, Manoharan R, Fitzmaurice M, Van Dam J, Feld MS. Appl. Opt. 1999;38:6628–6637. doi: 10.1364/ao.38.006628. [DOI] [PubMed] [Google Scholar]
- 64.Luker GD, Luker KE. J. Nucl. Med. 2008;49:1–4. doi: 10.2967/jnumed.107.045799. [DOI] [PubMed] [Google Scholar]
- 65.Balas C. Meas. Sci. Technol. 2009;20:104020–104031. [Google Scholar]
- 66.Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. Gastrointest. Endosc. 2005;62:686–695. doi: 10.1016/j.gie.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 67.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan WCW, Nie SM. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 69.Smith AM, Gao XH, Nie SM. Photochemistry and Photobiology. 2004;80:377–385. doi: 10.1562/0031-8655(2004)080<0377:QDNFIV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 70.Mulder WJM, Koole R, Brandwijk RJ, Storm G, Chin PTK, Strijkers GJ, Donega CD, Nicolay K, Griffioen AW. Nano Lett. 2006;6:1–6. doi: 10.1021/nl051935m. [DOI] [PubMed] [Google Scholar]
- 71.Wang S, Jarrett BR, Kauzlarich SM, Louie AY. J. Am. Chem. Soc. 2007;129:3848–3856. doi: 10.1021/ja065996d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 73.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 74.Werner EJ, Datta A, Jocher CJ, Raymond KN. Angew. Chem., Int. Ed. 2008;47:8568–8580. doi: 10.1002/anie.200800212. [DOI] [PubMed] [Google Scholar]
- 75.Pan D, Caruthers SD, Senpan A, Schmieder AH, Wickline SA, Lanza GM. Wiley Interdisciplinary Reviews. 2010 doi: 10.1002/wnan.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srinivas M, Heerschap A, Ahrens ET, Figdor CG, de Vries IJM. Trends Biotechnol. 2010;28:363–370. doi: 10.1016/j.tibtech.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janjic JM, Ahrens ET. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1:492–501. doi: 10.1002/wnan.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Solomon I. Phys. Rev. 1955;99:559–565. [Google Scholar]
- 79.Bloembergen N, Purcell EM, Pound RV. Phys. Rev. 1948;73:679–712. [Google Scholar]
- 80.Caravan P. Chem. Soc. Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 81.Laus S, Ruloff R, Toth E, Merbach AE. Chem.–Eur. J. 2003;9:3555–3566. doi: 10.1002/chem.200204612. [DOI] [PubMed] [Google Scholar]
- 82.Polasek M, Rudovsky J, Hermann P, Lukes I, Vander Else L, Muller RN. Chem. Commun. 2004:2602–2603. doi: 10.1039/b409996f. [DOI] [PubMed] [Google Scholar]
- 83.Costa J, Ruloff R, Burai L, Helm L, Merbach AE. J. Am. Chem. Soc. 2005;127:5147–5157. doi: 10.1021/ja0424169. [DOI] [PubMed] [Google Scholar]
- 84.Costa J, Toth E, Helm L, Merbach AE. Inorg. Chem. 2005;44:4747–4755. doi: 10.1021/ic0500309. [DOI] [PubMed] [Google Scholar]
- 85.Casali C, Janier M, Canet E, Obadia JF, Benderbous S, Corot C, Revel D. Acad. Radiol. 1998;5:S214–S218. doi: 10.1016/s1076-6332(98)80109-8. [DOI] [PubMed] [Google Scholar]
- 86.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Invest. Radiol. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 87.Port M, Corot C, Rousseaux O, Raynal I, Devoldere L, Idee JM, Dencausse A, Le Greneur S, Simonot C, Meyer D. Magnetic Resonance Materials in Physics Biology and Medicine. 2001;12:121–127. doi: 10.1007/BF02668093. [DOI] [PubMed] [Google Scholar]
- 88.Livramento JB, Toth E, Sour A, Borel A, Merbach AE, Ruloff R. Angew. Chem., Int. Ed. 2005;44:1480–1484. doi: 10.1002/anie.200461875. [DOI] [PubMed] [Google Scholar]
- 89.Guo HX, Yang HQ, Gallazzi F, Prossnitz ER, Sklar LA, Miao YB. Bioconjugate Chem. 2009;20:2162–2168. doi: 10.1021/bc9003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lewis MR, Kao JY, Anderson ALJ, Shively JE, Raubitschek A. Bioconjugate Chem. 2001;12:320–324. doi: 10.1021/bc0000886. [DOI] [PubMed] [Google Scholar]
- 91.Abiraj K, Jaccard H, Kretzschmar M, Helm L, Maecke HR. Chem. Commun. 2008:3248–3250. doi: 10.1039/b805281f. [DOI] [PubMed] [Google Scholar]
- 92.Mishra A, Pfeuffer J, Mishra R, Engelmann J, Mishra AK, Ugurbil K, Logothetis NK. Bioconjugate Chem. 2006;17:773–780. doi: 10.1021/bc050295b. [DOI] [PubMed] [Google Scholar]
- 93.De Leon-Rodriguez LM, Ortiz A, Weiner AL, Zhang SR, Kovacs Z, Kodadek T, Sherry AD. J. Am. Chem. Soc. 2002;124:3514–3515. doi: 10.1021/ja025511v. [DOI] [PubMed] [Google Scholar]
- 94.De Leon-Rodriguez LM, Kovacs Z. Bioconjugate Chem. 2008;19:391–402. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 95.LeveilleWebster CR, Rogers J, Arias IM. Hepatology. 1996;23:1631–1641. doi: 10.1002/hep.510230646. [DOI] [PubMed] [Google Scholar]
- 96.Artemov D, Mori N, Ravi R, Bhujwalla ZM. Cancer Research. 2003;63:2723–2727. [PubMed] [Google Scholar]
- 97.Mishra R, Su W, Pohmann R, Pfeuffer J, Sauer MG, Ugurbil K, Engelmann J. Bioconjugate Chem. 2009;20:1860–1868. doi: 10.1021/bc9000454. [DOI] [PubMed] [Google Scholar]
- 98.Poduslo JF, Curran GL, Peterson JA, McCormick DJ, Fauq AH, Khan MA, Wengenack TM. Biochemistry. 2004;43:6064–6075. doi: 10.1021/bi0359574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choi KS, Kim SH, Cai QY, Kim SY, Kim HO, Lee HJ, Kim EA, Yoon SE, Yun KJ, Yoon KH. Molecular Imaging and Biology. 2007;6:75. [PubMed] [Google Scholar]
- 100.Jun HY, Yin HH, Kim SH, Park SH, Kim HS, Yoon KH. Korean Journal of Radiology. 2010;11:449–456. doi: 10.3348/kjr.2010.11.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lauffer RB, Brady TJ. Magn. Reson. Imaging. 1985;3:11–16. doi: 10.1016/0730-725x(85)90004-9. [DOI] [PubMed] [Google Scholar]
- 102.Ogan MD, Schmiedl U, Moseley ME, Grodd W, Paajanen H, Brasch RC. Invest. Radiol. 1987;22:665–671. [PubMed] [Google Scholar]
- 103.Landon LA, Deutscher SL. J. Cell. Biochem. 2003;90:509–517. doi: 10.1002/jcb.10634. [DOI] [PubMed] [Google Scholar]
- 104.Santimaria M, Moscatelli G, Viale GL, Giovannoni L, Neri G, Viti F, Leprini A, Borsi L, Castellani P, Zardi L, Neri D, Riva P. Clinical Cancer Research. 2003;9:571–579. [PubMed] [Google Scholar]
- 105.Olafsen T, Kenanova VE, Sundaresan G, Anderson AL, Crow D, Yazaki PJ, Li L, Press MF, Williams LE, Wong JYC, Raubitschek AA, Shively JE, Wu AM. Cancer Res. 2005;65:5907–5916. doi: 10.1158/0008-5472.CAN-04-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orlova A, Magnusson M, Eriksson TLJ, Nilsson M, Larsson B, Hoiden-Guthenherg I, Widstrom C, Carlsson J, Tolmachev V, Stahl S, Nilsson FY. Cancer Res. 2006;66:4339–4348. doi: 10.1158/0008-5472.CAN-05-3521. [DOI] [PubMed] [Google Scholar]
- 107.Orlova A, Tolmachev V, Pehrson R, Lindborg M, Tran T, Sandstrom M, Nilsson FY, Wennborg A, Abrahmsen L, Feldwisch J. Cancer Res. 2007;67:2178–2186. doi: 10.1158/0008-5472.CAN-06-2887. [DOI] [PubMed] [Google Scholar]
- 108.Tolmachev V, Velikyan I, Sandstrom M, Orlova A. Eur. J. Nucl. Med. Mol. Imaging. 2010;37:1356–1367. doi: 10.1007/s00259-009-1367-7. [DOI] [PubMed] [Google Scholar]
- 109.Baum RP, Prasad V, Muller D, Schuchardt C, Orlova A, Wennborg A, Tolmachev V, Feldwisch J. J. Nucl. Med. 2010;51:892–897. doi: 10.2967/jnumed.109.073239. [DOI] [PubMed] [Google Scholar]
- 110.Fortin MA, Orlova A, Malmstrom PU, Tolmachev V. International Journal of Molecular Medicine. 2007;19:285–291. [PubMed] [Google Scholar]
- 111.Mankoff DA. Breast Cancer Res. 2008;10 doi: 10.1186/bcr2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jordan VC, Brodie AMH. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 114.Katzenellenbogen JA, Welch MJ, Dehdashti F. Anticancer Research. 1997;17:1573–1576. [PubMed] [Google Scholar]
- 115.Mankoff DA, Link JM, Linden HM, Sundararajan L, Krohn KA. J. Nucl. Med. 2008;49:149S–163S. doi: 10.2967/jnumed.107.045963. [DOI] [PubMed] [Google Scholar]
- 116.Huber MM, Staubli AB, Kustedjo K, Gray MHB, Shih J, Fraser SE, Jacobs RE, Meade TJ. Bioconjugate Chem. 1998;9:242–249. doi: 10.1021/bc970153k. [DOI] [PubMed] [Google Scholar]
- 117.Edwards WB, Xu B, Akers W, Cheney PP, Liang K, Rogers BE, Anderson CJ, Achilefu S. Bioconjugate Chem. 2008;19:192–200. doi: 10.1021/bc700291m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang ZR, Liang KX, Bloch S, Berezin M, Achilefu S. Bioconjugate Chem. 2005;16:1232–1239. doi: 10.1021/bc050136s. [DOI] [PubMed] [Google Scholar]
- 119.Zhang ZR, Achilefu S. Photochem. Photobiol. 2005;81:1499–1504. doi: 10.1562/2005-06-08-RA-568. [DOI] [PubMed] [Google Scholar]
- 120.Li C, Wang W, Wu QP, Shi K, Houston J, Sevick-Muraca E, Dong L, Chow D, Charnsangavej C, Gelovani JG. Nucl. Med. Biol. 2006;33:349–358. doi: 10.1016/j.nucmedbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 121.Pandey SK, Gryshuk AL, Sajjad M, Zheng X, Chen YH, Abouzeid MM, Morgan J, Charamisinau I, Nabi HA, Oseroff A, Pandey RK. J. Med. Chem. 2005;48:6286–6295. doi: 10.1021/jm050427m. [DOI] [PubMed] [Google Scholar]
- 122.Villaraza AJL, Bumb A, Brechbiel MW. Chem. Rev. 2010;110:2921–2959. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kellar KE, Fujii DK, Gunther WHH, Briley-Saebo K, Bjornerud A, Spiller M, Koenig SH. J. Magn. Reson. Imaging. 2000;11:488–494. doi: 10.1002/(sici)1522-2586(200005)11:5<488::aid-jmri4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 124.Maity D, Agrawal DC. J. Magn. Magn. Mater. 2007;308:46–55. [Google Scholar]
- 125.Dias MHM, Lauterbur PC. Magn. Reson. Med. 1986;3:328–330. doi: 10.1002/mrm.1910030218. [DOI] [PubMed] [Google Scholar]
- 126.Geraldes CFGC, Laurent S. Contrast Media Mol. Imaging. 2009;4:1–23. doi: 10.1002/cmmi.265. [DOI] [PubMed] [Google Scholar]
- 127.Veronese FM, Pasut G. Drug Discovery Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 128.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. N. Engl. J. Med. 2003;348:2491–U2495. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 129.Schmiedl U, Moseley ME, Ogan MD, Chew WM, Brasch RC. J. Comput.-Assisted Tomogr. 1987;11:306–313. doi: 10.1097/00004728-198703000-00023. [DOI] [PubMed] [Google Scholar]
- 130.Mulder WJM, Strijkers GJ, van Tilborg GAF, Griffioen AW, Nicolay K. NMR Biomed. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 131.Wu C, Brechbiel MW, Kozak RW, Gansow OA. Bioorg. Med. Chem. Lett. 1994;4:449. [Google Scholar]
- 132.Taylor KML, Rieter WJ, Lin WB. J. Am. Chem. Soc. 2008;130:14358–14359. doi: 10.1021/ja803777x. [DOI] [PubMed] [Google Scholar]
- 133.Sitharaman B, Kissell KR, Hartman KB, Tran LA, Baikalov A, Rusakova I, Sun Y, Khant HA, Ludtke SJ, Chiu W, Laus S, Toth E, Helm L, Merbach AE, Wilson LJ. Chem. Commun. 2005:3915–3917. doi: 10.1039/b504435a. [DOI] [PubMed] [Google Scholar]
- 134.Richard C, Doan BT, Beloeil JC, Bessodes M, Toth E, Scherman D. Nano Lett. 2008;8:232–236. doi: 10.1021/nl072509z. [DOI] [PubMed] [Google Scholar]
- 135.McDonald MA, Watkin KL. Acad. Radiol. 2006;13:421–427. doi: 10.1016/j.acra.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 136.Bridot JL, Faure AC, Laurent S, Riviere C, Billotey C, Hiba B, Janier M, Josserand V, Coll JL, Vander Elst L, Muller R, Roux S, Perriat P, Tillement O. J. Am. Chem. Soc. 2007;129:5076–5084. doi: 10.1021/ja068356j. [DOI] [PubMed] [Google Scholar]
- 137.Fortin MA, Petoral RM, Soderlind F, Klasson A, Engstrom M, Veres T, Kall PO, Uvdal K. Nanotechnology. 2007;18:395501–395509. [Google Scholar]
- 138.Evanics F, Diamente PR, van Veggel FCJM, Stanisz GJ, Prosser RS. Chem. Mater. 2006;18:2499–2505. [Google Scholar]
- 139.Hifumi H, Yamaoka S, Tanimoto A, Citterio D, Suzuki K. J. Am. Chem. Soc. 2006;128:15090–15091. doi: 10.1021/ja066442d. [DOI] [PubMed] [Google Scholar]
- 140.Na HB, Lee JH, An KJ, Park YI, Park M, Lee IS, Nam DH, Kim ST, Kim SH, Kim SW, Lim KH, Kim KS, Kim SO, Hyeon T. Angew. Chem., Int. Ed. 2007;46:5397–5401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 141.Shin JM, Anisur RM, Ko MK, Im GH, Lee JH, Lee IS. Angew. Chem., Int. Ed. 2009;48:321–324. doi: 10.1002/anie.200802323. [DOI] [PubMed] [Google Scholar]
- 142.Kim J, Lee JE, Lee SH, Yu JH, Lee JH, Park TG, Hyeon T. Adv. Mater. 2008;20:478–483. [Google Scholar]
- 143.Feng J, Song SY, Deng RP, Fan WQ, Zhang HJ. Langmuir. 2010;26:3596–3600. doi: 10.1021/la903008z. [DOI] [PubMed] [Google Scholar]
- 144.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. Cancer Research. 2003;63:8122–8125. [PubMed] [Google Scholar]
- 146.Glaus C, Rossin R, Welch MJ, Bao G. Bioconjugate Chem. 2010;21:715–722. doi: 10.1021/bc900511j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, Olson J, Zhang MQ. Nano Lett. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 148.Du GH, Liu ZL, Wang D, Xia X, Jia LH, Yao KL, Chu Q, Zhang SM. J. Nanosci. Nanotechnol. 2009;9:1304–1307. doi: 10.1166/jnn.2009.c143. [DOI] [PubMed] [Google Scholar]
- 149.Selvan ST, Patra PK, Ang CY, Ying JY. Angew. Chem., Int. Ed. 2007;46:2448–2452. doi: 10.1002/anie.200604245. [DOI] [PubMed] [Google Scholar]
- 150.Dosev D, Nichkova M, Dumas RK, Gee SJ, Hammock BD, Liu K, Kennedy IM. Nanotechnology. 2007;18:055102–055107. doi: 10.1088/0957-4484/18/5/055102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Choi JS, Park JC, Nah H, Woo S, Oh J, Kim KM, Cheon GJ, Chang Y, Yoo J, Cheon J. Angew. Chem., Int. Ed. 2008;47:6259–6262. doi: 10.1002/anie.200801369. [DOI] [PubMed] [Google Scholar]
- 152.Cho SJ, Jarrett BR, Louie AY, Kauzlarich SM. Nanotechnology. 2006;17:640–644. [Google Scholar]
- 153.Larson TA, Bankson J, Aaron J, Sokolov K. Nanotechnology. 2007;18:325101–325108. [Google Scholar]
- 154.Lin YS, Wu SH, Hung Y, Chou YH, Chang C, Lin ML, Tsai CP, Mou CY. Chem. Mater. 2006;18:5170–5172. [Google Scholar]
- 155.Santra S, Bagwe RP, Dutta D, Stanley JT, Walter GA, Tan W, Moudgil BM, Mericle RA. Adv. Mater. 2005;17:2165–2169. [Google Scholar]
- 156.Song Y, Xu XY, MacRenaris KW, Zhang XQ, Mirkin CA, Meade TJ. Angew. Chem., Int. Ed. 2009;48:9143–9147. doi: 10.1002/anie.200904666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Park JA, Kim HK, Kim JH, Jeong SW, Jung JC, Lee GH, Lee J, Chang Y, Kim TJ. Bioorg. Med. Chem. Lett. 2010;20:2287–2291. doi: 10.1016/j.bmcl.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 158.Empedocles SA, Neuhauser R, Shimizu K, Bawendi MG. Adv. Mater. 1999;11:1243–1256. [Google Scholar]
- 159.Jin T, Yoshioka Y, Fujii F, Komai Y, Seki J, Seiyama A. Chem. Commun. 2008:5764–5766. doi: 10.1039/b812302k. [DOI] [PubMed] [Google Scholar]
- 160.Gerion D, Herberg J, Bok R, Gjersing E, Ramon E, Maxwell R, Kurhanewicz J, Budinger TF, Gray JW, Shuman MA, Chen FF. J. Phys. Chem. C. 2007;111:12542–12551. [Google Scholar]
- 161.Zheng JZ, Perkins G, Kirilova A, Allen C, Jaffray DA. Invest. Radiol. 2006;41:339–348. doi: 10.1097/01.rli.0000186568.50265.64. [DOI] [PubMed] [Google Scholar]
- 162.Hak S, Sanders HMHF, Agrawal P, Langereis S, Grull H, Keizer HM, Arena F, Terreno E, Strijkers GJ, Nicolay K. Eur. J. Pharm. Biopharm. 2009;72:397–404. doi: 10.1016/j.ejpb.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 163.Elrod DB, Partha R, Danila D, Casscells SW, Conyers JL. Nanomed.: Nanotechnol., Biol. Med. 2009;5:42–45. doi: 10.1016/j.nano.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 164.Kamaly N, Kalber T, Ahmad A, Oliver MH, So PW, Herlihy AH, Bell JD, Jorgensen MR, Miller AD. Bioconjugate Chem. 2008;19:118–129. doi: 10.1021/bc7001715. [DOI] [PubMed] [Google Scholar]
- 165.Vuu K, Xie JW, McDonald MA, Bernardo M, Hunter F, Zhang YT, Li K, Bednarski M, Guccione S. Bioconjugate Chem. 2005;16:995–999. doi: 10.1021/bc050085z. [DOI] [PubMed] [Google Scholar]
- 166.Vucic E, Sanders HMHF, Arena F, Terreno E, Aimo S, Nicolay K, Leupold E, Dathe M, Sommerdijk NAJM, Fayad ZA, Mulder WJM. J. Am. Chem. Soc. 2009;131:406–407. doi: 10.1021/ja808310u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Talanov VS, Regino CAS, Kobayashi H, Bernardo M, Choyke PL, Brechbiel MW. Nano Lett. 2006;6:1459–1463. doi: 10.1021/nl060765q. [DOI] [PubMed] [Google Scholar]