Abstract
PURPOSE
We aimed to determine whether low-risk breast masses can be effectively managed with unenhanced magnetic resonance imaging (MRI) combining T2-weighted sequences with diffusion-weighted imaging (DWI) instead of immediate biopsy to decrease negative biopsy rates.
METHODS
After institutional review board and patient approvals, 141 consecutive women with 156 low-risk breast masses, who underwent unenhanced MRI and later on received a final diagnosis, were included in the study. There were 72 BI-RADS 3 masses in women with relative risk factors and 84 BI-RADS 4A masses, all referred for biopsy. Apparent diffusion coefficient (ADC) cutoff was 0.90×10-3 mm2/s. According to ADC values and T2-weighted imaging characteristics, masses were classified as either malignant or benign. Unenhanced MRI results were compared with final diagnoses obtained by histopathology or imaging surveillance, and diagnostic values were calculated.
RESULTS
Of 156 masses, 112 underwent biopsy. Four malignancies were diagnosed, three of which having ADC values lower than the cutoff. In women who rejected the biopsy, masses were stable during a follow-up of at least two years (n=44). MRI revealed 91% specificity and 99% negative predictive value (NPV) for detection of breast cancer.
CONCLUSION
Combination of T2-weighted imaging with DWI is a feasible method to further characterize breast masses with a low probability of malignancy. With the use of unenhanced MRI instead of immediate biopsy, it might be possible to decrease negative biopsy rates of low-risk breast masses.
The Breast Imaging Reporting and Data Systems (BI-RADS) lexicon (1) of American College of Radiology (ACR) provides an efficient and standardized assessment and management of breast lesions. It also stratifies breast cancer risk for a given lesion by classifying them into categories 1 through 5 according to the degree of suspicion.
According to this system, solid masses with a circumscribed margin, oval shape (including those with two or three gentle lobulations) and parallel orientation on ultrasonography (US) exam are classified as BI-RADS 3. These types of masses are commonly seen at diagnostic and screening examinations. In this category malignancy is highly unlikely (less than 2%) and a short interval follow-up is recommended (1). However, up to one-third of such masses undergo biopsy mainly because of radiologist, referring clinician, or patient concern about the substantial risk of malignancy (2–4). Many BI-RADS 3 masses are traditionally referred for biopsy if they are palpable, large in size, patient is of advanced age or has a positive family history for breast cancer.
The BI-RADS 4 assessment is reserved for findings that do not have the classic appearance of malignancy but are sufficiently suspicious to justify a recommendation for biopsy. This category is largely indeterminate and highly variable in outcome. Breast lesions in this category carry 2% to 95% risk for malignancy (1). Thus, almost all recommendations for breast biopsies come from assessments made using this category. According to BI-RADS classification; category 4 is subgrouped as 4A, 4B, and 4C to better inform the clinicians, pathologists, and patients of the degree of concern. However, the criteria for distinguishing among these subcategories have not been well delineated. BI-RADS 4A designates lesions with a low suspicion for malignancy. In this group, a benign pathologic diagnosis is expected and considered concordant (1). Studies of several institutions by the use of their internal criteria revealed positive predictive value (PPV) of 7%–9% for 4A lesions, and more than 50% of the suspicious lesions fall into this category. On the other hand, BI-RADS 4B and 4C designate lesions with moderate and high suspicion for malignancy and PPV in these categories were reported to be 19%–38% and 57%–82%, respectively (5–7).
Approximately 70%–80% of breast biopsies result in benign diagnosis (8, 9). Although the risk of malignancy is low, many BI-RADS 3 masses and all subcategory 4A masses are referred for biopsy. These two groups constitute the main source of negative biopsies which load unnecessary fear, anxiety, discomfort, pain, and financial cost to these patients.
Breast magnetic resonance imaging (MRI) is a well-established advanced technique for evaluation of the breast masses. Dynamic contrast-enhanced (DCE) imaging has high sensitivity, and it is the most proposed breast MRI method. However, this method is time consuming, needs contrast injection, has moderate specificity, and is relatively difficult to evaluate (10–12). DCE MRI evaluation of all low-risk lesions recommended for biopsy would not be cost effective. On the other hand, diffusion weighted imaging (DWI) is a newly proposed and highly effective MRI technique used for characterization of breast lesions, especially of masses, by measuring the random motion of free water protons in tissues. DWI is easy to evaluate, does not require contrast injection, has short imaging time and shows higher specificity (reported to be 84% in a meta-analysis) than DCE imaging (13).
The purpose of this study was to investigate the value of unenhanced MRI combining T2-weighted sequences with DWI for further characterization of breast masses having a low probability of being malignant (BI-RADS 3 and 4A). We hypothesized that unnecessary breast biopsies performed for benign masses might be decreased by evaluating these masses with unenhanced MRI.
Methods
Subjects
In this study, data were prospectively collected from 697 consecutive female patients who were referred to our department for biopsy of breast masses between March 2010 and February 2014. Conventional mammographic and sonographic imaging exams of most of these patients had been performed in our department and reported according to BI-RADS system by two radiologists (one with ten years of experience in breast imaging). If available, previous examinations were also evaluated for comparison. In case of patients who presented to our department for biopsy with prior studies performed elsewhere, imaging findings were reviewed, second-look US exams were performed and they were re-categorized according to BI-RADS. Senographe DS digital mammography (General Electric) and Acuson Antares (Siemens) ultrasonography equipment were used for that purpose.
Analysis of mammography and US findings
All noncalcified circumscribed solid masses seen at mammography were further evaluated with US. Solid masses with a circumscribed margin, oval shape (those with less than four gentle lobulations) and parallel orientation in US exam were classified as BI-RADS 3. Although in our routine practice the recommendation for BI-RADS 3 masses is short interval follow-up, for some of those masses biopsy was recommended by the radiologist or referring clinician due to presence of some relative risk factors such as palpability, large size of the mass, advanced age, or positive family history for breast cancer or patient preference. Breast masses with BI-RADS 3 sonographic features except for nonparallel orientation or irregular shape or microlobulated margin or exuberant vascularity were classified as BI-RADS 4A (14).
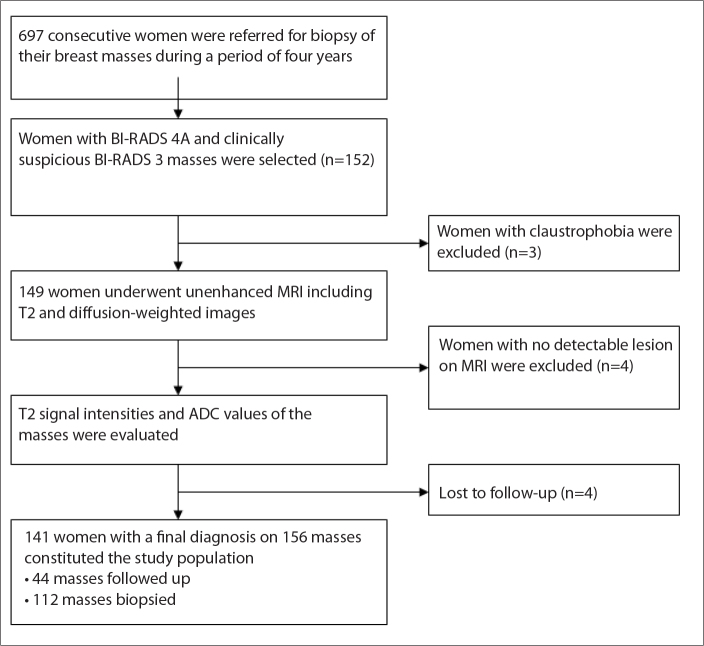
After informed consent was obtained, 149 women with 164 BI-RADS 3 and 4A masses were examined using unenhanced MRI. We recorded the BI-RADS categories of the masses and the possible causes for preference of biopsy in category 3 masses. Masses with prior histopathologic diagnosis and patients under treatment for breast cancer were not included in the study. Additionally, patients without detectable lesion on MRI corresponding to clinically or mammographically defined lesion (n=4) and without a final diagnosis of the lesion (lost to follow-up, n=4) were excluded from the study. A total of 156 masses in 141 women (age range, 13–77 years; mean age, 39 years) constituted the study population (Fig. 1). Approval for the study was obtained from the local ethics committee.
Figure 1.
Flow chart of the study.
MRI protocol
All patients were examined using a 1.5 T MRI unit (Magnetom, Symphony; Siemens) and dedicated double breast coil. Patients were placed in the prone position. Unenhanced MRI protocol included non–fat-suppressed T2-weighted turbo spin-echo sequence (TR/TE, 4500/97; matrix, 384×512; slice thickness, 3 mm) and two-dimensional (2D) spin-echo echo-planar imaging (EPI) sequence (TR/TE, 5400/94; matrix, 192×192; signal average, 3; slice thickness, 3 mm; distance factor, 20%; acquisition voxel size: 1.7×1.7×3 mm; b-values, 50, 400, and 1000 s/mm2) in the axial plane. The apparent diffusion coefficient (ADC) maps were created automatically by the system from the trace-weighted images with the use of b values 50, 400, 1000 s/mm2.
Image analysis
All MRI findings were interpreted on a workstation (Leonardo, Siemens, Germany) by a trained radiologist in breast MRI. During the interpretation, radiologist was blinded to the final diagnoses. Diffusion and T2-weighted images were used to identify and localize the masses that were referred for biopsy. Average tumor diameter measured on T2-weighted images was defined as the lesion size. T2 signal intensities of the masses were evaluated. Extremely dark signal intensity of a mass with a round or oval shape and smooth margins was taken as a sign of benign nature. ADC values of the masses were measured on ADC maps from the corresponding locations by using the circular region of interest (ROI) having the size of at least three pixels. Particular attention was paid to place the ROI to the solid portion of the lesion without fatty tissue contamination. At least three measurements were performed for each mass and the lowest mean value was selected. We used 0.90×10−3 mm2/s as the ADC cutoff value that was obtained from our previous DWI study of 285 cases (124 benign and 161 malignant tumors) (15).
Final diagnosis
Histopathologic examination was performed for 112 masses after MRI. The tissue samples were obtained either by surgical or 14–16-gauge (G) core-needle biopsy. Surgical biopsy was performed for six intraductal masses. Nine of the palpable masses also underwent surgical biopsy because of patient preference. We preferred to use 16G needles for 12 of our cases. Those were masses of smaller than 1 cm2 in dense breast. To be able to collect enough material at least four samples were taken. The remaining 85 cases underwent trucut biopsy with the use of 14G needles. We did not advise surgery for low-risk masses that were found to be benign on biopsy, and pathology results were considered definitive. However, periodic imaging surveillance by US was continued for needle biopsied cases. For 38 patients who refused to undergo biopsy after MRI, short-term US follow-up was performed. Forty-four masses in those 38 women were followed at 6-month intervals for the first year. The interval was extended to 12-month after the first year and continued at least for two years. Masses were considered benign if they remained stable for at least two years or decreased or resolved on follow-up.
Statistical analysis
All statistical analyses were performed with SPSS 13.0 software (SPSS Inc.). Data are presented as mean±standard deviation or median with range, as appropriate. To compare the continuous variables of two samples, Student’s t test or Mann-Whitney U test were used. Diagnostic performances of unenhanced MRI in the characterization of low-risk breast masses were calculated on the basis of final diagnoses. Also, the diagnostic accuracy of unenhanced MRI in BI-RADS 3 and 4A and in different age groups (≤40 years and >40 years) were compared with the use of Chi-square test. P < 0.05 was considered indicative of a significant difference.
Results
In the present study, 156 masses in 141 women (mean age 39.3±13 years; range, 13–77 years) were evaluated. Of 156 masses, 72 (46%) were prospectively classified as BI-RADS 3 and 84 (54%) as BI-RADS 4A. Mean size of the masses was significantly larger in BI-RADS 3 (median, 16.0 mm; range, 6–60 mm) than BI-RADS 4A (median, 12.0 mm; range, 6–35 mm, P = 0.002). Most common (74%) cause for preference of biopsy in BI-RADS 3 masses was determined as palpability (Table 1). Twelve masses with probably benign imaging features were categorized as BI-RADS 4A due to the presence of increase in the diameter of masses when compared with previous radiologic reports. All other BI-RADS 4A masses had some minor suspicious imaging findings.
Table 1.
Indication for biopsy in BI-RADS 3 masses
| (n=72) | n (%) |
|---|---|
| Palpability of the mass | 53 (74) |
| Advanced patient age | 11 (15) |
| Positive family history | 5 (7) |
| Patient preference | 3 (4) |
Definitive diagnoses were obtained through biopsy in 42 of 72 BI-RADS 3 (58%) and 70 of 84 BI-RADS 4A (83%) masses. Of the biopsied masses, four were malignant. Three masses were diagnosed as invasive ductal carcinomas (IDCs), one with accompanying ductal carcinoma in situ. Those were 21 mm, 10 mm, and 9 mm BI-RADS 4A masses in 31-, 40-, and 26-year-old women, respectively. Their ADCs were 0.70–0.90 ×10−3 mm2/s (Fig. 2). The fourth one was an 18 mm intraductal mass categorized as BI-RADS 4A and diagnosed as papilloma with a 4 mm invasive focus in a 31-year-old woman (ADC of the mass, 1.37×10−3 mm2/s). All other biopsied cases were benign with the following diagnoses: fibroadenoma (n=45, Fig. 3), fibrocystic changes (n=26), papilloma (n=6), epithelial hyperplasia (n=5), granulomatous mastitis (n=5), and others (n=21). All of the masses under imaging follow-up with US (30 BI-RADS 3 and 14 BI-RADS 4A) were stable for at least two years (n=42) or resolved on follow-up (n=2). Final diagnoses and relevant ADCs are given in Table 2.
Figure 2.
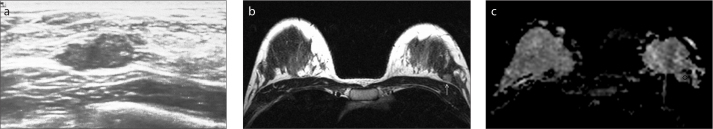
a–c. Invasive ductal carcinoma in a 40-year-old premenopausal woman with a positive family history. US image (a) shows a 6×12 mm irregular shaped, well-marginated, parallel oriented, hypoechoic, BI-RADS category 4A mass. On T2-weighted image (b), the mass was hyperintense (arrow). ADC map (c), revealed a low ADC value (0.70×10−3 mm2/s) for the mass.
Figure 3.
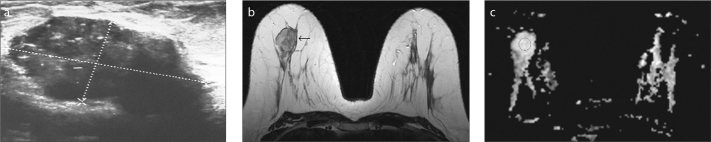
a–c. Fibroadenoma in a 37-year-old premenopausal woman with a palpable lump in her right breast. US image (a) shows a 16×32 mm oval shaped, well-marginated, parallel oriented, hypoechoic mass that was categorized as BI-RADS 4A due to increased size during the follow-up. T2-weighted image (b) shows rounded, hyperintense mass (arrow). ADC value was 1.48×10−3 mm2/s on the ADC map (c).
Table 2.
Final diagnoses and mean ADC values
| Final diagnoses | n (%) | Mean ADC values (×10−3 mm2/s) |
|---|---|---|
| IDC | 3 (2) | 0.83 |
| Papilloma with invasive focus | 1 (0.6) | 1.37 |
| FA | 45 (28.8) | 1.50 |
| FCC | 26 (16.7) | 1.26 |
| EH | 5 (3.2) | 1.21 |
| Papilloma | 6 (3.8) | 1.23 |
| Mastitis | 5 (3.2) | 0.91 |
| Other benign diagnosis | 21 (13.5) | 1.10 |
| Stable at follow-up | 42 (26.9) | 1.37 |
| Resolved during follow-up | 2 (1.3) | 1.33 |
ADC, apparent diffusion coefficient; IDC, invasive ductal carcinoma; FA, fibroadenoma; FCC, fibrocystic changes; EH, epithelial hyperplasia.
Malignancy rates were 0% for BI-RADS 3 masses and 4.8% for BI-RADS 4A masses. All malignant masses were iso-hypointense on T2-weighted images. Of the benign masses, 59% were hyperintense, 29% were iso-hypointense, and 12% were extremely hypointense. Mean ADCs of the benign masses were 1.34±0.35×10−3 mm2/s. Some demographic and imaging characteristics of benign and malignant masses were presented in Table 3.
Table 3.
Demographic and imaging characteristics of benign and malignant cases
| n | Agea (years) | Mass sizeb (mm) | ADC valuea (×10−3 mm2/s) | T2 signal intensity (n) | |
|---|---|---|---|---|---|
| Malignant | 4 | 32±7 | 14 (9–21) | 0.96±0.29 | Iso-hypointense (4) |
| Benign | 152 | 39±13 | 14 (6–60) | 1.34±0.35 | Iso-hypointense (44) Extremely hypointense (18) Hyperintense (90) |
Mean±standard deviation.
Median (range).
Three of the four malignant masses in the present study had ADC values lower than the cutoff (75% sensitivity). Of 152 benign masses, 139 had ADC values higher than the given cutoff and were classified correctly. All 13 false positive cases belonged to BI-RADS 4A group, except for one. Histopathologic details of these lesions were fibrocystic changes (n=4), epithelial hyperplasia (n=3), granulomatous inflammations (n=2), papilloma (n=1), fibroadenoma (n=1), and sclerosing adenosis (n=1). One of these cases refused biopsy and this BI-RADS 4A mass was stable at follow-up. Nine of the 13 false positive cases were more than 40-year-old. According to these results unenhanced MRI was determined to have a specificity of 91% (95% CI, 86%–95%) and a negative predictive value (NPV) of 99% (95% CI, 96%–100%). Diagnostic accuracy of unenhanced MRI was higher in BI-RADS 3 than in BI-RADS 4A (98.6% and 84.5%, respectively, P = 0.005) and in younger age group than older age group (93.8% and 87.5%, respectively, P = 0.294).
Discussion
In the present study, breast cancer was diagnosed in 2.6% of low-risk masses including BI-RADS 3 and 4A. The specificity and NPV of unenhanced MRI were 91% and 99%, respectively. Diagnostic efficacy was higher for BI-RADS category 3 lesions. If unenhanced MRI imaging were used in the diagnostic evaluation step before biopsy decision, number of low-risk masses referred for biopsy, which accounts for 20% of the masses biopsied during the study period, would have decreased by 90%.
It is not possible to differentiate all malignant breast lesions from benign ones using either mammography or US. Of breast malignancies, 10%–20% mimic benign nature with their morphologic features, including papillary, mucinous, medullary, metaplastic carcinomas, and malignant phyllodes tumors (16). A reasonable goal for the standard imaging modalities is to make risk stratification. Thereby, the subgroup of lesions that has a very low risk (<2%) of being malignant, namely BI-RADS 3, can be separated from the higher risk groups and short-interval follow-up can be offered instead of biopsy. Although cancer incidence among BI-RADS 3 masses is reported to be independent of larger size, advanced age, and palpability, in our practice and in many others, patients with these features are referred for biopsy instead of follow-up (17–19). In our limited study, none of the probably benign masses that were referred for biopsy due to such relative risk factors took a malignant diagnosis. Actually, we should rely on standard imaging findings and obey the classical approach of follow-up in case of BI-RADS 3 masses. However, unenhanced MRI with its high specificity in that category might also be used as an adjunct imaging method.
For breast lesions that have more than 2% risk of being malignant, risk is further stratified by categorizing them as BI-RADS 4A, 4B, 4C, and 5. However, in the present system no further action can be taken other than recommending biopsy for all these higher risk lesions regardless of whether they carry low, intermediate, or high risk (1).
Standard breast imaging modalities (mammography and US) are criticized for high false-positivity and associated benign biopsy rates, because of their contribution to morbidity, costs, and patient anxiety. The biopsy cannot be avoided for mammographic or sonographically detected moderate to high-risk breast lesions with high cancer expectancy, and further imaging evaluation would possibly not have an effect on their management. Therefore, in the present study we concentrated on lesions with a low probability of being malignant, namely, BI-RADS 3 and 4A masses that are referred for biopsy. Those occupy nearly one-fifth of the biopsy procedures in our practice. Although cancer expectancy can reach 2% for BI-RADS 3 and 10% for BI-RADS 4A, cancer was diagnosed in 0% and 4.8%, respectively, in the present study. Our lower cancer prevalence might be partially due to the low number of cases. A second factor might be that in the present study, most of the probably benign masses were referred for biopsy just because of their palpability. In some previous studies the prevalence of cancer among palpable masses with probably benign features was determined to be lower than that among nonpalpable ones (2, 20). As a third factor, the criteria defining the BI-RADS 4A masses are not well-defined. We used our internal criteria with reference to the study of Jales et al. (14) and it might not be compatible with other studies.
Here, we aimed to evaluate if unnecessary biopsies done for low-risk breast masses might be decreased with the use of an uncomplicated advanced imaging method. Compared to conventional methods, DWI has previously demonstrated higher specificity in differentiation of benign from malignant masses and is increasingly used in clinical practice (21–23). Use of DWI together with T2-weighted images created a good unenhanced imaging alternative to DCE-MRI in lesion detection and characterization. This method was previously used in some experimental clinical and reader studies and found to be useful for the diagnosis of breast cancer (24–27). Lastly, Trimboli et al. (28) reported 78% sensitivity and 87% specificity for breast cancer detection with unenhanced MRI of 67 women.
We used diffusion images to create ADC maps and to help localization of suspicious masses. Evaluation of DWI was performed quantitatively with the use of ADC values. It is well known that the calculated ADC value is clearly affected from the scanning parameters. Therefore, previous studies using different scanning parameters reported different cutoff values for the discrimination of malignant and benign tumors (13, 21–23). In our practice, we routinely apply DWI as a part of our standard breast MRI protocol and as ADC threshold we use 0.90 × 10−3 mm2/s, which is the cutoff value that was obtained from our previous DWI study (15). We also used this cutoff value in our present prospective study, and it provided good discrimination between malignant and benign masses.
We used T2-weighted images to evaluate the signal intensity and to localize the masses. T2 signal intensity can help in the characterization of breast masses but cannot specifically discriminate benign and malignant masses. High T2 signal can help diagnose active fibroadenomas most of the time. Fibroadenoma is most common diagnosis in low-risk breast masses and most prevalent lesion type that shows high ADC values (27). However, an uncommon malignancy of elderly women (mucinous cancers) also shows bright signal on T2-weighted images and can cause misdiagnosis. Iso-hypointense T2 signal can be seen in both benign and malignant masses and is not helpful for characterization (10). In the present study, extremely dark T2 signal was seen in 12% of smooth-marginated masses and it helped identify a typically benign pathology of sclerotic fibroadenomas.
In the present study, the specificity and NPV of unenhanced MRI were 91% (95% CI, 86%–95%) and 99% (95% CI, 96%–100%), respectively. Patient age did not have a significant effect on diagnostic efficacy of the method. If unenhanced MRI were used in the diagnostic evaluation step before biopsy decision, number of low-risk masses referred for biopsy, which accounts for 20% of the masses biopsied during the study period, would have decreased by 90%. Thus, unenhanced breast MRI may substantially decrease negative biopsy rates, especially in BI-RADS 3 masses. There was a missed cancer (papilloma with invasive focus) in our study population. In case of intraductal masses, it is not possible to differentiate papillomas from invasive carcinomas using MRI (29). Therefore, intraductal masses may not be suitable for characterization by unenhanced MRI. Also, it must be kept in mind that any act to reduce the number of unnecessary biopsies can increase the number of missed malignancies, as a consequence. Breast cancers are recognized by their high cellularity on DWI. Breast cancers with low cellularity such as hemorrhagic or necrotic tumors, mucinous cancers or a small malignant focus in a benign mass can be overlooked by unenhanced imaging. For masses that have benign features on unenhanced MRI, periodic imaging surveillance should be continued, so that missed malignant lesions can be identified during the follow-up.
In this study, our starting point was to decrease unnecessary biopsies. For this purpose we evaluated low-risk breast masses that were referred for biopsy. Another starting point might be the prevention of delayed cancer diagnosis which is possible for some masses that are initially followed-up as probably benign. Unfortunately, follow-up imaging compliance is not high, reported as 71% in the Digital Mammographic Imaging Screening Trial, and therefore recommendations do not work as intended (30). This is a great concern for the clinicians and it might be possible to diagnose these missed cancers immediately at the time of first diagnostic evaluation by further examination using unenhanced MRI. In the present study, the diagnostic efficacy of unenhanced MRI was higher for BI-RADS 3 lesions. However, the addition of another imaging technique to the diagnostic steps would not be cost-effective if the population that will benefit from the further imaging is not well-defined. Further studies are needed to evaluate the contribution of unenhanced MRI in different patient populations.
The most important limitation of our pilot study was the low number of cases. Larger studies and cost-benefit analyses are needed to validate the role of unenhanced MRI in further characterization of low-risk breast masses. Second limitation was the lack of histopathologic correlation for 44 breast masses (28%) in the study. Although, our study included cases referred for biopsy and MRI findings were not intentionally used to change the patient’s decisions, compliance with biopsy recommendation was low, especially in case of BI-RADS 3 masses. Third, due to lower diagnostic accuracy of DWI in non-mass lesions and possible difficulties that would be experienced in the localization of these lesions on unenhanced images, we did not include lesions other than masses (e.g., microcalcifications, focal asymmetries and distortions) in our study. Fourth, the areas of signal loss created by fat suppression and varying artifacts formed during the data acquisition, together with limited resolution, complicated the localization of four masses (3%) on the ADC maps. Fifth, calculated ADC value is affected from the scanning parameters, which is why different cutoff values are reported in breast DWI studies. Therefore, every MRI unit should find their own cutoff value according to their DWI sequence.
In conclusion, our study shows that unenhanced MRI applied for further characterization of low-risk breast masses has a high NPV and can successfully discriminate benign and malignant masses if the masses suitable for this evaluation are selected appropriately. Unenhanced MRI can be used to minimize unnecessary biopsies performed for breast cancer detection. However, more data based on larger studies would be needed to clearly document the clinical utility of unenhanced MRI.
Main points.
Approximately 70%–80% of breast biopsies result in benign diagnosis.
Low-risk breast masses constitute the main source of negative biopsies.
Unenhanced MRI applied for further characterization of low-risk breast masses has a high NPV and successfully discriminates benign from malignant masses.
Unenhanced MRI might be recommended instead of immediate biopsy to minimize unnecessary biopsies done for low-risk breast masses.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS) atlas. 5th ed. Reston, VA: American College of Radiology; 2013. [Accessed July 10, 2014]. Available at: www.acr.org. [Google Scholar]
- 2.Harvey JA, Nicholson BT, Lorusso AP, Cohen MA, Bovbjerg VE. Short-term follow-up of palpable breast lesions with benign imaging features: evaluation of 375 lesions in 320 women. AJR Am J Roentgenol. 2009;193:1723–1730. doi: 10.2214/AJR.09.2811. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Giess CS, Smeglin LZ, Meyer JE, Ritner JA, Bird-well RL. Risk of malignancy in palpable solid breast masses considered probably benign or low suspicion: implications for management. J Ultrasound Med. 2012;31:1943–1949. doi: 10.7863/jum.2012.31.12.1943. [DOI] [PubMed] [Google Scholar]
- 4.Raza S, Chikarmane SA, Neilsen SS, Zorn LM, Birdwell RL. BI-RADS 3, 4, and 5 lesions: value of US in management-follow-up and outcome. Radiology. 2008;248:773–781. doi: 10.1148/radiol.2483071786. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Wiratkapun C, Bunyapaiboonsri W, Wibulpolprasert B, Lertsithichai P. Biopsy rate and positive predictive value for breast cancer in BI-RADS category 4 breast lesions. J Med Assoc Thai. 2010;93:830–837. [PubMed] [Google Scholar]
- 6.Fu CY, Hsu HH, Yu JC, et al. Influence of age on PPV of sonographic BI-RADS categories 3, 4, and 5. Ultraschall Med. 2011;32:8–13. doi: 10.1055/s-0029-1245384. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Yoon JH, Kim MJ, Moon HJ, Kwak JY, Kim EK. Subcategorization of ultrasonographic BI-RADS category 4: positive predictive value and clinical factors affecting it. Ultrasound Med Biol. 2011;37:693–699. doi: 10.1016/j.ultrasmedbio.2011.02.009. http://dx.doi.org/10.1016/j.ultrasmedbio.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Liang W, Lawrence W, Burnett CB, et al. Acceptability of diagnostic tests for breast cancer. Breast Cancer Res Treat. 2003;79:199–206. doi: 10.1023/a:1023914612152. http://dx.doi.org/10.1023/A:1023914612152. [DOI] [PubMed] [Google Scholar]
- 9.Crowe JP, Jr, Rim A, Patrick R, et al. A prospective review of the decline of excisional breast biopsy. Am J Surg. 2002;184:353–355. doi: 10.1016/s0002-9610(02)00944-3. http://dx.doi.org/10.1016/S0002-9610(02)00944-3. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356–378. doi: 10.1148/radiol.2442051620. http://dx.doi.org/10.1148/radiol.2442051620. [DOI] [PubMed] [Google Scholar]
- 11.Heywang-Köbrunner SH, Viehweg P, Heinig A, Kuchler C. Contrast enhanced MRI of the breast: accuracy, value, controversies, solutions. Eur J Radiol. 1997;24:94–108. doi: 10.1016/s0720-048x(96)01142-4. http://dx.doi.org/10.1016/S0720-048X(96)01142-4. [DOI] [PubMed] [Google Scholar]
- 12.Warren RML, Pointon L, Thompson D, et al. Reading protocol for dynamic contrast- enhanced MR images of the breast: sensitivity and specificity analysis. Radiology. 2005;236:779–788. doi: 10.1148/radiol.2363040735. http://dx.doi.org/10.1148/radiol.2363040735. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZL. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693–704. doi: 10.1186/1471-2407-10-693. http://dx.doi.org/10.1186/1471-2407-10-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jales RM, Sarian LO, Torresan R, Marussi EF, Alvares BR, Derchain S. Simple rules for ultrasonographic subcategorization of BI-RADS 4 breast masses. Eur J Radiol. 2013;82:1231–1235. doi: 10.1016/j.ejrad.2013.02.032. http://dx.doi.org/10.1016/j.ejrad.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Kul S, Eyuboglu I, Cansu A, Alhan E. Diagnostic efficacy of the diffusion weighted imaging in the characterization of different types of breast lesions. J Magn Reson Imaging. 2014;40:1158–1164. doi: 10.1002/jmri.24491. http://dx.doi.org/10.1002/jmri.24491. [DOI] [PubMed] [Google Scholar]
- 16.Yoo JL, Woo OH, Kim YK, et al. Can MR imaging contribute in characterizing well-circumscribed breast carcinomas? Radiographics. 2010;30:1689–1702. doi: 10.1148/rg.306105511. http://dx.doi.org/10.1148/rg.306105511. [DOI] [PubMed] [Google Scholar]
- 17.Graf O, Helbich TH, Fuchsjaeger MH, et al. Follow-up of palpable circumscribed noncalcified solid breast masses at mammography and US: can biopsy be averted? Radiology. 2004;233:850–856. doi: 10.1148/radiol.2333031845. http://dx.doi.org/10.1148/radiol.2333031845. [DOI] [PubMed] [Google Scholar]
- 18.Barr RG, Zhang Z, Cormack JB, Mendelson EB, Berg WA. Probably benign lesions at screening breast US in a population with elevated risk: prevalence and rate of malignancy in the ACRIN 6666 trial. Radiology. 2013;269:701–712. doi: 10.1148/radiol.13122829. http://dx.doi.org/10.1148/radiol.13122829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojcinski S, Boehme E, Farrokh A, Soergel P, Degenhardt F, Hillemanns P. Ultrasound real-time elastography can predict malignancy in BI-RADS®-US 3 lesions. BMC Cancer. 2013;13:159. doi: 10.1186/1471-2407-13-159. http://dx.doi.org/10.1186/1471-2407-13-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerlikowske K, Smith-Bindman R, Abraham LA, et al. Breast cancer yield for screening mammographic examinations with recommendation for short-interval follow-up. Radiology. 2005;234:684–692. doi: 10.1148/radiol.2343031976. http://dx.doi.org/10.1148/radiol.2343031976. [DOI] [PubMed] [Google Scholar]
- 21.Marini C, Iacconi C, Giannelli M, Cilotti A, Moretti M, Bartolozzi C. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol. 2007;17:2646–2655. doi: 10.1007/s00330-007-0621-2. http://dx.doi.org/10.1007/s00330-007-0621-2. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Cai YQ, Cai ZL, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16:172–178. doi: 10.1002/jmri.10140. http://dx.doi.org/10.1002/jmri.10140. [DOI] [PubMed] [Google Scholar]
- 23.Rubesova E, Grell AS, De Maertelaer V, Metens T, Chao SL, Lemort M. Quantitative diffusion imaging in breast cancer: A clinical prospective study. J Magn Reson Imaging. 2006;24:319–324. doi: 10.1002/jmri.20643. http://dx.doi.org/10.1002/jmri.20643. [DOI] [PubMed] [Google Scholar]
- 24.Baltzer PA, Benndorf M, Dietzel M, Gajda M, Camara O, Kaiser WA. Sensitivity and specificity of unenhanced MR mammography (DWI combined with T2-weighted TSE imaging, ueMRM) for the differentiation of mass lesions. Eur Radiol. 2010;20:1101–1110. doi: 10.1007/s00330-009-1654-5. http://dx.doi.org/10.1007/s00330-009-1654-5. [DOI] [PubMed] [Google Scholar]
- 25.Yabuuchi H, Matsuo Y, Sunami S, et al. Detection of non-palpable breast cancer in asymptomatic women by using unenhanced diffusion-weighted and T2-weighted MR imaging: comparison with mammography and dynamic contrast-enhanced MR imaging. Eur Radiol. 2011;21:11–17. doi: 10.1007/s00330-010-1890-8. http://dx.doi.org/10.1007/s00330-010-1890-8. [DOI] [PubMed] [Google Scholar]
- 26.Kuroki-Suzuki S, Kuroki Y, Nasu K, Nawano S, Moriyama N, Okazaki M. Detecting breast cancer with non-contrast MR imaging: combining diffusion-weighted and STIR imaging. Magn Reson Med Sci. 2007;6:21–27. doi: 10.2463/mrms.6.21. http://dx.doi.org/10.2463/mrms.6.21. [DOI] [PubMed] [Google Scholar]
- 27.Parsian S, Rahbar H, Allison KH, et al. Non-malignant breast lesions: ADCs of benign and high-risk subtypes assessed as false-positive at dynamic enhanced MR imaging. Radiology. 2012;265:696–706. doi: 10.1148/radiol.12112672. http://dx.doi.org/10.1148/radiol.12112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trimboli RM, Verardi N, Cartia F, Carbonaro LA, Sardanelli F. Breast cancer detection using double reading of unenhanced MRI including T1-weighted, T2-weighted STIR, and diffusion-weighted imaging: a proof of concept study. AJR Am J Roentgenol. 2014;203:674–681. doi: 10.2214/AJR.13.11816. http://dx.doi.org/10.2214/AJR.13.11816. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Zhang S, Liu P, Lu H, Xu Y, Yang WT. Solitary intraductal papillomas of the breast: MRI features and differentiation from small invasive ductal carcinomas. AJR Am J Roentgenol. 2012;199:936–942. doi: 10.2214/AJR.12.8507. http://dx.doi.org/10.2214/AJR.12.8507. [DOI] [PubMed] [Google Scholar]
- 30.Baum JK, Hanna LG, Acharyya S, et al. Use of BI-RADS 3-probably benign category in the American College of Radiology imaging network digital mammographic imaging screening trial. Radiology. 2011;260:61–67. doi: 10.1148/radiol.11101285. http://dx.doi.org/10.1148/radiol.11101285. [DOI] [PMC free article] [PubMed] [Google Scholar]