Abstract
Background
Limited data suggest one or two doses of the HPV vaccines confer similar protection to the three-dose regimen. This study aimed to further evaluate the question of reduced-dose efficacy of the HPV-16/18 vaccine.
Methods
Summary-level data from the Costa Rica Vaccine Trial (CVT; NCT00128661) and the PApilloma TRIal against Cancer In young Adults (PATRICIA; NCT001226810), two phase III controlled, randomized, double-blind, clinical trials of the HPV-16/18 AS04-adjuvanted vaccine among young women, were combined in a post-hoc analysis (GSK e-track 202142) to investigate efficacy of fewer doses of the HPV-16/18 vaccine after four years of follow-up. Women were randomly assigned to receive three doses of the HPV-16/18 vaccine or to a control vaccine; yet some received fewer doses. After excluding women with <12-months follow-up or those HPV16/18 DNA-positive at enrollment (for the HPV16/18 endpoint), vaccine efficacy (VE) was calculated against one-time detection of incident HPV infections after three (n=11,110 HPV:11,217control), two (n=611:574), and one (N=292:251) dose(s). The main aim of the study was to ascertain HPV16/18 VE in both full and naïve cohorts, as well as to explore protection conferred against non-vaccine HPV types, by number of doses received.
Findings
VE against incident HPV16/18 infections for three doses was 77·0% (95%CI 74·7 to 79·1%), two doses was 76·0% (95%CI 62·0 to 85·3%), and one dose was 85·7% (95%CI 70·7 to 93·7%). VE against incident HPV31/33/45 infections for three doses was 59·7% (95%CI 56·0 to 63·0%), two doses was 37·7% (95%CI 12·4 to 55·9%), and one dose was 36·6% (95%CI −5·4 to 62·2%). However, two-dose women who received their second dose at six months, but not those receiving it at one month, had efficacy estimates against HPV 31/33/45 similar to the three-dose group (VE 68·1%, 95%CI 27·0 to 87·0%; CVT data only).
Interpretation
Four years following vaccination of women aged 15 to 25 years, one and two dose(s) of the HPV16/18 vaccine appear to protect against cervical HPV16/18 infections, similar to the protection provided by the three-dose schedule. Two doses separated by six months additionally provided limited cross-protection. These data argue for a direct evaluation of one-dose efficacy of the HPV16/18 vaccine.
Funding
The CVT trial was sponsored and funded by the US National Cancer Institute, NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health, and done with the support from the Ministry of Health of Costa Rica. Vaccine was provided for CVT by GlaxoSmithKline Biologicals SA, under a Clinical Trials Agreement with the NCI. GlaxoSmithKline Biologicals SA provided support for aspects of the trial associated with regulatory submission needs of the company under US Food and Drug Administration BB-IND 7920. The PATRICIA trial was sponsored by GlaxoSmithKline Biologicals SA.
Introduction
Cervical cancer remains a leading cause of cancer mortality among women worldwide, though the burden disproportionately affects women in low-income nations.1 Human papillomavirus (HPV) type 16 causes approximately 50% of cervical cancers, followed by HPV 18 (20%), and the remaining 30% are caused primarily by 10 other carcinogenic types.2 Preventing HPV acquisition, especially types 16 and 18, could dramatically reduce cervical cancer incidence and mortality.
Prophylactic HPV vaccination with three doses administered via a prime/prime/boost schedule over a six-month period of either of two commercially-available vaccines (HPV16/18 vaccine Cervarix® [GSK group of companies, Rixensart, Belgium] and HPV6/11/16/18 vaccine Gardasil® [Merck & Co., Whitehouse Station, NJ]) is highly efficacious in preventing cervical HPV16/18 infections and related diseases.3,4 Costs and infrastructure complexities associated with a three-dose program are barriers in many world regions.5 Based on immunological non-inferiority, 2-dose schedules of the HPV 16/18 and HPV 6/11/16/18 vaccines are now licensed in pre-teens/adolescents in a number of countries.
The only published data on efficacy of fewer doses comes from a post-hoc analysis in the NCI-sponsored Costa Rica Vaccine Trial (CVT) among women who did not complete the three-dose regimen.6 Amongst women HPV16/18 DNA-negative at the time of first vaccination, HPV16/18 vaccine efficacy (VE) was uniformly high against incident HPV16/18 infections that persisted 6+ months for recipients of one (100%, 95%CI 79 to 100%), two (81%, 95%CI 53 to 94%) or three doses (84%, 95%CI 77 to 89%) throughout the four years post-vaccination. Women who received fewer-than-three doses also had strong and stable antibody responses that persisted during this period, although titers among recipients of one dose were almost four-times lower than for two or three doses.7 Similar analyses from other studies showed stable plateau titers after their decrease in the immediate post-vaccination period after administration of one, two and three doses.8–10
Since the original publication from CVT, new data on reduced-dose protection have come from non-inferiority immunogenicity studies, where the minimal titer required for protection remains unknown as a consequence of the high VE.8–10 Here, we aimed to confirm the initial CVT dose-stratified VE findings in the PApilloma TRIal against Cancer In young Adults (PATRICIA trial). Summary-level data from these trials were combined to: 1) evaluate HPV16/18 VE of fewer doses among HPV-naïve women, and 2) determine whether protection against HPV31, 33, 45, demonstrated among women who received three doses, is present with fewer doses.11
Methods
Study design and participants
Data from CVT (Clinical Trial number NCT00128661) and PATRICIA (Clinical Trial number NCT001226810), the only two large-scale phase III controlled, randomized, double-blind, clinical trials of the HPV-16/18 vaccine among young women, were used to evaluate the study aims.3,12–15 Trials of the quadrivalent, and now nonavalent, HPV vaccines, were considered out of scope of this work because it is currently unknown whether it is the VLP that induces the strong response, in which case both vaccines may be efficacious with a single dose, or whether the special adjuvant of the HPV16/18 vaccine is the cause of one-dose efficacy. Moreover, there were differences in the trial designs between the HPV16/18 vaccine programs and the quadrivalent/nanovalent program, as it relates to criteria for inclusion, assays for HPV DNA and serology, and colposcopy algorithms. Consequently, this manuscript focused only on the HPV16/18 vaccine.
The CVT and PATRICIA trials are registered with ClinicalTrials.gov. For CVT12, 7466 young women were enrolled between 28 June 2004 and 21 December 2005 from Costa Rica; the main eligibility requirements were age 18–25 years, good general health, and neither pregnant nor breastfeeding. Women were excluded if they had pre-existing medical conditions that preclude vaccination, had history of hepatitis A or previous vaccination against it, or were unwilling to use contraception during the vaccination period. For PATRICIA14, enrollment occurred between 6 May 2004 to 27 June 2005; 18644 women aged 15–25 years were enrolled and vaccinated. Women who reported no more than six lifetime sexual partners before study enrolment (in some countries this criteria was not considered for minors), who agreed to adequate contraception (barrier methods in combination with a spermicide or hormonal contraception) over the vaccination period, and had an intact cervix, were eligible for inclusion. Main exclusion criteria included history of hepatitis A or previous vaccination against it, history of colposcopy, pregnancy or breastfeeding, chronic or autoimmune disease or immunodeficiency.
Investigators from each trial generated study-specific analyses independently according to a pre-specified analytic plan; the summary-level data were merged to generate the combined analytic estimates. These studies, which have been described previously, were generally harmonized at the design phase; thus increasing the validity of the combined analysis (GSK e-track 202142).11–14 Importantly, the same vaccines were administered on the same schedules, the baseline characteristics were comparable (i.e.: age), HPV DNA testing and serologic analyses were done using the same assays conducted at the same laboratories, thus eliminating potential differences in outcome misclassification for virological endpoints, the main outcome measures for this analysis; referral algorithms for women who needed additional work up were also similar. The main difference between the 2 studies as it related to this paper is that PATRICIA saw women biennially, whereas CVT saw women annually, although more frequent visits occurred when clinically indicated. The clinical protocols and other materials were approved by independent ethics committees or institutional review boards.
Procedures
At the enrollment visit following informed consent/assent, each participant underwent a risk factor interview, blood collection, and if sexually-active, a pelvic examination where cervical cells were collected for cytology and HPV DNA detection/genotyping. Women were then randomly assigned to receive either the HPV-16/18 AS04 adjuvanted vaccine (Cervarix®) or an active-control hepatitis A vaccine (HAV), administered at 0, 1, and 6 months. Women not vaccinated within the pre-specified dosing windows did not receive the scheduled dose. In both studies, pregnancy was a contraindication for vaccination. Women who were pregnant at vaccination visits did not receive that dose if the vaccination window closed. In CVT only, women referred to colposcopy missed the dose if the vaccination window was closed when they returned to the regular study visits. Data safety monitoring groups reviewed safety data during the vaccination phase and as needed during the follow-up period.
The protocols required all women to be seen every 6 months (PATRICIA) or annually (CVT) during the four year follow-up. At each visit, clinicians collected exfoliated cervical cells from sexually-active women for cytologic evaluation and HPV DNA testing. Women found to have low-grade squamous intraepithelial neoplasia (LSIL) or carcinogenic HPV-positive atypical squamous cells of undetermined significance (HPV+ ASC-US) underwent intensified follow-up (missing/inadequate cytology was similarly sent to intensified follow-up in CVT). Women with high-grade disease or persistent low-grade abnormalities were referred to colposcopy for evaluation and directed biopsy, with treatment if needed. HPV DNA testing was performed as previously described.16–18
Statistical analysis
This post hoc analysis followed a statistical analysis plan prepared prior to the analysis. CONSORT diagrams show the sample size for CVT (Supplementary figure 1) and PATRICIA (Supplementary figure 2). General characteristics and reasons for not receiving all three vaccine doses were described within dose, arm, and study, as was follow-up time from enrollment within each study (restricted to women with ≥12 months of follow-up; time-total vaccinated cohort, T-TVC; Figure 1).
Figure 1. CONSORT diagram for the Costa Rica Vaccine Trial and PATRICIA trial combined.
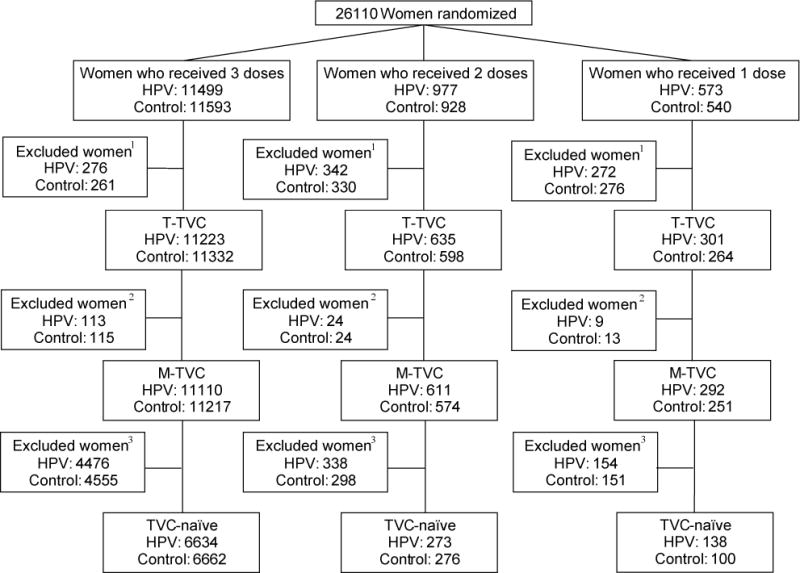
This CONSORT contains women who were randomized into both trials, stratified by vaccine arm and number of doses received. 1Women were excluded if they had less than 12 months follow-up time (time total vaccinated cohort, T-TVC). 2Women were additionally excluded if their enrollment cervical status was HPV16 AND 18 DNA-positive (or missing) or they had fewer than 300 days between first and last PCR result; modified total vaccinated cohort (M-TVC) evaluating HPV16/18-related endpoints. 3Women were further excluded from the TVC-naïve if their cervical status at enrollment was HPV DNA-positive for any oncogenic type (or missing), or HPV16 or 18 seropositive (or missing), or had enrollment cytology abnormal (or missing).
All endpoints were evaluated for both HPV16/18 and HPV31/33/45, as well as by individual HPV type within these groups. The primary study endpoint was one-time detection of first incident HPV infections accumulated over the follow-up phase. Women with multiple events were only counted once at the time of the first event, at which time her person-time stopped. Secondary endpoints included incident HPV infections that persisted for ≥6 months (defined as two or more type-specific positive tests at least 150 days apart, with no intervening negatives) and ≥12 months (similarly for >300 days). The distinction between the endpoints of one-time detection of incident HPV infection and persistent infection is that the latter criteria is met when a 2nd detection of the HPV type in question occurs within the interval specified, without an HPV negative test (for the HPV type in question) occurring between the two positive tests; this is not to say that a one-time detection did not also persist, but that the criteria for an incident event was met when an infection was detected a single time.
Endpoints were evaluated in two analytic cohorts: 1) modified total vaccinated cohort (M-TVC), which excluded women who were HPV DNA-positive for the type in question at the enrollment visit; consequently each M-TVC can have differing sample sizes due to differing number of excluded women, and 2) total vaccinated cohort-naïve (TVC-naïve), which excluded women who were HPV DNA-positive for any of 14 high-risk types, HPV16/18 seropositive (even in analyses of protection by non-HPV16/18 types), and who had positive cytology at the enrollment visit (women who were negative for carcingeonic HPV types by Hybrid Capture II, but were ASCUS positive were considered negative and included in the TVC-naïve cohort).
For both analytic cohorts, person time was counted starting one day post-enrollment. The number of vaccine study visits (i.e.: visits at enrollment, month 1, and month 6) were directly linked to the number of doses a woman received (i.e.: women receiving only one or two vaccine doses in most instances didn’t attend the month 1 or month 6 vaccine study visits [or both of these visits] and as a result did not receive the full vaccine series). Outcome assessment therefore began at the 12-month study visit (301 or more days after enrollment), because this visit was the first study visit potentially attended by women in all dose groups. Enrollment HPV results were used to exclude events if the HPV type detected in follow-up was also present at enrollment. Women with unknown HPV DNA status at baseline were excluded. HPV results between 1 and 300 days after enrollment were ignored, due to the bias by number of doses in sample availability (and thus HPV ascertainment) in this time frame. Subjects were required to have a cervical sample available (so HPV DNA status could be determined) at least 301 days after the start of outcome assessment. Follow-up ended at the time of an event (e.g., the date of the first HPV-positive test in the sequence of HPV-positive tests that defined the event). For women who did not have an event, follow-up ended on the date of the last negative test (including untested visits for virgins) to ensure parallelism in outcome assessment between women who did and did not have events.
For each group, event rates expressed per 100 woman-years were calculated as the ratio of number of events and the total follow-up time. Analyses were performed separately on subjects who received one-dose only, two-doses only regardless of the schedule, and the full three-dose regimen (which served as a benchmark to interpret the fewer-dose VE estimates). As an additional, exploratory analysis, further stratification of the two-dose VE by timing of the second dose (at one- or six-months) was evaluated in CVT; PATRICIA was excluded as only 26 women received two doses on the 0/6 month schedule.
The main analysis estimated differences in attack rates between the HPV-vaccinated and HAV-vaccinated women, by number of doses received. Numbers in the numerators were combined across the two studies, as were the denominators, and these summary counts were used to generate a combined VE by dose for each endpoint. Study-specific VEs are presented as supplementary tables, as are individual-HPV-type VEs combined across the two trials. VE was defined as the percentage reduction in endpoint related to vaccine administration, estimated as the complement of the ratio of the attack rates in the HPV and control arms. The analysis was conditioned on the total number of events, and the 95% confidence interval (95%CI) around VE was derived from the ratio of the events in the vaccine arm to the total events using a mid-p option for the exact confidence interval calculation. Significant VE was defined as the lower bound of the 95%CI greater than 0. Statistical adjustment of VE estimates was not used to account for underlying risk differences by dose and arm because strong differences were not observed in baseline characteristics. A Poisson regression model with an interaction term for vaccination arm and number of doses was used to test for heterogeneity in the trend parameters between the two arms. Mid-P adjusted exact methods were used in all instances except when they failed because the sample size was too large; in those instances, standard asymptotic methods were used to calculate p values.
Heterogeneity in VE between the two studies was tested using a Poisson regression model with an interaction term for vaccination arm by trial. All calculations were performed using SAS version 9.2.
Role of the Funding Source
The PATRICIA trial was funded by GlaxoSmithKline Biologicals SA (GSK), who designed the study in collaboration with investigators, and coordinated collection, analysis, and interpretation of data, and preparation of the manuscript. Investigators from the PATRICIA Study Group collected data for the trial and cared for the subjects. The trial is registered with clinicaltrials.gov (NCT00122681).
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the NCI. The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline Biologicals SA (GSK) provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the four-year, randomized blinded phase of our study. The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of samples and data. Registered with Clinicaltrials.gov NCT00128661.
Publishing related costs (publication charges) were covered by the NCI.
In this analysis, all authors (ARK, FS, MRDRR, AH, SRS, SW, SMG, RH, MPD, CMW, SJ, PGonzález, DRL, LAP, CP, ACR, M Safaeian, M Schiffman, JTS, JS, M Sherman, FXB, XC, AC, SNC, DD, FDM, GD, MJG, DMH, DJML, GL, PN, KP, WAJP, BR, BR, JS, TFS, JCT, WT) had full access to all the trial data for the trial they participated in and access to summary-level trial data for both trials.
Results
In the T-TVC cohort (Figure 1 CONSORT), the most common reason for missing doses was pregnancy in PATRICIA (for all arms and dose groups, range: 48·6 (53/109) to 66·2% (143/216)) and CVT two-dose women (35·8 (150/419) and 37·5% (142/379) for HPV and control arm, respectively); CVT one-dose women missed due primarily to colposcopy referral (21·6 (40/185) to 28·6% (55/192)) and then pregnancy (Supplementary table 1). Mean follow-up time was similar by arm and dose group in CVT (range of means: 54·1 to 54·5 months) and PATRICIA (43·0 to 44·9) (Table 1); overall mean time of follow-up was 47.6-months (Standard Deviation=8.8 months). In both studies and for all dosage groups, women who received the HPV versus control vaccine were comparable with respect to mean age at entry (CVT: 20·9 to 21·4 years and PATRICIA: 19·9 to 21.3). Number of non-vaccine study visits was balanced for all dose groups in CVT (range of means: 4·3 to 4·7) and for one- and two-dose women in PATRICIA (4·4 to 4·8); three-dose women in PATRICIA had on average one additional study visit, though balance was present by arm (mean 5·5 visits for HPV and HAV arms among three-dose women). For each study, HPV16/18 status at enrollment appeared balanced by arm within dose, though fluctuations were present given the small numbers in some cells; differences were observed in enrollment HPV16/18 status across studies. As a further demonstration of balance in underlying HPV risk by dose group within the HPV and control arms over the 4 year follow-up period, cumulative incident HPV infections for types against which the vaccine does not protect11 were assessed as composites of oncogenic and non-oncogenic HPV types. Similar attack rates were observed in this assessment in both PATRICIA and CVT studies across and within all treatment groups (1/2/3 vaccine doses) (Supplementary table 2).
Table 1.
Descritptive characteristics of study population, stratified by study, number of vaccine doses received and vaccine arm using available data in the total vaccinated cohort among women with 12 or more months of follow-up time (T-TVC).
| Costa Rica Vaccine Trial | PATRICIA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 dose | 2 doses | 3 doses | 1 dose | 2 doses | 3 doses | |||||||
| HPV N=192 |
Control N=185 |
HPV N=419 |
Control N=379 |
HPV N=2927 |
Control N=2992 |
HPV N=109 |
Control N=79 |
HPV N=216 |
Control N=219 |
HPV N=8296 |
Control N=8340 |
|
| Age at enrollment1 | 21·3 (2·3) | 21·4 (2·4) | 20·9 (2·3) | 20·9 (2·4) | 21·1 (2·3) | 21·1 (2·3) | 21·3 (2·7) | 21·0 (3·0) | 21·1 (2·9) | 21·3 (2·8) | 19·9 (3·1) | 19·9 (3·1) |
| Follow-up time (months)1 | 54·2 (8·8) | 54·5 (9·1) | 54·2 (10·0) | 54·1 (10·3) | 54·1 (8·3) | 54·3 (8·3) | 43·8 (9·3) | 43·1 (9·4) | 43·0 (9·2) | 43·1 (8·6) | 44·9 (7·2) | 44·9 (7·4) |
| #non-vaccine study visits1 | 4·7 (1·5) | 4·5 (1·5) | 4·3 (1·3) | 4·4 (1·5) | 4·4 (1·2) | 4·5 (1·3) | 4·8 (1·7) | 4·5 (1·7) | 4·4 (1·6) | 4·4 (1·7) | 5·5 (1·2) | 5·5 (1·2) |
| HPV16 status at enrollment | ||||||||||||
| Negative | 122 (63·5) | 116 (62·7) | 278 (66·3) | 265 (69·9) | 2098 (71·7) | 2091 (69·9) | 92 (84·4) | 60 (75·9) | 165 (76·4) | 176 (80·4) | 6669 (80·4) | 6682 (80·1) |
| Positive2 | 66 (34·4) | 63 (34·1) | 131 (31·3) | 106 (28·0) | 791 (27·0) | 849 (28·4) | 17 (15·6) | 18 (22·8) | 46 (21·3) | 39 (17·8) | 1553 (18·7) | 1565 (18·8) |
| HPV18 status at enrollment | ||||||||||||
| Negative | 129 (67·2) | 122 (65·9) | 290 (69·2) | 273 (72·0) | 2152 (73·5) | 2196 (73·4) | 100 (91·7) | 66 (83·5) | 180 (83·3) | 191 (87·2) | 7177 (86·5) | 7218 (86·5) |
| Positive2 | 57 (29·7) | 56 (30·3) | 118 (28·2) | 95 (25·1) | 710 (24·3) | 729 (24·4) | 9 (8·3) | 13 (16·5) | 33 (15·3) | 27 (12·3) | 1054 (12·7) | 1051 (12·6) |
Mean (SD);
Positive indicates positivity for HPV serology and/or DNA for the type in question; N (%).
In the PATRICIA trial alone, VE against one-time detection of incident HPV16/18 infections for three doses was 76·8% (95%CI 74·2 to 79·2%), two doses was 73·3% (95%CI 40·4 to 89·2%), and one dose was 72·2% (95%CI 13·6 to 92·4%), thereby confirming the original report from CVT of high VE against HPV16/18 regardless of dose group. In the current analysis, there was no evidence for heterogeneity by study in dose-stratified VEs against HPV16/18 (Supplementary table 3).
In both trials combined, the analysis in the M-TVC cohort evaluating HPV16/18-related endpoints included (for one-time detection) 22,327 women who received three doses (11,110 HPV: 11,217 control), 1,185 women who received two doses (611:574), and 543 women who received a single dose (292:251). This cohort excluded women for inadequate follow-up (either the women had no 12+ month visit or there was less than 300 days between the 12+ month visit and the last visit) consisting of 537 women who received three doses (276 HPV: 261 control), 672 women who received two doses (342:330), and 548 women who received a single dose (272:276). It also excluded women for having positive or missing PCR results for both HPV 16 and HPV 18 at enrollment consisting of 228 women who received three doses (113:115), 48 women who received two doses (24:24), and 22 women who received a single dose (9:13) (Figure 1). In the M-TVC, VE against one-time detection of incident HPV16/18 infections for three doses was 77·0% (95%CI 74·7 to 79·1%), two doses was 76·0% (95%CI 62·0 to 85·3%), and one dose was 85·7% (95%CI 70·7 to 93·7%) (Table 2); no significant difference was present in VE by dose (p for trend=0·36). Study-specific dose-stratified VEs were also similar (minimum p value=0·15), indicating consistency of the findings (Figure 2; Supplementary table 3). Combined VEs were similar by dose for the 6-month and 12-month persistent HPV16/18 infection endpoints (Table 2). VEs for incident HPV type 16 alone, as well as incident HPV type 18 alone, were high regardless of dose and endpoint (Figure 3; Supplementary table 5).
Table 2.
Dose-stratified vaccine efficacy against incident HPV16/18 infection (one-time detection, six-month persistence, and 12-month persistence) for women in the modified total vaccinated cohort (CVT and PATRICIA combined).
| Doses, no. | Arm | Women, no. | Events, no. | Person years | Rate per 100 PY (95%CI) | Vaccine Efficacy |
|---|---|---|---|---|---|---|
| Incident one-time detection of HPV16/18 | ||||||
| 3 (standard regimen) | HPV | 11110 | 529 | 43140 | 1·23 (1·12 to 1·34) | 77·0% (74·7 to 79·1%) |
| Control | 11217 | 2172 | 40682 | 5·34 (5·12 to 5·57) | ||
| 2 | HPV | 611 | 22 | 2538 | 0·87 (0·56 to 1·29) | 76·0% (62·0 to 85·3%) |
| Control | 574 | 82 | 2271 | 3·61 (2·89 to 4·46) | ||
| 1 | HPV | 292 | 8 | 1220 | 0·66 (0·30 to 1·25) | 85·7% (70·7 to 93·7%) |
| Control | 251 | 45 | 982 | 4·58 (3·38 to 6·08) | ||
| Incident detection of HPV16/18 that persisted ≥6 months | ||||||
| 3 | HPV | 11104 | 114 | 43706 | 0·26 (0·22 to 0·31) | 89·1% (86·8 to 91·0%) |
| Control | 11209 | 1000 | 41913 | 2·39 (2·24 to 2·54) | ||
| 2 | HPV | 611 | 4 | 2573 | 0·16 (0·05 to 0·38) | 89·7% (73·3 to 96·9%) |
| Control | 574 | 35 | 2308 | 1·52 (1·07 to 2·09) | ||
| 1 | HPV | 292 | 1 | 1234 | 0·08 (0·00 to 0·40) | 96·6% (81·7 to 99·8%) |
| Control | 250 | 24 | 1017 | 2·36 (1·55 to 3·46) | ||
| Incident detection of HPV16/18 that persisted ≥12 months | ||||||
| 3 | HPV | 11104 | 84 | 43775 | 0·19 (0·15 to 0·24) | 87·0% (83·7 to 89·7%) |
| Control | 11203 | 627 | 42589 | 1·47 (1·36 to 1·59) | ||
| 2 | HPV | 611 | 3 | 2576 | 0·12 (0·03 to 0·32) | 89·6% (68·9 to 97·5%) |
| Control | 574 | 26 | 2324 | 1·12 (0·75 to 1·62) | ||
| 1 | HPV | 292 | 1 | 1234 | 0·08 (0·00 to 0·40) | 95·1% (73·2 to 99·8%) |
| Control | 249 | 17 | 1021 | 1·67 (1·00 to 2·61) | ||
Figure 2.
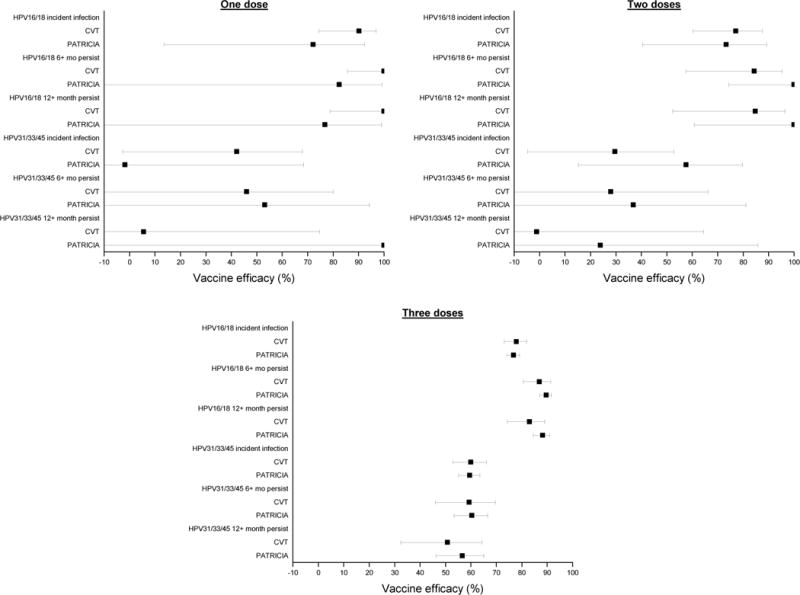
Vaccine efficacy by study (CVT and PATRICIA) for multiple endpoints in the modified total vaccinated cohort, by dose (M-TVC).
Figure 3.
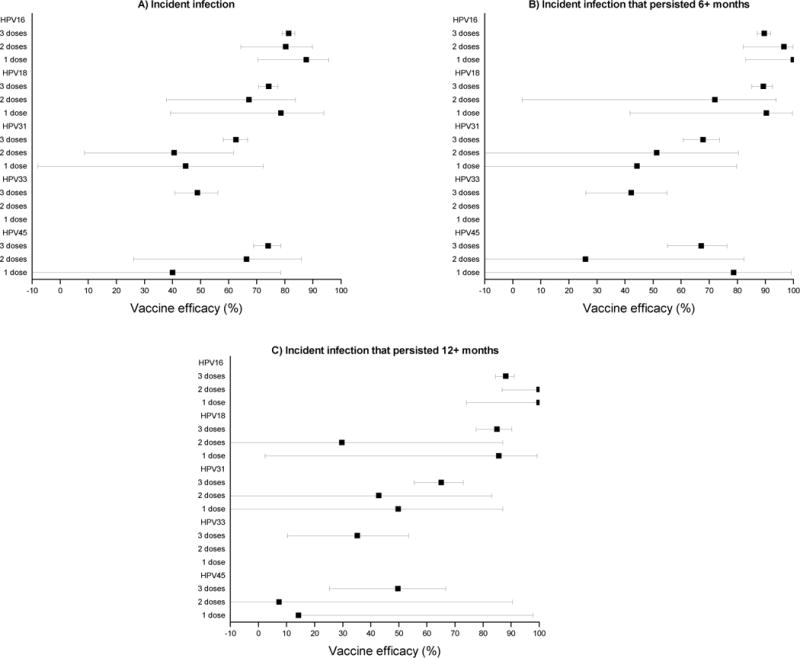
Vaccine efficacy for individual HPV types by dose, for incident HPV infections that were detected one time (panel A), that persisted 6+ months (panel B), and that persisted 12+ months (panel C) (M-TVC).
The TVC-naïve cohort included 13,296 women who received three doses (6634 HPV: 6662 control), 549 women who received two doses (273:276), and 238 women who received a single dose (138:100). In this cohort, VE for three doses against one-time detection of incident HPV16/18 infections was 81·4% (95%CI 78·7 to 83·8%), for two doses was 81·2% (95%CI 59·5 to 92·3%), and for one dose was 87·5% (95%CI 60·9 to 97·1%) (Table 3). In this analytic cohort, which was subset to women who were HPV-negative at enrollment, VEs were similar and high (all >80%) by dose for the 6-month and 12-month persistent HPV16/18 infection endpoints (Table 3), and again were consistent across studies (Supplementary table 4).
Table 3.
Dose-stratified vaccine efficacy against incident HPV16/18 infection (one-time detection, six-month persistence, and 12-month persistence) for women in the total vaccinated cohort naïve, which excluded women HPV DNA-positive for any of 14 high-risk types, HPV16/18 seropositive, or had greater than negative cytology at the enrollment visit, CVT and PATRICIA combined.
| Doses, no. | Arm | Women, no. | Events, no. | Person years | Rate per 100 PY (95%CI) | Vaccine Efficacy |
|---|---|---|---|---|---|---|
| Incident one-time detection of HPV16/18 | ||||||
| 3 (standard regimen) | HPV | 6634 | 241 | 25750 | 0·94 (0·82 to 1·06) | 81·4% (78·7 to 83·8%) |
| Control | 6662 | 1220 | 24275 | 5·03 (4·75 to 5·32) | ||
| 2 | HPV | 273 | 7 | 1114 | 0·63 (0·27 to 1·24) | 81·2% (59·5 to 92·3%) |
| Control | 276 | 36 | 1074 | 3·35 (2·38 to 4·59) | ||
| 1 | HPV | 138 | 3 | 556 | 0·54 (0·14 to 1·47) | 87·5% (60·9 to 97·1%) |
| Control | 100 | 17 | 394 | 4·32 (2·60 to 6·77) | ||
| Incident detection of HPV16/18 that persisted ≥6 months | ||||||
| 3 | HPV | 6634 | 38 | 26046 | 0·15 (0·10 to 0·20) | 93·6% (91·2 to 95·5%) |
| Control | 6660 | 567 | 24818 | 2·28 (2·10 to 2·48) | ||
| 2 | HPV | 273 | 2 | 1121 | 0·18 (0·03 to 0·59) | 87·9% (54·0 to 98·1%) |
| Control | 276 | 16 | 1089 | 1·47 (0·87 to 2·33) | ||
| 1 | HPV | 138 | 0 | 562 | 0·00 (0·00 to 0·53) | 100% (67·4 to 100%) |
| Control | 100 | 8 | 403 | 1·99 (0·92 to 3·77) | ||
| Incident detection of HPV16/18 that persisted ≥12 months | ||||||
| 3 | HPV | 6634 | 27 | 26073 | 0·10 (0·07 to 0·15) | 92·6% (89·2 to 95·1%) |
| Control | 6656 | 351 | 25186 | 1·39 (1·25 to 1·55) | ||
| 2 | HPV | 273 | 2 | 1121 | 0·18 (0·03 to 0·59) | 83·7% (35·7 to 97·5%) |
| Control | 276 | 12 | 1093 | 1·10 (0·59 to 1·87) | ||
| 1 | HPV | 138 | 0 | 562 | 0·00 (0·00 to 0·53) | 100% (41·1 to 100%) |
| Control | 99 | 5 | 403 | 1·24 (0·45 to 2·75) | ||
Cross-protective efficacy was evaluated in the M-TVC excluding women who were HPV DNA-positive for HPV31/33/45 infections at the enrollment visit. VE against one-time detection of incident HPV31/33/45 infections for three doses was 59·7% (95%CI 56·0 to 63·0%), for two doses was 37·7% (95%CI 12·4 to 55·9%), and for one dose was 36·6% (95%CI −5·4 to 62·2%) (Table 4). For the 6-month and 12-month persistent HPV31/33/45 infection endpoints, only the three-dose VE attained statistical significance. Consistency was observed across studies (Supplementary table 5).
Table 4.
Dose-stratified vaccine efficacy against incident HPV31/33/45 infection (one-time detection, six-month persistence, and 12-month persistence) for women in the modified total vaccinated cohort in CVT and PATRICIA combined.
| Doses, no. | Arm | Women, no. | Events, no. | Person years | Rate per 100 PY (95%CI) | Vaccine Efficacy |
|---|---|---|---|---|---|---|
| Incident one-time detection of HPV31/33/45 | ||||||
| 3 (standard regimen) | HPV | 11156 | 710 | 42990 | 1·65 (1·53 to 1·78) | 59·7% (56·0 to 63·0%) |
| Control | 11272 | 1713 | 41837 | 4·09 (3·90 to 4·29) | ||
| 2 | HPV | 615 | 55 | 2490 | 2·21 (1·68 to 2·85) | 37·7% (12·4 to 55·9%) |
| Control | 577 | 81 | 2285 | 3·54 (2·83 to 4·38) | ||
| 1 | HPV | 293 | 26 | 1185 | 2·19 (1·46 to 3·17) | 36·6% (−5·4 to 62·2%) |
| Control | 253 | 35 | 1012 | 3·46 (2·45 to 4·75) | ||
| Incident detection of HPV31/33/45 that persisted ≥6 months | ||||||
| 3 | HPV | 11150 | 266 | 43507 | 0·61 (0·54 to 0·69) | 60·1% (54·0 to 65·4%) |
| Control | 11269 | 659 | 42997 | 1·53 (1·42 to 1·65) | ||
| 2 | HPV | 615 | 18 | 2549 | 0·71 (0·43 to 1·09) | 30·7% (−27·9 to 63·0%) |
| Control | 577 | 24 | 2355 | 1·02 (0·67 to 1·49) | ||
| 1 | HPV | 293 | 9 | 1222 | 0·74 (0·36 to 1·35) | 48·8% (−16·9 to 78·5%) |
| Control | 253 | 15 | 1043 | 1·44 (0·84 to 2·32) | ||
| Incident detection of HPV31/33/45 that persisted ≥12 months | ||||||
| 3 | HPV | 11150 | 175 | 43682 | 0·40 (0·34 to 0·46) | 54·9% (46·2 to 62·3%) |
| Control | 11266 | 386 | 43447 | 0·89 (0·80 to 0·98) | ||
| 2 | HPV | 615 | 11 | 2569 | 0·43 (0·23 to 0·74) | 7·6% (−117·8 to 60·8%) |
| Control | 577 | 11 | 2373 | 0·46 (0·24 to 0·81) | ||
| 1 | HPV | 293 | 5 | 1230 | 0·41 (0·15 to 0·90) | 46·1% (−66·8 to 84·0%) |
| Control | 253 | 8 | 1061 | 0·75 (0·35 to 1·43) | ||
In a post hoc, additional analysis, VE for two-doses was further stratified in the M-TVC of CVT (Table 5): no VE was observed against incident HPV31/33/45 for women who received their second dose one-month post-dose one (VE 10·2%, 95%CI −42·0 to 43·3%), whereas women who received their second dose six-months post-dose one (VE 68·1%, 95%CI 27·0 to 87·0%) had a higher efficacy estimate (p value comparing the VEs among two-dose women by the timing of the 2nd dose =0·029). In the same cohort, for the endpoint of incident HPV16/18, VE was high regardless of two dose timing (0/1: VE 75·3% [95%CI 54·2 to 87·5%]; 0/6: VE 82·6% [95%CI 42·3 to 96·1%]).
Table 5.
Two-dose vaccine efficacy stratified by timing of the second dose. Modified total vaccinated cohort in the Costa Rica Vaccine Trial only.
| Timing of 2nd doses | Arm | Women, no. | Events, no. | Person years | Rate per 1000 PY (95%CI) | Vaccine Efficacy |
|---|---|---|---|---|---|---|
| Incident one-time detection of HPV16/18 | ||||||
| 1 month | HPV | 300 | 12 | 1334 | 0·90 (0·49 to 1·53) | 75·3% (54·2 to 87·5%) |
| Control | 283 | 44 | 1208 | 3·64 (2·68 to 4·85) | ||
| 6 months | HPV | 97 | 3 | 441 | 0·68 (0·17 to 1·85) | 82·6% (42·3 to 96·1%) |
| Control | 72 | 12 | 307 | 3·91 (2·12 to 6·65) | ||
| Incident one-time detection of HPV31/33/45 | ||||||
| 1 month | HPV | 303 | 36 | 1304 | 2·76 (1·96 to 3·78) | 10·2% (−42·0 to 43·3%) |
| Control | 286 | 38 | 1236 | 3·07 (2·21 to 4·18) | ||
| 6 months | HPV | 98 | 8 | 431 | 1·86 (0·86 to 3·53) | 68·1% (27·0 to 87·0%)* |
| Control | 72 | 17 | 292 | 5·82 (3·50 to 9·13) | ||
The observed difference in the cross-protective VEs was significantly greater among women who received their second dose at six months compared to one month (p= 0·029).
For individual HPV types for which cross-protection has been reported,10 there was significant VE for three doses against HPV31, HPV33, and HPV45, and for two doses for HPV31 and 45 (Supplementary table 6).
There was absence of heterogeneity in VE by study for all cohorts and endpoints (minimum p =0·15) in all analyses except one (two-dose women, TVC-naïve analysis of HPV 31/33/45 infections [data not shown]; p= 0.035).
Discussion
Findings in the PATRICIA trial confirm in an independent RCT the original report in CVT that two and one dose(s) of the HPV16/18 vaccine afford protection against cervical HPV16/18 infections similar to the protection provided by the three-dose schedule over four years of post-vaccination follow-up6 and extend the findings to efficacy against HPV types not included in the vaccine. Further, in analyses combining data from the 2 trials, the result of high HPV16/18 VE regardless of dose was replicated in a cohort of women naïve to HPV16/18 infection at the time of vaccination, which alleviates concerns that the initial findings may have been due to vaccination boosting of natural immunity in our cohort of older-aged women in CVT (n.b.: the original analysis lacked power to create a HPV-naïve cohort).6 Thus, these results are likely relevant to girls in the preferred age range for HPV vaccination (i.e.: 11 to 12 years old).
Two doses of the HPV16/18 AS04-adjuvanted vaccine protected against a composite endpoint of HPV31, 33, and 45 when the second dose was administered six months after the initial vaccine. Yet, based on these new data, a single dose or two priming doses separated by a short interval (i.e.: one month) may not be adequate for inducing measurable cross-protection. This finding is supported by observations in CVT that antibody levels for two doses, when given at least six months apart, are very close to those for three doses.19,20 A study with an investigational HPV16/18 vaccine showed that a 0/2 month schedule among girls aged 9–14 y did not achieve immunological non-inferiority compared with the licensed three-dose schedule in women aged 15–25 years, indicating that the interval between the prime and boost doses is an important factor for the induction of immune response necessary to afford cross-protection with a two-dose formulation.21
One-time detection of incident infection was our primary end-point, rather than persistent infection or disease. Results using this endpoint indicate that, within the limits of the PCR assay, the vaccine conferred sterilizing immunity (defined as protection not only against clinical disease, but also infection) for most HPV16/18 exposures, even for women who received a single vaccine dose. Immunogenicity data from CVT indicate that single-dose vaccine recipients had antibody titers between months 6–48 that were lower than those elicited with two or three doses, but the titers were stable and several times higher than those observed for natural immunity.20
We can now infer that these lower, vaccine-induced antibody titers provide as strong HPV prevention as the titers from two or three doses, at least in the short term. Compared with persistent infection, one-time detection has the limitation of including both short-term infection that regresses spontaneously in addition to persistent infections, which have a higher risk of progression to cervical lesions. Furthermore, some outcomes might have resulted from undetected infections present before vaccination, which explains why efficacy estimates for this endpoint are generally lower than those for persistent infection. Yet, results using persistence as an endpoint buttressed the one-time detection findings. Additional analysis of efficacy and immunogenicity data from one-dose recipients may also aid in the identification of an immune-correlate of protection, given the lack of efficacy observed against related HPV types; analyses are being considered and will be the subject of a future manuscript.
The structure of the HPV virus-like particles (VLPs), the key component of HPV prophylactic vaccines, present closely-spaced, repetitive epitopes to the immune system that induce highly-potent, protective antibody responses, which may reduce or even eliminate the need for doses beyond the priming dose.20,22–24 Further, the immune-stimulatory effects of a toll-like receptor agonist adjuvant in the HPV-16/18 vaccine may also contribute to the magnitude and durability of the immune response to this vaccine. If the protective effect afforded by a single dose is primarily due to the repetitive display of the VLP, this result may be attained for the quadrivalent (and nonavalent; Merck and Co, Inc.) HPV vaccines as well.25 Alternatively, if the protective effect is mainly due to the adjuvant used (or differences in manufacturing of the VLPs), strong VE among fewer doses could be unique to the HPV16/18 AS04-adjuvanted vaccine.
Our data for estimating the efficacy of fewer than three doses, summarized here, has important limitations. The biggest concerns for this post-hoc, non-randomized study are that one-dose women have 1) the possibility of increasingly greater immune response, and 2) lower risk of infection which could introduce biases. In earlier published work from CVT, we evaluated these possibilities directly and showed that 1) antibody levels after the first dose among one-, two- and three-dose women are equivalent and 2) HPV infection rates are also equivalent in the control arm by dose group.6,7 In this analysis, we are further reassured that biases do not explain these findings because 1) the most common reason for missing doses appeared unrelated to vaccination arm (i.e. pregnancy) rather than immune-related events (such as syncope or erythema), which could have indicated differential immune response by number of doses eventually received, 2) follow-up time was equivalent in all groups in both CVT and PATRICIA and 3) risk of HPV acquisition in the control arm was generally similar in the groups who had similar number of study visits (i.e. all control women in one- and two-dose groups, as well as three-dose CVT control women). Finally, despite pooling data across two large trials, the number of women who received one dose was small, allowing for evaluation of virological endpoints but not histologic endpoints. And, most women received their second of only two doses one-month after the first, a schedule now recognized as inferior to two doses administered six months apart.26 Continued active surveillance of fewer-dose women beyond the four-year study period is essential to ensure longer-term duration of protection.
Now, post-hoc analyses in two RCTs independently find similar results for efficacy against cervical HPV16/18 infections, regardless of the number of doses, over the four-year study period. By combining summary-level data, we provide further support for the possibility that the benefit of VE against heterologous HPV types is retained with two doses administered six months apart, but perhaps not with administration of a single dose or two closely-spaced, priming doses. Because of the non-randomized nature of these analyses, the small sample size in the one-dose group, and the use of incident infection as the primary endpoint of this analysis, an endpoint not accepted by regulators as a surrogate for cervical cancer, it is unlikely that policy change moving to a single dose will occur in response to this work. Yet, these new data argue strongly for additional evaluations of this question.27 Long-term population-effectiveness studies of girls vaccinated at young ages will be informative, but trial evaluations to directly evaluate the VE of one dose will likely be necessary to motivate policy change. We recognize that decisions about implementation of HPV vaccination and cervical cancer screening are region-specific, and typically require some level of micro-costing or cost-effectiveness modeling. If one-dose HPV vaccine administration provides strong protection against HPV16/18 for the long term, this approach may be what is necessary to overcome the barriers currently prohibiting vaccine uptake in many world regions.
Supplementary Material
Panel: Research in context.
Systematic Review
There are now convincing immunogenicity data suggesting that two doses of the HPV vaccines administered to adolescents six months apart evoke immune responses comparable to that of three doses, for at least four years. Only one study published to date has evaluated the efficacy of fewer than three doses—the Costa Rica Vaccine Trial—in a post-hoc analysis nested in this randomized controlled trial. Its findings suggested that strong efficacy was provided by the HPV-16/18 vaccine regardless of the number of doses received.
Interpretation
These new data show similar findings of protection for four years regardless of the number of doses received in the PATRICIA trial. Further, by combining the Costa Rica Vaccine Trial and PATRICIA trial data, we provide new evidence suggesting that two and one dose(s) of the HPV-16/18 vaccine afford protection against cervical HPV16/18 infections, similar to the protection provided by the full three-dose schedule for four years following vaccination. Two doses administered six months apart also appeared to provide partial protection against HPV31/33/45, similar to that observed for three doses. These data strongly argue for a direct evaluation of one-dose efficacy of the HPV16/18 vaccine.
Acknowledgments
Editing and publication co-ordinating service was provided by J Andersson, PhD (CROMSOURCE Ltd, UK) on behalf of GSK Vaccines, Wavre, Belgium. The authors thank Mr. David Check, Biostatistics Branch, DCEG, NCI, NIH, for development of the forest plots.
CVT
We would like to extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. We also acknowledge the tremendous effort and dedication of the staff in Costa Rica involved in this project. In the United States we extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort. We would like to specifically acknowledge the invaluable contributions made by J Cyr, J Buckland and B Befano. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants in our trial (SG Self, Chair, A Benavides, L Diego Calzada, R Karron, R Nayar, and N Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (JM Cain, DD Davey, F Fuster, A Gershon, E Holly, S Lara, H Raventós, W Rida and L Rosero-Bixby).
CVT Protocol available at http://proyectoguanacaste.org
PATRICIA
We thank all study participants and their families. We gratefully acknowledge the work of the central and local study coordinators, and staff members of the sites that participated in this study.
The HPV PATRICIA Principal investigators/co-principal investigator collaborators:
Australia S M Garland, A Mindel, S R Skinner. Belgium P De Sutter, W A J Poppe, W Tjalma. Brazil N S De Carvalho, P Naud, J C Teixeira. Canada F Y Aoki, F Diaz-Mitoma, L Ferguson, M Miller, K Papp, B Ramjattan, B Romanowski, P H Orr Finland D Apter, T Karppa, N Kudjoi, M Lehtinen, K Lönnberg, J Palmroth, J Paavonen, T Petaja, L Tuomivaara. Germany T Gent, T Grubert, W D Höpker, K Peters, K Schulze, TF Schwarz, R Waddell. Mexico J Salmerón. Philippines C Crisostomo, MR Del Rosario-Raymundo, JE Raymundo, M J Germar, G Limson, G Villanueva, S Villanueva, J D Zamora. Spain J Bajo-Arenas, J Bayas, M Campins, X Castellsagué, M Castro, C Centeno, ML Rodríguez de la Pinta, A Torné, J A Vidart. Taiwan S N Chow, M H Yu. Thailand S Angsuwathana, U Jaisamrarn. UK M Cruickshank, E A Hakim, D Lewis, A Szarewski (deceased). USA R Ackerman, M Caldwell, C Chambers, A Chatterjee, L DeMars, P Fine, W Huh, T Klein, J Lalezari, L Leeman, S Luber, M Martens, C Peterson, J Rosen, L Seidman, R Sperling, M Stager, J Stapleton, A Waldbaum, C M Wheeler.
Contributors (Group co-authors)
The Costa Rica Vaccine Trial Study Group: Silvia Jiménez, M.Sc. (Proyecto Epidemiologico Guanacaste, San José, Costa Rica), Paula González, MD (Proyecto Epidemiologico Guanacaste, San José, Costa Rica), Douglas R Lowy, MD (National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA), Ligia A Pinto, Ph.D. (National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA), Carolina Porras, M.Sc. (Proyecto Epidemiologico Guanacaste, San José, Costa Rica), Ana Cecilia Rodriguez, MD (Proyecto Epidemiologico Guanacaste, San José, Costa Rica), Mahboobeh Safaeian, Ph.D. (National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA), Mark Schiffman, MD (National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA), John T Schiller, Ph.D. (National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA), John Schussler, BChE (Information Management Systems, Silvier Spring, Maryland, USA), Mark E Sherman, MD (National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA)
The PATRICIA Study Group: Prof. F Xavier Bosch, MD (Network on Cooperative Cancer Research (RTICC) and Unit of Infections and Cancer, Cancer Epidemiology Research Program, Institut Català d’Oncologia, L’Hospitalet de Llobregat, IDIBELL, Barcelona, Catalonia, Spain), Xavier Castellsague, MD (Unit of Infections and Cancer, Cancer Epidemiology Research Program, Institut Català d’Oncologia, L’Hospitalet de Llobregat, IDIBELL, Biomedical Research Centre Network for Epidemiology and Public Health (CIBER-ESP), L’Hospitalet de Llobregat Catalonia, Spain), Prof. Archana Chatterjee, Ph.D. (Department of Pediatrics, University of South Dakota, Sanford School of Medicine and Sanford Children’s Specialty Clinic, South Dakota, USA), Prof. Song-Nan Chow, Ph.D. (Department of Obstetrics and Gynecology, College of Medicine and the Hospital, National Taiwan University, Taipei, Taiwan), Dominique Descamps, MD (GSK Vaccines, Wavre, Belgium), Francisco Diaz-Mitoma, MD (Advanced Medical Research Institute of Canada, Sudbury, Ontario, Canada), Gary Dubin, MD (GSK Vaccines, King of Prussia, PA, USA), Maria Julieta Germar, MD (University of the Philippines College of Medicine, Philippine General Hospital, Manila, Philippines), Prof. Diane M Harper, MD (Geisel School of Medicine at Dartmouth, Hanover, NH, USA), Prof. David J M Lewis, MD (Clinical Research Centre, University of Surrey, Guildford, UK), Prof. Genara Limson, MD (University of the Philippines, College of Medicine, Philippine General Hospital, Makati Medical Centre, Makati City, Philippines), Prof. Paulo Naud, MD (University Federal of Rio Grande do Sul, Hospital de Clínica de Porto Alegre, Porto Alegre, Brazil), Klaus Peters, MD (Facharzt für Frauenheilkunde und Geburtshilfe, Hamburg Germany), Prof. Willy AJ Poppe, Ph.D. (Department of Gynaecology, University Hospital KU Leuven Gasthuisberg, Leuven, Belgium), Brian Ramjattan, MD (First Line Medical Services Ltd., St. John’s, NL Canada), Prof. Barbara Romanowski, FRCPC (University of Alberta, Edmonton, AB, Canada), Prof. Jorge Salmeron, MD (Unidad de Investigación Epidemiológica y en Servicios de Salud, Instituto Mexicano del Seguro Social, Morelos, Mexico), Prof. Tino F Schwarz, Prof. (Central Laboratory and Vaccination Centre, Stiftung Juliusspital, Academic Teaching Hospital of the University of Wuerzburg, Wuerzburg, Germany), Julio C Teixeira, Ph.D. (Departamento de Tocoginecologia da Unicamp, University of Campinas, Campinas, Sao Paulo, Brazil), Prof. Wiebren AA Tjalma, Ph.D. (Multidisciplinary Breast Clinic – Gynecological Oncology Unit, Department of Obstetrics and Gynecology, Antwerp University Hospital – University of Antwerp, Antwerpen, Belgium)
Conflict of Interest
D Descamps, F Struyf, G Dubin and M-P David are employees of GSK group of companies. MP David, D Descamps, G Dubin and F Struyf own stock shares and stock options in the GSK group of companies and G Dubin holds a relevant patent; CM Wheeler received funding to conduct the clinical trial and reimbursement for travel from GSK group of companies via University of New Mexico, and reagents and equipment for HPV genotyping studies was provided to the University of New Mexico by Roche Molecular Systems; SM Garland reports she was a member of the Merck Cervical Cancer Global Advisory Board, Merck Scientific Advisory Board and received fare and accommodation related to her participation, grants from GSK group of companies paid via her institution to perform phase three clinical trials for HPV vaccines, researcher initiated grant from Merck Sharp & Dohme to perform surveillance RRP in Australia post HPV vaccine, and grant to her institution from CSL Bio for research on HPV and cancers; SR Skinner has received, via her institution, grants for clinical trials, travel reinbursements and honoraria to attend Advisory Board meetings and to present at educational meetings from GSK group of companies; X Bosch has received grants for clinical trials and speaker fees from Merck Sharp & Dohme, Sanofi Pasteur MSD and GSK group of companies, and grants from Merck Sharp & Dohme and GSK group of companies for educational presentations; X Castellsague has received grants from Merck Sharp & Dohme, Sanofi Pasteur MSD and GSK group of companies, and he has received non-financial support from Sanofi Pasteur MSD; A Chatterjee has received honoraria for speaking engagements from Merck Sharp & Dohme and GSK group of companies; D Lewis has received grants from GSK group of companies via St George’s University of London and University of Surrey for vaccine trials in Innovative Medicines Initiative project BIOVACSAFE; DR Lowy report that as a part of his US government supported research at the National Cancer Institute/National Institute of Health, he is the inventor of technology that underlies the L1-based prophylactic virus-like particle (VLP) HPV vaccine and technology that underlies an L2-based candidate prophylactic HPV vaccine. The NIH has licensed the technology for the LI VLP vaccine to Merck, the manufacturer of Gardasil, to GSK group of companies, the manufacturer of Cervarix®, and Indian Immunologicals Ltd. The L2-based vaccine technology is the subject of a cooperative research and development agreement between the NCI, John Hopkins University, and Shantha Biotech, and has been licensed to Shantha, PanVax, Acambis Inc, and GSK group of companies. US Federal law entitles D Lowy to a limited share of royalties the NIH receives for their technologies; K Peters has received grants from GSK group of companies for conducting the study; MR Del Rosario-Raymundo has received honorarium, travel support and payment for lectures including speakers bureaus from GSK group of Companies; B Romanowski has received grants for clinical trials from Merck Sharp & Dohme and GSK group of companies and support for travel to meetings for the study or other purposes and consulting fees/honoraria paid via B Romanowski corporation; J Salmeron grants from Merck Sharp & Dohme, GSK group of companies and Qiagen; TF Schwarz has received honoraria from GSK group of companies for being a member of advisory boards, lecturing and conducting clinical trials; J Schiller, is the inventor on U.S. government owned patents and has several patents related to HPV VLP technology with royalties paid by GSK group of companies and Merck Sharp & Dohme; J Teixeira reports grants, personal fees and non-financial support from GSK group of companies; A A Hildesheim, AR Kreimer, S Wacholder, R Herrero, S-N Chow, F Diaz Mitoma, MJV Germar, P Gonzalez, D Harper, S Jimenez, G Limson, P Naud, LA Pinto, C Porras, W Poppe, B Ramjattan, AC Rordiguez, M Safaeian, ME Sherman, M Schiffman, J Schussler and W Tjalma have nothing to disclose.
Other contributors
GSK Vaccines clinical study support: B Colau, S Genevrois, N Martens, N Houard, S Poncelet, C Provenzano, A Tonglet, C Van Hoof (Xpe Pharma), D Descamps, K Hardt, V Xhenseval, T Zahaf.
Laboratory contribution: E Alt, B Iskaros, A Limaye, R D Luff, M McNeeley, B Winkler (Quest Diagnostics Clinical Trials, Teterboro, NJ, USA). A Molijn, W Quint, L Struijk, M Van de Sandt, L J Van Doorn (DDL Diagnostic Laboratory, Voorburg, The Netherlands).
Endpoint committee: K P Klugman, P Nieminen.
Independent Data Monitoring Committee: C Bergeron, E Eisenstein, R Karron, R Marks, T Nolan, S K Tay.
CERVARIX is a registered trademark of the GSK group of companies.
GARDASIL is a registered trademark of Merck & Co Inc.
Contributors
ARK, FS, MRDRR, AH, SRS, SW, SMG, RH, MPD and CMW formed the manuscript core writing team. All authors (ARK, FS, MRDRR, AH, SRS, SW, SMG, RH, MPD, CMW, SJ, PGonzález, DRL, LAP, CP, ACR, M Safaeian, M Schiffman, JTS, JS, M Sherman, FXB, XC, AC, SNC, DD, FDM, GD, MJG, DMH, DJML, GL, PN, KP, WAJP, BR, BR, JS, TFS, JCT, WT) have qualified for authorship in adherence with the ICMJE guidelines and have reviewed and commented upon a draft, and gave final approval and had final responsibility for the decision to submit for publication. All authors contributed towards study design, acquisition of data or statistical analyses, and interpretation of data. In this analysis, the authors had full access to all the trial data for the trial they participated in and access to summary-level trial data for both trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 2.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 3.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 4.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–271. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 5.Levin A, Wang SA, Levin C, Tsu V, Hutubessy R. Costs of introducing and delivering HPV vaccines in low and lower middle income countries: inputs for GAVI policy on introduction grant support to countries. PLoS One. 2014;9:e101114. doi: 10.1371/journal.pone.0101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreimer AR, Rodriguez AC, Hildesheim A, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103(19):1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safaeian M, Porras C, Pan Y, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013;6(11):1242–50. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamontagne D, Mugisha E, Pan Y, et al. Immunogenicity of bivalent HPV vaccine among partially vaccinated young adolescent girls in Uganda. Vaccine. 2014 doi: 10.1016/j.vaccine.2014.08.071. Epubg ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. Jama. 2013;309(17):1793–802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 10.Romanowski B, Schwarz TF, Ferguson LM, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination Results from a randomized study. Human Vaccines & Immunotherapeutics. 2014;10(5):1–11. doi: 10.4161/hv.28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler CM, Castellsague X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–10. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 12.Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26(37):4795–4808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrero R, Wacholder S, Rodriguez AC, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 2011;1:408–19. doi: 10.1158/2159-8290.CD-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 15.Lehtinen M1, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 16.Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153(6):1731–1739. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37(8):2508–2517. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44(9):3292–3298. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp TJ1, Hildesheim A, Safaeian M, et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011;29(11):2011–4. doi: 10.1016/j.vaccine.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safaeian M, Porras C, Pan Y, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013;6:1242–50. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romanowski B1, Schwarz TF, Ferguson LM, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin. 2011;7(12):1374–86. doi: 10.4161/hv.7.12.18322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–96. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 23.Takacs P, Chatterjee A, Sperling R, et al. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: end-of-study analysis of a Phase III randomized trial. Hum Vaccin Immunother. 2014 doi: 10.4161/hv.36121. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiller JT, Lowy DR. Raising Expectations For Subunit Vaccine. J Infect Dis. 2014 doi: 10.1093/infdis/jiu648. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joura E, Giuliano A, Iversen OE, et al. Efficacy of a 9-Valent HPV Vaccine against genital infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Summary of the SAGE April 2014 meeting. The World Health Organization’s (WHO) Strategic Advisory Group of Experts (SAGE) to endorse a two-dose vaccination strategy for young adolescents 2014. E-pub ahead of print. [Google Scholar]
- 27.Kreimer AR, Sherman ME, Sahasrabuddhe VV, Safaeian M. The case for conducting a randomized clinical trial to assess the efficacy of a single dose of prophylactic HPV vaccines among adolescents. J Natl Cancer Inst. 2015;107(3) doi: 10.1093/jnci/dju436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


