Abstract
Pulmonary embolism (PE) is a potentially life threatening condition requiring adequate diagnosis and treatment. Computed tomography pulmonary angiography (CTPA) is excellent for including and excluding PE, therefore CT is the first-choice diagnostic imaging technique in patients suspected of having acute PE. Due to its wide availability and low invasiveness, CTPA tends to be overused. Correct implementation of clinical decision rules in diagnostic workup for PE improves adequate use of CT. Also, CT adds prognostic value by evaluating right ventricular (RV) function. CT-assessed RV dysfunction and to lesser extent central emboli location predicts PE-related mortality in normotensive and hypotensive patients, while PE embolic obstruction index has limited prognostic value. Simple RV/left ventricular (LV) diameter ratio measures >1.0 already predict risk for adverse outcome, whereas ratios <1.0 can safely exclude adverse outcome. Consequently, assessing the RV/LV diameter ratio may help identify patients who are potential candidates for treatment at home instead of treatment in the hospital. A minority of patients develop chronic thromboembolic pulmonary hypertension (CTEPH) following acute PE, which is a life-threatening condition that can be diagnosed by CT. In proximal CTEPH, involving the more central pulmonary arteries, thrombectomy usually results in good outcome in terms of both functional status and long-term survival rate. CT is becoming the imaging method of choice for diagnosing CTEPH as it can identify patients who may benefit from thrombectomy. New CT developments such as distensibility measurements and dual-energy or subtraction techniques may further refine diagnosis and prognosis for improved patient care.
Acute pulmonary embolism (PE) is the third most common cardiovascular condition, after coronary artery disease and stroke (1). Due to lack of specific sets of symptoms that accurately predict or exclude the diagnosis of acute PE, the diagnosis strongly relies on noninvasive imaging techniques. Diagnostic strategies for evaluating PE have undergone important changes over the past decades (2). Due to rapid technical advances in speed and spatial resolution, the utility of computed tomography (CT) angiography has been recognized in vascular imaging. Particularly, after the development of multidetector row CT in 1998 (3), CT pulmonary angiography (CTPA) has become the imaging method of choice in the diagnosis of acute PE (4). CTPA has advantages over conventional invasive X-ray pulmonary angiography and nuclear ventilation-perfusion (V/Q) imaging. CT is a widely available, fast and noninvasive technique, has the capability to directly visualize emboli, and may provide alternative diagnoses (4).
Despite adequate diagnosis and anticoagulant therapy, death rate after a diagnosis of acute PE is still 8%–15% (5, 6). The prognosis of acute PE mainly depends on residual pulmonary circulation and the severity of right ventricular (RV) dysfunction (7). Recent studies have shown that CT permits the assessment of acute right-sided heart failure. Furthermore, CT can predict adverse clinical outcome by using the RV/left ventricular (LV) diameter ratio (8, 9) or RV ejection fraction in patients with PE (10).
The aim of this review is to discuss the developments of CT in PE diagnosis, and to analyze the added value of CT in estimating PE severity and prognosis. Furthermore, CT findings of chronic thromboembolic pulmonary arterial hypertension (CTEPH) as a complication of acute PE will be discussed.
CT developments in PE diagnosis
Historically, pulmonary angiography and nuclear planar V/Q-imaging were the main imaging methods used for diagnosing PE. Both methods have recognized limitations. Pulmonary angiography has previously been regarded as reference standard, but the method is invasive and involves right heart catheterization. Moreover, its sensitivity is not 100% as recurrent venous thromboembolic events have been observed in a limited number of patients with normal angiograms (11). Interobserver agreement rates for detection of subsegmental emboli with pulmonary angiography ranged from 45% to 66% (12, 13). For multidetector CT this is in the range of 56%–85% (14, 15).
Until the 1990s, planar V/Q-imaging was the imaging method of choice in patients with suspected PE. However, the PIOPED I study showed that with V/Q-imaging in more than 65% of cases an indeterminate probability for PE was reported, and reliable diagnosis of PE could not be made (16). Of note, in as many as 40% of indeterminate V/Q-scans, PE was shown by invasive pulmonary angiography (17). Nowadays, planar V/Q-imaging has largely been replaced by CTPA. Since the emergence of multiple-head cameras, V/Q single-photon emission CT (SPECT) was proposed to improve the diagnostic performance with better sensitivity and/or specificity and lower nonconclusive test results (18). However, its advantages have not yet been validated by outcome studies that use V/Q SPECT as part of a diagnostic strategy to rule out PE (18).
With the introduction of fast-speed helical CT in the early nineties, its potential in vascular imaging and diagnosing PE has been recognized (19). Multidetector CT enabled isotropic voxel imaging that led to routine utilization of three-dimensional imaging. CT angiography has now become an established technique for minimally invasive vascular imaging (20). The 4- or 16-slice CTPA already provides a high level of image quality resulting in a diagnostic performance that equals or surpasses that of conventional pulmonary angiography (21), with sensitivity and specificity varying between 83%–100% and 89%–96%, respectively (22–24). Also, the sensitivity of CT for identifying PE in small pulmonary arteries (92%) is superior to that of invasive pulmonary angiography (56%) (22, 25). Reassessment of 20 causes of discordant CTPA readings from the PIOPED II study showed PE detection sensitivity of 87% for CT and 32% for angiography (26). Because of its high sensitivity and specificity, CTPA has nowadays replaced invasive pulmonary angiography as the reference standard for diagnosing PE (4).
CT diagnosis of PE
CTPA is currently the imaging test of choice for diagnosing PE (23). Due to the high sensitivity of CT, the number of false negatives is low. If the test is negative, PE is ruled out. Also, because of the high specificity, the proportion of false positives is low. If the CTPA test is positive, PE is diagnosed and anticoagulant therapy can be started (27).
With an overall PE prevalence of 15%–38%, the negative predictive value of CTPA for ruling out PE is 96.2%–99.1%; a negative CTPA alone can safely exclude PE in patients with high clinical pretest probability or low to intermediate clinical probability and elevated D-dimer levels (28, 29). The afterward incidence of venous thromboembolism (VTE) has been shown to be about 1%. Furthermore, the three-month fatal risk after a normal CTPA has been shown to be very low (0.41%–0.6%), making it safe to withhold anticoagulant therapy in case of negative CTPA results (28, 29). The CTPA studies show much better negative predictive values for ruling out PE (96.2%) than those that can be obtained by V/Q imaging (75.9%), which is clinically important as to safely withhold anticoagulant therapy (30, 31).
CT diagnosis of subsegmental PE
With the use of multidetector CTPA instead of single-detector CT techniques, the number of subsegmental PE diagnoses has increased (32). Clinical relevance of subsegmental PE (presence of PE on subsegmental level only) has been the topic of discussion for many years. The definition of subsegmental PE has yet to be standardized and a single subsegmental PE probably does not have the same clinical relevance as multiple subsegmental emboli (33). One meta-analysis that included 2657 patients with PE showed that multidetector CTPA increased the proportion of subsegmental PE diagnoses to 9.4%, as compared to 4.7% with single-detector CTPA, but without lowering the three-month risk of VTE in patients with normal multidetector CTPA. The authors therefore speculated that subsegmental PE may not be clinically relevant (32). A recent multicenter study of 3728 consecutive patients with clinically suspected PE identified 748 patients with proven PE, 116 of whom having subsegmental PE, and directly investigated the outcome for three-month follow-up risk of recurrent VTE and mortality. Between patients with subsegmental PE and those with more proximal PE, no statistical differences were seen in the prevalence of VTE risk factors, the three-month risk of recurrent VTE (3.6% vs. 2.5%), or mortality (10.7% vs. 6.5%). Also, when compared with patients without PE, patients with subsegmental PE were at an increased risk of VTE during follow-up (hazard ratio, 3.8; 95% CI, 1.3–11.1). The study indicates that in contrast to the common belief that subsegmental PE represents a benign subset of VTE, patients with symptomatic subsegmental PE have comparable prognosis as patients with segmental or more proximal PE regarding short-term clinical course (34). Thus, subsegmental PE is clinically relevant and should be reported when present. However, the positive predictive value of CT and interobserver agreement is low. The European Society of Cardiology (ESC) 2014 guidelines suggest that compression ultrasonography of the legs may be used to identify deep vein thrombosis (DVT) that would require treatment. In case of an isolated subsegmental PE and no DVT, the decision on whether to treat should be made on an individual basis, taking into account the clinical probability and the bleeding risk (33).
CT in clinical decision rules for diagnosing PE
The high accuracy and easy accessibility of CT increased the numbers of CTPA examinations up to five-fold within six years, but also resulted in a decrease in PE prevalence (35). In the U.S., the percentage of positive PE diagnoses decreased from 15% in the year 2000 to around 7%–8% in 2005 (36). Clinical decision rules (CDRs) that use pretest probability estimations for the presence of PE are highly effective in selecting patients for further work-up by CTPA. It has been shown that the implementation of a CDR decreases the number of CTPA requests by 20%, and increases the number of positive CTPA examinations by 69% (35). The best validated and widely used CDRs are the Wells and Geneva scores (Table 1), mainly valid for outpatients (37, 38). Since 2008, the European Society of Cardiology guidelines on the diagnosis and management of acute PE recommend using a pretest clinical probability test like the Wells score (21). Standardized assessments of pretest clinical probability allow patients to be classified into three groups. After diagnostic testing (V/Q imaging or compression ultrasonography of the legs or invasive pulmonary angiography), the estimated prevalence of PE is ≤10% for low clinical probability, 30% as a midpoint for intermediate clinical probability, and ≥70% for high clinical probability (37, 39). While the Geneva score and the original Wells score classify PE probability into three groups, the revised Well score uses a dichotomized version i.e., PE likely (score of >4), or PE unlikely (score of ≤4) (40).
Table 1.
The Wells rule and the revised Geneva score
| Wells rule | Score | Revised Geneva score | Score |
|---|---|---|---|
| Clinical signs of DVT | 3 | Age >65 years | 1 |
|
| |||
| Previous DVT or PE | 1.5 | Previous DVT or PE | 3 |
|
| |||
| Surgery or immobilization within 4 weeks | 1.5 | Surgery or fracture within 1 month | 2 |
|
| |||
| Active malignancy | 1 | Active malignancy | 2 |
|
| |||
| Alternative diagnosis less likely than PE | 3 | Unilateral lower limb pain | 3 |
|
| |||
| Hemoptysis | 1 | Hemoptysis | 2 |
|
| |||
| Heart rate >100 beats/min | 1.5 | Heart rate 74–95 beats/min | 3 |
| Heart rate >95 beats/min | 5 | ||
| Pain on lower limb deep vein palpation and unilateral edema | 4 | ||
|
| |||
| Clinical probability (revised Wells score) | Clinical probability | ||
|
| |||
| Low | <2 | Low | 0–3 |
| Intermediate | 2–6 | Intermediate | 4–10 |
| High | >6 | High | ≥11 |
|
| |||
| PE unlikely | ≤4 | ||
| PE likely | >4 | ||
DVT, deep venous thrombosis; PE, pulmonary embolism.
The value of CT in diagnosing PE after applying CDRs has been evaluated in many studies (41). The largest study to date was PIOPED II (824 patients), where Wells score and CTPA results were compared with V/Q imaging, invasive pulmonary angiography or ultrasonography for DVT (23). PIOPED II concluded that the predictive value of CTPA for diagnosing or excluding PE is high in case of concordant clinical probability assessment, but additional testing is needed when clinical probability is inconsistent with the CTPA results, although these results are mainly applicable for four-detector row CT (23).
In the Christopher study the effectiveness of revised Wells score, D-dimer testing, and CT was assessed (42). PE was safely excluded by revised Wells score ≤4 and low D-dimer testing (<500 ng/mL); only one DVT (0.1%) and four nonfatal PEs (0.4%) occurred among 1028 untreated patients. They concluded that patients with high Wells score (>4), or low Wells score (≤4) but positive D-dimer test should undergo CTPA. In such cases, a negative CTPA was shown to safely exclude PE, with a low incidence of DVT (0.6%), nonfatal PE (0.2%), and fatal PE (42–44). Of note, among patients with a high pretest probability for PE but normal D-dimer, the CTPA showed PE in 19%–28% of patients when using quantitative rapid ELISA technique for determining D-dimers or even 24%–36% when using a semiquantitative latex agglutination technique (45, 46). Therefore, a negative D-dimer cannot be used for safely excluding PE in patients with a high pretest probability.
In the current diagnostic work-up of patients with suspected PE, the combination of low clinical probability and normal D-dimer level safely excludes PE (47). A high (>500 ng/mL) plasma D-dimer level is a highly sensitive but nonspecific screening test for suspected PE. Elevated D-dimer levels are present in almost all patients with PE but are also present in many other conditions, including advanced age, pregnancy, trauma, postoperative period, inflammation, and cancer (48).
A low D-dimer rules out PE in 60% of patients below 40 years of age, but only in 5% of patients older than 80 years. Recently an age-adjusted D-dimer cutoff (age × 10 μg/L, above 50 years) was associated with a higher number of patients in whom PE could be ruled out (from 6.4% [95% CI, 4.8%–8.5%] to 29.7% [95% CI, 26.4%–33.3%]), with a low likelihood of subsequent clinical VTE. The age-adjusted D-dimer cutoff can help excluding the need for CTPA in the elderly with compromised renal function (49).
Radiation exposure in CTPA
Since CTPA has replaced V/Q imaging, and the number of CTPA examinations has increased exponentially, concern has been raised about the risks that are associated with the radiation exposure. CTPA delivers a higher absorbed dose to breast tissue than V/Q imaging (typically 10–70 mSv for CTPA vs. <1.5 mSv for V/Q imaging) (50). The variation in values is related to CT parameter settings, differences in size and configuration of breast tissue, and the methods to calculate or directly measure radiation dose. For comparison, these doses greatly exceed the American College of Radiology recommendation of an equivalent dose in breast tissue of 3 mSv or less for standard two-view mammography (51). Patient risk also depends on age, with lower risk for patients above 40 years (52), due to the relative shorter life expectancy after 40 years and the latency period of radiation-induced tumors.
Actual data on the carcinogenic potential at the relatively low dose level at which CTPA is performed are lacking. Most authors have drawn conclusions of increased cancer risk based on the outcomes of victims surviving the atomic bombings in Hiroshima and Nagasaki by extrapolating effects that have been observed at a relatively high dose to supposed effects at a low dose (51). The excess stochastic risk of fatal cancer induction in a standard person is estimated as five deaths per 100,000 persons per mSv (53), meaning 15–30 excess deaths per 100,000 persons undergoing CTPA with the current effective dose of 3–6 mSv for CTPA (54). There have been substantial achievements in dose reduction for CT in general and for CTPA in particular. Automatic exposure control adapts the output of the scanner to the size and build (posture) of each individual patient, over ranging of helical scans can be reduced with dynamic collimators, iterative reconstructions and advanced noise reduction allows for radiation reduction whilst retaining image quality, and CT scans with intravenous iodine contrast like CTPA, can be optimized by using a lower tube voltage (e.g., 80 or 100 kV). Lowering the tube voltage and tube current and anatomic coverage can lower the dose by 81% (55). Mean effective chest radiation dose reduction of 27.6% in patients more than 60 kg can be achieved when adaptive statistical iterative reconstruction is used (56).
Major risks of radiation exposure during CTPA are developing breast and lung carcinoma. The lifetime attributable risk for breast cancer has been estimated by phantom studies to be 20 excess cases per 100,000 in 55-year-old women undergoing a single CTPA examination. These risk calculations are suggested to be higher in young peripartum women (57). Both CTPA and a nuclear medicine perfusion imaging have high diagnostic performance in pregnant women and can be used without substantial risk to the fetus (21). However, there is evidence that CTPA results in lower radiation dose to the fetus in the first or second trimester as compared to nuclear perfusion imaging (24). The estimated equivalent dose for the fetus in first, second, and third trimester of pregnancy is 0.003–0.02 mSv, 0.008–0.08 mSv, 0.0051–0.13 mSv, respectively, in case of CTPA, while this is 0.06–0.12 mSv for nuclear lung perfusion imaging (21). In young women, some authors recommend use of V/Q imaging above CTPA because of the lower radiation dose to the breast (50). In pregnant women, the current guidelines on the diagnosis and management of acute PE favor nuclear lung perfusion imaging as far as exposure of the breasts to radiation is concerned (21). However, the evidence to recommend either CTPA or nuclear lung perfusion is complex and effects to the mother and fetus should be taken into consideration. As an initial imaging study a chest film is recommended by the American Thoracic Society (58), and compression ultrasonography of the legs may be suggested in case of leg symptoms; if the ultrasound exam is positive for deep-vein thrombosis the need for radiographic imaging is eliminated (21). When the chest film is normal a nuclear lung scintigraphy is recommended; however, if the chest film is abnormal or the lung scintigraphy is nondiagnostic, a CTPA is recommended (58). CTPA and nuclear perfusion scanning have equivalent clinical negative predictive value (99% for CTPA and 100% for perfusion scanning) and have equivalent image quality in the care of pregnant patients. Overall, the PE prevalence in series of PE suspicion during pregnancy is lower than in the general population (only 3.7% as compared to 5%–25% in the general population) (59). There are no known mutagenic or teratogenic effects of iodinated contrast. Recent study has shown that a single, high dose in utero exposure to iodinated contrast is unlikely to give suppression of neonatal thyroid function (60).
Potential adverse effects related to the use of iodinated contrast agent administration include contrast material allergy and contrast material induced nephropathy. V/Q imaging may be an alternative imaging tool in patients with allergy to iodine or severe renal insufficiency (21).
CT in estimating PE severity and prognosis
PE obstruction index and embolus location
Several studies have focused on the performance of multidetector CT in the diagnosis of PE and its ability to determine the embolus burden, which can be calculated by either applying conventional angiographic scores adapted for CT (Miller and Walsh scores) or dedicated CT scores (Qanadli and Mastora scores) (61).
The prognostic role of embolic burden as a marker for short-term clinical outcome is a topic of discussion (62). In a meta-analysis, exploring short-term (30-day or three-month) prognostic value of embolic burden on CT by Qanadli obstruction index (16 studies, 3884 patients) and by embolic location, classified as central (at least lobar branches) or distal (segmental and subsegmental branches), three studies involving 1309 patients demonstrated that the location of emboli in the central pulmonary artery branches was associated with a two-fold increased risk of 30-day mortality, (OR, 2.24; 95% CI, 1.29–3.89) (62). However, no association was found between the Qanadli obstruction index and 30-day or three-month mortality. The meta-analysis included a variety of studies, mostly with hemodynamically stable and some with hemodynamically unstable patients. The main difference between the two methods (embolic burden by Qanadli obstruction index and embolic location) is that the Qanadli score includes the total number of pulmonary vascular segments and the degree of embolic obstruction that allows calculation of the percentage of blocked pulmonary arteries. The location method includes the more proximal emboli, but disregards the degree of obstruction. According to the authors, the differences in prognostic value observed in this study between the location of emboli and Qanadli score may reflect their effect on RV dilatation (62). While multiple small emboli by Qanadli score may not have been able to induce RV dysfunction, central emboli may present more often with RV dysfunction (62). Thus, the location of emboli seems to be more important in predicting the short-term mortality than the percent embolic obstruction of the pulmonary arterial bed.
RV dysfunction
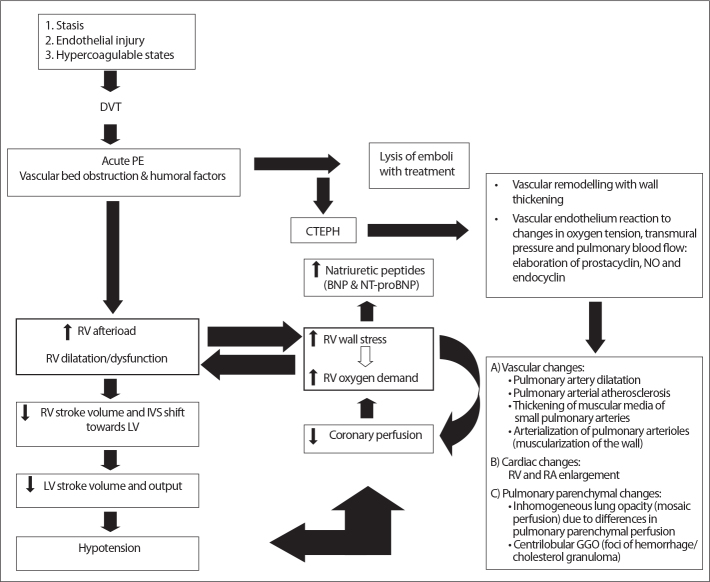
Whereas prognosis in PE patients is only weakly correlated with embolus-load, it is highly dependent on RV function (Fig. 1). Pulmonary artery obstruction causes an increase in RV afterload, release of neurohumoral factors, and RV enlargement and dysfunction (63). Elevated RV pressure leads to interventricular septum shift and compression of the left ventricle. This may result in LV diastolic failure, underfilling, and ultimately in LV systolic dysfunction and cardiogenic shock (64, 65). Secondary to increased RV afterload, wall stress and myocardial damage, cardiac troponins and brain natriuretic protein (BNP) or its precursors (NT-pro-BNP) increase (66). The mechanism and pathophysiologic pathway of PE and RV dysfunction are shown in Fig. 2.
Figure 1.
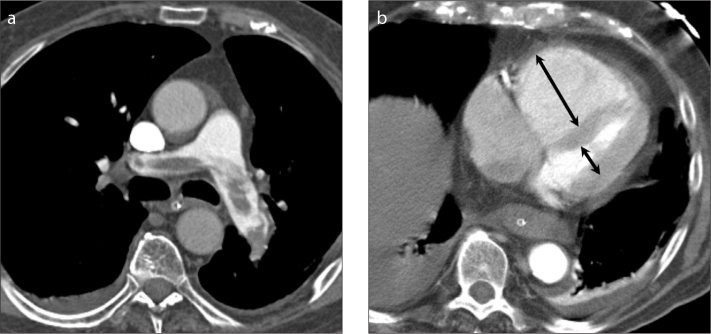
a, b. A 82-year-old man presenting with hypoxemia, hypotension, sinus tachycardia and ECG-changes suggesting right ventricular strain. Multidetector CT (a) shows a saddle embolism, with signs of right ventricular dysfunction with high RV/LV diameter ratio of 2.8 (arrows in b; arrow in the LV cavity is partly overlying the papillary muscle). The patient was treated with thrombolysis which resolved the pulmonary embolism (b), however, the patient finally died of ventilation-associated pneumonia and septic shock.
Figure 2.
Pathophysiology of hemodynamic instability due to pulmonary embolism, development of CTEPH, and its cardiovascular and pulmonary parenchymal changes. Predisposing factors may cause DVT that can dislodge and cause pulmonary embolism. Pulmonary embolism can cause RV failure directly or indirectly after inadequate lysis of emboli and development of CTEPH. Right lower box shows morphologic changes that may be observed on CT. DVT, deep vein thrombosis; PE, pulmonary embolism; RV, right ventricular; LV, left ventricular; IVS, interventricular septal; CTEPH, chronic thromboembolic pulmonary hypertension; BNP, brain natriuretic peptide; NT-proBNP, aminoterminal-probrain natriuretic peptide; NO, nitric oxide; RA, right atrial; GGO, ground glass opacity.
International echocardiographic multicenter studies have shown that in PE patients, RV dysfunction is a major determinant of short-term mortality (67, 68). RV dysfunction can also be recognized on CT, e.g., by measuring the ratio of RV/LV diameters (8, 9) or by volumetric measurements, that can be obtained with or without electrocardiography (ECG)-gating (69, 70). Several multidetector CT studies have shown the prognostic value of RV dysfunction that may help to identify those patients at risk and may facilitate selecting therapeutic strategies (9).
A RV/LV diameter ratio cutoff value of >1.0 is commonly considered to represent RV dysfunction and has been shown to predict short-term adverse outcome and mortality (10, 71, 72). Axial and four-chamber view measurements of RV/LV diameter ratio >1.0 are comparable for predicting 30-day mortality (10, 73). Normotensive patients with PE and a RV/LV diameter ratio <1.0 have excellent short-term outcomes with a negative predictive value (NPV) of 98%–100% (10, 73). This means that patients without RV dysfunction have a very low risk for in-hospital death or adverse outcome and could be candidate for home-treatment. Conversely, normotensive PE patients with a RV/LV diameter ratio >1.0 have been associated with a three-month mortality risk of 3%–15%, but only a low positive predictive value (PPV) of 9%–15% was observed. This implies that many patients with RV/LV diameter ratio >1.0 will not have severe short-term complications (10, 73).
It has been suggested that volumetric measurements, obtained with or without ECG-gating may be more useful as a marker for RV dysfunction in patients with acute PE (69, 71). In a study with 260 PE patients, a RV/LV volume ratio >1.2 resulted in a six-fold increased risk for 30-day mortality, while 97.8% survived with a ratio <1.2 (71). Non-ECG-gated volumetric analysis seems to be slightly superior to identify high-risk patients with adverse clinical outcome compared to RV/LV diameter ratio measurements (71, 74). Furthermore, axial and four-chamber view measurements of RV/LV diameter ratio have shown considerable interobserver variability, while volumetric measurements have shown better reproducibility (71). In another study of 60 patients, a RV/LV volumetric ratio >1.28 measured in non-ECG-gated CTPA showed 89% sensitivity, 75% specificity, 42% PPV and 97% NPV to predict adverse clinical outcome (74).
A recent study, in which an additional low-dose ECG-gated cardiac scan was performed in 113 patients with PE, showed that RV ejection fraction <47% was the best predictor of adverse clinical outcome, with a sensitivity of 100% (95% CI, 52%–100%) and specificity of 60% (95% CI, 50%–70%). The PPV was 13% (95% CI, 5%–26%) and NPV 100% (95% CI, 93%–100%) (10). However, no significant additional value of RV ejection fraction <47% compared with axial RV/LV diameter ratio >1.0 was shown (10). Thus, measuring simple RV/LV diameter ratio >1.0 on standard axial CTPA was shown to be as prognostic as RV ejection fraction <47% by ECG-gated cardiac CT. The limited PPV of adverse outcome will probably not outweigh the additional radiation dose associated with ECG-gating for function analysis. Thus, it can be helpful to estimate patient prognosis in PE by RV/LV diameter ratio measures that can be made directly on the CTPA investigation. By ESC consensus criteria, RV/LV diameter ratio <1 in combination with advantageous clinical parameters may be used as to help select patients who may be treated at home instead of hospital (21). However, the recent Hestia Study has suggested that hemodynamically stable patients with asymptomatic RV dysfunction as assessed by RV/LV diameter ratio >1.0 may also be treated safely at home when appropriately selected based on the Hestia criteria risk checklist (75).
Many other secondary CT signs of RV dysfunction such as interventricular septum bowing, inferior vena cava reflux, and pulmonary artery diameter exceeding that of the aorta have been recognized, although without direct association with short- term mortality in acute PE (76).
To conclude, regarding prognosis in PE, RV function is more important than embolus load. RV function represented by RV/LV diameter ratio can be helpful in estimating prognosis and, with further selection based on clinical parameters, this may be used for safely selecting patients for treatment at home.
Prognostic value of cardiac biomarkers
Elevated cardiac biomarkers like NT-pro-BNP, secondary to increased RV afterload and wall stress have shown a higher discriminative power and clinical utility as predictor of adverse events after PE than the RV/LV diameter ratio (77). NT-pro-BNP increased to > 600 pg/mL may be superior to RV/LV ratio >1.0 in predicting adverse events after PE. The NPV is excellent for both (99% vs. 98%) (77).
CT findings of CTEPH as a complication of acute PE
CTEPH is defined as mean pulmonary artery pressure greater than 25 mmHg that persists six months after an acute PE and pulmonary vascular resistance of 3 Wood units (240 dyne·s/cm5) or greater with persistent pulmonary arterial thrombotic obstruction despite at least three months of effective, uninterrupted anticoagulation therapy (78). The pathogenesis of CTEPH is poorly understood, although a clinical history of VTE has been recorded in 80% of patients. Inadequate anticoagulation, large thrombus mass, residual thrombi and recurrence of VTE may contribute to development of CTEPH (79).
Pathologically, CTEPH is characterized by wall-adherent or intraluminal organized thrombus, forming vascular channels interspersed with connective tissue (80). Peculiar finding is that a history of symptomatic VTE is lacking in 31%–42% of patients diagnosed with CTEPH (81). A prospective follow-up study of 834 consecutive patients suspected for PE (and 320 proven PE) reported a CTEPH incidence of 1% (82). Another study comprised 866 unselected, consecutive patients after acute PE (83). Patients without known pulmonary hypertension underwent echocardiography. Patients with echocardiographically suspected pulmonary hypertension had total diagnostic work-up for CTEPH, including V/Q imaging and invasive pulmonary artery pressure measurements (83). After an average follow-up period of 34 months of all 866 patients, CTEPH incidence was found as 0.57%.
Diagnosing CTEPH is relevant to guide potential treatment options. Patients with predominantly proximal vascular distribution may be treated with pulmonary artery endarterectomy, while more distally located CTEPH is not amenable to surgery and must be treated medically (84). Endarterectomy can lead to major health improvement. Studies have shown that before thrombectomy, over 95% of CTEPH patients were in poor functional New York Heart Association (NYHA) class III or IV, i.e., only comfortable at rest or severe limitation with symptoms even at rest (85). One year after thrombectomy, 93%–95% of patients were found to have NYHA class I or II, i.e., no limitations or mild symptoms during normal activity (85, 86). Thrombectomy improved not only the functional status, but also long-term survival (six-year survival of 75%) (86).
CT diagnosis of CTEPH
CT imaging is the method of choice for diagnosis and follow-up CTEPH (87). CT sensitivity (86%) exceeds that of invasive pulmonary angiography (70%) and MRI (27%–44%) (88), and CT has been found more specific than nuclear scintigraphy (89). CT has advantages in evaluating CTEPH patients as it provides direct information over wall-adherent thrombus, RV function and changes in lung parenchyma (88).
The primary diagnostic CT criteria for CTEPH are listed in Table 2. These diagnostic criteria include the demonstration of intraluminal filling defects (intravascular web), signs of pulmonary hypertension such as dilatation of the pulmonary arteries and RV, and RV hypertrophy (87). The peripheral pulmonary vessels show decreased diameters (80).
Table 2.
Summary of diagnostic criteria for CTEPH
| Criteria | Pathologic findings | CT findings |
|---|---|---|
| Primary (87) | ||
| Changes in pulmonary arteries: | Organizing thrombus |
|
| Changes due to PH: | Intravascular webs (due to recanalization) | Linear intraluminal filling defects |
| Dilatation and atherosclerotic changes (due to increased pressure and turbulent flow) | ||
|
| ||
| Secondary (85) | Small-vessel arteriopathy due to shear stress, pressure, inflammation, and the release of cytokines | Mosaic lung perfusion (due to decreased perfusion) and variation in the size of segmental vessels |
Secondary signs of CTEPH include mosaic perfusion pattern, referring to heterogeneous lung attenuation due to heterogeneous blood flow (90), and enlargement of bronchial or non-bronchial collateral systemic arteries (Fig. 3) (85). Not all features are required to be diagnosed as CTEPH (84).
Figure 3.
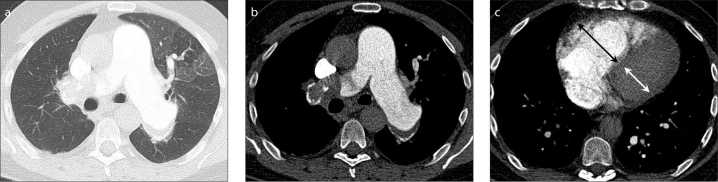
a–c. CTPA and HRCT reconstruction in CTEPH and mosaic perfusion. A 53-year-old man with CTEPH who presented with persistent dyspnea and dyspnea on exertion lasting several months, known with a history of adequately treated acute PE six years before. Blood analysis was unremarkable except elevated NT-proBNP (1212 ng/mL), echocardiography showed severe RV and right atrial dilatation with moderate tricuspid insufficiency and an estimated pulmonary artery pressure of 62 mmHg. Right-sided catheter measurements showed a pulmonary vascular resistance of 781 dyne·s/cm5 and a mean pulmonary artery pressure at rest of 46 mmHg. CTPA with high resolution reconstructions in lung setting (a) shows mosaic perfusion with hyperperfused pulmonary areas of high attenuation associated with larger vessels, and areas of hypoperfusion with low attenuation that contain smaller vessels. In soft tissue setting (b), the diameter ratio of the main pulmonary artery to the aorta is >1, indicative for pulmonary hypertension. Also note the wall-adherent thrombus and atherosclerotic calcification of the main pulmonary arteries, as signs of pulmonary hypertension. There is intraluminal web in the segmental artery to the left upper lobe. Dilated RV and flattening of the ventricular septum indicate RV dysfunction (c). Figure is published with patient’s permission and courtesy of Dr. I. Bahce and Dr. A. Boonstra, Department of Pulmonary Diseases at the VU University Medical Center Amsterdam, the Netherlands.
Regarding the size of the main pulmonary artery at the level of pulmonary artery bifurcation, a variety of “upper limits” of normal diameter have been published (91, 92). A practical rule-of-thumb is the diameter ratio of the main pulmonary artery to that of the (normal) aorta measured on CT. A ratio >1 strongly suggests pulmonary hypertension, with 92% specificity and 96% PPV (93). The main pulmonary artery (PA) diameter is measured at the level of the bifurcation of the right PA and perpendicular to its long axis. This is easy to define anatomically and highly reproducible on axial CT images (92). At the same location, the ascending aorta can be measured to provide the ratio of PA diameter to that of the aorta (92). A PA diameter of 29 mm or more was determined to have 97% PPV, 87% sensitivity, and 89% specificity for the presence of pulmonary hypertension (94). Specificity increases to 100% if accompanied by findings of a segmental artery-to-bronchus ratio greater than one in three of four pulmonary lobes (94). However, a diameter less than 29 mm does not rule out pulmonary hypertension (94).
Recently, the Framingham Heart Study reported a sex-specific 90th percentile cutoff value with risk for pulmonary hypertension above a PA diameter of 29 mm in men and 27 mm in women on ECG-gated CT. Increase with diameter was associated with higher risk for self-reported dyspnea (adjusted odds ratio, 1.31) (92). Regarding patient size, only a moderate correlation has been observed between body surface area and PA diameter (for men: r=0.41; for women: r=0.42). Moreover, the correlation between PA diameter and age was poor (men: r=0.10; women: r=0.07), indicating that the PA size does not significantly increase with age (92).
Mosaic perfusion is common and occurs in 77%–100% of patients with CTEPH (95). Distinction of mosaic pattern in chronic PE from that of small airway disease may be made by assessing the diameter of the main PA that may be typically dilated in chronic PE (88), but is usually normal in patients with airway disease (96). Both airway disease and CTEPH have decreased size and number of vessels in lucent lungs compared with higher-attenuation lung. Associate signs that help differentiate are direct signs of airways disease such as bronchial wall thickening and bronchiectasis (97). Also, air trapping will be present in airway disease but not in CTEPH (98). In CTEPH, chronic thrombi may be observed.
As compared to patients with CTEPH, mosaic perfusion is seen significantly less often in patients with idiopathic or cardiac or pulmonary disease-associated pulmonary arterial hypertension (90).
The normal pulmonary circulation is a low-pressure, low-resistance, and highly distensible system (99). In CTEPH, pulmonary vascular remodeling results in increased vascular resistance and decreased vascular compliance (i.e., increased wall stiffness) (100). Consequently, the distensibility of the pulmonary arteries will decrease. The duration and the extent of increased PA pressure seem crucial in vessel wall remodeling. PA distensibility has been suggested to be a sensitive (83%) and specific (82%) marker for PH, even in early or mild clinical stages (101, 102). Moreover, decreased PA distensibility was shown to be related to mortality (99). Measurement of PA distensibility is possible from ECG-gated CT and can be calculated from the change in cross-sectional PA area between diastole and systole, as the maximal cross-sectional area minus the minimal cross-sectional area divided by the maximal cross-sectional area (103). Studies have demonstrated that the PA distensibility was lowest in PH (6.0%±2.7%), but also decreased in PE (12.9%±3.4%) as compared with normal controls (25.9%±5.7%) (102). In PE patients, anticoagulant therapy significantly improves PA distensibility (102). Including functional approach of PA distensibility evaluation next to anatomic CT measurements of main PA diameter may better identify patients with PH (102). In patients with PH, CT measurements of PA distensibility have shown good interobserver reproducibility (r>0.7) (104). Measuring PA distensibility may be helpful in patients with CTEPH. These patients eventually die due to RV failure. Diagnosing decreased distensibility could help to identify CTEPH patients who may benefit from thrombectomy by reducing RV afterload (105).
Some studies have suggested that innovative CT techniques such as contrast-enhanced dual-energy or subtraction techniques may provide further functional information on blood volume by assessing the pulmonary iodine distribution as a marker for pulmonary perfusion (106). CT-perfusion may help differentiate between various forms of pulmonary hypertension, i.e., chronic thromboembolic vs. nonembolic pulmonary hypertension (107). Iodine mapping by using dual-energy may be complementary to thin-section CTPA and may be used to study the impact of CTEPH on regional pulmonary perfusion, which seems strongly correlated to mosaic attenuation patterns, where iodine mapping may also identify systemic collateral supply in CTEPH (108). The ability to characterize perfusion parameters next to anatomic evaluation by dual-energy or subtraction CT techniques may further refine diagnostic accuracy and prognosis in the assessment of acute PE and CTEPH (108).
Conclusion
PE is a potentially life threatening disease with a challenging diagnosis. CTPA requests from emergency departments are increasing. Considering the risk of X-ray induced malignancy (e.g., breast carcinoma in women of child-bearing age), the indication for CTPA should outweigh its risk. To avoid useless patient radiation exposure, CTPA should not be overused. Correct implementation of clinical decision rules in diagnostic workup for PE improves adequate use of CT. CTPA is excellent for including and excluding PE, therefore CT is the first-choice diagnostic imaging technique in patients suspected of having acute PE. Also, CT adds prognostic value by evaluating RV function. CT-assessed RV dysfunction and to lesser extent central emboli location predicts PE-related mortality in normotensive and hypotensive patients, while PE embolic obstruction index has limited prognostic value. Simple RV/LV diameter ratio measures >1.0 already predict risk for adverse outcome, whereas ratios <1.0 can safely exclude adverse outcome. Consequently, assessing the RV/LV diameter ratio may help identify patients who are potential candidates for treatment at home instead of treatment in the hospital. A minority of patients develop CTEPH following acute PE, which is a life-threatening condition that can be diagnosed by CT. In proximal CTEPH involving the more central pulmonary arteries, thrombectomy usually results in good outcome in terms of both functional status and long-term survival rate. CT is becoming the imaging method of choice for diagnosing CTEPH as it can identify patients who may benefit from thrombectomy. New CT developments such as distensibility measurements and dual-energy or subtraction techniques may further refine diagnosis and prognosis for improved patient care.
Main points.
CT pulmonary angiography (CTPA) is the first-choice diagnostic imaging technique in the diagnostic algorithm for pulmonary embolism.
The high accuracy and easy accessibility increased the CTPA orders; however, the indication for CTPA should outweigh its potential risk of X-ray induced malignancy.
Implementation of clinical decision rules (like Wells and Geneva score) can decrease CTPA orders and increase the number of positive CTPA and therefore is recommended by the European Society of Cardiology guidelines.
CT adds prognostic value by evaluating right ventricular (RV) function; simple RV/left ventricular (LV) diameter ratio > 1.0 predicts risk for adverse outcome, whereas ratios <1.0 can safely exclude adverse outcome.
CT is becoming the imaging method of choice for diagnosis and follow-up of chronic thromboembolic pulmonary hypertension (CTEPH) as it can identify patients who may benefit from thrombectomy.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Giuntini C, Di Ricco G, Marini C, Melillo E, Palla A. Pulmonary embolism: epidemiology. Chest. 1995;107(Suppl 1):3S–9S. doi: 10.1378/chest.107.1_supplement.3s. http://dx.doi.org/10.1378/chest.107.1_Supplement.3S. [DOI] [PubMed] [Google Scholar]
- 2.Stein PD, Woodard PK, Hull RD, et al. Gadolinium-enhanced magnetic resonance angiography for detection of acute pulmonary embolism: an in-depth review. Chest. 2003;124:2324–2328. doi: 10.1016/s0012-3692(15)31694-9. [DOI] [PubMed] [Google Scholar]
- 3.Wintersperger BJ, Nikolaou K. Basics of cardiac MDCT: techniques and contrast application. Eur Radiol. 2005;15( Suppl 2):B2–B9. doi: 10.1007/s10406-005-0090-0. http://dx.doi.org/10.1007/s10406-005-0090-0. [DOI] [PubMed] [Google Scholar]
- 4.Remy-Jardin M, Pistolesi M, Goodman LR, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology. 2007;245:315–329. doi: 10.1148/radiol.2452070397. http://dx.doi.org/10.1148/radiol.2452070397. [DOI] [PubMed] [Google Scholar]
- 5.Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–1245. doi: 10.1056/NEJM199205073261902. http://dx.doi.org/10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 6.Stein PD, Kayali F, Olson RE. Trends in the use of diagnostic imaging in patients hospitalized with acute pulmonary embolism. Am J Cardiol. 2004;93:1316–1317. doi: 10.1016/j.amjcard.2004.02.022. http://dx.doi.org/10.1016/j.amjcard.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Kjaergaard J, Schaadt BK, Lund JO, Hassager C. Quantification of right ventricular function in acute pulmonary embolism: relation to extent of pulmonary perfusion defects. Eur J Echocardiogr. 2008;9:641–645. doi: 10.1093/ejechocard/jen033. http://dx.doi.org/10.1093/ejechocard/jen033. [DOI] [PubMed] [Google Scholar]
- 8.Quiroz R, Kucher N, Schoepf UJ, et al. Right ventricular enlargement on chest computed tomography: prognostic role in acute pulmonary embolism. Circulation. 2004;109:2401–2404. doi: 10.1161/01.CIR.0000129302.90476.BC. http://dx.doi.org/10.1161/01.CIR.0000129302.90476.BC. [DOI] [PubMed] [Google Scholar]
- 9.Reid JH, Murchison JT. Acute right ventricular dilatation: a new helical CT sign of massive pulmonary embolism. Clin Radiol. 1998;53:694–698. doi: 10.1016/s0009-9260(98)80297-3. http://dx.doi.org/10.1016/S0009-9260(98)80297-3. [DOI] [PubMed] [Google Scholar]
- 10.van der Bijl N, Klok FA, Huisman MV, et al. Measurement of right and left ventricular function by ECG-synchronized CT scanning in patients with acute pulmonary embolism: usefulness for predicting short-term outcome. Chest. 2011;140:1008–1015. doi: 10.1378/chest.10-3174. http://dx.doi.org/10.1378/chest.10-3174. [DOI] [PubMed] [Google Scholar]
- 11.van Beek EJ, Brouwerst EM, Song B, Stein PD, Oudkerk M. Clinical validity of a normal pulmonary angiogram in patients with suspected pulmonary embolism--a critical review. Clin Radiol. 2001;56:838–842. doi: 10.1053/crad.2001.0778. http://dx.doi.org/10.1053/crad.2001.0778. [DOI] [PubMed] [Google Scholar]
- 12.Diffin DC, Leyendecker JR, Johnson SP, Zucker RJ, Grebe PJ. Effect of anatomic distribution of pulmonary emboli on interobserver agreement in the interpretation of pulmonary angiography. AJR Am J Roentgenol. 1998;171:1085–1089. doi: 10.2214/ajr.171.4.9763002. http://dx.doi.org/10.2214/ajr.171.4.9763002. [DOI] [PubMed] [Google Scholar]
- 13.Stein PD, Henry JW, Gottschalk A. Reassessment of pulmonary angiography for the diagnosis of pulmonary embolism: relation of interpreter agreement to the order of the involved pulmonary arterial branch. Radiology. 1999;210:689–691. doi: 10.1148/radiology.210.3.r99mr41689. http://dx.doi.org/10.1148/radiology.210.3.r99mr41689. [DOI] [PubMed] [Google Scholar]
- 14.Brunot S, Corneloup O, Latrabe V, Montaudon M, Laurent F. Reproducibility of multi-detector spiral computed tomography in detection of sub-segmental acute pulmonary embolism. Eur Radiol. 2005;15:2057–2063. doi: 10.1007/s00330-005-2844-4. http://dx.doi.org/10.1007/s00330-005-2844-4. [DOI] [PubMed] [Google Scholar]
- 15.Ghaye B, Szapiro D, Mastora I, et al. Peripheral pulmonary arteries: how far in the lung does multi-detector row spiral CT allow analysis? Radiology. 2001;219:629–636. doi: 10.1148/radiology.219.3.r01jn32629. http://dx.doi.org/10.1148/radiology.219.3.r01jn32629. [DOI] [PubMed] [Google Scholar]
- 16.Stein PD, Hull RD, Raskob GE. Withholding treatment in patients with acute pulmonary embolism who have a high risk of bleeding and negative serial noninvasive leg tests. Am J Med. 2000;109:301–306. doi: 10.1016/s0002-9343(00)00508-8. http://dx.doi.org/10.1016/S0002-9343(00)00508-8. [DOI] [PubMed] [Google Scholar]
- 17.Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED) The PIOPED Investigators. JAMA. 1990;263:2753–2759. doi: 10.1001/jama.1990.03440200057023. http://dx.doi.org/10.1001/jama.1990.03440200057023. [DOI] [PubMed] [Google Scholar]
- 18.Duc-Pennec A, Le Roux PY, Cornily JC, et al. Diagnostic accuracy of single-photon emission tomography ventilation/perfusion lung scan in the diagnosis of pulmonary embolism. Chest. 2012;141:381–387. doi: 10.1378/chest.11-0090. http://dx.doi.org/10.1378/chest.11-0090. [DOI] [PubMed] [Google Scholar]
- 19.Remy-Jardin M, Remy J, Wattinne L, Giraud F. Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single-breath-hold technique--comparison with pulmonary angiography. Radiology. 1992;185:381–387. doi: 10.1148/radiology.185.2.1410342. http://dx.doi.org/10.1148/radiology.185.2.1410342. [DOI] [PubMed] [Google Scholar]
- 20.Duddalwar VA. Multislice CT angiography: a practical guide to CT angiography in vascular imaging and intervention. Br J Radiol. 2004;77:S27–S38. doi: 10.1259/bjr/25652856. http://dx.doi.org/10.1259/bjr/25652856. [DOI] [PubMed] [Google Scholar]
- 21.Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29:2276–2315. doi: 10.1093/eurheartj/ehn310. http://dx.doi.org/10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 22.Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology. 2000;217:447–455. doi: 10.1148/radiology.217.2.r00nv01447. http://dx.doi.org/10.1148/radiology.217.2.r00nv01447. [DOI] [PubMed] [Google Scholar]
- 23.Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–2327. doi: 10.1056/NEJMoa052367. http://dx.doi.org/10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 24.Winer-Muram HT, Boone JM, Brown HL, Jennings SG, Mabie WC, Lombardo GT. Pulmonary embolism in pregnant patients: fetal radiation dose with helical CT. Radiology. 2002;224:487–492. doi: 10.1148/radiol.2242011581. http://dx.doi.org/10.1148/radiol.2242011581. [DOI] [PubMed] [Google Scholar]
- 25.Cronin P, Weg JG, Kazerooni EA. The role of multidetector computed tomography angiography for the diagnosis of pulmonary embolism. Semin Nucl Med. 2008;38:418–431. doi: 10.1053/j.semnuclmed.2008.07.002. http://dx.doi.org/10.1053/j.semnuclmed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Wittram C, Waltman AC, Shepard JA, Halpern E, Goodman LR. Discordance between CT and angiography in the PIOPED II study. Radiology. 2007;244:883–889. doi: 10.1148/radiol.2443061693. http://dx.doi.org/10.1148/radiol.2443061693. [DOI] [PubMed] [Google Scholar]
- 27.Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-based medicine: how to practice and teach EBM. London: Churchill-Livingstone; 2000. [Google Scholar]
- 28.Galipienzo J, Garcia dT, Flores J, Alvarez C, Alonso-Viteri S, Ruiz A. Safety of withholding anticoagulant therapy in patients with suspected pulmonary embolism with a negative multislice computed tomography pulmonary angiography. Eur J Intern Med. 2010;21:283–288. doi: 10.1016/j.ejim.2010.05.006. http://dx.doi.org/10.1016/j.ejim.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Mos IC, Klok FA, Kroft LJ, de Roos A, Dekkers OM, Huisman MV. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491–1498. doi: 10.1111/j.1538-7836.2009.03518.x. http://dx.doi.org/10.1111/j.1538-7836.2009.03518.x. [DOI] [PubMed] [Google Scholar]
- 30.Blachere H, Latrabe V, Montaudon M, et al. Pulmonary embolism revealed on helical CT angiography: comparison with ventilation-perfusion radionuclide lung scanning. AJR Am J Roentgenol. 2000;174:1041–1047. doi: 10.2214/ajr.174.4.1741041. http://dx.doi.org/10.2214/ajr.174.4.1741041. [DOI] [PubMed] [Google Scholar]
- 31.van Beek EJ, Reekers JA, Batchelor DA, Brandjes DP, Buller HR. Feasibility, safety and clinical utility of angiography in patients with suspected pulmonary embolism. Eur Radiol. 1996;6:415–419. doi: 10.1007/BF00182453. http://dx.doi.org/10.1007/BF00182453. [DOI] [PubMed] [Google Scholar]
- 32.Carrier M, Righini M, Wells PS, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010;8:1716–1722. doi: 10.1111/j.1538-7836.2010.03938.x. http://dx.doi.org/10.1111/j.1538-7836.2010.03938.x. [DOI] [PubMed] [Google Scholar]
- 33.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069. doi: 10.1093/eurheartj/ehu283. http://dx.doi.org/10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 34.den Exter PL, van Es J, Klok FA, et al. Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood. 2013;122:1144–1149. doi: 10.1182/blood-2013-04-497545. http://dx.doi.org/10.1182/blood-2013-04-497545. [DOI] [PubMed] [Google Scholar]
- 35.Raja AS, Ip IK, Prevedello LM, et al. Effect of computerized clinical decision support on the use and yield of CT pulmonary angiography in the emergency department. Radiology. 2012;262:468–474. doi: 10.1148/radiol.11110951. http://dx.doi.org/10.1148/radiol.11110951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weir ID, Drescher F, Cousin D, et al. Trends in use and yield of chest computed tomography with angiography for diagnosis of pulmonary embolism in a Connecticut hospital emergency department. Conn Med. 2010;74:5–9. [PubMed] [Google Scholar]
- 37.Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–420. [PubMed] [Google Scholar]
- 38.Wicki J, Perneger TV, Junod AF, Bounameaux H, Perrier A. Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score. Arch Intern Med. 2001;161:92–97. doi: 10.1001/archinte.161.1.92. http://dx.doi.org/10.1001/archinte.161.1.92. [DOI] [PubMed] [Google Scholar]
- 39.Miniati M, Prediletto R, Formichi B, et al. Accuracy of clinical assessment in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med. 1999;159:864–871. doi: 10.1164/ajrccm.159.3.9806130. http://dx.doi.org/10.1164/ajrccm.159.3.9806130. [DOI] [PubMed] [Google Scholar]
- 40.Klok FA, Kruisman E, Spaan J, et al. Comparison of the revised Geneva score with the Wells rule for assessing clinical probability of pulmonary embolism. J Thromb Haemost. 2008;6:40–44. doi: 10.1111/j.1538-7836.2007.02820.x. http://dx.doi.org/10.1111/j.1538-7836.2007.02820.x. [DOI] [PubMed] [Google Scholar]
- 41.Prologo JD, Gilkeson RC, Diaz M, Asaad J. CT pulmonary angiography: a comparative analysis of the utilization patterns in emergency department and hospitalized patients between 1998 and 2003. AJR Am J Roentgenol. 2004;183:1093–1096. doi: 10.2214/ajr.183.4.1831093. http://dx.doi.org/10.2214/ajr.183.4.1831093. [DOI] [PubMed] [Google Scholar]
- 42.van Belle A, Buller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172–179. doi: 10.1001/jama.295.2.172. http://dx.doi.org/10.1001/jama.295.2.172. [DOI] [PubMed] [Google Scholar]
- 43.Geersing GJ, Erkens PM, Lucassen WA, et al. Safe exclusion of pulmonary embolism using the Wells rule and qualitative D-dimer testing in primary care: prospective cohort study. BMJ. 2012;345:e6564. doi: 10.1136/bmj.e6564. http://dx.doi.org/10.1136/bmj.e6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srivali N, Ratanapo S, Cheungpasitporn W, Chongnarungsin D. State of the art: practical approach for diagosis of pulmonary embolism. Am Med J. 2012;3:141–146. http://dx.doi.org/10.3844/amjsp.2012.141.146. [Google Scholar]
- 45.Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140:589–602. doi: 10.7326/0003-4819-140-8-200404200-00005. http://dx.doi.org/10.7326/0003-4819-140-8-200404200-00005. [DOI] [PubMed] [Google Scholar]
- 46.Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med. 2006;119:1048–1055. doi: 10.1016/j.amjmed.2006.05.060. http://dx.doi.org/10.1016/j.amjmed.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 47.Cate-Hoek AJ, Prins MH. Management studies using a combination of D-dimer test result and clinical probability to rule out venous thromboembolism: a systematic review. J Thromb Haemost. 2005;3:2465–2470. doi: 10.1111/j.1538-7836.2005.01556.x. http://dx.doi.org/10.1111/j.1538-7836.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 48.Kelly J, Rudd A, Lewis RR, Hunt BJ. Plasma D-dimers in the diagnosis of venous thromboembolism. Arch Intern Med. 2002;162:747–756. doi: 10.1001/archinte.162.7.747. http://dx.doi.org/10.1001/archinte.162.7.747. [DOI] [PubMed] [Google Scholar]
- 49.Righini M, van Es J, den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311:1117–1124. doi: 10.1001/jama.2014.2135. http://dx.doi.org/10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]
- 50.Schembri GP, Miller AE, Smart R. Radiation dosimetry and safety issues in the investigation of pulmonary embolism. Semin Nucl Med. 2010;40:442–454. doi: 10.1053/j.semnuclmed.2010.07.007. http://dx.doi.org/10.1053/j.semnuclmed.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Parker MS, Hui FK, Camacho MA, Chung JK, Broga DW, Sethi NN. Female breast radiation exposure during CT pulmonary angiography. AJR Am J Roentgenol. 2005;185:1228–1233. doi: 10.2214/AJR.04.0770. http://dx.doi.org/10.2214/AJR.04.0770. [DOI] [PubMed] [Google Scholar]
- 52.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. http://dx.doi.org/10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 53.International Commission on Radiological Protection. The 2007 recommendations of the International Commission on Radiological Protection. Oxford: Pergamon press; 2007. [Google Scholar]
- 54.Mayo JR. Radiation dose issues in longitudinal studies involving computed tomography. Proc Am Thorac Soc. 2008;5:934–939. doi: 10.1513/pats.200808-079QC. http://dx.doi.org/10.1513/pats.200808-079QC. [DOI] [PubMed] [Google Scholar]
- 55.Litmanovich D, Boiselle PM, Bankier AA, Kataoka ML, Pianykh O, Raptopoulos V. Dose reduction in computed tomographic angiography of pregnant patients with suspected acute pulmonary embolism. J Comput Assist Tomogr. 2009;33:961–966. doi: 10.1097/RCT.0b013e318198cd18. http://dx.doi.org/10.1097/RCT.0b013e318198cd18. [DOI] [PubMed] [Google Scholar]
- 56.Prakash P, Kalra MK, Digumarthy SR, et al. Radiation dose reduction with chest computed tomography using adaptive statistical iterative reconstruction technique: initial experience. J Comput Assist Tomogr. 2010;34:40–45. doi: 10.1097/RCT.0b013e3181b26c67. http://dx.doi.org/10.1097/RCT.0b013e3181b26c67. [DOI] [PubMed] [Google Scholar]
- 57.Hurwitz LM, Reiman RE, Yoshizumi TT, et al. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: implications for cancer induction. Radiology. 2007;245:742–750. doi: 10.1148/radiol.2453062046. http://dx.doi.org/10.1148/radiol.2453062046. [DOI] [PubMed] [Google Scholar]
- 58.Leung AN, Bull TM, Jaeschke R, et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline--Evaluation of suspected pulmonary embolism in pregnancy. Radiology. 2012;262:635–646. doi: 10.1148/radiol.11114045. http://dx.doi.org/10.1148/radiol.11114045. [DOI] [PubMed] [Google Scholar]
- 59.Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195:W214–W220. doi: 10.2214/AJR.09.3506. http://dx.doi.org/10.2214/AJR.09.3506. [DOI] [PubMed] [Google Scholar]
- 60.Bourjeily G, Chalhoub M, Phornphutkul C, Alleyne TC, Woodfield CA, Chen KK. Neonatal thyroid function: effect of a single exposure to iodinated contrast medium in utero. Radiology. 2010;256:744–750. doi: 10.1148/radiol.10100163. http://dx.doi.org/10.1148/radiol.10100163. [DOI] [PubMed] [Google Scholar]
- 61.Ghaye B, Ghuysen A, Bruyere PJ, D’Orio V, Dondelinger RF. Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics. 2006;26:23–39. doi: 10.1148/rg.261055062. http://dx.doi.org/10.1148/rg.261055062. [DOI] [PubMed] [Google Scholar]
- 62.Vedovati MC, Germini F, Agnelli G, Becattini C. Prognostic role of embolic burden assessed at computed-tomography angiography in patients with acute pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost. 2013;11:2092–2102. doi: 10.1111/jth.12429. http://dx.doi.org/10.1111/jth.12429. [DOI] [PubMed] [Google Scholar]
- 63.Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877–905. doi: 10.1378/chest.121.3.877. http://dx.doi.org/10.1378/chest.121.3.877. [DOI] [PubMed] [Google Scholar]
- 64.Belenkie I, Dani R, Smith ER, Tyberg JV. Ventricular interaction during experimental acute pulmonary embolism. Circulation. 1988;78:761–768. doi: 10.1161/01.cir.78.3.761. http://dx.doi.org/10.1161/01.CIR.78.3.761. [DOI] [PubMed] [Google Scholar]
- 65.Lualdi JC, Goldhaber SZ. Right ventricular dysfunction after acute pulmonary embolism: pathophysiologic factors, detection, and therapeutic implications. Am Heart J. 1995;130:1276–1282. doi: 10.1016/0002-8703(95)90155-8. http://dx.doi.org/10.1016/0002-8703(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 66.Masotti L, Righini M, Vuilleumier N, et al. Prognostic stratification of acute pulmonary embolism: focus on clinical aspects, imaging, and biomarkers. Vasc Health Risk Manag. 2009;5:567–575. doi: 10.2147/vhrm.s4861. http://dx.doi.org/10.2147/VHRM.S4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. http://dx.doi.org/10.1016/S0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 68.Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J. 1997;134:479–487. doi: 10.1016/s0002-8703(97)70085-1. http://dx.doi.org/10.1016/S0002-8703(97)70085-1. [DOI] [PubMed] [Google Scholar]
- 69.Dogan H, Kroft LJ, Huisman MV, van der Geest RJ, de Roos A. Right ventricular function in patients with acute pulmonary embolism: analysis with electrocardiography-synchronized multi-detector row CT. Radiology. 2007;242:78–84. doi: 10.1148/radiol.2421052089. http://dx.doi.org/10.1148/radiol.2421052089. [DOI] [PubMed] [Google Scholar]
- 70.Lu MT, Cai T, Ersoy H, et al. Comparison of ECG-gated versus non-gated CT ventricular measurements in thirty patients with acute pulmonary embolism. Int J Cardiovasc Imaging. 2009;25:101–107. doi: 10.1007/s10554-008-9342-0. http://dx.doi.org/10.1007/s10554-008-9342-0. [DOI] [PubMed] [Google Scholar]
- 71.Kang DK, Thilo C, Schoepf UJ, et al. CT signs of right ventricular dysfunction: prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging. 2011;4:841–849. doi: 10.1016/j.jcmg.2011.04.013. http://dx.doi.org/10.1016/j.jcmg.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Kumamaru KK, Lu MT, Ghaderi NS, Hunsaker AR. Right ventricular enlargement in acute pulmonary embolism derived from CT pulmonary angiography. Int J Cardiovasc Imaging. 2013;29:705–708. doi: 10.1007/s10554-012-0126-1. http://dx.doi.org/10.1007/s10554-012-0126-1. [DOI] [PubMed] [Google Scholar]
- 73.Becattini C, Agnelli G, Vedovati MC, et al. Multidetector computed tomography for acute pulmonary embolism: diagnosis and risk stratification in a single test. Eur Heart J. 2011;32:1657–1663. doi: 10.1093/eurheartj/ehr108. http://dx.doi.org/10.1093/eurheartj/ehr108. [DOI] [PubMed] [Google Scholar]
- 74.Apfaltrer P, Bachmann V, Meyer M, et al. Prognostic value of perfusion defect volume at dual energy CTA in patients with pulmonary embolism: correlation with CTA obstruction scores, CT parameters of right ventricular dysfunction and adverse clinical outcome. Eur J Radiol. 2012;81:3592–3597. doi: 10.1016/j.ejrad.2012.02.008. http://dx.doi.org/10.1016/j.ejrad.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Zondag W, Vingerhoets LM, Durian MF, et al. Hestia criteria can safely select patients with pulmonary embolism for outpatient treatment irrespective of right ventricular function. J Thromb Haemost. 2013;11:686–692. doi: 10.1111/jth.12146. http://dx.doi.org/10.1111/jth.12146. [DOI] [PubMed] [Google Scholar]
- 76.Furlan A, Aghayev A, Chang CC, et al. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012;265:283–293. doi: 10.1148/radiol.12110802. http://dx.doi.org/10.1148/radiol.12110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klok FA, van der BN, Eikenboom HC, et al. Comparison of CT assessed right ventricular size and cardiac biomarkers for predicting short-term clinical outcome in normotensive patients suspected of having acute pulmonary embolism. J Thromb Haemost. 2010;8:853–856. doi: 10.1111/j.1538-7836.2010.03780.x. http://dx.doi.org/10.1111/j.1538-7836.2010.03780.x. [DOI] [PubMed] [Google Scholar]
- 78.Mehta S, Helmersen D, Provencher S, et al. Diagnostic evaluation and management of chronic thromboembolic pulmonary hypertension: a clinical practice guideline. Can Respir J. 2010;17:301–334. doi: 10.1155/2010/704258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lang IM, Simonneau G, Pepke-Zaba JW, et al. Factors associated with diagnosis and operability of chronic thromboembolic pulmonary hypertension. A case-control study. Thromb Haemost. 2013;110:83–91. doi: 10.1160/TH13-02-0097. http://dx.doi.org/10.1160/TH13-02-0097. [DOI] [PubMed] [Google Scholar]
- 80.Gotway MB. Pulmonary hypertension. In: Webb WR, Higgins CB, editors. Thoracic imaging: pulmonary and cardiovascular radiology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2011. pp. 683–699. [Google Scholar]
- 81.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345:1465–1472. doi: 10.1056/NEJMra010902. http://dx.doi.org/10.1056/NEJMra010902. [DOI] [PubMed] [Google Scholar]
- 82.Miniati M, Monti S, Bottai M, et al. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine (Baltimore) 2006;85:253–262. doi: 10.1097/01.md.0000236952.87590.c8. http://dx.doi.org/10.1097/01.md.0000236952.87590.c8. [DOI] [PubMed] [Google Scholar]
- 83.Klok FA, van Kralingen KW, van Dijk AP, Heyning FH, Vliegen HW, Huisman MV. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica. 2010;95:970–975. doi: 10.3324/haematol.2009.018960. http://dx.doi.org/10.3324/haematol.2009.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCann C, Gopalan D, Sheares K, Screaton N. Imaging in pulmonary hypertension, part 2: large vessel diseases. Postgrad Med J. 2012;88:317–325. doi: 10.1136/postgradmedj-2011-130274. http://dx.doi.org/10.1136/postgradmedj-2011-130274. [DOI] [PubMed] [Google Scholar]
- 85.Moser KM, Auger WR, Fedullo PF, Jamieson SW. Chronic thromboembolic pulmonary hypertension: clinical picture and surgical treatment. Eur Respir J. 1992;5:334–342. [PubMed] [Google Scholar]
- 86.Archibald CJ, Auger WR, Fedullo PF, et al. Long-term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med. 1999;160:523–528. doi: 10.1164/ajrccm.160.2.9808109. http://dx.doi.org/10.1164/ajrccm.160.2.9808109. [DOI] [PubMed] [Google Scholar]
- 87.Castaner E, Gallardo X, Ballesteros E, et al. CT diagnosis of chronic pulmonary thromboembolism. Radiographics. 2009;29:31–50. doi: 10.1148/rg.291085061. http://dx.doi.org/10.1148/rg.291085061. [DOI] [PubMed] [Google Scholar]
- 88.Bergin CJ, Sirlin CB, Hauschildt JP, et al. Chronic thromboembolism: diagnosis with helical CT and MR imaging with angiographic and surgical correlation. Radiology. 1997;204:695–702. doi: 10.1148/radiology.204.3.9280245. http://dx.doi.org/10.1148/radiology.204.3.9280245. [DOI] [PubMed] [Google Scholar]
- 89.Wittram C, Maher MM, Yoo AJ, Kalra MK, Shepard JA, McLoud TC. CT angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. Radiographics. 2004;24:1219–1238. doi: 10.1148/rg.245045008. http://dx.doi.org/10.1148/rg.245045008. [DOI] [PubMed] [Google Scholar]
- 90.Sherrick AD, Swensen SJ, Hartman TE. Mosaic pattern of lung attenuation on CT scans: frequency among patients with pulmonary artery hypertension of different causes. AJR Am J Roentgenol. 1997;169:79–82. doi: 10.2214/ajr.169.1.9207504. http://dx.doi.org/10.2214/ajr.169.1.9207504. [DOI] [PubMed] [Google Scholar]
- 91.Karazincir S, Balci A, Seyfeli E, et al. CT assessment of main pulmonary artery diameter. Diagn Interv Radiol. 2008;14:72–74. [PubMed] [Google Scholar]
- 92.Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging. 2012;5:147–154. doi: 10.1161/CIRCIMAGING.111.968610. http://dx.doi.org/10.1161/CIRCIMAGING.111.968610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging. 1999;14:270–278. doi: 10.1097/00005382-199910000-00007. http://dx.doi.org/10.1097/00005382-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 94.Tan RT, Kuzo R, Goodman LR, Siegel R, Haasler GB, Presberg KW. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest. 1998;113:1250–1256. doi: 10.1378/chest.113.5.1250. http://dx.doi.org/10.1378/chest.113.5.1250. [DOI] [PubMed] [Google Scholar]
- 95.Schwickert H, Schweden F, Schild H, Duber C, Iversen S. Detection of chronic recurrent pulmonary emboli using spiral-CT. Rofo. 1993;158:308–313. doi: 10.1055/s-2008-1032655. http://dx.doi.org/10.1055/s-2008-1032655. [DOI] [PubMed] [Google Scholar]
- 96.King MA, Ysrael M, Bergin CJ. Chronic thromboembolic pulmonary hypertension: CT findings. AJR Am J Roentgenol. 1998;170:955–960. doi: 10.2214/ajr.170.4.9530043. http://dx.doi.org/10.2214/ajr.170.4.9530043. [DOI] [PubMed] [Google Scholar]
- 97.Hansell DM, Rubens MB, Padley SP, Wells AU. Obliterative bronchiolitis: individual CT signs of small airways disease and functional correlation. Radiology. 1997;203:721–726. doi: 10.1148/radiology.203.3.9169694. http://dx.doi.org/10.1148/radiology.203.3.9169694. [DOI] [PubMed] [Google Scholar]
- 98.Stern EJ, Swensen SJ, Hartman TE, Frank MS. CT mosaic pattern of lung attenuation: distinguishing different causes. AJR Am J Roentgenol. 1995;165:813–816. doi: 10.2214/ajr.165.4.7676972. http://dx.doi.org/10.2214/ajr.165.4.7676972. [DOI] [PubMed] [Google Scholar]
- 99.Gan CT, Lankhaar JW, Westerhof N, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest. 2007;132:1906–1912. doi: 10.1378/chest.07-1246. http://dx.doi.org/10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 100.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: an important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ. 2011;1:212–223. doi: 10.4103/2045-8932.83453. http://dx.doi.org/10.4103/2045-8932.83453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanz J, Kariisa M, Dellegrottaglie S, et al. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2:286–295. doi: 10.1016/j.jcmg.2008.08.007. http://dx.doi.org/10.1016/j.jcmg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 102.Wu DK, Hsiao SH, Lin SK, et al. Main pulmonary arterial distensibility: different presentation between chronic pulmonary hypertension and acute pulmonary embolism. Circ J. 2008;72:1454–1459. doi: 10.1253/circj.cj-08-0223. http://dx.doi.org/10.1253/circj.CJ-08-0223. [DOI] [PubMed] [Google Scholar]
- 103.Revel MP, Faivre JB, Remy-Jardin M, Delannoy-Deken V, Duhamel A, Remy J. Pulmonary hypertension: ECG-gated 64-section CT angiographic evaluation of new functional parameters as diagnostic criteria. Radiology. 2009;250:558–566. doi: 10.1148/radiol.2502080315. http://dx.doi.org/10.1148/radiol.2502080315. [DOI] [PubMed] [Google Scholar]
- 104.Abel E, Jankowski A, Pison C, Luc BJ, Bouvaist H, Ferretti GR. Pulmonary artery and right ventricle assessment in pulmonary hypertension: correlation between functional parameters of ECG-gated CT and right-side heart catheterization. Acta Radiol. 2012;53:720–727. doi: 10.1258/ar.2012.120009. http://dx.doi.org/10.1258/ar.2012.120009. [DOI] [PubMed] [Google Scholar]
- 105.Lankhaar JW, Westerhof N, Faes TJ, et al. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J. 2008;29:1688–1695. doi: 10.1093/eurheartj/ehn103. http://dx.doi.org/10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 106.Wildberger JE, Klotz E, Ditt H, Spuntrup E, Mahnken AH, Gunther RW. Multislice computed tomography perfusion imaging for visualization of acute pulmonary embolism: animal experience. Eur Radiol. 2005;15:1378–1386. doi: 10.1007/s00330-005-2718-9. http://dx.doi.org/10.1007/s00330-005-2718-9. [DOI] [PubMed] [Google Scholar]
- 107.Fink C, Johnson TR, Michaely HJ, et al. Dual-energy CT angiography of the lung in patients with suspected pulmonary embolism: initial results. Rofo. 2008;180:879–883. doi: 10.1055/s-2008-1027724. http://dx.doi.org/10.1055/s-2008-1027724. [DOI] [PubMed] [Google Scholar]
- 108.Hoey ET, Mirsadraee S, Pepke-Zaba J, Jenkins DP, Gopalan D, Screaton NJ. Dual-energy CT angiography for assessment of regional pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: initial experience. AJR Am J Roentgenol. 2011;196:524–532. doi: 10.2214/AJR.10.4842. http://dx.doi.org/10.2214/AJR.10.4842. [DOI] [PubMed] [Google Scholar]
- 109.Baque-Juston MC, Wells AU, Hansell DM. Pericardial thickening or effusion in patients with pulmonary artery hypertension: a CT study. AJR Am J Roentgenol. 1999;172:361–364. doi: 10.2214/ajr.172.2.9930782. http://dx.doi.org/10.2214/ajr.172.2.9930782. [DOI] [PubMed] [Google Scholar]