Abstract
BACKGROUND
MicroRNA-223 (miR-223) is a hematopoietic lineage cell-specific microRNA. However, a significant amount of miR-223 has been identified in vascular smooth muscle cells (VSMCs) and vascular walls that should not have endogenous miR-223.
OBJECTIVES
This study sought to determine the sources of miR-223 in normal and atherosclerotic arteries and the role of miR-223 in atherogenesis.
METHODS
The levels and sources of miR-223 in blood cells (leukocytes and platelets), serum, blood microparticles, VSMCs, and vascular walls were determined. Both in vivo and in vitro studies were conducted to evaluate miR-223 secretion by blood cells and the ability of miR-223 to enter VSMCs and vascular walls. Subsequent changes in and the effects of miR-223 levels on serum and arteries in atherosclerotic animals and patients were investigated.
RESULTS
Blood cells were able to secrete miR-223 into serum. MicroRNA-223 from blood cells was the most abundant cell-free miRNA in blood. Blood cell-secreted miR-223 could enter VSMCs and vascular walls, which produced strong biological effects via its target genes. In both atherosclerotic apolipoprotein-E knockout mice and patients with atherosclerosis, miR-223 levels were significantly increased in serum and atherosclerotic vascular walls. The atherosclerotic lesions in apolipoprotein-E knockout mice were exacerbated by miR-223 knockdown. The effect of miR-223 on atherogenesis was verified using miR-223 knockout mice.
CONCLUSIONS
Blood cell-secreted miR-223 enters vascular cells and walls, and appears to play important roles in VSMC function and atherogenesis. As a novel endocrine genetic signal between blood cells and vascular cells, miR-223 may provide a novel mechanism and new therapeutic target for atherosclerosis.
Keywords: atherosclerosis, bone marrow, insulin-like growth factor 1 receptor, microRNAs
It is well established that inflammation is the principal underlying mechanism in atherogenesis, in which blood cells such as leukocytes and platelets play a central role. A large body of evidence supports the role of leukocytes and platelets in the development, progression, and complications of atherosclerosis. However, the molecular mechanisms responsible for atherogenesis induced by blood cells and any related new therapeutic targets remain to be defined (1).
Micro-ribonucleic acids (miRNAs) are a class of endogenous, small, noncoding RNAs that directly regulate >30% of genes in a cell (2,3). Once thought to exist only within cells, recent studies from our groups and others have demonstrated that miRNAs can be exported from cells (4-6). MicroRNAs are found in many bodily fluids, including circulating blood and urine. Moreover, extracellular miRNAs are stable due to binding with microparticles. These extracellular miRNAs may enter into other tissues and cells to serve as novel cell-to-cell communicators (7).
MicroRNA-223 is a hematopoietic lineage, cell-specific miRNA. Recent studies have revealed that miR-223 is nearly exclusively expressed in hematopoietic cells at bone marrow and in bone marrow-derived blood cells, mainly in blood platelets and leukocytes, and has a low level in red blood cells (8). Although vascular cells should have no endogenous miR-223 expression, a significant amount of miR-223 has been identified in normal vascular walls, as has been demonstrated previously (9).
We hypothesized that miR-223 could be secreted into the circulating blood by bone marrow-derived blood cells such as platelets and leukocytes. The blood cell-secreted miR-223 could enter into vascular smooth muscle cells (VSMCs) as a novel endocrine genetic signal to regulate their functions and begin atherogenesis via its target genes. If verified, miR-223 might be a novel link between the inflammatory blood cells and vascular cells that control vascular cell functions and the development of vascular disease.
METHODS
VSMCs from the aortas of male Sprague-Dawley rats were cultured with Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. For the platelet study, mouse (C57BL/6) platelets were isolated using platelet-rich plasma and the gel filtration method (10). For the leukocyte study, mouse monocytes from blood were used and isolated as previously described (11). We also used human monocytic cell line THP-1 cells (ATCC, Manassas, Virginia).
Microparticles from serum were isolated by ultra-centrifugation (4,5). RNAs were isolated with a reagent (TRIzol, Life Technologies, Carlsbad, California) (5,9). Levels of miRNAs and messenger RNAs were determined by real-time reverse transcription-polymerase chain reaction (qRT-PCR). The absolute quantification of a miRNA was on the basis of qRT-PCR and a standard curve using a series of concentrations of synthesized mature miRNA (4,5), which were expressed as picomoles per liter, copies per cell, or copies per 15-pg tissue RNA.
The profiles of miRNAs in serum, blood cells (platelets and leukocytes), and blood microparticles were performed using Rodent MicroRNA low-density array, whereas human miRNA profiling was performed using a human low-density array (both TaqMan, Life Technologies).
The expression of miR-223 was down-regulated by its inhibitor and up-regulated via its mimics.
Culture medium was collected before and after 12 h of THP-1 macrophage culture, and miR-223 levels in the collected medium were determined. In addition, the collected medium after 12 h of THP-1 macrophage culture was added to the cultured medium of VSMCs. After 12 h, levels of miR-223 in VSMCs were determined.
THP-1 macrophages were seeded in the upper chamber of a co-culture system and then transfected with Cy3-marked miR-223. VSMCs were added into the lower chamber. Secretion of Cy3-marked miR-223 and its ability to enter into VSMCs were monitored by fluorescence microscopy.
In vivo, mice miR-223 expression was knocked down by the locked nucleic acid miRNA inhibitor for miR-223 (LN-anti-miR-223) (30 mg/kg, intraperitoneal, biweekly; Exiqon, Vedbaek, Denmark).
ASSAYS AND ANALYSIS
Proteins were isolated from cultured VSMCs and determined by Western blot analysis using antibodies for the insulin-like growth factor-1 receptor (IGF-1R), Akt, and phospho-Akt (Cell Signaling Technology, Danvers, Massachusetts). Glyceraldehyde-3-phosphate dehydrogenase antibody was used as a loading control.
A fragment of the 3′-untranslated region (UTR) of IGF-1R mRNA, which contained the conserved miR-223 binding sequence with a firefly luciferase reporter, was transfected into HEK 293 cells. The cells were co-transfected with a vehicle, an empty plasmid (pDNR-CMV), and a plasmid expressing miR-223 (pmiR-223). Relative luciferase activity was measured.
VSMC proliferation was induced by platelet-derived growth factor (PDGF) (20 ng/ml) and determined by MTT assay and 5-ethynyl-2′-deoxyuridine assay (12). VSMC migration was determined by a modified Boyden chamber assay (12), and VSMC apoptosis in cultured cells was measured by terminal deoxynucleotidyl transferase dUTP nick end labeling assay (12).
The well-established leukocyte depletion and platelet depletion models in male wild-type C57BL/6 mice (3 months old) were used as described previously (13,14).
Atherosclerosis was induced in the aortas of apolipoprotein E (apoE) knockout male mice on a C57BL/6 background (The Jackson Laboratory, Bar Harbor, Maine) by giving them a Western diet (13).
Atherosclerotic lesions were measured by oil red O staining using en face preparation of whole aortas (13, 14). Carotid artery balloon injury was induced in male Sprague-Dawley rats (230 to 300 g) as previously described (9).
Right carotid artery ligation injury was induced in male wild-type C57BL/6 mice and age-matched miR-223 knockout mice as described in our previous study (15).
Morphometric analysis via computerized image analysis system (NIS Elements BR 3.0, Nikon Instruments, Inc., Melville, New York) was performed in sections with hematoxilyn and eosin staining (9, 15).
All animal protocols were approved by the Institutional Animal Care and Use Committee at Rush University and were consistent with the Guide for the Care and Use of Laboratory Animals (updated version of the National Institutes of Health guidelines, 2011). The study was also approved by the research ethics committee of the Sun Yat-sen University and performed in accordance with the Declaration of Helsinki. Human serum samples were from healthy subjects (n = 8) and from patients with atherosclerosis (n = 8). The therosclerotic arteries (n = 6) were obtained from patients with arteriosclerosis obliterans, and the normal lower limb artery samples (n = 6) were acquired from donors without arteriosclerosis obliterans. All subjects provided written informed consent.
STATISTICAL ANALYSIS
All data are presented as mean ± SE. For relative gene expression, the mean value of the vehicle control group is defined as 100% or 1. Two-tailed unpaired Student t tests and analyses of variance were used for statistical evaluation of the data. SPSS (version 17.0; IBM, Armonk, New York) was used for data analysis. A p value < 0.05 was considered significant.
Additional methodological details are described in the Online Appendix.
RESULTS
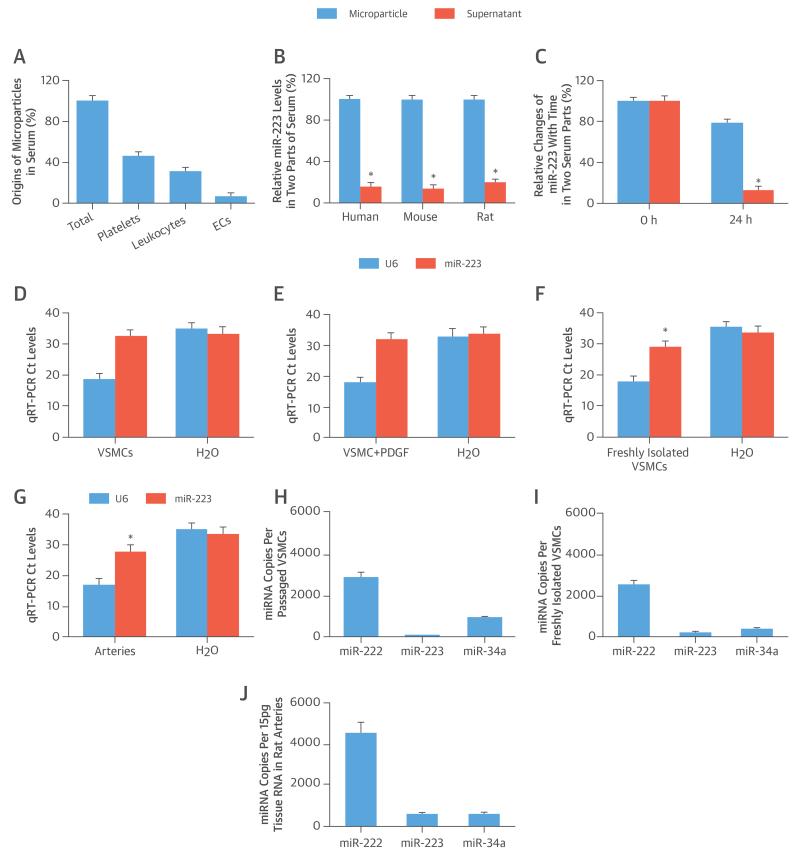
The results of the miRNA arrays demonstrated that miR-223 is the most abundant miRNA in platelets, leukocytes (monocytes), and blood microparticles from humans, mice, and rats. The 5 most abundant miRNAs in mice are shown in Online Table 1. The origins of isolated microparticles from serum were discriminated by flow cytometry according to the expression of membrane-specific antigens. The majority of circulating microparticles were from platelets, followed by leukocytes and endothelial cells (Figure 1A); the remainder (18%) were from other sources.
FIGURE 1. miR-223 in Circulating Serum, VSMCs, and Vessels.
(A) Microparticles arise from different sources in serum (n = 6). (B) The extracellular distribution of micro-ribonucleic acid (miR)-223 in 2 parts of serum. (C) MicroRNA-223 exhibits varying stability in microparticles and supernatant parts within 24 h. (D) The cycle threshold (Ct) levels of miR-223 and U6 in water (H2O) (negative control) and in passaged vascular smooth muscle cells (VSMCs) cultured in serum-free medium. (E) Platelet-derived growth factor (PDGF) (20 ng/ml) stimulation does not induce any miR-223 expression in passaged VSMCs. (F) A significant amount of miR-223 can be found in freshly isolated VSMCs. (G) A significant amount of miR-223 can be found in normal vascular walls. Absolute quantification of miR-223, miR-222, and miR-34a in (H) passaged VSMCs, (I) freshly isolated VSMCs, and (J) normal vascular walls. *p < 0.05 compared with control subjects. EC = endothelial cell.
The expression levels of miRNA in mouse and rat serum were profiled by miRNA arrays. The top 5 miRNAs (Online Table 2) in serum were further verified by qRT-PCR. To increase the translational feature of our study, we determined the miRNA profile in normal human serum samples. (The top 5 miRNAs in human serum are listed in Online Table 2.) Clearly, miR-223 was the most abundant miRNA in both animal and human serum.
To identify the distribution of extracellular miR-223 in blood, mouse serum was divided into 2 parts, supernatant and microparticle, as described previously (4, 5), and the majority of the miR-223 was located in the latter (Figure 1B). To assess the stability of miR-223, the levels of miR-223 were determined immediately and 24 h later at 4°C. MicroRNA-223 in microparticles was very stable (Figure 1C), unlike the miR-223 outside the microparticles, which was not stable.
As shown in Figure 1D, it was difficult to find miR-223 in passaged VSMCs cultured with serum-free medium by qRT-PCR (fetal bovine serum had a high level of miR-223). Its cycle threshold level was very close to water control (negative control). The negative result of miR-223 in passaged VSMCs was not related to their growth status, because the PDGF stimulation did not change miR-223 levels in serum-free cultured VSMCs (Figure 1E). However, a significant amount of miR-223 was identified in freshly isolated VSMCs and in normal vascular walls (Figures 1F and 1G). For further confirmation, we determined the absolute quantification of miR-223, miR-222 (an abundant miRNA in VSMCs and vessels), and miR-34a (another well-studied miRNA in VSMCs and vessels) in these cells and vessels. Again, a significant amount of miR-223 was found in freshly isolated VSMCs and in normal vascular walls (Figures 1I and 1J), but was not found in passaged VSMCs (Figure 1H). In freshly isolated VSMCs and in normal vascular walls, the levels of miR-223 were lower than the abundant levels of miR-222, but was similar to the levels of miR-34a (Figures 1I and 1J).
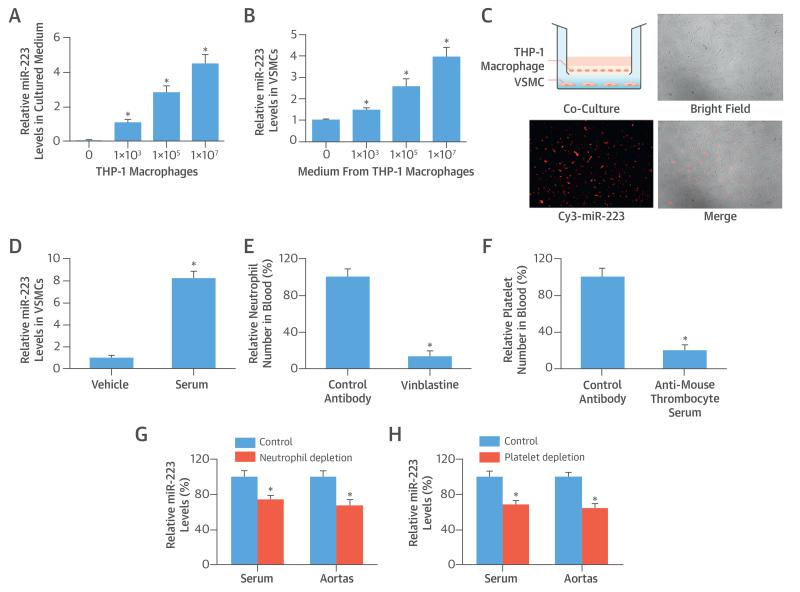
To test whether blood cells could secrete miR-223 into the extracellular space, THP-1 macrophages were seeded into cell culture wells with different cell numbers but with a fixed amount of serum-free medium. At 12 h after culture, miR-223 levels in the medium were determined. No miR-223 was found in the medium without THP-1 macrophages. However, a significant amount of miR-223 was identified in THP-1 macrophage-cultured medium (Figure 2A). These culture media were then added into the VSMC culture medium. At 12 h after culture, the VSMCs were collected to determine the levels of miR-223 inside the VSMCs. Blood cell-secreted miR-223 could enter into VSMCs, as demonstrated by the increased levels of miR-223 in these VSMCs, which were cultured with miR-223-containing medium (Figure 2B). To provide direct evidence that the blood cell-released miR-223 could enter into VSMCs apart from the blood cells, a co-culture system (Transwell, Corning, Tewksbury, Massachusetts) was used. After seeding into the upper chamber, THP-1 macrophages were transfected with Cy3-marked miR-223. VSMCs were added into the lower chamber. The THP-1 macrophage-released, Cy3-marked miR-223 could enter the VSMCs (Figure 2C), although these 2 groups of different cells did not contact each other. To test whether serum miR-223 could enter the VSMCs, fresh serum isolated from rats was added into the culture medium (at 10%) of VSMCs for 24 h. The results revealed that the serum miR-223 could enter VSMCs (Figure 2D).
FIGURE 2. Blood Cell-Secreted miR-223 in Extracellular Space, VSMCs, and Vascular Walls.
(A) MiR-223 levels rose in culture medium of THP-1 macrophages with increasing cell numbers. (B) Culture medium of THP-1 macrophages were added into culture medium of VSMCs; miR-223 levels were increased in VSMCs with miR-223–containing medium. (C) THP-1 macrophage-secreted, Cy3-marked miR-223 (red color) in the upper chamber of a co-culture system could enter VSMCs in the lower chamber through the extracellular medium. (D) MicroRNA-223 levels were higher in the group in which fresh serum isolated from rats was added into a culture medium (at 10%) of VSMCs for 24 h. (E) Neutrophils in blood were depleted by vinblastine (2.5 mg/kg intraperitoneally). (F) Platelets in blood were depleted by antimouse thrombocyte serum (50 μl intraperitoneally). (G) The levels of miR-223 in serum and aortas were decreased in mice that had depletion of neutrophils. (H) MicroRNA-223 levels in serum and aortas decreased in mice with depletion of platelets. *p < 0.05 compared with control groups. Abbreviations as in Figure 1.
To further verify these findings with regard to miR-223 sources and mobility, we applied mouse models of leukocyte (neutrophil) or platelet depletion. As expected, the leukocytes or platelets in blood were successfully depleted by their special antibodies, as evidenced by the decreased numbers of these blood cells in mice (Figures 2E and 2F). Seven days after depletion, the serum and aortas from these mice were collected to measure the miR-223 levels, which were significantly decreased in mice with the depletion of leukocytes or platelets (Figures 2G and 2H). The results suggest that blood cells are the major source of miR-223 in serum and vascular walls.
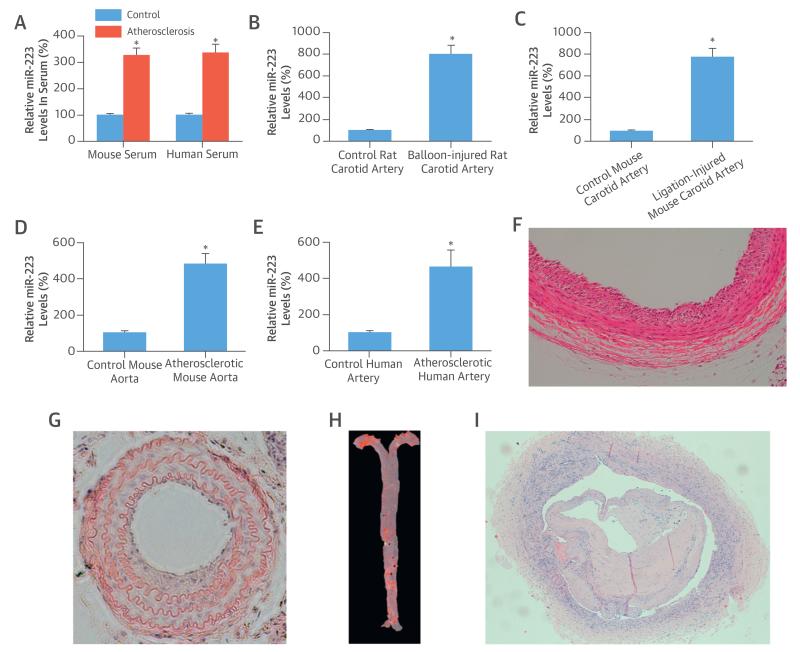
We also found that the serum levels of miR-223 from atherosclerotic apoE knockout mice and patients with atherosclerosis were significantly higher compared with the levels from normal control subjects (Figure 3A).
FIGURE 3. miR-223 Levels from Subjects With Atherosclerosis and in Injured Arteries.
(A) Serum levels of miR-223 from atherosclerotic apolipoprotein E knockout mice and from patients with higher atherosclerosis levels compared with normal control subjects. (B) MiR-223 levels rose in balloon-injured rat carotid arteries, (C) ligation-injured mouse carotid arteries, (D) atherosclerotic aortas from apolipoprotein E knockout mice, and (E) atherosclerotic human arteries compared with normal controls. (F) The successful rat carotid artery balloon injury, (G) mouse carotid artery ligation injury in mice, (H) mouse atherosclerotic aorta, and (I) human atherosclerotic artery were confirmed by histology. *p < 0.05 compared with control groups. Abbreviations as in Figure 1.
As shown in Figures 3B to 3E, qRT-PCR results revealed that the miR-223 levels in balloon-injured rat arteries, ligation-injured mouse arteries, mouse atherosclerotic aortas, and human atherosclerotic arteries were significantly higher than those in matching normal control vessels. The successful rat carotid artery balloon injury (Figure 3F), mouse carotid artery ligation injury (Figure 3G), mouse atherosclerotic aorta (Figure 3H), and human atherosclerotic artery (Figure 3I) were confirmed by histology.
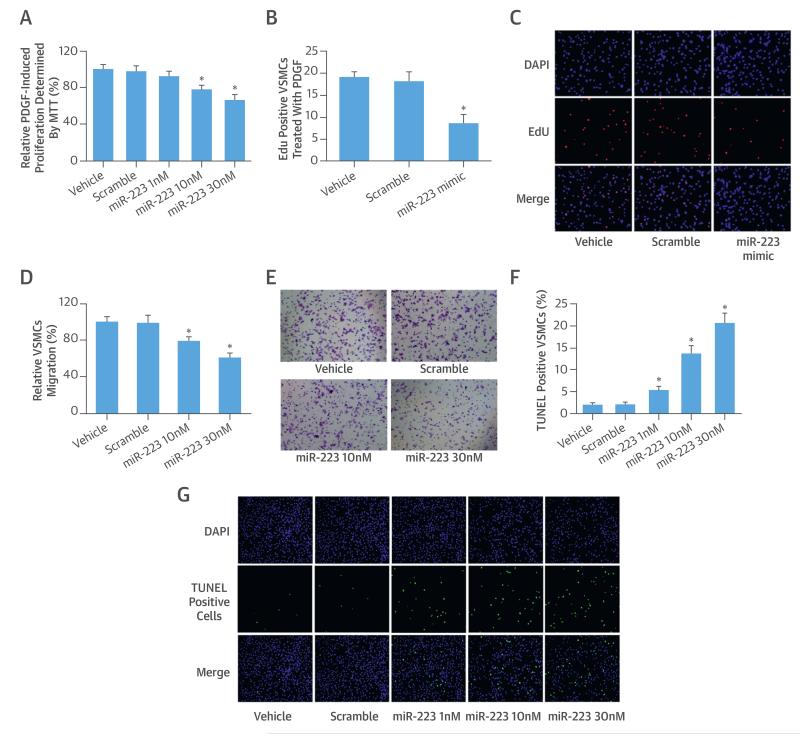
The effects of miR-223 on VSMC proliferation, migration, and apoptosis were determined using our well-established cell models. Because VSMCs have no or negligible endogenous miR-223, we could only use the gain-of-function approach to test them. To increase the levels of miR-223, different concentrations of miR-223 mimic (1 to 30 nM) were used. We found that miR-223 had a strong negative effect on VSMC proliferation as determined by MTT (Figure 4A) and 5-ethynyl-2′-deoxyuridine assay (Figures 4B and 4C). Also, the miR-223 mimic inhibited the VSMC migration in a dose-dependent manner (Figures 4D and 4E). In contrast, miR-223 overexpression significantly increased VSMC apoptosis (Figures 4F and 4G).
FIGURE 4. Cellular Functions of miR-223 in VSMCs.
The effect of miR-223 on PDGF-induced VSMC proliferation as determined by (A) MTT assay and by (B) 5-ethynyl-2′-deoxyuridine (EdU) method. (C) The EdU-positive VSMCs from different groups. The effect of miR-223 on (D) VSMC migration determined by the Boyden chamber assay seen in (E) representative images from different groups. (F) The effect of miR-223 on VSMC apoptosis determined by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis, seen in (G) representative images from different groups. *p < 0.05 compared with control groups. DAPI = 4′,6-diamidino-2-phenylindole; other abbreviations as in Figure 1.
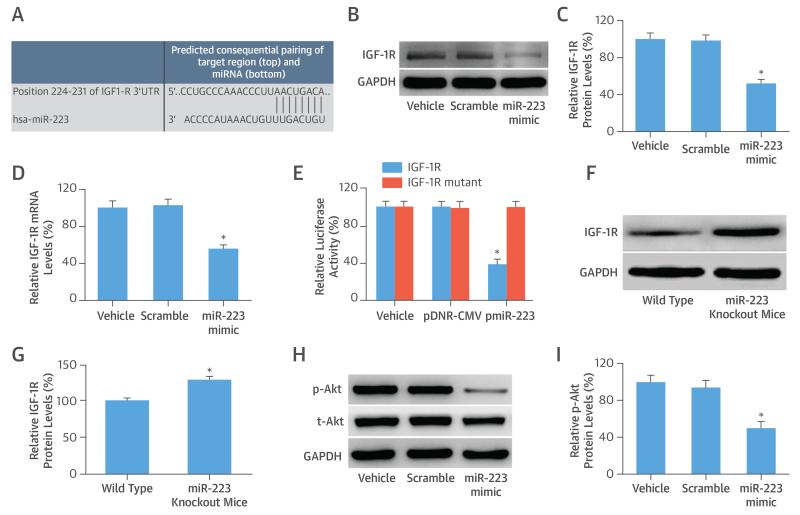
Computational analysis suggested that human IGF-1R has a miR-223 binding site in its 3′-UTR, which is conserved in mice, rats, and humans (Figure 5A). IGF-1R is thus a potential direct target gene in VSMCs. To test this, the miR-223 mimic was transfected into VSMCs, and the IGF-1R expression was determined at both the protein and mRNA levels. The expression of IGF-1R was down-regulated in VSMCs (Figures 5B to 5D).
FIGURE 5. IGF-1R/PI3K-Akt: Downstream Signaling Pathway of miR-223 in VSMCs.
(A) Computational analysis suggests that human insulin-like growth factor 1 receptor (IGF-1R) has an miR-223 binding site in its 3′-UTR. (B) Representative Western blots show IGF-1R protein levels from different groups. Overexpression of miR-223 decreased the expression of IGF-1R at (C) the protein level and the (D) messenger RNA (mRNA) level in VSMCs. (E) Luciferase assay in HEK 293 cells co-transfected with a fragment of the 3′-UTR of IGF-1R mRNA containing the conserved miR-223 binding sequence, and either vehicle, an empty plasmid (pDNR-CMV), or a plasmid expressing miR-223 (pmiR-223). An IGF-1R 3′-UTR mutated fragment was used as a mutated control group. (F) Representative Western blots show IGF-1R protein levels in aortas from miR-223 knockout mice and wild-type control mice. (G) IGF-1R expression in aortas from miR-223 knockout mice was higher than that from wild-type control mice. (H) Representative Western blots show p-AKT and total AKT (t-AKT) from different groups. (I) Overexpression of miR-223 decreased expression of p-AKT in VSMCs. *p < 0.05 compared with control groups. GADPH = glyceraldehyde-3-phosphate dehydrogenase; hsa = human; other abbreviations as in Figure 1.
To further confirm that miR-223 is able to directly bind to IGF-1R and inhibit its expression, a fragment of the 3′-UTR of IGF-1R mRNA that contained the conserved miR-223 binding sequence was cloned into a firefly luciferase reporter construct and was transfected into HEK 293 cells. As expected, pmiR-223, but not pDNR-CMV, the empty control plasmid, inhibited luciferase activity (Figure 5E). In the IGF-1R mutated control groups, the inhibitory effect of pmiR-223 disappeared (Figure 5E). The results suggested that miR-223 was able to bind to IGF-1R directly and inhibit their expression. Finally, IGF-1R, as a target gene of miR-223, was verified using knockout mice. As shown in Figures 5F and 5G, IGF-1R expression in aortas from miR-223 knockout mice was significantly higher than that from wild-type control mice.
PI3K-Akt is reported to be the downstream signaling pathway of IGF-1R and has strong cellular effects on VSMCs (16). To test the potential involvement of PI3K-Akt in miR-223-mediated effects on VSMCs, we overexpressed miR-223 via its mimic; miR-223 could inhibit the PI3K-Akt pathway, as shown by the decreased expression of p-Akt (Figures 5H and 5I).
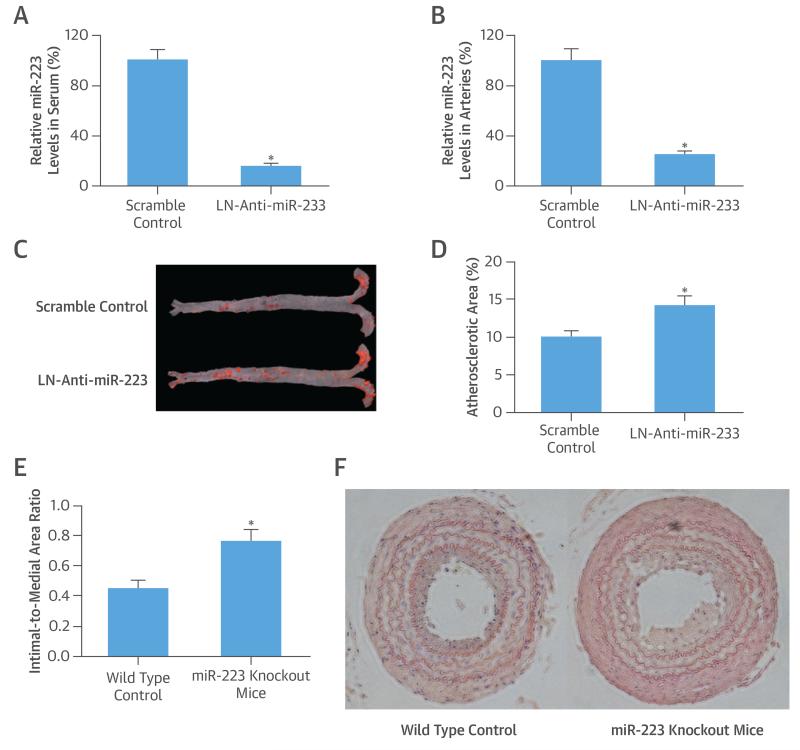
To test miR-223 effects on atherosclerosis, we applied a well-established mouse atherosclerosis model, in which atherosclerotic lesions were induced in apoE knockout mice by a Western diet. We confirmed that LN-anti-miR-223 (30 mg/kg), given both intravenously (tail vein) (Online Figures 1 and 2) or intraperitoneally (Figures 6A and 6B) biweekly could successfully knock down the miR-223 both in serum and vascular walls. We thus applied an intraperitoneal approach in this study. Our results demonstrated that miR-223 inhibition significantly increased atherogenesis, as shown by the increased atherosclerotic area determined via oil red O staining (Figures 6C and 6D).
FIGURE 6. Effects of miR-223 on Atherogenesis.
(A) Serum levels and (B) vascular levels of miR-223 in mice were knocked down by locked nucleic acid miRNA inhibitor for miR-223 (LN-anti-miR-223; 30 mg/kg intraperitoneally biweekly). (C) Atherosclerotic lesion areas (red color by oil red O staining) in aortas from apolipoprotein-E knockout mice treated with LN-anti-miR-223 or scramble control. (D) MicroRNA-223 inhibition by LN-anti-miR-223 significantly increased atherosclerotic lesion areas in apolipoprotein-E knockout mice with a Western diet. (E) Vascular neointimal lesion growth in miR-223 knockout mice was significantly increased compared with wild-type control mice in a model of carotid artery ligation injury. (F) Vascular neointimal formation in carotid arteries from wild-type C57BL/6 mice and miR-223 knockout mice. *p < 0.05 compared with control groups. Abbreviations as in Figures 1 and 3.
To verify the effect of miR-223 on atherogenesis, miR-223 knockout mice were used, with miR-223 knockout confirmed by qRT-PCR (Online Figure 3). To induce vascular neointimal growth, carotid artery ligation injury was performed in miR-223 knockout mice and in wild-type mice of the same age. Histological analysis revealed that the vascular neointimal growth in knockout mice at 4 weeks post-ligation injury was significantly increased compared with that in wild-type mice (Figures 6E and 6F).
DISCUSSION
MicroRNA-223 is a hematopoietic lineage, cell-specific miRNA. Theoretically, VSMCs should not produce or generate only negligible amounts of endogenous miR-223. The negative result was not induced by growth stopping under the serum-free condition, because in PDGF-stimulated, proliferative VSMCs, miR-223 still could not be detected. However, a significant amount of miR-223 could be found both in freshly isolated VSMCs and in normal vascular walls, but this miR-223 should mainly be from other sources. In this study, we found that blood cell-secreted miR-223 in serum could enter VSMCs, and that bone marrow-derived blood cells are the major sources of miR-223 in VSMCs and vascular walls.
More importantly, we identified that the extracellular serum miR-223 could not only enter into VSMCs, but also had strong biological functions in VSMCs via its target gene. Only 2 previous studies sought to determine the biological roles of miR-223 in VSMCs, but for unclear reasons, they reached opposite conclusions (17, 18). In this study, we identified that miR-223 had antiproliferative and antimigratory effects on VSMCs. No previous study has tested the effect of miR-223 on VSMC apoptosis, but we identified that overexpression of miR-223 via a miR-223 mimic markedly enhanced VSMC apoptosis.
It is well known that miRNA obtains its cellular effects via its multiple target genes. Previous studies have suggested that IGF-1R could be a direct target gene of miR-223 (16). In this study, IGF-1R was a target gene of miR-223, and along with its downstream signaling pathway PI3K-Akt, was involved in miR-223–mediated effects on VSMCs (Central Illustration). Other potential target genes from bioinformatics analysis (e.g., CDH11 and 14-3-3-gamma, which are involved in miR-223-mediated effects) should be tested in future studies.
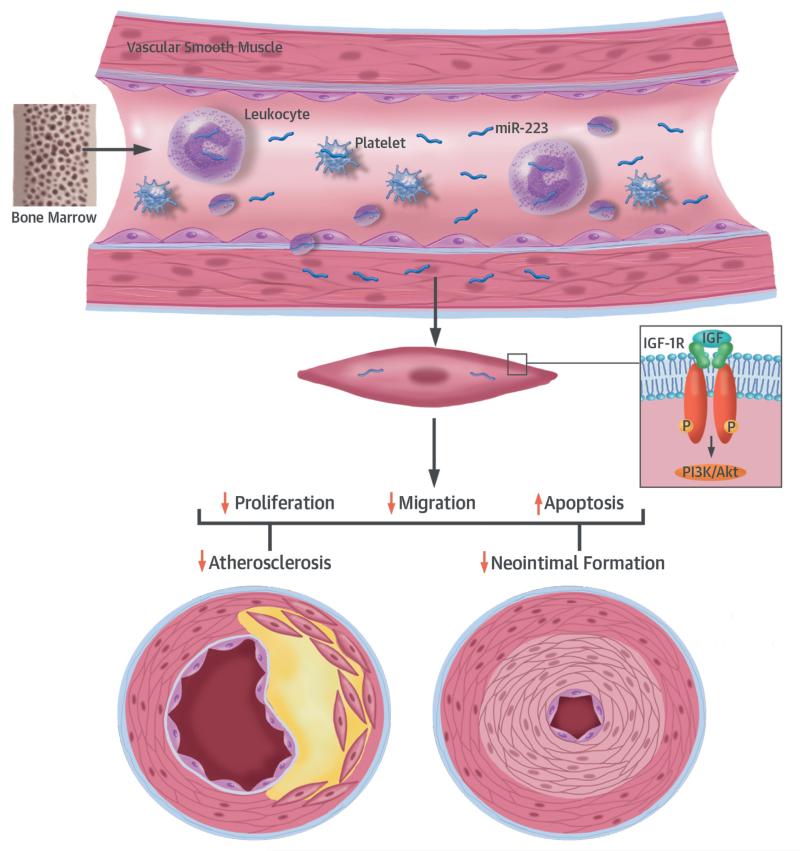
CENTRAL ILLUSTRATION. Blood Cell-Secreted miR-223 in Atherosclerotic Vascular Disease.
Inflammatory blood cells, such as leukocytes and platelets, which are originally from hematopoietic cells in bone marrow, can secrete the hematopoietic lineage, cell-specific micro-ribonucleic acid (miR)-223 into circulating serum. This miR-223 can then enter vascular cells (e.g., vascular smooth muscle cells [VSMCs] and vascular walls) and work as a novel endocrine genetic signal (like a hormone) in VSMCs to regulate their biological functions, such as proliferation, migration, and apoptosis via target genes such as insulin-like growth factor 1 receptor (IGF-1R) and the P13K-Akt pathway. Under pathological conditions, such as atherosclerosis and vascular injury, blood cells release more miR-223, which results in increased levels of miR-223 in VSMCs and vascular walls. The increased miR-223 helps protect against atherosclerotic vascular disease (vascular neointimal formation and atherosclerosis). P = phosphate.
It is well established that VSMC proliferation, migration, and apoptosis are key cellular events in atherosclerosis development. Because miR-223 had strong effects on these cellular events of VSMCs, we hypothesized that miR-223 might play a role in atherogenesis, and found that miR-223 in serum and arteries from atherosclerotic mice and patients were significantly higher than those from normal control subjects. Interestingly, the atherosclerotic lesions in apoE knockout mice on a Western diet were significantly exacerbated by miR-223 knockdown. Thus, increased miR-223 might help protect against atherosclerosis. This protective role in atherogenesis has been identified in other miRNAs, such as exogenous miR-145, a down-regulated miRNA in human atherosclerotic lesions (19).
Although we focused on VSMCs, blood cell-secreted miR-223 may also enter other vascular cells. Recent studies have found that high-density lipoprotein–binding miR-223 is able to enter other cells, including vascular endothelial cells, without endogenous miR-223 (20, 21). However, the exogenous miR-223 delivered via high-density lipoprotein reduces expression of intercellular adhesion molecule 1 in endothelial cells (21).
Finally, to verify the role of miR-223 in atherogenesis, we assessed vascular neointimal lesion formation induced by carotid artery ligation in miR-223 knockout mice. Compared with that in wild-type mice, the vascular neointimal formation in miR-223 deficient mice was markedly increased. The result regarding the effect of miR-223 on vascular neointimal growth from a gene knockout approach was consistent with that from pharmacological approaches on atherogenesis.
STUDY LIMITATIONS
The biological functions of miR-223 in other vascular cells and in blood inflammatory cells should be tested in future studies. In addition, cross-breeding of Apo E knockout mice with miR-223 knockout or miR-223 transgenic mice will provide additional evidence for the role of miR-223 in atherogenesis.
CONCLUSIONS
On the basis of our findings, inflammatory blood cells, such as leukocytes and platelets that are originally from hematopoietic cells in bone marrow, can secrete hematopoietic lineage, cell-specific miR-223 into circulating serum. This miR-223 could then enter vascular cells, such as VSMCs and vascular walls, and work as a novel endocrine genetic signal (like a hormone) in VSMCs to regulate biological functions, such as proliferation, migration, and apoptosis. Under pathological conditions, such as atherosclerosis and vascular injuries, blood cells are activated to release more miR-223 into serum and subsequently increase miR-223 levels in VSMCs and vascular walls, which helps protect against atherogenesis.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Micro-RNAs, such as miR-223, which are secreted from blood cells that modulate genetic endocrine signals in bone marrow, circulating inflammatory cells, and vascular tissue, may play a role in atherosclerosis.
TRANSLATIONAL OUTLOOK
Future studies should aim to define the role of blood cell–secreted miRNAs in atherosclerosis and explore the potential therapeutic value of interventions that influence their activity in patients at risk of atherosclerosis.
Acknowledgments
C. Zhang was supported by the National Institutes of Health (grants HL095707, HL109656, and NR013876 and Qingdao Ling Jun Ren Cai Fund # 13-CX-3). This work was supported in part by grants from the National Natural Science Foundation of China to Dr. Li (81370368) and Dr. Wang (81270378).
ABBREVIATIONS AND ACRONYMS
- apo-E
apolipoprotein-E
- IGF-1R
insulin-like growth factor-1 receptor
- miRNA
micro ribonucleic acid
- PDGF
platelet-derived growth factor
- qRT-PCR
real-time reverse transcription-polymerase chain reaction
- VSMC
vascular smooth muscle cell
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Shan, Qin, and Li contributed equally to this work and should be considered joint first authors.
APPENDIX For an expanded Methods section and supplemental tables and figures, please see the online version of this article.
REFERENCES
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Liang M. MicroRNA: a new entrance to the broad paradigm of systems molecular medicine. Physiol Genomics. 2009;38:113–5. doi: 10.1152/physiolgenomics.00080.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C. MicroRNAs in vascular biology and vascular disease. J Cardiovasc Transl Res. 2010;3:235–40. doi: 10.1007/s12265-010-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y, Tan N, Yang J, et al. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin Sci (Lond) 2010;119:87–95. doi: 10.1042/CS20090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Y Cheng, X Wang, J Yang, et al. A translational study of urine miRNAs in acute myocardial infarction. J Mol Cell Cardiol. 2012;53:668–76. doi: 10.1016/j.yjmcc.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deddens JC, Colijn JM, Oerlemans MI, et al. Circulating microRNAs as novel biomarkers for the early diagnosis of acute coronary syndrome. J Cardiovasc Transl Res. 2013;6:884–98. doi: 10.1007/s12265-013-9493-9. [DOI] [PubMed] [Google Scholar]
- 7.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–95. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 8.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–6. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 9.Ji R, Cheng Y, Yue J, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–88. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 10.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–7. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Zhang W, Zhu C, et al. Inactivation of the adenosine A2A receptor protects apolipoprotein E-deficient mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1046–52. doi: 10.1161/ATVBAHA.109.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Cheng Y, Yang J, et al. Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. J Mol Cell Cardiol. 2012;52:245–55. doi: 10.1016/j.yjmcc.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer M, Lefort J, Vargaftig BB. Granulocyte depletion and dexamethasone differentially modulate airways hyperreactivity, inflammation, mucus accumulation, and secretion induced by rmIL-13 or antigen. Am J Respir Cell Mol Biol. 2002;26:74–84. doi: 10.1165/ajrcmb.26.1.4618. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan BP, Wang R, Tawfik O, et al. Protective and damaging effects of platelets in acute cholestatic liver injury revealed by depletion and inhibition strategies. Toxicol Sci. 2010;115:286–94. doi: 10.1093/toxsci/kfq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotha J, Zhang C, Longhurst CM, et al. Functional relevance of tetraspanin CD9 in vascular smooth muscle cell injury phenotypes: a novel target for the prevention of neointimal hyperplasia. Atherosclerosis. 2009;203:377–86. doi: 10.1016/j.atherosclerosis.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:435–44. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 17.Song L, Duan P, Guo P, et al. Downregulation of miR-223 and miR-153 mediates mechanical stretch-stimulated proliferation of venous smooth muscle cells via activation of the insulin-like growth factor-1 receptor. Arch Biochem Biophys. 2012;528:204–11. doi: 10.1016/j.abb.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Rangrez AY, M’Baya-Moutoula E, Metzinger-Le Meuth V, et al. Inorganic phosphate accelerates the migration of vascular smooth muscle cells: evidence for the involvement ofmiR-223. PLoS One. 2012;7:e47807. doi: 10.1371/journal.pone.0047807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovren F, Pan Y, Quan A, et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation. 2012;126(11 Suppl 1):S81–90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- 20.Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabet F, Vickers KC, Cuesta Torres LF, et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun. 2014;5:3292. doi: 10.1038/ncomms4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.