Abstract
Background
Smoking contributes to reasons for hospitalisation, and the period of hospitalisation may be a good time to provide help with quitting.
Objectives
To determine the effectiveness of interventions for smoking cessation that are initiated for hospitalised patients.
Search methods
We searched the Cochrane Tobacco Addiction Group register which includes papers identified from CENTRAL, MEDLINE, EMBASE and PsycINFO in December 2011 for studies of interventions for smoking cessation in hospitalised patients, using terms including (hospital and patient*) or hospitali* or inpatient* or admission* or admitted.
Selection criteria
Randomized and quasi-randomized trials of behavioural, pharmacological or multicomponent interventions to help patients stop smoking, conducted with hospitalised patients who were current smokers or recent quitters (defined as having quit more than one month before hospital admission). The intervention had to start in the hospital but could continue after hospital discharge. We excluded studies of patients admitted to facilities that primarily treat psychiatric disorders or substance abuse, studies that did not report abstinence rates and studies with follow-up of less than six months. Both acute care hospitals and rehabilitation hospitals were included in this update, with separate analyses done for each type of hospital.
Data collection and analysis
Two authors extracted data independently for each paper, with disagreements resolved by consensus.
Main results
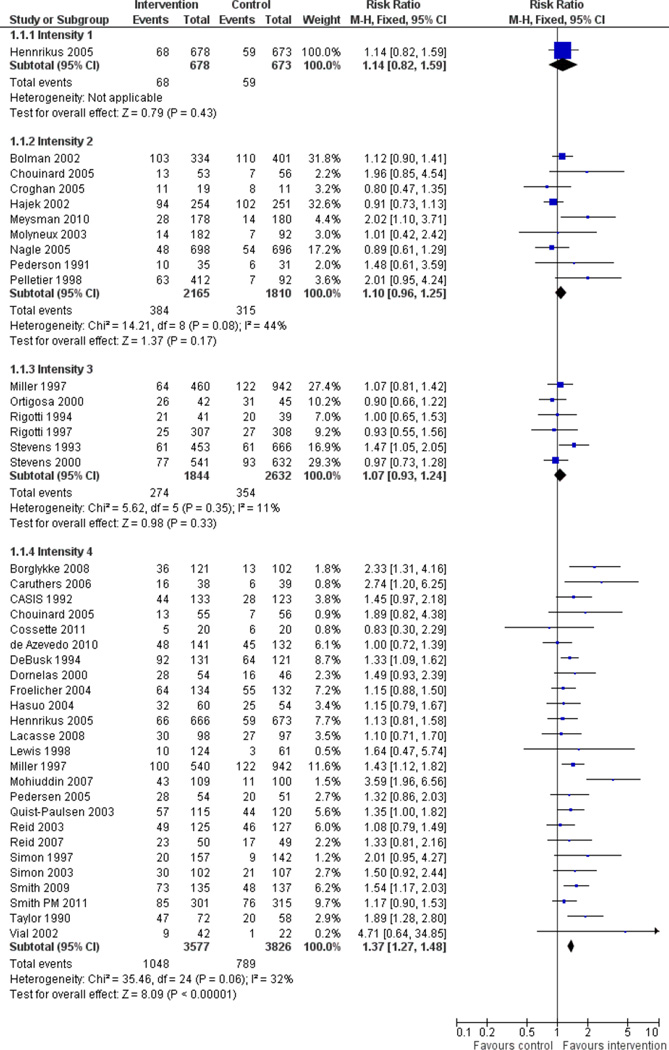
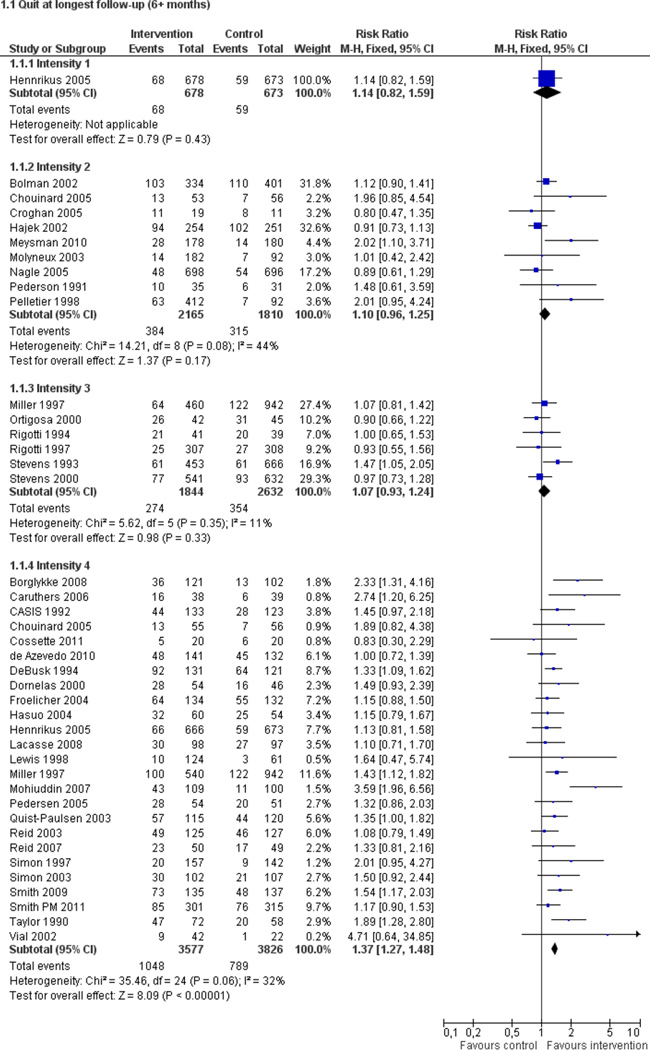
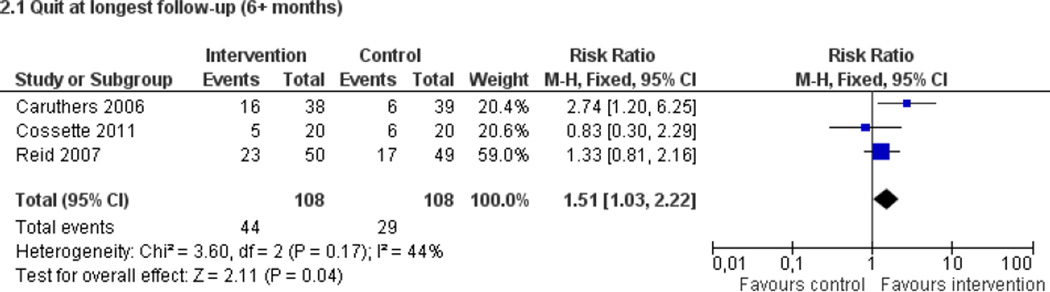
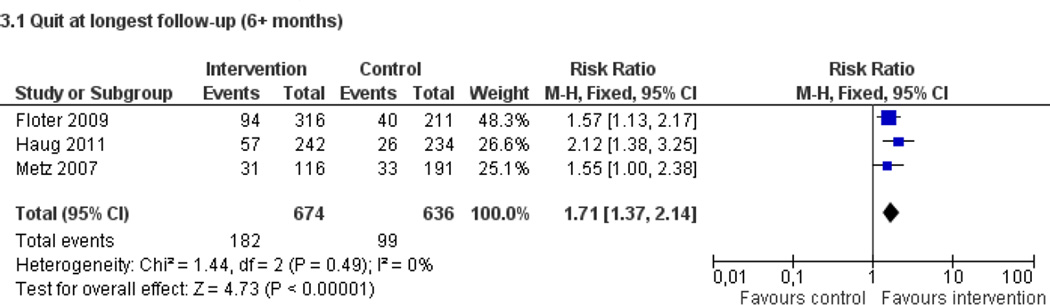
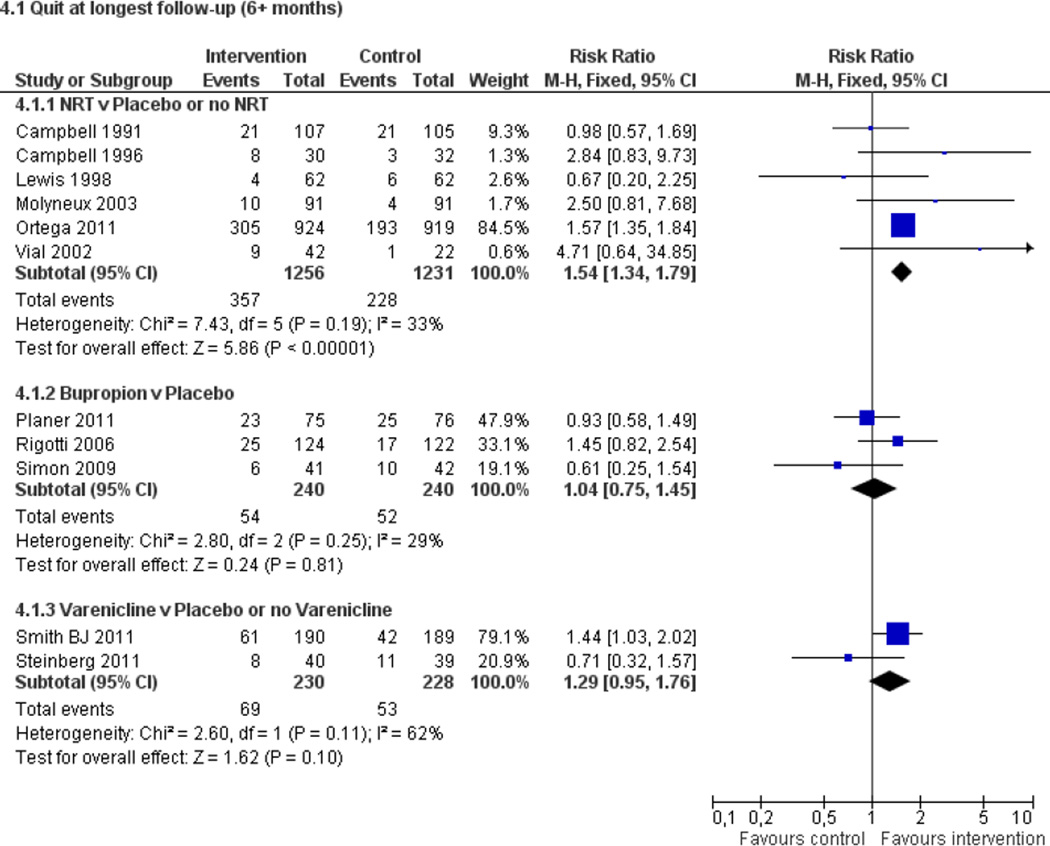
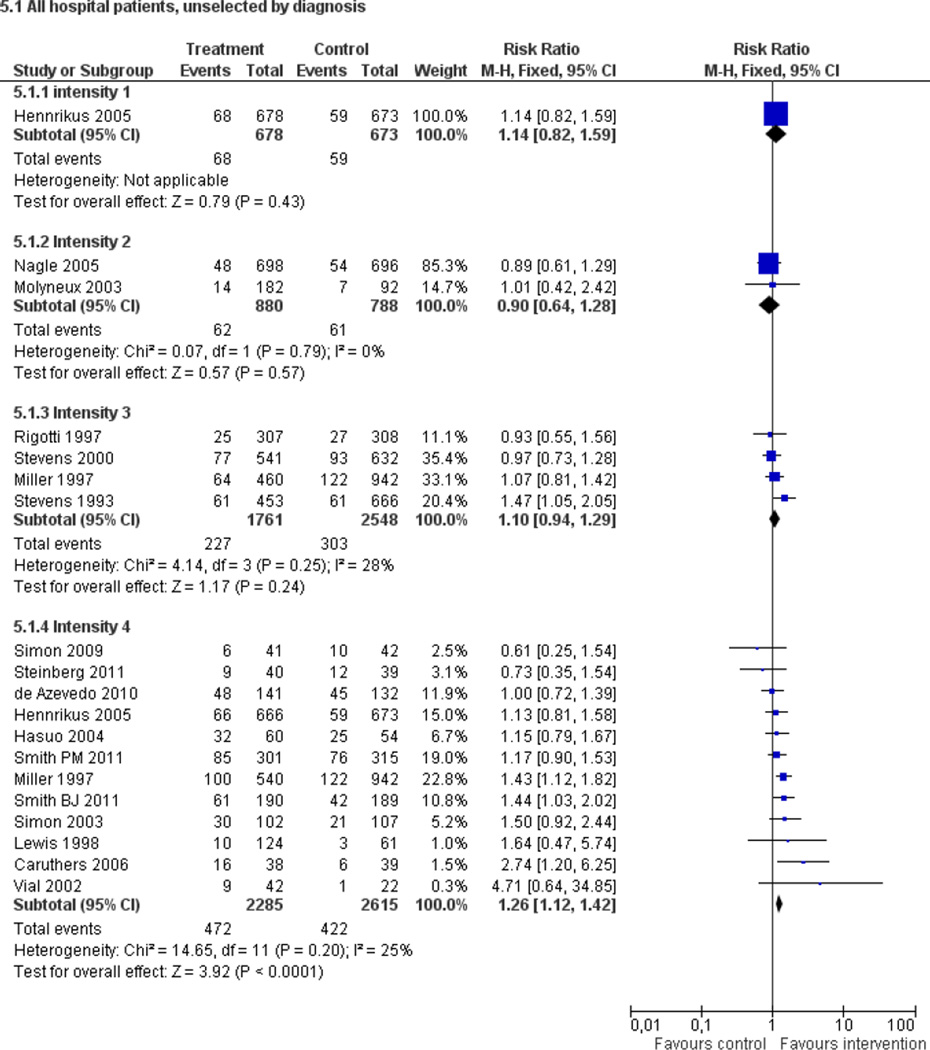
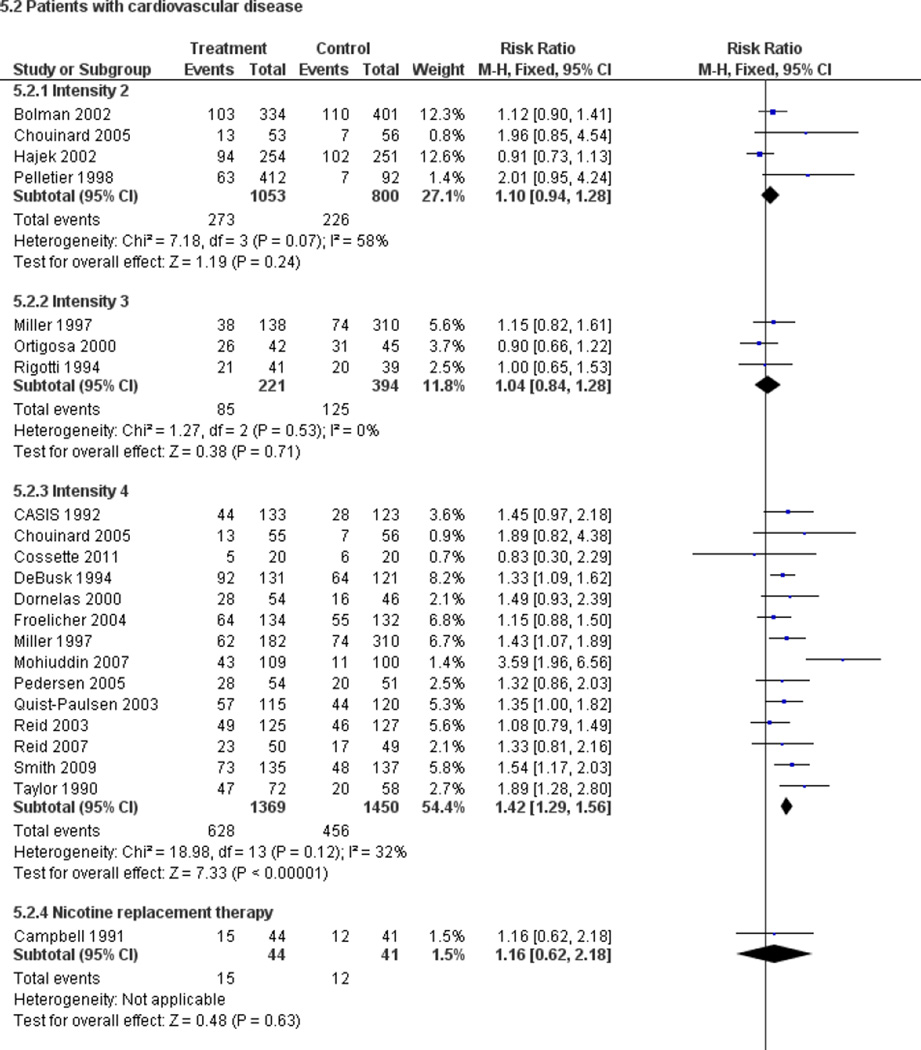
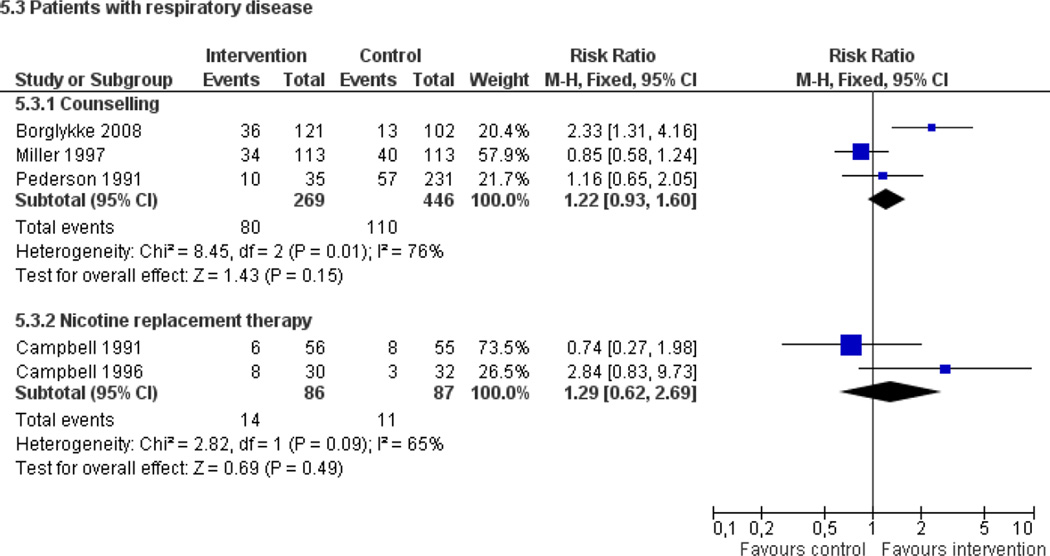
Fifty trials met the inclusion criteria. Intensive counselling interventions that began during the hospital stay and continued with supportive contacts for at least one month after discharge increased smoking cessation rates after discharge (risk ratio (RR) 1.37, 95% confidence interval (CI) 1.27 to 1.48; 25 trials). A specific benefit for post-discharge contact compared with usual care was found in a subset of trials in which all participants received a counselling intervention in the hospital and were randomly assigned to post-discharge contact or usual care. No statistically significant benefit was found for less intensive counselling interventions. Adding nicotine replacement therapy (NRT) to an intensive counselling intervention increased smoking cessation rates compared with intensive counselling alone (RR 1.54, 95% CI 1.34 to 1.79, six trials). Adding varenicline to intensive counselling had a non-significant effect in two trials (RR 1.28, 95% CI 0.95 to 1.74). Adding bupropion did not produce a statistically significant increase in cessation over intensive counselling alone (RR 1.04, 95% CI 0.75 to 1.45, three trials). A similar pattern of results was observed in a subgroup of smokers admitted to hospital because of cardiovascular disease (CVD). In this subgroup, intensive intervention with follow-up support increased the rate of smoking cessation (RR 1.42, 95% CI 1.29 to 1.56), but less intensive interventions did not. One trial of intensive intervention including counselling and pharmacotherapy for smokers admitted with CVD assessed clinical and health care utilization endpoints, and found significant reductions in all-cause mortality and hospital readmission rates over a two-year follow-up period. These trials were all conducted in acute care hospitals. A comparable increase in smoking cessation rates was observed in a separate pooled analysis of intensive counselling interventions in rehabilitation hospitals (RR 1.71, 95% CI 1.37 to 2.14, three trials).
Authors' conclusions
High intensity behavioural interventions that begin during a hospital stay and include at least one month of supportive contact after discharge promote smoking cessation among hospitalised patients. These interventions are effective regardless of the patient's admitting diagnosis and are effective in rehabilitation settings as well as acute care hospitals. lnterventions of lower intensity or shorter duration have not been shown to be effective in this setting. This update found that adding NRT to intensive counselling significantly increases cessation rates over counselling alone. There is insufficient direct evidence to conclude that adding bupropion or varenicline to intensive counselling increases cessation rates over what is achieved by counselling alone.
Plain language summary
Interventions started during hospitalisation to help people to stop smoking
Smoking contributes to many health problems including cancers, cardiovascular disease and lung diseases. Smoking also increases the risk associated with hospitalisation for surgery. People who are in hospital because of a smoking-related illness are likely to be more receptive to help to give up smoking. Our review of fifty trials found that effective programmes to stop smoking are those that begin during a hospital stay and include counselling with follow-up support for at least one month after discharge. Such programmes are effective when administered to all hospitalised smokers, regardless of the reason why they were admitted to hospital, and in the subset of smokers who are admitted to hospital with cardiovascular disease. Adding nicotine replacement therapy to a counselling program increases the success rate of a program for hospitalised smokers.
Background
Smoking contributes to many of the health problems leading to hospitalisation, particularly vascular disease, respiratory illness and many cancers. In addition, smoking increases the risk associated with hospitalisations for surgical procedures. Hospitalisation, especially for a tobacco-related illness, may boost receptivity to smoking cessation messages by increasing perceived vulnerability, a so-called 'teachable moment'. Illness also brings smokers to the healthcare setting, where they have contact with health professionals who can provide a smoking cessation message or intervention. In addition, procedures such as coronary arteriography that provide detail of the patient's cardiac status may minimise subsequent denial of cardiac risk by the patient (Ockene 1992). Many hospitals restrict or prohibit smoking by patients to protect patients and staff from secondhand smoke exposure. This smoke-free environment may also provide an opportunity for smokers to try out tobacco abstinence away from the usual environmental cues to smoke. For these reasons, providing (or at least initiating) tobacco dependence treatments in hospitals may be an effective preventive health strategy.
A number of studies have evaluated smoking cessation services provided or initiated in hospital. The interventions have included behavioural counselling of different forms and intensity (including post-hospitalisation contacts), pharmacological therapies (such as nicotine replacement therapy [NRT], bupropion and varenicline), and combinations of counselling and pharmacotherapy. The aim of this review is to evaluate the effectiveness of smoking cessation interventions initiated during a hospital stay. In order to inform policy, we aimed to identify the components of effective programmes. In addition, we aimed to explore whether there is a difference in effect according to the reason for hospitalisation or whether the effect holds for patients with a variety of admission diagnoses, and whether the effect of interventions in acute care hospitals is also observed in rehabilitation hospitals.
Objectives
The primary objective was to determine the efficacy of any type of smoking cessation programme for hospitalised patients. Our hypotheses were that:
Systematic behavioural intervention (brief advice, individual counselling, provision of self-help materials, group therapy) increases quit rates more than usual care, and intensive intervention increases quit rates more than brief intervention.
Interventions that occur both in hospital and after discharge increase quit rates more than interventions limited to the hospital stay, and longer post-discharge follow-up increases quit rates more than short follow-up.
Adding pharmacotherapy (such as NRT, bupropion or varenicline) to a behavioural intervention increases quit rates more than placebo or no medication, and combining pharmacotherapy with a behavioural intervention increases quit rates more than either alone.
A secondary objective was to explore the possibility that the efficacy of interventions differed for patients with different diagnoses. This was done using subgroup analysis of trials that recruited patients from more than one specialty, and by indirect comparison of trials that recruited patients from within one disease category. The primary review focuses on interventions for smokers who are admitted to an acute care hospital. Studies of interventions for smokers in rehabilitation hospitals have now been published. This update includes a new separate review of the efficacy of smoking interventions initiated during a stay in a rehabilitation hospital.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi-randomized controlled trials.
Types of participants
Participants were patients who were hospitalised and who were currently smoking (defined as having smoked within one month of hospital admission) or had recently quit (defined as having quit more than one month before hospital admission). We excluded trials of secondary prevention or cardiac rehabilitation that did not recruit on the basis of smoking history, and trials in patients hospitalised in facilities that primarily treated psychiatric disorders or substance abuse (including inpatient tobacco addiction programmes). We included interventions that began in either acute care hospitals or rehabilitation hospitals. We included trials that recruited all hospitalised smokers and those limited to patients who planned to quit smoking after hospital discharge. Trials in which 'recent quitters' were classified as smokers were included, but a sensitivity analysis was performed on these data to determine whether they differed from trials that excluded such individuals.
Types of interventions
Any intervention that was initiated during hospitalisation and that aimed to increase motivation to quit, to assist a quit attempt, or to help recent quitters avoid relapse was included. Interventions that began in hospital and continued after discharge were included. The intervention could be delivered by physicians, nursing staff, psychologists, smoking cessation counsellors or other hospital staff. The intervention could include advice, more intensive behavioural therapy, or smoking cessation pharmacotherapy, with or without continued contact after hospital discharge. The control intervention could be any less intensive intervention, such as brief advice to quit, or it could be usual care. Studies that provided identical treatment consisting of more than usual or minimal care during the hospital stay and then randomly assigned participants to different post-discharge interventions were analysed separately in a sensitivity analysis. We included studies of smoking interventions that were part of a broader risk reduction or rehabilitation programme only if it was possible to extract data on the outcome effects of the smoking cessation component specifically, and if details of the nature of the intervention and control were explicitly stated. We included studies that reported the use of NRT, bupropion, varenicline, or other pharmacotherapy for smoking cessation.
We categorised behavioural interventions during the hospital stay according to whether they included follow-up after discharge. Within these categories we further defined both the hospital and follow-up interventions by level of intensity. This led to four categories of intervention intensity:
Single contact in hospital lasting <= 15 minutes, no follow-up support.
One or more contacts in hospital lasting in total > 15 minutes, no follow-up support.
Any hospital contact plus follow-up <=1 month.
Any hospital contact plus follow-up > 1 month.
Types of outcome measures
The principal outcome measure was abstinence from smoking at least six months after the start of the intervention. We used the most conservative measure of quitting at the longest follow-up, i.e. we preferred a biochemically validated quit rate to self-reported abstinence, and preferred continuous or sustained abstinence to point prevalence abstinence. We used abstinence at 12-month follow-up in preference to abstinence at six-month follow-up. We counted participants lost to followup as continuing smokers.
Search methods for identification of studies
We searched the Tobacco Addiction Group trials register in December 2011. This specialised register is regularly updated by electronic searches of databases including CENTRAL (2011 Issue 4), MEDLINE (via OVID to update 20111104.ud), EMBASE (via Ovid to update 20111104.em), PsycINFO (via OVID to 2011 November week 4) and handsearching of conference abstracts. Searches for the register cover smoking cessation, nicotine dependence, nicotine addiction and tobacco use. To identify papers potentially relevant to this reivew we searched for (hospital and patient*) or hospitali* or inpatient* or admission* or admitted in the title or abstract. In addition, we searched CINAHL (EBSCO to March 2012, search strategy in Appendix 1). We searched the Centers for Disease Control Smoking and Health database for the original review but since it did not retrieve any additional studies we did not use it for the update. We asked individuals with expertise in the area of smoking cessation for details of conference abstracts and studies in press. We hand-checked bibliographies of studies generated by the search for further studies.
Data collection and analysis
Identification of studies and data extraction
Three authors checked studies identified by the search strategies for relevance. Two authors extracted data independently. Disagreements were resolved by consensus. We noted reasons for the exclusion of studies. For each study we extracted the following data:
author(s) and year of publication,
methods (country of origin, recruitment, randomization and participants),
description of intervention(s) and control, including a designation of intensity for behavioural interventions (1–4),
outcomes (length of follow-up, definition of abstinence, validation technique).
If necessary we contacted the original authors for clarification of data.
We reported the following information about each trial in the Characteristics of included studies table:
Country
Reasons for hospitalisation or specialty of admission
Criteria for recruitment (e.g. current smokers only or recent quitters) and whether selected according to willingness to make a quit attempt
Method of randomization and adequacy of concealment
Smoking behaviour and characteristics of participants
Therapist types
Description of experimental and control interventions and classification by length of in-hospital contact and post-discharge support
Outcome measures (definition of abstinence used in review, use of biochemical validation), number of deaths.
Characteristics of studies.
Characteristics of included studies
| Bolman 2002 | |||
| Methods | Country: Netherlands Recruitment: Cardiac ward patients in 11 hospitals Selection: All eligible patients asked to participate by ward nurses |
||
| Participants | Participants: 789 smokers who had smoked in previous week Number smoked: not stated Age: 56 yrs average Therapists: Physician, nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained at 12m Validation: None Died: 25 at 12m |
||
| Notes | Included in CVD subcategory Numbers in meta-analysis adjusted to approximate the OR reported from a logistic regression analysis on continuous abstinence (OR 1.17, 90% CI 0.85 to 1.61) |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | High risk | Cluster randomized by hospital, 4/11 self-selected intervention condition, although exclusion of these did not change results. | |
| Allocation concealment (selection bias) | High risk | Participants identified by ward nurses. Possibility of selection bias although control group nurses said to be blind to condition. | |
| Incomplete outcome data (attrition bias) | Unclear risk | 25 deaths, 38 refusals, 64 missing baseline data excluded from analysis denominator. | |
| Borglykke 2008 | |||
| Methods | Country:Denmark Recruitment: patients admitted with symptoms of acute exacerbation of COPD in 1 university hospital Selection: all eligible patients asked to participate |
||
| Participants | Participants: 223 current smokers Diagnosis: COPD Age: 65.9 yrs av. Gender: 35% male Willingness to quit: not reported Therapists: nurses |
||
| Interventions |
|
||
| Outcomes | Abstinence: self-reported PP at 12m Validation: CO (in 84% of patients) Died: none reported |
||
| Notes | Category: pulmonary patients Only 48/105 intervention patients received intervention OR adjusted for sex, age and duration of COPD: 2.83 (1.40–5.74) |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | High risk | Not randomized. Participants assigned to intervention ward or control ward based on vacancy. | |
| Allocation concealment (selection bias) | Low risk | "On hospital admission, the patients were met by the medical officer in charge of the distribution of patients…who had no knowledge of the study being conducted. The medical officer randomly assigned the patients to one of the hospital wards by … vacancy." | |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow-up at 1 year. | |
| Campbell 1991 | |||
| Methods | Country: UK Recruitment: Inpatients with smoking-related diseases Selected: Invited to participate |
||
| Participants | Participants: 212 current smokers Number smoked: not stated Most had heart or lung disease Therapists: Physician and non-specialist counsellor |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 6 and 12m Validation: Expired air CO Died: None reported |
||
| Notes | Not included in analysis by counselling intensity because arms differed only by use of NRT Heart disease, lung disease and other given separately in analysis by diagnosis. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | "those who had agreed were given packages of identical appearance randomly containing either nicotine (2mg) or placebo gum" | |
| Allocation concealment (selection bias) | Unclear risk | No details reported | |
| Incomplete outcome data (attrition bias) | Unclear risk | "Non-attenders were classified as failures"; rate of drop-outs not reported. | |
| Campbell 1996 | |||
| Methods | Country: UK Recruitment: Inpatients with respiratory or cardiovascular disease Selected: Prepared to make quit attempt |
||
| Participants | Participants: 62 current smokers Age: not stated Approx. 75% had respiratory disease Therapists: Physician and non-specialist counsellor |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 3, 6, 12m Validation: Expired air CO Died: None reported |
||
| Notes | Only data on inpatients extracted from study. Included in respiratory disease subcategory. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method not described. | |
| Allocation concealment (selection bias) | Unclear risk | No details reported. | |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analysis, but number lost to follow-up in inpatient-only group not specified. | |
| Caruthers 2006 | |||
| Methods | Country:USA Recruitment: smokers admitted to a medical/surgical unit Selection: A convenience sample of 80 participants of 106 individuals screened for participation |
||
| Participants | Participants: 80 smokers (smoking at least one cigarette within 30 days of their hospital admission) Diagnosis: med and surgical Age: 51 yrs av. Gender: 40% male Willingness to quit: 79/80 indicated a desire to quit Therapists: nurses |
||
| Interventions |
|
||
| Outcomes | Abstinence: self-reported 7-day PP at 12 and 24 wks (6m) post-discharge Validation: CO confirmed Died: 3 (2 in intervention, 1 in control group) |
||
| Notes | Additional information provided by 1st author 2/2012 | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | Adaptive randomization by minimization was computer generated via a program which provided stratification by gender, ethnicity, and whether the participant was admitted with or without tobacco related disorder(s) | |
| Allocation concealment (selection bias) | Unclear risk | not described | |
| Incomplete outcome data (attrition bias) | Low risk | 10 lost to follow-up in control group and 2 in intervention but ITT analyses conducted | |
| CASIS 1992 | |||
| Methods | Country: USA Recruitment: Inpatients with coronary artery stenosis confirmed by catheterization. Selected: Invited to participate. |
||
| Participants | Participants: 267 current smokers or recent quitters (50%, defined as at least 5 cpd at any time in previous 2m) Number smoked: 25 cpd Age: 53 yrs av. 78 had acute MI, 21 recent MI, 152 other symptoms Therapists: Masters level health educators |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 6m, 12m Validation: Expired air CO. Died: None reported. |
||
| Notes | Patients admitted with MI more likely to be quitters at 6m (74%). Evidence of interaction between intervention and illness. Included in CVD subcategory |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described | |
| Allocation concealment (selection bias) | Unclear risk | No details reported | |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of losses to follow-up. All survivors included in denominators. | |
| Chouinard 2005 | |||
| Methods | Country: Canada Recruitment: Inpatients with cardiovascular disease (MI, angina, CHF) or PVD Selected: Not by motivation |
||
| Participants | Participants: 168 past-month smokers Number smoked: not stated Age: 56 yrs av Therapist: nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 2 & 6m Validation: Urine cotinine or expired air CO Died: 3 in 1. 1 in 2. 0 in 3. |
||
| Notes | Two interventions compared separately to control in intensity subgroups Included in CVD subcategory | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "Cluster randomization was used… by first randomly assigning individuals to predetermined clusters of three to six subjects. The group assignment was then randomly assigned to each of these clusters." | |
| Allocation concealment (selection bias) | Unclear risk | "Individuals not familiar with the study were in charge of the randomization procedure, which included inserting the information into envelopes that were sealed and would be opened by the investigator only at the time of treatment." Comment: no other information on envelopes provided |
|
| Incomplete outcome data (attrition bias) | Low risk | 4 deaths and 3 not meeting follow-up criteria excluded from meta-analysis; all other dropouts and those lost to follow-up counted as smokers; similar numbers in all arms. | |
| Cossette 2011 | |||
| Methods | Country:Canada Recruitment: smokers admitted to 1 specialized cardiac hospital Selection: all smokers who were hospitalised were asked to participate by the study nurse |
||
| Participants | Participants: 40 current daily smokers Diagnosis: cardiovascular diseases Age: 57.1 yrs av. Gender: 60% male Willingness to quit: yes (most in preparation stage, 1 in contemplation stage in control group) Therapists: nurse specialized in smoking cessation |
||
| Interventions |
|
||
| Outcomes | Abstinence: self-reported abstinence at 6m Validation: only for one participant Died: 0 |
||
| Notes | Included in post-discharge intervention category (randomization after discharge) | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | Not specified, but generated by a centre for randomized controlled trials | |
| Allocation concealment (selection bias) | Low risk | opaque sealed envelopes | |
| Incomplete outcome data (attrition bias) | Low risk | missing data similar in both groups and analyses are ITT, participants lost to follow-up considered smokers | |
| Croghan 2005 | |||
| Methods | Country: USA Recruitment: Inpatients having surgical resection of lung or oesophageal cancers Selected: unclear |
||
| Participants | Participants: 30 smokers admitted for surgery for newly diagnosed lung or oesophageal cancer Age: not stated Therapist: doctor, nurse and trained smoking counsellor |
||
| Interventions |
|
||
| Outcomes | Abstinence: 7-day PP at 6m Validation: expired air CO or saliva tobacco alkaloid Died: 1 in 6m |
||
| Notes | |||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not stated | |
| Allocation concealment (selection bias) | Unclear risk | No details reported | |
| Incomplete outcome data (attrition bias) | Low risk | 2 lost to follow-up in control group considered smokers. 1 death in intervention group excluded from MA. | |
| de Azevedo 2010 | |||
| Methods | Country:Brazil Recruitment: patients admitted to 1 public university hospital Selection: research team approached all patients admitted to the hospital wards (except for ICU and psychiatric unit) |
||
| Participants | Participants: 273 current smokers (smoked ≥ 1 cpd in month prior to admission) Diagnosis: all (exclude ICU and psychiatric units) Age: not reported Gender: 63.7% male Willingness to quit: any Therapists: trained smoking cessation counsellor (psychologists, nurses, occupational therapist) |
||
| Interventions |
|
||
| Outcomes | Abstinence:self-reported 7-day PP at 6m Validation: none Died: 28 |
||
| Notes | In the article, analyses excluded lost to follow-up and death. Extra control arm not randomized and not included in data extraction. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "An allocation sequence based on a random-number table was used to randomly assign all enrolled subjects" | |
| Allocation concealment (selection bias) | Low risk | "The allocation was maintained in a serially numbered, opaque envelope, which was opened at the Phase 2 interview to prevent counsellor bias." | |
| Incomplete outcome data (attrition bias) | Low risk | 32% lost to follow-up in intervention and 22% in control but if analysis are done ITT low risk of bias | |
| DeBusk 1994 | |||
| Methods | Country: USA Recruitment: Inpatients with acute MI Selected: Invited to participate if prepared to make a quit attempt |
||
| Participants | Participants: 252 current smokers or recent quitters (proportion not stated, defined as any tobacco use in previous 6m). Number smoked: not stated Age: 57 yrs av. First year after MI Therapists: Physician and nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 6 and 12m Validation: Expired air CO and plasma cotinine Died: None reported |
||
| Notes | Included in CVD subcategory | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned using a computer program that achieved a balanced allocation to the two management conditions within each hospital." | |
| Allocation concealment (selection bias) | Low risk | "Randomization was done centrally; nurses were notified of the assignments by telephone calls from the coordinating centre staff." | |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear what percentage of smokers were lost to follow-up. "Among participants who did not relapse before death or dropout, censoring occurred at the last point at which they reported not smoking." | |
| Dornelas 2000 | |||
| Methods | Country: USA Recruitment: Inpatients with acute MI Selected: Invited to participate |
||
| Participants | Participants: 100 current smokers. Number smoked: 29 cpd Age: 54 yrs av. Therapists: Psychologist |
||
| Interventions |
|
||
| Outcomes | Abstinence: PP at 12m Validation: Significant other Died: 5 at 12m |
||
| Notes | Validation by significant other only in 70% of cases. Included in CVD subcategory |
||
| Risk of bias table | |||
| Bias | Authors' judgement | Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified | |
| Allocation concealment (selection bias) | Unclear risk | "drawing random numbers from an envelope" Comment: no further details provided |
|
| Incomplete outcome data (attrition bias) | Low risk | 20 (20%) lost to follow-up included in ITT analysis | |
| Feeney 2001 | |||
| Methods | Country: Australia Recruitment: Inpatients admitted for acute MI to coronary care unit of 1 hospital Selected: Invited to participate |
||
| Participants | Participants: 198 current smokers (smoked in past week) Age: 54 yrs av. Therapists: Physician and nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 1, 3, 12m. Validation: Urinary cotinine (limit not stated) Died: 9 at 12m |
||
| Notes | Very large treatment effect (31/92 vs 1/97) but risk of attrition bias. Excluded from meta-analyses because of heterogeneity. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "A random list of odd and even numbers was generated and a sequence of 200 sealed envelopes created." | |
| Allocation concealment (selection bias) | Unclear risk | "With patient consent an envelope was opened and they were assigned to either programme." Comment: no other detail on envelopes provided. |
|
| Incomplete outcome data (attrition bias) | High risk | Only participants who attended basic ADAU follow-up programme assessed, so large number of drop-outs. More drop-outs in group 2 (79%) than group 1 (51%), so treating drop-outs as smokers may overestimate treatment effect. 9 deaths (4/5) excluded from denominator in analysis. | |
| Floter 2009 | |||
| Methods | Country: Germany Recruitment: women admitted in 21 prevention or rehabilitation clinics Selection: all women who smoked were offered a smoking cessation course |
||
| Participants | Participants: 527 smokers ≥ 1 cigarette during the 30 days preceding hospitalisation Diagnosis: women hospitalised with their children for medical, psycho physiologic or psychiatric reasons Age: 35.9 Gender: 100% female Willingness to quit: all stages Therapists: social workers, psychologists, physicians or nurses and counsellors |
||
| Interventions |
|
||
| Outcomes | Abstinence: self-reported abstinence during the past 30 days at 6m Validation: none Died: none reported |
||
| Notes | Category: post discharge intervention and rehabilitation centre OR adjusted for age, single mother (yes/no), education, weight concern, smoking dependence, self efficacy, depression, perceived social support:
|
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | not described | |
| Allocation concealment (selection bias) | Unclear risk | not described | |
| Incomplete outcome data (attrition bias) | Unclear risk | According to the text there are 80 lost to follow-up and according to table 2 there are 53 lost to follow-up. Not specified in which group. | |
| Froelicher 2004 | |||
| Methods | Country: USA Recruitment: Inpatients with CVD or PVD admitted to 10 hospitals Selected: Willing to make quit attempt |
||
| Participants | Participants: 277 current smokers or recent quitters (smoked in past month), willing to make serious quit attempt at discharge Gender: All females Number smoked: 20 cpd Age: 61 yrs av. Therapists: Physician and nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: 7-day PP at 12m Validation: Saliva cotinine < 14 ng/ml OR family/friend verification Died: 11 at 12m |
||
| Notes | Included in CVD subcategory | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "Randomization was by random permuted blocks, stratified by hospital, with an equal chance of assignment to the usual-care group or the intervention group." | |
| Allocation concealment (selection bias) | Low risk | Centralized randomization | |
| Incomplete outcome data (attrition bias) | Low risk | 20 participants (13 intervention; 7 control) lost to follow-up included in meta-analysis as smokers. 11 deaths excluded from meta-analysis. | |
| Hajek 2002 | |||
| Methods | Country: UK Recruitment: Inpatients with acute MI Selected: Invited to participate |
||
| Participants | Participants: 540 current smokers. Number smoked: 23 cpd Age: 56 yrs av. Therapists: cardiac rehab nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: PP at 12m, with visit to self-reported non-smoker Validation: Expired air CO and salivary cotinine Died: 35 at 12m |
||
| Notes | Included in CVD subcategory | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "Participants…were randomised to the intervention or control group on a 1:1 ratio by nurses opening a serially numbered… envelope." | |
| Allocation concealment (selection bias) | Low risk | Nurses opened a "serially numbered, opaque, sealed envelope designating the patient's allocation." | |
| Incomplete outcome data (attrition bias) | Low risk | No significant differences in numbers lost to follow-up or patients who had died or moved away. Those who had died or moved away excluded from outcome data; those lost to follow-up counted as smokers. | |
| Hasuo 2004 | |||
| Methods | Country: Japan Recruitment: Inpatients (all diagnoses) to 1 hospital Selected: Intending to be quit on day of discharge |
||
| Participants | Participants: 120 current smokers or recent quitters (smoked in past month) Diagnoses include cancer (n=37), cardiac (n=57) Age: not stated Therapists: Nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: Abstinence at 12m (type not stated) Validation: urinary cotinine at 12m Died: 6 at 12m |
||
| Notes | Not clear whether results are self-report or cotinine-validated. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | Computerized randomization stratified by smoking status, FTND, and self-efficacy | |
| Allocation concealment (selection bias) | Low risk | Computerized programme randomly assigned individual participants | |
| Incomplete outcome data (attrition bias) | Unclear risk | More control participants missing outcome data at 12m than intervention group (9 versus 5). MA denominators exclude 6 deaths, but include 8 who were still smoking on day of discharge. This gives marginally larger relative effect. | |
| Haug 2011 | |||
| Methods | Country: Germany Recruitment: patients admitted in 3 German inpatient rehabilitation centres Selection: all consecutively admitted patients were assessed by a medical doctor or a nursing staff for inclusion criteria |
||
| Participants | Participants: 477 current smokers (at least 1 cpd) and recent former smokers (quit for <= 6 months and used to smoke at least 1 cpd) Diagnosis: various acute or chronic disorders (stroke, CHD, cancer, diabetes, asthma,…) Age: 46.5 yrs av. Gender: 48% male Willingness to quit: all stages of change Therapists: computer expert system |
||
| Interventions |
|
||
| Outcomes | Abstinence: self-reported 7-day point prevalence smoking abstinence at 6m Validation: no Died: 1 (in control group) |
||
| Notes | Category: rehabilitation centres Not clear if intervention began during hospitalisation but probably OR (Intention to treat analyses) adjusted for rehabilitation centre, baseline stage of change and baseline self-efficacy: 2.0 (95% CI 1.1–3.7) |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | High risk | Quasi randomized study. Randomly assigned to intervention or control group based on the calendar week of admission. | |
| Allocation concealment (selection bias) | High risk | See above | |
| Incomplete outcome data (attrition bias) | Low risk | Similar in both groups and ITT analyses | |
| Hennrikus 2005 | |||
| Methods | Country: USA Recruitment: Inpatients (all diagnoses) admitted to 4 hospitals Selected: Invited to participate |
||
| Participants | Participants: 2095 current smokers (smoked in past week and considered self to be regular smoker in month before admission) Age: 47 yrs av. Therapists: Physician and nurse. |
||
| Interventions |
|
||
| Outcomes | Abstinence: 7-day PP at 12m Validation: Saliva cotinine (<15 ng/ml) Died: 78 at 12m |
||
| Notes | High and differential levels of refusal to provide validation/mis-reporting | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified | |
| Allocation concealment (selection bias) | Unclear risk | "Research assistants… randomized [participants] to one of three treatment conditions by looking up the next available group assignment on a list on which the three conditions were randomly ordered within blocks of 30 assignments." | |
| Incomplete outcome data (attrition bias) | Low risk | 78 deaths and ineligible (too ill) for follow-up excluded from denominators; all other participants missing data at final follow-up counted as smokers. Similar numbers lost to follow-up in all groups. | |
| Lacasse 2008 | |||
| Methods | Country: Canada Recruitment: patients with expected LOS >=36 hours in 1 tertiary cardiopulmonary centre Selection: Eligible patients who accepted to participate were immediately assigned to one group |
||
| Participants | Participants: 196 current smokers Diagnosis: Mainly cardiology (63%) and pneumology (27%) Age: 52 yrs av. Gender: 64–68% male Willingness to quit: yes, patients in the precontemplation stage of change were excluded Therapists: counsellors (no further definition) |
||
| Interventions |
|
||
| Outcomes | Abstinence:7-day PP at 6 and 12 months Validation: urinary cotinine (<200 ng/ml) but non validated quit rates used in the meta-analysis Died: 1 in intervention group |
||
| Notes | Study stopped early because of lack of efficacy. Non validated quit rates used in this meta-analysis instead of cotinine validated because only half of participants had validation. Mainly cardiac and pulmonary patients but can't separate results. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "table of random numbers" | |
| Allocation concealment (selection bias) | Unclear risk | "Those who were eligible and who accepted to participate were immediately assigned to either the intervention or the control group by one of the hospital pharmacists." Comment: Method not specified |
|
| Incomplete outcome data (attrition bias) | Low risk | Similar numbers lost in both groups (14/99 intervention, 13/97 usual care), "analyses were run according to the intention-to-treat principle." | |
| Lewis 1998 | |||
| Methods | Country: USA Recruitment: Inpatients excluding certain cardiac conditions Selected: Prepared to make quit attempt |
||
| Participants | Participants: 185 current smokers. Number smoked: 24 cpd Age: 43 yrs av. 12 ICD-9 diagnostic categories Therapists: Physician and nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: PP at 6m Validation: Expired air CO Died: None reported |
||
| Notes | 1 vs 2 for effect of NRT. 1+2 vs 3 for behavioural counselling intervention analysis. Highest quit rates found in patients with respiratory disease. |
||
| Risk of bias table | |||
| Bias | Authors' judgement | Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "using a predetermined computer-generated randomization code" | |
| Allocation concealment (selection bias) | Low risk | Central allocation | |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop-out rates not reported, but analyses conducted as ITT | |
| Metz 2007 | |||
| Methods | Country: Germany Recruitment: patients with length of stay of at least 3 weeks in 13 rehabilitation hospitals treating respiratory diseases, CVD, cancer or DM Selection: not reported |
||
| Participants | Participants: 307 smokers ≥1 cigarette during the past month Diagnosis: Diverse disease (stroke, CHD, cancer, pulmonary disease diabetes, etc) Age: not reported Gender: 58.6% male Willingness to quit: all stages (12.5% precontemplation, 54.6% contemplation, 17.9% preparation, 15% action) Therapists: therapeutic staff with 3-day training performed the in-hospital interventions and 2 specially trained psychologists performed the telephone sessions |
||
| Interventions |
|
||
| Outcomes | Abstinence:self-reported 7-day PP at 3, 6 and 12m Validation: none Died: none reported |
||
| Notes | Category: Rehabilitation centres and postdischarge intervention OR (not ITT): 2.18 (95% CI 1.21–3.93) |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | Randomized, 1:2 ratio, method not described | |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not specified | |
| Incomplete outcome data (attrition bias) | High risk | 17/316 randomized to I excluded, no contact post discharge. Differential drop-out from remainder, 17% Int, 40% Cont. No detected differences in characteristics of drop-outs. Sensitivity analyses excluding losses to follow-up removes significance. | |
| Meysman 2010 | |||
| Methods | Country: Belgium Recruitment: patients admitted on surgical wards in 4 university hospitals Selection: inpatients admitted on surgical wards, recruited within 24 hours of admission |
||
| Participants | Participants: 358 current smokers of > 10 cpd Diagnosis: surgical patients (orthopaedics, traumatology, ENT, head and neck surgery and neurosurgery) Age: 43.2 year av. Gender: 63% male Willingness to quit: all stages of change (precontemplation 25%, contemplation 56%, preparation and action 19%) Therapists: nurse and counsellor |
||
| Interventions |
|
||
| Outcomes | Abstinence:self-reported continuous abstinence at 6m Validation: none Died: none reported |
||
| Notes | category: surgical patients | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | Participants stratified by stage of change. Method of randomization not specified. | |
| Allocation concealment (selection bias) | Unclear risk | Not stated | |
| Incomplete outcome data (attrition bias) | Unclear risk | Patients lost to follow-up counted as smokers, exact numbers not provided. | |
| Miller 1997 | |||
| Methods | Country: USA Recruitment: Inpatients excluding obstetric and psychiatric patients Selected: Prepared to make quit attempt, those wishing to do so alone excluded |
||
| Participants | Participants: 1942 current smokers. Number smoked: 20 cpd Age: 51 yrs av. 32% with cardiovascular, 12% pulmonary diagnosis Therapists: Physician and nurse counsellor |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 3, 6 & 12m Validation: Plasma cotinine or family member corroboration Died: 82 at 12m |
||
| Notes | 1 vs 3 in intensive comparison, 2 vs 3 in minimal comparison 12 months abstinence (PP) 1+2 vs 3 separately for cardiovascular, pulmonary and other diagnosis. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified | |
| Allocation concealment (selection bias) | Low risk | "Nurses opened sealed envelopes in front of patients to determine patients' assignments." | |
| Incomplete outcome data (attrition bias) | Low risk | Deaths excluded from MA denominator; all others lost to follow-up considered smokers; similar loss to follow-up across all groups (10%). | |
| Mohiuddin 2007 | |||
| Methods | Country: USA Recruitment: Inpatients with diagnosis of acute coronary syndrome (including MI) or decompensated CHF, admitted to CCU of 1 hospital Selected: Invited to participate. |
||
| Participants | Participants: 209 current smokers who had smoked for 5+ yrs, FTND>7 Number smoked: 24 cpd Age: 55 yrs av. Therapists: Physician and trained tobacco counsellor or nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 3, 6, 12m. (note: sustained abstinence to 24m reported but not used in pooling) Validation: CO Died: 15 at 12m (12 control, 3 intervention) |
||
| Notes | 1 vs 2 in intensity 4 subgroup. Same in-hospital intervention; differed in follow-up component only. Included in CVD subcategory |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | "Consenting patients were then randomly assigned using simple randomization without block assignment." Comment: method not specified |
|
| Allocation concealment (selection bias) | Unclear risk | Method not specified | |
| Incomplete outcome data (attrition bias) | Low risk | Similar number lost to follow-up in both groups (5/109 intervention, 4/100 control). Participants lost to follow-up counted as smokers. | |
| Molyneux 2003 | |||
| Methods | Country: UK Recruitment: Medical and surgical inpatients admitted to 1 hospital Selected: Invited to participate. |
||
| Participants | Participants: 274 current smokers (smoked in past month) Number smoked: 17 cpd Age: 50 yrs av. Therapists: Physician or nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 3, 12m Validation: CO <10 ppm at 12m Died: not stated |
||
| Notes | 1+2 vs 3 for intensity 2 comparison, 2 vs 1v for NRT comparison. Deaths not stated so not excluded from main analysis. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised … using a list generated for each centre, allocating equally in random permuted blocks of nine". | |
| Allocation concealment (selection bias) | Unclear risk | Not stated | |
| Incomplete outcome data (attrition bias) | Low risk | Large number lost to follow-up but similar across all groups. Losses to follow-up counted as continuing smokers. All losses fully detailed in flow chart. | |
| Nagle 2005 | |||
| Methods | Country: Australia Recruitment: Inpatients (all diagnoses) admitted to 1 teaching hospital (excluded intensive care units) Selected: Invited to participate |
||
| Participants | Participants: 1422 current smokers or quitters (smoked in past 12m) Age: not stated Therapists: nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: 7-day PP at 12m (Continuous self-reported abstinence also given) Validation: Saliva cotinine <=15 ng/ml Died: 28 at 12m |
||
| Notes | Study includes recent quitters (smoked in past year but not in past month); results not stratified by baseline smoking status. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | Computerised. "Randomization was based on blocks of 20 patients… Stratification into recent smoker and recent quitter categories occurred prior to randomization." |
|
| Allocation concealment (selection bias) | Low risk | "Patients who reported smoking within the last 12 months were entered by the research assistant at the patient's bedside into the LAPSMOKE program on a laptop computer, which gave an immediate random allocation to either control or intervention that could not be changed." | |
| Incomplete outcome data (attrition bias) | Low risk | "At 12 months no difference for completed surveys or for loss to follow-up existed between the intervention group and the control group." 28 deaths at 12m excluded from denominator, all other participants missing data counted as smokers. | |
| Ortega 2011 | |||
| Methods | Country: Spain Recruitment: patients admitted in 1 hospital Selection: all hospitalised smokers were asked to enter in the smoking cessation protocol |
||
| Participants | Participants: 1843 current smokers (smoked > 100 cigarettes lifetime) Diagnosis: medicine and surgery patients Age: 61–66 Gender: 83–88% males Willingness to quit: all stages Therapists: nurse |
||
| Interventions |
Pharmacotherapy: NRT (patches or chewing gums) in intervention group |
||
| Outcomes | Abstinence: smoking abstinent at 1 year (not more specified) Validation: CO in subgroup only Died: none reported |
||
| Notes | Category: pharmacotherapy 1 extra arm = control who refuse to enter study but not eligible for this meta-analysis No blinding, no placebo. Used in NRT comparison only as both arms offered same counselling. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using a "computerized algorithm." | |
| Allocation concealment (selection bias) | Unclear risk | Not described | |
| Incomplete outcome data (attrition bias) | Unclear risk | Number not specified. Participants lost to follow-up included as smokers in outcome data. | |
| Ortigosa 2000 | |||
| Methods | Country: Spain Recruitment: Inpatients with acute MI Selected: Invited to participate |
||
| Participants | Participants: 90 current smokers Number smoked: 25 cpd Age: 57 yrs av Therapists: Physician |
||
| Interventions |
|
||
| Outcomes | Abstinence: PP at 12m. Validation: Expired air CO. Died: 3 at 12m. |
||
| Notes | Intervention not delivered by specialist counsellor. Included in CVD subcategory. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomization, method of sequence generation not specified | |
| Allocation concealment (selection bias) | Unclear risk | Method not specified | |
| Incomplete outcome data (attrition bias) | Low risk | No participants lost to follow-up. 3 deaths excluded from the analysis. | |
| Pedersen 2005 | |||
| Methods | Country: Denmark Recruitment: Inpatients with cardiac disease Selected: Invited to participate |
||
| Participants | Participants: 105 current smokers (not defined) Age: not stated Therapists: not stated |
||
| Interventions |
|
||
| Outcomes | Abstinence: Abstinence (probably PP) at 12m Validation: none Died: not stated |
||
| Notes | Included in CVD subcategory | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | Not specified | |
| Allocation concealment (selection bias) | Unclear risk | After enrolling, patients drew an envelope containing an allocation. No further details about the envelope provided. | |
| Incomplete outcome data (attrition bias) | Low risk | 10 participants lost to follow-up (7 intervention, 3 control) counted as smokers in final analysis. | |
| Pederson 1991 | |||
| Methods | Country: USA Recruitment: Inpatients with COPD. Selected: Invited to participate |
||
| Participants | Participants: 74 current smokers Number smoked: 25 cpd Age: 53 yrs av. 43% chronic bronchitis, 57% emphysema Therapists: Non-specialist trained in counselling |
||
| Interventions |
|
||
| Outcomes | Abstinence: PP at 6m Validation: Serum COHb (in sample) Died: 8 at 6m |
||
| Notes | |||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation selection bias) | Unclear risk | Details not provided | |
| Allocation concealment (selection bias) | Unclear risk | Details not provided | |
| Incomplete outcome data (attrition bias) | Low risk | 8 deaths excluded, 8 lost to follow-up included and counted as smokers. Similar number lost to follow-up in both groups. | |
| Pelletier 1998 | |||
| Methods | Country: Canada Recruitment: Inpatients with acute MI Selected: Invited to participate |
||
| Participants | Participants: 504 current smokers Age: not stated Therapists: Nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: self-reported PP at 12m Validation: None Died: Not stated |
||
| Notes | Included in CVD subcategory | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | High risk | Quasi experimental design. 2 control hospitals, 1 experimental hospital. | |
| Allocation concealment (selection bias) | High risk | See above | |
| Incomplete outcome data (attrition bias) | Unclear risk | Number lost to follow-up not stated. | |
| Planer 2011 | |||
| Methods | Country: Israel Recruitment: patients hospitalised for ACS in 2 separate campuses in Jerusalem Selection: all smokers hospitalised for acute coronary syndrome were approached on their 2nd day of hospitalisation by the study nurse |
||
| Participants | Participants: 151 smokers of > 10 cpd Diagnosis: acute coronary syndrome Age: 51.9 yrs av. Gender: 79.9% male Willingness to quit: yes, patients required to exhibit intention to quit smoking Therapists: study physician and research nurse |
||
| Interventions |
Pharmacotherapy: Bupropion during 2m in the intervention group |
||
| Outcomes | Abstinence: self-reported continuous abstinence at 12m Validation: none Died: none reported |
||
| Notes | Category: pharmacotherapy and cardiac patients Study stopped early after interim analysis indicated no benefit OR adjusted for age, sex, invasive procedure, risk factors, Fagerstrome score, cpd: 0.90 (95% CI 0.39–2.09) |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | "Randomized," method not specified | |
| Allocation concealment (selection bias) | Unclear risk | not specified | |
| Incomplete outcome data (attrition bias) | Low risk | 1 lost to follow-up in each group | |
| Quist-Paulsen 2003 | |||
| Methods | Country: Norway Recruitment: Inpatients admitted to cardiac ward of 1 general hospital (Diagnoses: MI, unstable angina, post-CABG care) Selected: Invited to participate |
||
| Participants | Participants: 240 current smokers (smoked daily before symptoms began). Number smoked: 15 cpd Age: 57 yrs av. Therapists: Nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: PP at 12m Validation: Urine cotinine <2.0 mmol/mol creatinine Died: 5 at 12m |
||
| Notes | Included in CVD subcategory | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was in blocks of varying sizes." Method not specified. | |
| Allocation concealment (selection bias) | Low risk | "The nurses were given a serially numbered sealed envelope from a secretary who was otherwise uninvolved in the study." | |
| Incomplete outcome data (attrition bias) | Unclear risk | Meta-analysis does not include 5 deaths; all other losses to follow-up considered to be smoking but differential loss to follow-up (15 in intervention group, 2 in control group). | |
| Reid 2007 | |||
| Methods | Country: Canada Recruitment: patients admitted in 1 tertiary care cardiac facility Selection: current smokers who met eligibility criteria were recruited within 24 hours of admission. Patients living > 1 hour away were excluded |
||
| Participants | Participants: 99 current smokers ≥ 5 cpd Diagnosis: ACS, elective PCI or diagnostic catheterization related to CHD Age: 54 Gender: 61–75% male Willingness to quit: not assessed Therapists: nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: 7-day PP at 12m Validation: none Died: 1 in control group |
||
| Notes | Category: post discharge intervention | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "mediated through the Clinical Epidemiology Unit’s data centre, using a computer generated randomization list" | |
| Allocation concealment (selection bias) | Low risk | "Research staff were unaware of the treatment allocation prior to randomization" | |
| Incomplete outcome data (attrition bias) | Low risk | ~15% lost to follow-up, similar between groups. 1 Control death excluded, others included | |
| Rigotti 1994 | |||
| Methods | Country: USA Recruitment: Inpatients scheduled for CABS Selected: Invited to participate |
||
| Participants | Participants: 87 current smokers or recent quitters (38%, defined as at least 1 pack/cigarettes in previous 6m) Number smoked: 33 cpd Age: 58 yrs av. 82% of all CABG surgery Therapists: Nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 4, 8, 12m. Validation: Salivary cotinine Died: 7 at 12m |
||
| Notes | Abstinence rates include smokers who had quit prior to surgery. Included in CVD subcategory. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to control or intervention groups after surgery." Method not specified. | |
| Allocation concealment (selection bias) | Unclear risk | Not specified | |
| Incomplete outcome data (attrition bias) | Low risk | 7 deaths not counted in final meta-analysis. No other patients lost to follow-up at 12m. | |
| Rigotti 1997 | |||
| Methods | Country: USA Recruitment: Inpatients in medical or surgical services. Selected: Invited to participate |
||
| Participants | Participants: 615 current smokers or recent quitters (proportion not stated, defined as at least 1 cigarette in previous month) Number smoked: 24 cpd Age: 48 yrs av. 23% had cardiac or pulmonary diagnosis Therapists: Research assistant and nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: PP at 6m. Validation: Salivary cotinine Died: 35 at 12m |
||
| Notes | 50% of patients could recall being given physician advice. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | Each day's list of eligible smokers put in random order and patients recruited consecutively in this order. Randomized by research assistant. | |
| Allocation concealment (selection bias) | Unclear risk | See above | |
| Incomplete outcome data (attrition bias) | Low risk | 73 (22.4%) lost to follow-up included in ITT analysis, no evidence of differential loss. 35 (5.4%) deaths excluded. | |
| Rigotti 2006 | |||
| Methods | Country: USA Recruitment: Inpatients with cardiovascular disease (MI, unstable angina, CHF) or PVD admitted to 5 hospitals Selected: Invited to participate |
||
| Participants | Participants: 254 current smokers (smoked in past month) and willing to consider smoking cessation at discharge (no commitment required) Number smoked: 23/21 cpd Age: 56 yrs av. Therapists: Nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: Continuous abstinence at 2, 4, 12, 52 wks. Validation: Saliva cotinine at 12 and 52 wks, CO at 2 and 4 wks Died: 2 at 12m |
||
| Notes | Used for bupropion comparison and CV diagnosis, not for comparison of counselling intensity because both groups had same counselling. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "Using a computer program, the study statistician generated a sequence of randomly-permuted blocks of 4 within strata formed by study site and daily cigarette consumption (10 vs 10)." | |
| Allocation concealment (selection bias) | Low risk | "The study pharmacist used this sequence, concealed from enrolment staff, to assign participants to study arm. Subjects and study personnel, except the statistician and pharmacist, were blind to treatment assignment." | |
| Incomplete outcome data (attrition bias) | Low risk | "Subjects were considered smokers if they were lost to follow-up…"; same percentage lost to follow-up in both groups | |
| Simon 1997 | |||
| Methods | Country: USA Recruitment: Inpatients undergoing non-cardiac surgery Selected: Prepared to make quit attempt |
||
| Participants | Participants: 299 current smokers Number smoked: 20 cpd Age: 54 yrs av. Most cardiovascular or respiratory disease Therapists: Public health educator |
||
| Interventions |
|
||
| Outcomes | Abstinence: PP at 12m Validation: Serum or salivary cotinine or corroboration by significant other Died: 25 at 12m |
||
| Notes | Appro× 65% intervention and 17% control used NRT. Not associated with quitting in either group. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Unclear risk | "Random list of assignments" | |
| Allocation concealment (selection bias) | Low risk | "Sealed opaque envelopes opened on formal enrolment" | |
| Incomplete outcome data (attrition bias) | Low risk | 28 lost to follow-up included in ITT analysis, 25 deaths excluded from denominator. | |
| Simon 2003 | |||
| Methods | Country: USA Recruitment: Inpatients (all diagnoses) admitted to 1 hospital for military veterans Selected: Invited to participate |
||
| Participants | Participants: 223 current smokers (smoked >=20 cigarettes in wk before admission), contemplation or action stage of change, able to use NRT. Number smoked: 23 cpd Age: 55 yrs av. Therapists: Nurse or health educator |
||
| Interventions |
|
||
| Outcomes | Abstinence: 7-day PP at 12m Validation: Saliva cotinine <15 ng/ml OR spousal corroboration Died: 14 at 12m. |
||
| Notes | Study tests marginal efficacy of counselling in setting of NRT. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned using computerized algorithm" | |
| Allocation concealment (selection bias) | Unclear risk | No details provided | |
| Incomplete outcome data (attrition bias) | Low risk | 7 (3%) lost to follow-up included in ITT analysis, 14 (6%) died & excluded from denominator | |
| Simon 2009 | |||
| Methods | Country: United States Recruitment: patients admitted in 1 VA hospital Selection: all smokers hospitalised ≥ 24h screened for eligibility (exclusion criteria: CI to Bupropion, admitted for ACS, terminally ill, serious unstable psychiatric illness, family history of seizure, women pregnant or lactating, history of drug abuse consumption of >= 3 alcoholic beverages/d) |
||
| Participants | Participants: 85 smokers ≥ 5cpd during previous year and smoking the week prior admission Diagnosis: not specified Age: 56 yrs av. Gender: 96% male Willingness to quit: not assessed Therapists: public health educator |
||
| Interventions |
|
||
| Outcomes | Abstinence: 7-day PP at 6m Validation: salivary cotinine Died: 2 (1 in each group) |
||
| Notes | Category: pharmacotherapy Not used in primary meta-analysis by counselling intensity as both arms received same counselling. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "computer algorithm to generate a random list of treatment assignments." | |
| Allocation concealment (selection bias) | Unclear risk | "All study personnel engaged in providing interventions to participants were blinded to treatment assignment." Comment: not explicit that this included enrolment staff. |
|
| Incomplete outcome data (attrition bias) | Low risk | Similar number lost to follow-up in both groups. All except deaths included in MA. | |
| Smith 2009 | |||
| Methods | Country: Canada Recruitment: patients admitted in 4 cardiac units in a large urban hospital Selection: sequential patients admitted for acute MI or CABG who met inclusion criteria were included |
||
| Participants | Participants: 276 patients who used tobacco in the month before admission Diagnosis: acute MI or CABG Age: 54 yrs av. Gender: 82–83% male Willingness to quit: ranged from 3–7, mean 6.8 (on a 1–7 scale, with 7 = full intention) Therapists: nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: 7-day PP and continuous abstinence at 12m Validation: proxy corroboration at 12m only for 7-day PP only Died: 4 (2 in each group) |
||
| Notes | Category: cardiac patients For the meta-analysis we used validated 7-day PP |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "… randomization code using a computer random-number generator to select random permuted blocks of 10… stratified by acute MI and CABG." | |
| Allocation concealment (selection bias) | Unclear risk | The nurse "opened the randomization envelope and informed the patients of intervention assignment (intensive or minimal)." Comment: no details of envelope |
|
| Incomplete outcome data (attrition bias) | Low risk | Participants lost to follow-up counted as smokers. Similar percentage lost to follow-up in both groups (9.4% control, 8.8% intervention). | |
| Smith BJ 2011 | |||
| Methods | Country: Australia Recruitment: patients with smoking-related diseases admitted on pulmonary, cardiology, neurology, vascular or general medicine wards in 3 hospitals Selection: patients who agree to make a serious smoking cessation attempt, plan to return home were included |
||
| Participants | Participants: 392 current smoker (≥ 10 cpd on average in past year) Diagnosis: smoking related in respiratory, cardiology, neurology, or vascular medicine Age: 18–75 Gender: both Willingness to quit: yes (only patients willing to make a serious smoking cessation attempt were included) Therapists: Quitline counsellors at Quit SA (South Australia quitline) |
||
| Interventions |
|
||
| Outcomes | Abstinence: continuous abstinence (2–52 weeks) defined as <=5 cigarettes during 12 months Validation: a random subsample was CO validated, but we use self report Died: 13 (7 in control and 6 in intervention group) |
||
| Notes | Category: pharmacotherapy. Data from abstract and unpublished manuscript only. Open label (varenicline vs. no varenicline). Not included in primary meta-analysis as both arms received same counselling. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | A pre-defined, central, computer-generated randomisation sequence was used to assign subjects in a 1:1 ratio to either intervention or control | |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved using opaque, sealed envelopes with consecutive numbers | |
| Incomplete outcome data (attrition bias) | Low risk | low risk (>80% follow-up in both groups) | |
| Smith PM 2011 | |||
| Methods | Country: Canada Recruitment: patients admitted in 3 community hospitals Selection: study nurses approached eligible patients |
||
| Participants | Participants: 643 current smokers (tobacco use in the last 30 days) Diagnosis: diverse (CVD, pulmonary, other internal medicine, cancer, orthopaedic, gynaecology, non cardiac surgery) Age: 49 yrs av. Gender: 49.3% male Willingness to quit: yes Therapists: nurses |
||
| Interventions |
|
||
| Outcomes | Abstinence: self-reported 7 day point-prevalence abstinence at 12m Validation: with saliva cotinine (< 15 ng/mL) or proxy confirmation at 1 year only Died: 27 (19 in control and 8 in intervention group) |
||
| Notes | |||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | computerized random number generator | |
| Allocation concealment (selection bias) | Unclear risk | not specified, randomization envelopes | |
| Incomplete outcome data (attrition bias) | Low risk | Participants lost to follow-up counted as smokers but not specified, deaths excluded from final denominators | |
| Steinberg 2011 | |||
| Methods | Country: USA Recruitment: patients admitted in 1 university-based hospital Selection:patients approached within 24–48h after admission, hospital computer system identified all patients |
||
| Participants | Participants: 79 smokers (smoking ≥ 10 cpd within the past month) Diagnosis: various diagnoses (CVD, orthopedic, pulmonary, other) Age: 51 yrs av. Gender: 59 % male Willingness to quit: not specified Therapists: tobacco treatment specialist |
||
| Interventions |
|
||
| Outcomes | Abstinence: sustained abstinence at 6 months (abstinent at 4w, 12w & 6m visits) Validation: expired CO (<8ppm) Died: 0 |
||
| Notes | OR adjusted for age, race, education and level of dependence 0.34 (95% CI 0.10–1.23) | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "randomized in a 1:1 ratio through centralized telephone randomization process by the study statistician and hospital research pharmacist" | |
| Allocation concealment (selection bias) | Low risk | See above | |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis conducted; unvalidated smoking status included where ascertained for non-attenders; lost to follow-up the same in 2 groups | |
| Stevens 1993 | |||
| Methods | Country: USA Recruitment: Inpatients with stay >36 hrs excluding postpartum and psychiatric patients. Selected: Invited to participate |
||
| Participants | Participants: 1119 current smokers or recent quitters (5%, defined as smoking regularly at any time in previous 3m) Number smoked: 20 cpd Age: 44 yrs av. 17% cardiovascular or respiratory diagnosis Therapists: Masters level cessation counsellors |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 3 and 12m Validation: None (low success in obtaining cotinine returns) Died: None reported |
||
| Notes | No significant baseline differences between patient characteristics in intervention and control. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | High risk | Not random, intervention alternated between hospitals on a monthly basis in order to avoid contamination | |
| Allocation concealment (selection bias) | High risk | Intervention or control status of hospital known when patients recruited | |
| Incomplete outcome data (attrition bias) | Low risk | 6% loss to follow-up, no difference by group, included in ITT analysis | |
| Stevens 2000 | |||
| Methods | Country: USA Recruitment: Inpatients with stay >36 hours excluding postpartum and psychiatric patients Selected: Invited to participate |
||
| Participants | Participants: 1173 current smokers or recent quitters (proportion not stated, defined as smoking regularly at any time in previous 3m) Numbers smoked: 19 cpd Age: 47 yrs av. Therapists: Respiratory therapist |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 6 and 12m Validation: None Died: None reported |
||
| Notes | Only 68% of intervention group actually offered intervention. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | High risk | "Eligible smokers in each hospital were assigned to treatment or usual care by a random digit in their HMO member number." | |
| Allocation concealment (selection bias) | High risk | See above. | |
| Incomplete outcome data (attrition bias) | Unclear risk | Number lost to follow-up not specified. All not contacted at 1 year were counted as smokers. | |
| Taylor 1990 | |||
| Methods | Country: USA Recruitment: Inpatients with acute MI. Selected: Invited to participate if prepared to make a quit attempt |
||
| Participants | Participants: 173 current smokers (within last 6m) Number smoked: 25 cpd Age: 58 yrs av. 10% previous MI Therapists: Nurse |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 3 and 12m. Validation: Serum thiocyanate, expired air CO Died: 7 at 12m |
||
| Notes | NRT gum prescribed to 5 patients. | ||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "A random list of odd and even numbers was generated" | |
| Allocation concealment (selection bias) | Low risk | "a sequence of numbers sealed in envelopes was created…the nurse assessing the intervention called the nurse coordinator who opened the next envelope to determine the condition to which the patient would be assigned" | |
| Incomplete outcome data (attrition bias) | High risk | 14/86 patients in intervention group and 29/87 patients in control group missing data at 12m follow-up. Higher loss to follow-up in control group increases apparent effect of intervention when using ITT approach, so denominators in MA based on numbers followed-up. | |
| Vial 2002 | |||
| Methods | Country: Australia Recruitment: Inpatients (medical and surgical wards) of 1 teaching hospital Selected: Willing to stop smoking |
||
| Participants | Participants: 102 current smokers (>= 10 cpd) Number smoked: not stated Age: not stated Therapists: Pharmacist |
||
| Interventions |
|
||
| Outcomes | Abstinence: Sustained abstinence at 3, 6, 12m. Validation: CO test 'whenever possible' - frequency not stated Died: not stated |
||
| Notes | Smoking cessation counselling not clearly done (pharmacist consultation about NRT) ; deletion of study does not change results. 1&2 compared to 3 in both the intensity analysis and the NRT efficacy analysis. |
||
| Risk of bias table | |||
| Bias |
Authors' judgement |
Support for judgement | |
| Random sequence generation (selection bias) | Low risk | "consenting patients were randomized in blocks of ten using computer-generated random numbers" | |
| Allocation concealment (selection bias) | Low risk | Centralized, see above. | |
| Incomplete outcome data (attrition bias) | Unclear risk | Follow-up incomplete due to time constraints; analysis therefore does not include all participants randomized. 64 out of 102 participants included in 12m data, 19 of whom lost to follow-up. | |
Footnotes
Intensity of intervention: 1. Single contact in hospital lasting <= 15 mins, no follow-up support. 2. One or more contacts in hospital lasting in total > 15 mins, no follow-up support. 3. Any hospital contact plus follow-up <=1 month. 4. Any hospital contact plus follow-up > 1 month.
Abbreviations: ACS: acute coronary syndrome; av: average; CABG/S: coronary artery bypass graft/surgery; CCU: coronary care unit; CHD: coronary heart disease; CHF: congestive heart failure; CI: confidence interval; CO: carbon monoxide; COPD: Chronic Obstructive Pulmonary Disease; cpd: cigarettes per day; CVD: cardiovascular disease; FTND: Fagerstrom Test for Nicotine Dependence; m: month(s); MI: myocardial infarction; NRT: nicotine replacement therapy; OR: odds ratio; PCI: percutaneous coronary intervention; PP: point prevalence; PVD: peripheral vascular disease; yrs: years
Assessment of risk of bias in included studies
We judged risk of bias on the basis descriptions of the randomization and allocation concealment procedure, as this is the main source of bias which has been empirically associated with over-estimation of treatment effects (Schulz 1995). We also assessed whether the studies reported validation of self-reported smoking cessation, and assessed the studies for attrition bias, including how they handled patients lost to follow-up, since these are possible sources of bias in smoking cessation studies. We also assessed the extent to which study populations consisted of current smokers and recent quitters.
Analysis of the data
We used statistical methods for pooling using a Mantel-Haenszel fixed-effect method, with 95% confidence intervals. This summary statistic replaced the Peto method (Yusuf 1985) used in earlier versions of this review, since the Mantel-Haenszel method is now recommended for Cochrane reviews (Higgins 2011). Differences in results using the two methods are small, and most likely to be apparent where numbers are unbalanced between groups, in which case the Peto method may give biased results. Where there was substantial heterogeneity between studies we explored possible reasons using sensitivity analyses or considered the impact of outliers. We express results as a risk ratio (intervention risks/control risks) for achieving abstinence from smoking together with the 95% confidence interval for this estimate. To investigate statistical heterogeneity we used the I2 statistic, given by the formula [(Q - df)/Q] × 100%, where Q is the Chi2 statistic and df is its degrees of freedom (Higgins 2003). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). A value greater than 50% may be considered to indicate moderate to substantial heterogeneity.
We calculated quit rates based on the numbers of patients randomized to an intervention, excluding any deaths. Those who dropped out or were lost to follow-up were counted as continuing smokers. Most studies verified self-reported smoking status with a biochemical test. In these studies, self-reported nonsmokers who did not pass the verification procedure were counted as smokers. We noted the number of deaths in Characteristics of included studies.
We analysed data according to our pre-determined classification of four levels of intensity (see Types of interventions, above). Where we included studies that were judged to be more prone to bias, we planned sensitivity analyses to assess whether their inclusion altered our findings. We also planned sensitivity analyses to explore, where possible, the contribution of different components to an overall effect (for example, the role of NRT in a multicomponent intervention) and to determine whether the effects were different when the study population was restricted to those wishing to stop. Another sensitivity analysis explored the efficacy of interventions that differed only after hospital discharge. In these studies participants received an identical intervention in the hospital and were randomly assigned to different post-discharge treatments.
In an exploratory analysis, we evaluated the effects of interventions in patients admitted to hospital because of the following diagnoses: cardiovascular disease, respiratory disease and cancer. We also assessed the effects of interventions that were designed to be delivered to all (or nearly all) of the smokers who were admitted to hospital regardless of the smoker's admitting diagnosis. Where there were insufficient data for meta-analysis, the results were tabulated. In cases where a single study reported data on patients from different categories, we pooled the data only when it was possible to extract data by disease category. Otherwise we included only those studies reporting data from patients in a single disease category. A separate analysis included studies that met inclusion criteria but were conducted in rehabilitation hospitals rather than acute care hospitals. These studies were not included in the main analysis.
We include the Tobacco Addiction Group glossary of tobacco-specific terms (Appendix 2).
Results
Description of studies
Fifty trials conducted in the United States, the United Kingdom, Australia, Belgium, Brazil, Canada, Denmark, Germany, Israel, Japan, the Netherlands, Norway, and Spain between 1990 and 2011 met the inclusion criteria and contributed to the review. The previous version of this review included 33 trials published between 1990 and 2007; this update includes 17 new studies. All but 12 of the 50 studies contributed to the main comparison of a behavioural counselling intervention, classified by intensity, versus control. Eight that did not contribute (Campbell 1991; Campbell 1996; Ortega 2011; Rigotti 2006; Planer 2011; Simon 2009; Smith BJ 2011; Steinberg 2011) did not include a control group of usual care or less intensive counselling; the intervention tested in those studies was pharmacotherapy as an adjunct to behavioural support. Three studies that were performed in rehabilitation hospitals (Floter 2009; Haug 2011; Metz 2007) were also analysed separately from the studies conducted in acute care hospitals. Twenty-six studies (Bolman 2002; Borglykke 2008; Campbell 1991; Campbell 1996; CASIS 1992; Chouinard 2005; Cossette 2011; Croghan 2005; DeBusk 1994; Dornelas 2000; Feeney 2001; Froelicher 2004; Hajek 2002; Miller 1997; Mohiuddin 2007; Ortigosa 2000; Pedersen 2005; Pederson 1991; Pelletier 1998; Planer 2011; Quist-Paulsen 2003; Reid 2003; Reid 2007; Rigotti 1994; Rigotti 2006; Smith 2009; Taylor 1990) provided separate data by disease and contributed to the comparison of intervention versus control in different disease categories. We describe each intervention in Characteristics of included studies. We excluded 66 studies which appeared relevant but did not meet all inclusion criteria (see Characteristics of excluded studies). We did not include two studies (Brunner-Frandsen 2010; Jimenez 2007) for which there was insufficient data to make a decision, despite our efforts to contact the authors for additional information; these remain in the Characteristics of studies awaiting classification.
Characteristics of excluded studies
| Agewall 2001 | |
| Reason for exclusion | Multifactorial intervention. No smoking cessation outcomes reported. |
| Allen 1998 | |
| Reason for exclusion | Intervention not delivered in inpatient setting. |
| Asfar 2005 | |
| Reason for exclusion | Intervention not delivered in inpatient setting. |
| Avanzini 2011 | |
| Reason for exclusion | Case control study. |
| Becker 2003 | |
| Reason for exclusion | Participants admitted to observation unit for less than 24 hour hospital stay. Insufficient data. |
| Bize 2006 | |
| Reason for exclusion | Not randomized (uses historical controls). |
| Blom 2005 | |
| Reason for exclusion | Intervention not delivered in inpatient setting. |
| BTS 1983 | |
| Reason for exclusion | Included both inpatient and outpatient data (results for inpatients alone not available). |
| Burt 1974 | |
| Reason for exclusion | Not randomized. |
| Chan 2003 | |
| Reason for exclusion | Intervention not delivered in inpatient setting. |
| Chan 2010 | |
| Reason for exclusion | Coronary heart disease patients recruited in outpatient clinics. |
| Choo 2004 | |
| Reason for exclusion | Short follow-up (1m). |
| Colby 1998 | |
| Reason for exclusion | Short follow-up (3m). Enrolled only adolescents. |
| Cole 2001 | |
| Reason for exclusion | Review article (no new data). |
| Dale 1995 | |
| Reason for exclusion | Intervention not delivered in inpatient setting (some participants admitted to inpatient unit for smoking intervention). |
| Davies 2005 | |
| Reason for exclusion | Insufficient data on cessation outcome. |
| Elsony 2005 | |
| Reason for exclusion | Intervention not delivered in inpatient setting. |
| Emmons 2000 | |
| Reason for exclusion | Baseline and pharmacy data from a trial. Main outcomes not reported. |
| Freund 2009 | |
| Reason for exclusion | Smoking behavior is not measured as outcome. |
| Fung 2005 | |
| Reason for exclusion | Not randomized. |
| Gadomski 2011 | |
| Reason for exclusion | Not randomized. |
| Galvin 2001 | |
| Reason for exclusion | Intervention not delivered in inpatient setting. |
| Gariti 2002 | |
| Reason for exclusion | Participants were inpatients in a substance abuse treatment unit. |
| Gies 2008 | |
| Reason for exclusion | Follow-up too short (3 months). |
| Gritz 1993 | |
| Reason for exclusion | Intervention not delivered in inpatient setting (only recruitment carried out in hospital setting). |
| Hand 2002 | |
| Reason for exclusion | Included both inpatient and outpatient data (results for inpatients alone not available). |
| Hanssen 2007 | |
| Reason for exclusion | Intervention after hospital discharge, intervention was secondary prevention (general cardiac rehab intervention), not limited to smokers. |
| Hasan 2007 | |
| Reason for exclusion | Not randomized (patients could chose their treatment), tested hypnotherapy |
| Hilleman 2004 | |
| Reason for exclusion | Not randomized. |
| Holmes-Rovner 2008 | |
| Reason for exclusion | Recruitment after hospital discharge, intervention was secondary prevention, not limited to smokers, and insufficient data in article to calculate quit rates. |
| Horn 2008 | |
| Reason for exclusion | Intervention in emergency department, not inpatients, teens. No smoking cessation outcome. |
| Jeong 2002 | |
| Reason for exclusion | Multifactorial intervention with little smoking cessation content. |
| Johnson 1999 | |
| Reason for exclusion | Not randomized. |
| Jones 2001 | |
| Reason for exclusion | Intervention delivered after discharge from ITU. |
| Joseph 2004 | |
| Reason for exclusion | Participants inpatients for substance abuse treatment. |
| Joseph 2005 | |
| Reason for exclusion | Intervention goal smoking reduction, not cessation (enrolled only smokers who do not plan to quit). |
| Kalman 2001 | |
| Reason for exclusion | Participants inpatients for alcohol dependence treatment. |
| Lewis 2009 | |
| Reason for exclusion | Enrolled outpatients and inpatients; inpatients not analysed separately in article. |
| Lisspers 1999 | |
| Reason for exclusion | Intervention delivered after discharge following PTCA. |
| McHugh 2001 | |
| Reason for exclusion | Multicomponent intervention delivered prior to hospitalisation for CABG. |
| Meenan 1998 | |
| Reason for exclusion | Not randomised. |
| Moller 2002 | |
| Reason for exclusion | Intervention delivered prior to hospital admission. |
| Mosca 2010 | |
| Reason for exclusion | Does not only recruit smokers; insufficient data to calculate quit rates. Assesses the impact of a systematic hospital-based educational intervention among women hospitalised with CHD. |
| Nackaerts 2009 | |
| Reason for exclusion | No data for 6m quit rates |
| Nasell 2010 | |
| Reason for exclusion | Outcome is postoperative complications and not smoking cessation. |
| Ong 2005 | |
| Reason for exclusion | Not an RCT. |
| Ranote 2003 | |
| Reason for exclusion | Not an RCT (quasi-experimental design). Abstract only. Insufficient data. |
| Ratner 2004 | |
| Reason for exclusion | Intervention delivered prior to hospital admission. |
| Regan 2011 | |
| Reason for exclusion | Follow-up too short (12 wks). |
| Reid 2006 | |
| Reason for exclusion | Not an RCT (uncontrolled cohort study). |
| Richman 2000 | |
| Reason for exclusion | Patients not admitted to hospital, follow-up 3m. |
| Rissel 2000 | |
| Reason for exclusion | Intervention delivered to outpatients. Not randomized. |
| Schmitz 1999 | |
| Reason for exclusion | No control / usual care group. |
| Smith 2002 | |
| Reason for exclusion | Not an RCT (evaluates real world effect of intervention used in DeBusk 1994, Miller 1997 and Taylor 1990). |
| Strecher 1985 | |
| Reason for exclusion | Not randomized. |
| Sundblad 2008 | |
| Reason for exclusion | Intervention starts before hospital stay; this is a residential smoking cessation program. |
| Takahashi 2006 | |
| Reason for exclusion | Intervention not delivered in inpatient setting. |
| Targhetta 2011 | |
| Reason for exclusion | No follow-up assessment after discharge. |
| Taylor 2005 | |
| Reason for exclusion | Not an RCT (observational study only). |
| Thomsen 2010 | |
| Reason for exclusion | Does not start in hospital but before hospitalisation. |
| Wakefield 2004 | |
| Reason for exclusion | Intervention not delivered in inpatient setting. |
| Warner 2005 | |
| Reason for exclusion | Intervention not delivered in inpatient setting (prior to hospital admission). |
| Wewers 1994 | |
| Reason for exclusion | Short follow-up (5 wks). |
| Winickoff 2010 | |
| Reason for exclusion | Subjects are not hospital patients but the parents of hospitalised children. |
| Wolfenden 2005 | |
| Reason for exclusion | Intervention not delivered in inpatient setting (begun pre-operatively). |
| Wolfenden 2008 | |
| Reason for exclusion | Not an RCT, starts before hospital admission. |
Footnotes
CABG: coronary artery bypass graft; ITU: Intensive Therapy Unit; m: month(s); PTCA: percutaneous transluminal coronary angioplasty; wk(s): week(s)
Characteristics of studies awaiting classification
| Brunner-Frandsen 2010 | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | |
| Jimenez 2007 | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | |
Footnotes
Counselling interventions
Advice to quit smoking and/or behavioural counselling was provided in all 50 studies. In 48 of them, a nurse or counsellor provided stop-smoking advice and/or behavioural counselling. Twelve studies included physician advice to quit (Campbell 1991; Campbell 1996; Croghan 2005; DeBusk 1994; Feeney 2001; Froelicher 2004; Hennrikus 2005; Lacasse 2008; Lewis 1998; Miller 1997; Ortigosa 2000; Pelletier 1998;), and in one study physician advice was offered prior to admission (Pederson 1991). In three studies, the patient chart was stamped with a prompt to remind the physician to offer smoking cessation advice (Rigotti 1997; Smith 2009; Smith PM 2011). Counselling ranged in duration from less than five minutes to two hours. In nine studies, counselling was delivered on more than one occasion during the hospitalisation period (Borglykke 2008; CASIS 1992; Cossette 2011; Floter 2009; Metz 2007; Nagle 2005; Ortega 2011; Pederson 1991; Rigotti 1994). Most studies also included materials such as self-help booklets, relaxation audio tapes and video tapes. In Haug 2011, participants were provided with access to an internet-based smoking cessation program and no face-to-face counselling was performed. In Smith BJ 2011, patients were referred to a quitline and were called by a quitline counsellor.
Forty-two of 50 studies (all except Bolman 2002; Croghan 2005; Hajek 2002; Hennrikus 2005; Molyneux 2003; Nagle 2005; Pederson 1991; Pelletier 1998) offered follow-up support following discharge. Of these, 29 offered support by telephone (Caruthers 2006; CASIS 1992; Chouinard 2005; Cossette 2011; de Azevedo 2010; DeBusk 1994; Dornelas 2000; Floter 2009 ; Froelicher 2004; Hasuo 2004; Hennrikus 2005; Lacasse 2008; Lewis 1998; Metz 2007; Miller 1997; Ortigosa 2000; Quist-Paulsen 2003; Rigotti 1994; Rigotti 1997; Rigotti 2006; Simon 1997; Simon 2003; Simon 2009; Smith 2009; Smith BJ 2011; Smith PM 2011; Stevens 1993; Stevens 2000; Taylor 1990), nine provided in-person support in various settings (Borglykke 2008; Campbell 1991; Campbell 1996; Meysman 2010; Mohiuddin 2007; Pedersen 2005; Reid 2003; Steinberg 2011; Vial 2002), and two offered support by telephone and/or in-person (Ortega 2011; Planer 2011). Two studies used newer technologies to contact patients after discharge: Haug 2011 provided individual feedback letters, an internet-based smoking cessation program and offered email support and Reid 2007 used an Interactive Voice Response (IVR) system. The duration of extended support ranged from one week to 12 months from discharge.
Pharmacotherapy
No studies tested the efficacy of pharmacotherapy with nicotine replacement therapy (NRT), bupropion, or varenicline versus placebo in the absence of a counselling intervention. However, six studies tested the marginal value of adding NRT to a counselling intervention (Campbell 1991; Campbell 1996; Lewis 1998; Molyneux 2003; Ortega 2011; Vial 2002), three studies tested the marginal value of adding bupropion to a counselling intervention (Planer 2011; Rigotti 2006; Simon 2009), and two trials tested the marginal value of adding varenicline to a counselling intervention (Smith BJ 2011; Steinberg 2011). One trial (Simon 2003) tested the marginal value of adding counselling to pharmacotherapy with NRT. In a number of other studies, particularly the newer ones, pharmacotherapy was allowed as part of the intervention or available to participants in the trial but was not specifically offered to all participants in one group and to none in another. Fourteen studies that reported providing NRT to a subgroup of patients did not specify the extent of its use (Borglykke 2008; Caruthers 2006; Chouinard 2005; DeBusk 1994; Froelicher 2004; Lacasse 2008; Pedersen 2005; Quist-Paulsen 2003; Reid 2003; Reid 2007; Rigotti 1997; Simon 1997; Simon 2003; Taylor 1990). Two studies included bupropion in a similar fashion (Chouinard 2005; Mohiuddin 2007) and in one study NRT, bupropion or varenicline were suggested during hospitalisation and follow-up (Cossette 2011).
Other study characteristics
Three studies compared two durations of post-discharge follow-up with a usual care control (Chouinard 2005; Hennrikus 2005; Miller 1997). Results from each arm of these studies were included separately in the analysis by intervention intensity. In four other studies that compared two intervention arms to a usual care control, the behavioural support offered in the two arms was comparable and results of the two intervention arms were combined in the analysis by intensity subgroups (Floter 2009; Lewis 1998; Molyneux 2003; Vial 2002). In Lewis 1998 and Molyneux 2003, the two intervention arms differed by the presence or absence of nicotine replacement, and these arms were directly compared in the pooled analysis of the effect of NRT. In Vial 2002, both intervention arms included the use of NRT, and compared follow-up from either a hospital or community pharmacist. In Floter 2009, both intervention arms included group counselling sessions during hospitalisation and follow-up with proactive telephone calls but the post-discharge intervention differed in style. In one study the smoking cessation intervention was part of a multicomponent risk intervention for patients with cardiovascular disease (DeBusk 1994); in this case the smoking cessation intervention was well-defined and met our inclusion criteria. Two studies had a third arm consisting of control patients who were not assigned randomly (de Azevedo 2010; Ortega 2011). We excluded the nonrandomised patients from analyses of these studies.
Most studies (37 of 50) assessed cigarette abstinence 12 months after hospital discharge. Thirteen studies reported a shorter follow-up period of six months (Caruthers 2006; Cossette 2011; Croghan 2005; de Azevedo 2010; Floter 2009; Haug 2011; Lewis 1998; Meysman 2010; Pedersen 2005; Pederson 1991; Rigotti 1997; Simon 2009; Steinberg 2011). Fewer than half of the studies (20 of 50) used the preferred outcome measure, sustained abstinence. Twenty-eight studies used point prevalence abstinence as the outcome measure and two studies did not specify how abstinence was defined (Cossette 2011 ; Ortega 2011). One study reported sustained abstinence rates for overall cessation but point prevalence rates by diagnosis (Miller 1997).
All but two studies included both males and females; the exceptions (Floter 2009; Froelicher 2004) included only females. All studies included adults who smoked cigarettes currently or recently (e.g., within the past month). Seven studies included recent quitters as well as current smokers (CASIS 1992; DeBusk 1994; Haug 2011; Nagle 2005; Rigotti 1994; Stevens 1993; Stevens 2000).
Risk of bias in included studies
Fifteen of the fifty studies reported procedures for both random sequence generation and allocation concealment that we judged likely to avoid selection bias (Cossette 2011; de Azevedo 2010; DeBusk 1994; Froelicher 2004; Hajek 2002; Hasuo 2004; Lewis 1998; Nagle 2005; Reid 2003; Reid 2007; Rigotti 2006; Smith BJ 2011; Steinberg 2011; Taylor 1990; Vial 2002). Seventeen studies did not report the method of randomization and concealment in enough detail to judge the risk of selection bias, nine studies had low risk of selection bias for random sequence generation but unclear risk for allocation concealment, and three studies had unclear risk for random sequence generation and low risk for allocation concealment. Six studies did not allocate treatment at the individual patient level (Borglykke 2008; Bolman 2002; Haug 2011; Pelletier 1998; Stevens 1993 ; Stevens 2000). Two of them allocated treatment by alternating the intervention condition between hospitals over time (Stevens 1993, Stevens 2000) and one study employed a quasi-experimental design with one intervention and two control hospitals (Pelletier 1998). One study used a quasi-experimental design and assigned participants to intervention or control group according to bed availability in two wards of the same hospital (Borglykke 2008) while another used a quasirandomized design where participants were assigned to control or intervention groups based on the calendar week of admission (Haug 2011). One other study (Bolman 2002) was not fully randomized; seven of 11 participating hospitals were randomized to condition, but four others selected their study arm. All six of these studies share the potential problems of recruitment bias and of underestimation of confidence limits due to intracluster correlation. Therefore, we conducted sensitivity analyses on the effect of excluding them.
The majority of studies (33 out of 50) reported numbers lost to follow-up and methods for addressing incomplete outcome data that we judged at low risk of attrition bias. Fourteen studies did not report enough information to be assessed for incomplete outcome data and were hence rated as unclear. Three studies were rated at high risk of attrition bias: Feeney 2001 assessed only those participants who attended a follow-up programme and hence had a large and unequal percentage of losses to follow-up; in Metz 2007, sensitivity analysis excluding losses to follow-up removes the significance of the study findings due to differential drop-out rates between study arms; and differential drop-out rates in Taylor 1990 increased the apparent effect of the intervention when using an intent-to-treat approach.
Most studies (41 of 50) used a method to validate subjects' self-reports of quitting at the follow-up assessment. Biochemical validation of smoking status was done in 32 studies, using expired air carbon monoxide in 15 studies (Campbell 1991; Campbell 1996; Caruthers 2006; CASIS 1992; Croghan 2005; DeBusk 1994; Hajek 2002; Lewis 1998; Mohiuddin 2007; Molyneux 2003; Ortigosa 2000; Reid 2003; Steinberg 2011; Taylor 1990; Vial 2002), and using plasma, salivary, or urinary cotinine in 17 studies (Chouinard 2005; DeBusk 1994; Feeney 2001; Froelicher 2004; Hajek 2002; Hasuo 2004; Hennrikus 2005; Lacasse 2008; Miller 1997; Nagle 2005; Quist-Paulsen 2003; Rigotti 1994; Rigotti 1997; Rigotti 2006; Simon 1997; Simon 2003; Simon 2009). Two studies used "corroboration by significant other" as the only validation method (Dornelas 2000; Smith 2009), and five other studies used "corroboration by significant other" in cases where a plasma or salivary cotinine measure was not available (Froelicher 2004; Lewis 1998; Miller 1997; Simon 2003; Smith 2009). Thirteen studies (Bolman 2002; Cossette 2011; de Azevedo 2010; Floter 2009; Haug 2011; Metz 2007; Meysman 2010; Pedersen 2005; Pelletier 1998; Planer 2011; Reid 2007; Stevens 1993; Stevens 2000) did not validate self-reported quitting at the follow-up assessment for any participants. Five others (Borglykke 2008; Ortega 2011; Pederson 1991; Reid 2003; Vial 2002) did not validate the smoking status of all participants who self-reported abstinence. Four studies used more than one means of validation other than corroboration by significant other (Chouinard 2005; DeBusk 1994; Rigotti 2006; Taylor 1990). In one study (Smith BJ 2011), validation of smoking abstinence with expired air carbon monoxide was performed only in a subsample but we used self-reported smoking abstinence rates in the analyses.
Most studies recruited participants on the basis of a convenience sample, with randomization being to group (intervention or control) rather than to initial inclusion. Participation rates (i.e., the proportion of those approached who agreed to take part in the trial) were also seldom recorded. Most studies recorded those lost to follow-up as continuing smokers. In one study (Stevens 2000), the intervention was offered inconsistently, with only 68% of those eligible for the intervention actually being approached.
Effects of interventions
Effect of counselling interventions categorised by intensity Figure 1
Figure 1.
Forest plot of comparison: 1 Intervention v Control, by intensity of counselling intervention, outcome: 1.1 Quit at longest follow-up (6+ months).
Only one included study (Hennrikus 2005) reported on the effect of a brief intervention in hospitalised patients with no followup after discharge (intensity 1). That study had a large sample size (>650 subjects per study arm). The brief intervention was no more effective than usual care (RR 1.14, 95% CI 0.82 to 1.59) although the confidence limits did not exclude the possibility of a benefit. Nine studies (Bolman 2002; Chouinard 2005; Croghan 2005; Hajek 2002; Meysman 2010; Molyneux 2003; Nagle 2005; Pederson 1991; Pelletier 1998) used a more intensive intervention in hospital but had no follow-up intervention component after discharge (intensity 2). There was no evidence of a significant benefit from pooling these studies and the confidence intervals suggest that any effect is likely to be small (RR 1.10, 95% CI 0.96 to 1.25, I2 = 44%). Similar lack of statistically significant benefit was observed in a pooled analysis of the six studies that tested the effect of an intervention that began during hospitalisation and continued for up to 1 month after discharge (intensity 3). The risk ratio and confidence interval for the estimate of the effect of this level of intervention (RR 1.07, 95% CI 0.93 to 1.24, I2 = 11%) is almost identical to that produced by the intensity 2 intervention.
In this update, we added eight new included studies that tested the highest intensity intervention (intensity 4), consisting of counselling that began in the hospital and continued for more than one month after discharge. The pooled estimate showed a statistically significant increase in quit rates that was similar to the previous review (RR 1.37, 95% CI 1.27 to 1.48, 25 studies) and heterogeneity remained relatively low (I2 = 32%). This estimate excludes one study (Feeney 2001) which was an extreme outlier reporting a very large effect. In this trial amongst 198 patients admitted to a coronary care unit there was a very high drop out rate (79%) and low quit rate (1%) at 12 months in the usual care condition whilst the dropout rate was 55% and the quit rate 34% in the intervention group. The intervention group quit rate was comparable to that of other trials in the intensity 4 subgroup, but control group quit rates in the other trials were typically over 10%. This suggested that the difference in relative effect might have been due to characteristics of the support given to the control group and we decided to exclude this trial from the meta-analysis.
Three of the eight newly identified studies testing the most intensive intervention had a different design from the other trials (Caruthers 2006; Cossette 2011; Reid 2007). In these studies, all participants received the same intervention while in the hospital but were randomly assigned to interventions that differed after hospital discharge. We conducted a subgroup analysis limited to these trials in order to isolate the specific effect of a post-discharge intervention. The pooled estimate was a statistically significant 51% increase in smoking cessation rates (RR 1.51, 95% CI 1.03 to 2.22, I2 = 44% Analysis 2.1) with the addition of a post-discharge counselling intervention.
The studies included in the preceding analyses were all conducted in acute care hospitals. We also identified three trials conducted in rehabilitation hospitals (Floter 2009; Haug 2011; Metz 2007), where patients are less acutely ill and length of stays are longer. Because of these differences, we chose to analyse studies from rehabilitation hospitals separately from studies in acute care hospitals. The interventions provided by studies based in the rehabilitation hospitals were of similar intensity; each provided over one month of follow-up contact after discharge (intensity 4), allowing us to pool the results. The pooled estimate was a statistically significant 71% increase in smoking cessation rates (RR 1.71, 95% CI 1.37 to 2.14, I2 = 0% Analysis 3.1).
Sensitivity analyses
Some studies of behavioural counselling also included the option of pharmacotherapy, principally NRT. A sensitivity analysis excluding eighteen studies that reported the use of NRT within the highest intervention intensity did not suggest that the efficacy of these interventions was due to the use of NRT. The result from pooling only the seven trials that did not include the option of pharmacotherapy (CASIS 1992; de Azevedo 2010; Dornelas 2000; Hasuo 2004; Hennrikus 2005; Smith 2009; Smith PM 2011) though smaller, remained statistically significant (RR 1.24, 95% CI 1.09 to 1.41, I2 = 0%).
Another sensitivity analysis excluded studies that did not randomly assign subjects to condition. Within studies that did not provide follow-up (intensity 2) we performed a sensitivity analysis excluding data reported by two studies that did not fully randomise patients (Bolman 2002; Pelletier 1998). The conclusion did not change (RR 1.03, 95% CI 0.87 to 1.22, I2 = 44%). Within the group of studies that delivered an intervention with minimal follow-up (intensity 3) a sensitivity analysis excluding the data reported by two studies that did not randomise patients (Stevens 1993, Stevens 2000) slightly modified the point estimate, but did not substantially affect the confidence intervals (RR 1.01, 95% CI 0.83 to 1.21, I2 = 0%). Within the group of studies with highest level of intensity of intervention (intensity 4), a sensitivity analysis excluding the data from one study that used a quasi-experimental design (Borglykke 2008) did not change the effect estimate (RR 1.35, 95% CI 1.25 to 1.46, I2= 28%).
Approximately half of the studies that delivered the highest intervention intensity (intensity 4) excluded smokers who were not willing to attempt cessation after discharge. We performed a sensitivity analysis excluding the data reported by 13 studies in which participants were selected on the basis of their willingness to make a quit attempt (Caruthers 2006; Cossette 2011; DeBusk 1994; Froelicher 2004; Hasuo 2004; Lacasse 2008; Lewis 1998; Miller 1997; Reid 2003; Simon 1997; Smith PM 2011; Taylor 1990; Vial 2002). An intervention effect persisted in the remaining 12 studies (RR 1.44, 95% CI 1.28 to 1.62, I2 = 43%).
We performed a sensitivity analysis excluding studies that enrolled former smokers (defined as having not smoked for more than one month before admission) as well as current smokers (CASIS 1992; DeBusk 1994; Nagle 2005; Rigotti 1994; Stevens 1993; Stevens 2000, Taylor 1990;). For intensity 3 (studies delivering a minimal intensity intervention with short-term follow-up), limiting the analysis to current smokers produced little change in the result (RR 1.01, 95% CI 0.82 to 1.24, I2 = 0%). For studies delivering the highest intervention intensity (intensity 4), a statistically significant increase in quitting remained even after the exclusion of studies that included quitters, and the point estimate changed little (RR 1.35, 95% CI 1.24 to 1.48, I2 = 35%). In the update, only one new study included both current smokers and recent former smokers who had quit for six months or less (Haug 2011), and this study was performed in a rehabilitation setting. Excluding this study from the analysis did not significantly change the estimate but only two studies remained in the analysis for rehabilitation centres (RR 1.56, 95% CI 1.20 to 2.03, I2 = 0%).
We performed a sensitivity analysis excluding 15 studies that did not validate self-reported smoking cessation outcomes (Bolman 2002; Borglykke 2008; Cossette 2011; de Azevedo 2010; Floter 2009; Haug 2011; Metz 2007; Meysman 2010; Pedersen 2005; Pelletier 1998; Planer 2011; Reid 2007; Smith BJ 2011; Stevens 1993; Stevens 2000). This did not alter the results. The point estimates for the lower intensity interventions declined, but confidence intervals remained wide and conclusions did not change (intensity 2 RR 0.96, 95% CI 0.81 to 1.14, I2 = 0%; intensity 3 RR 1.01, 95% CI 0.83 to 1.21, I2 = 0%). Five studies in the most intensive intervention category (intensity 4) did not validate self-reported smoking cessation (Borglykke 2008; Cossette 2011; de Azevedo 2010; Pedersen 2005; Reid 2007). Excluding them did not alter the point estimate or statistical significance of the effect (RR 1.38, 95% CI 1.28 to 1.50, I2 = 32%).
Effect of pharmacotherapy
The effect of pharmacotherapy compared with placebo as a single intervention in the absence of counselling has not been tested. Several trials have tested the effect of adding pharmacotherapy to a counselling intervention or, conversely, of adding counselling to a pharmacotherapy intervention.
NRT
Six trials (Campbell 1991, Campbell 1996, Lewis 1998; Molyneux 2003; Ortega 2011; Vial 2002) tested the marginal effect of NRT added to counselling. In these trials, NRT was compared with placebo NRT or no NRT and all subjects received a counselling intervention. Pooled analysis of these studies produced a significant RR of 1.54 (95% CI 1.34 to 1.79, I2 = 33%, Analysis 4.1.1). This result is consistent with the effect of NRT seen in other settings (Stead 2008b). One trial compared the effect of adding intensive counselling versus minimal counselling to an NRT intervention (Simon 2003). The study had an RR of 1.68 for sustained abstinence, but the confidence limits of that estimate missed statistical significance (95% CI 0.80, 3.53). However, the result was consistent with the impact of intensive counselling observed in the absence of pharmacotherapy.
Bupropion
Three studies systematically compared the use of bupropion with placebo among hospitalised smokers who also received intensive smoking cessation counselling (Planer 2011; Rigotti 2006; Simon 2009). The pooled analysis did not detect a statistically significant effect of the drug over intensive counselling alone (RR 1.04, 95% CI 0.75 to 1.45, I2 = 29%, Analysis 4.1.2). While the confidence limits were wide, they do not encompass the confidence limits for the effect of bupropion in other settings (OR 1.94, 95% CI 1.72 to 2.19, Hughes 2007), suggesting that bupropion may not be effective, or may be less effective, when started in the hospital.
Varenicline
Two studies of varenicline compared the use of varenicline with placebo (Steinberg 2011) or counselling alone (Smith BJ 2011). The pooled estimate suggested an effect of the drug over intensive counselling alone (RR 1.29, 95% CI 0.95 to 1.76) but the wide confidence limits reflect the small numbers of participants (580) and the result was not statistically significant. There was also heterogeneity between the two studies (I2= 62%), with Steinberg 2011 reporting lower quit rates in the varenicline group.
Effect of intervention by diagnosis
The included studies were heterogeneous in the types of hospitalised patients who were recruited. Because of this diagnostic heterogeneity, we examined the results of interventions within the following diagnostic groups, keeping the same intensity subgroups where the number of studies justified this approach. Seventeen studies enrolled hospital patients with a wide range of admitting diagnoses. These studies tested smoking intervention programs that were implemented hospital-wide (Caruthers 2006; de Azevedo 2010; Hasuo 2004; Hennrikus 2005; Lewis 1998; Miller 1997; Molyneux 2003; Nagle 2005; Rigotti 1997; Simon 2003; Simon 2009; Smith BJ 2011; Smith PM 2011; Steinberg 2011; Stevens 1993; Stevens 2000; Vial 2002). Twenty-two studies reported on the effects of interventions in patients hospitalised with a cardiovascular diagnosis (Bolman 2002; Campbell 1991; CASIS 1992; Chouinard 2005; Cossette 2011; DeBusk 1994; Dornelas 2000; Froelicher 2004 ; Hajek 2002; Miller 1997; Mohiuddin 2007; Ortigosa 2000; Pedersen 2005; Pelletier 1998; Planer 2011; Quist-Paulsen 2003; Reid 2003; Reid 2007; Rigotti 1994; Rigotti 2006; Simon 2009; Taylor 1990). Five studies reported on interventions in patients with a respiratory diagnosis (Borglykke 2008; Campbell 1991;Campbell 1996; Miller 1997; Pederson 1991). Only one small pilot study was found that recruited hospitalised patients admitted for a cancer diagnosis (Croghan 2005).
The pattern of effect across intervention intensities was similar for the eighteen studies that enrolled patients with all admitting diagnoses (Analysis 5.1). Interventions categorized as intensity 4 were effective in a pooled analysis of twelve studies in this subgroup (RR 1.26, 95% CI 1.12 to 1.42, I2 = 25%). The risk ratio was lower than the effect of the intensity 4 intervention in the overall analysis, but the confidence intervals overlap and we cannot conclude that intensive interventions are less effective in this subgroup. Pooled analysis of less intensive interventions demonstrated no effect and did not differ from the overall analysis (intensity 2: RR 0.90, 95% CI 0.64 to 1.28, I2 = 0%, 2 studies; intensity 3, RR 1.10, 95% CI 0.94 to 1.29, I2 = 28%, 4 studies).
The estimate of the effect for each level of intervention intensity among patients with a cardiovascular diagnosis was also very similar to that for the entire sample of hospitalised patients (Analysis 5.2). Pooled analysis of 14 studies reporting on the effect of the most intensive intervention (intensity 4) found a statistically significant effect (RR 1.42, 95% CI 1.29 to 1.56, I2 = 32%, CASIS 1992; Chouinard 2005; Cossette 2011; DeBusk 1994; Dornelas 2000; Froelicher 2004; Miller 1997; Mohiuddin 2007; Pedersen 2005; Quist-Paulsen 2003; Reid 2003; Reid 2007; Smith 2009; Taylor 1990). The point estimate of the effect was similar to that for overall analysis (RR 1.37, 95% CI 1.27 to 1.48), the confidence intervals overlap substantially, and we cannot conclude that interventions in patients hospitalised for cardiovascular disease are more effective than in the general hospital population. No statistically significant effect was found for interventions of lower intensity. Pooled analysis of four studies of in-hospital counselling without follow-up after discharge (intensity 2) found no intervention effect (RR 1.10, 95% CI 0.94 to 1.28, I2 = 58%, Bolman 2002; Chouinard 2005; Hajek 2002; Pelletier 1998). Pooled analysis of three studies that provided in-hospital counselling and brief follow-up contact after discharge (intensity 3) also found no intervention effect (RR 1.04, 95% CI 0.84 to 1.28, I2 = 0%, Miller 1997; Ortigosa 2000; Rigotti 1994).
One of the trials that tested an intensity 4 smoking intervention in the cardiovascular subgroup (Mohiuddin 2007) also assessed all-cause mortality and hospital readmission rates as endpoints. Over a two-year follow-up, the intervention produced a relative risk reduction of 0.77 (95% CI 0.27 to 0.93, p=.014) in all-cause mortality and a relative risk reduction of 0.44 (95% CI 0.16 to 0.63, p=.007) in hospital readmissions.
Five studies provided interventions to patients hospitalised with a respiratory diagnosis. Two of these studies evaluated NRT (Campbell 1991; Campbell 1996) and three other studies of counselling interventions used different intensity interventions (Borglykke 2008; Miller 1997; Pederson 1991). We estimated a separate pooled effect for the NRT studies (RR 1.29, 95% CI 0.62 to 2.69, I2 = 65%) and for the counselling studies (RR 1.22, 95% CI 0.93 to 1.60, I2 = 76%).
One pilot study reported on the effects of a hospital-based intervention for patients with cancer (Croghan 2005). It found no evidence of efficacy but the sample size was very small and the confidence limits were very broad.
Discussion
The results of this review indicate that smoking cessation counselling interventions delivered during a period of hospitalisation and including follow-up support that lasts at least one month after discharge increase smoking cessation rates. The estimated effect of such interventions was to increase the smoking cessation rate by 37% at six to 12 months after hospital discharge. This finding was robust. It remained statistically significant in sensitivity analyses that excluded studies of lower quality (e.g., those that did not validate self-reported smoking cessation at outcome or those that were not randomized). Neither the exclusion of studies that included recent quitters as well as current smokers nor those that included patients selected for motivation significantly affected the relative effect of intervention over control. This review found no evidence to support the efficacy of less intensive counselling interventions, such as those delivered only during hospitalisation or those which include less than one month of follow-up support after discharge. Therefore, post-discharge follow-up support appears to be an important component of interventions that begin during hospitalisation. We caution that the effect sizes observed in all these studies may be artificially modest because in many cases the "control" condition was more intensive than usual care or simply brief advice.
The counselling intervention in these studies was generally delivered by a research nurse or trained smoking cessation counsellor, not by a nurse responsible for other aspects of the patients' clinical care. Physician advice was a component of the intervention in many trials. There is no specific evidence from this review that brief physician advice to quit is effective by itself in the hospital setting, although evidence from trials in primary care settings support the efficacy of physician advice to quit (Stead 2008a). Pharmacotherapy with nicotine replacement therapy (NRT), bupropion, or varenicline was included in some of the counselling studies, especially the more recent ones. In most of these trials, the pharmacotherapy was not systematically provided to all subjects in the intervention arm or excluded from all subjects in the control arm. The efficacy of counselling interventions remained after excluding those studies that reported the use of NRT, suggesting that counselling alone is effective.
This update includes a new finding regarding pharmacotherapy. In hospitalised smokers the effect of pharmacotherapy by itself, compared to placebo or no pharmacotherapy in the absence of counselling, cannot be determined because no such trials have been conducted. However, the marginal effect of NRT, bupropion, or varenicline when added to counselling in the hospital setting has been tested. Pooled analysis of six studies found a statistically significant 54% increase in the smoking cessation rate when NRT was added to counselling alone. This finding is new since the 2007 update, at which time there was a non-significant trend toward finding the addition of NRT to counselling to be efficacious in the hospital setting. The current estimate of the effect of NRT in the hospital setting is within the confidence intervals of the estimated RRs from the Cochrane review of NRT: 1.43 (95% CI 1.33 to 1.53) for nicotine gum and 1.66 (95% CI 1.53 to 1.81) for nicotine patch (Stead 2008b). Hence these data support NRT's usefulness in appropriate patients during and following hospitalisation. Starting NRT before discharge was associated with a higher rate of NRT use two weeks after discharge in a non-randomized observational trial in one hospital (Regan 2011). The marginal effect of counselling when added to NRT begun in the hospital was tested in only one study (Simon 2003). Intensive counselling increased the rate of smoking cessation over that achieved by NRT alone, but the result was not statistically significant (RR 1.68,95% CI 0.80, 3.53). However, the result was consistent with the pooled estimate from this review of the effect of intensive counselling without pharmacotherapy.
Fewer data are available to assess the benefit of bupropion or varenicline as adjuncts to smoking cessation counselling that starts in the hospital setting. This update identified two new trials of bupropion to add to one previous trial (Rigotti 2006). All three trials tested the marginal efficacy of bupropion over placebo among smokers who all received intensive counselling in the hospital setting. Bupropion was not more effective than placebo in the pooled analysis (RR 1.06, 95% CI 0.68 to 1.63). This finding contrasts with evidence that bupropion is effective for smoking cessation in other populations (Hughes 2007). A possible explanation for the lack of efficacy of bupropion in the context of hospitalisation is the long half-life of sustained-release bupropion. Steady-state bupropion blood levels occur only after five to seven days of drug administration. This is generally after hospital discharge. Consequently, hospitalised smokers may be discharged into an environment filled with cues to smoke before they have sufficient levels of bupropion for it to be effective.
This update also identified the first randomized controlled trials that tested the efficacy of initiating varenicline in the hospital setting. Two trials compared the efficacy of adding varenicline (versus placebo or no drug) to counselling in the hospital setting. The pooled result of these studies produced an estimated 28% increase in the rate of smoking cessation with varenicline, but the result was not statistically significant and confidence intervals were wide due to the small sample sizes of the trials (95% CI 0.88 to 2.26). This contrasts with strong evidence of efficacy and a higher estimate of the effect size of varenicline that has been found in a systematic review of varenicline (RR 2.27, 95% CI 2.02 to 2.55, Cahill 2012).
The analyses by diagnosis demonstrate that the intensive counselling intervention is effective in the subgroup of patients admitted to hospital with a cardiovascular diagnosis, as it is for the overall group of hospitalised smokers who are not selected by diagnosis. The absolute cessation rates amongst smokers admitted with cardiovascular disease tended to be higher than amongst smokers not selected by diagnosis, but the relative effect of an intensive counselling intervention was not significantly greater in CVD patients. The potential benefit of intensive intervention in smokers with CVD was illustrated in the one study that assessed health care utilization and mortality outcomes (Mohiuddin 2007). That study found a large increase in smoking cessation in the intervention group, and at two-year follow-up, a substantial decline in hospital readmission and all-cause mortality rates. There was a possibility of confounding due to better control of blood pressure and cholesterol and better medication compliance in the intervention group. Among smokers hospitalised for myocardial infarction, intensive counselling begun in the hospital is highly cost effective, even when the cost of a course of pharmacotherapy is included in the calculation (Ladapo 2011). The effectiveness of smoking cessation interventions for patients who are admitted to hospital with a respiratory diagnosis is less clear, in part because of a small number of studies in this subgroup. Overall, there is no strong evidence for a differential effect of the intensive counselling intervention by diagnosis. These data support offering hospital-based interventions to all smokers, regardless of admitting diagnosis.
Determining how to translate these findings effectively and consistently into routine clinical practice is the next challenge for this field. The intervention in most of the trials included in this review was delivered by research staff. The effectiveness of implementing the intervention in routine clinical practice, where interventions will be delivered by clinical staff, needs to be demonstrated. Feasible models that can be readily implemented in hospital settings are needed. Current evidence on this point is limited. Two studies included in this review illustrate this challenge (Stevens 1993; Stevens 2000). Both studies provided a similar counselling intervention in a similar setting, but counselling was delivered by research staff (masters-level psychologists) in the first study and by clinical staff (trained respiratory therapists) in the second study. The intervention efficacy was demonstrated in the first study but did not persist in the second study. The feasibility of maintaining an efficacious intervention after the conclusion of a research trial was investigated for another study included in this systematic review (Miller 1997). The counselling intervention was maintained in the same hospitals for three years after the clinical trial ended. During that time approximately half of the smokers accepted the offer of intervention, and those smokers had a cessation rate comparable to that achieved in the randomized trial. These results suggested that programme effectiveness was maintained (Smith 2002). More studies are needed to demonstrate the feasibility and effectiveness of hospital-initiated smoking cessation interventions in routine practice.
Finally, this update includes the first studies conducted in rehabilitation hospitals. The results of the pooled analysis finds that smoking interventions in these settings are effective and extend the effect from acute care hospitals to a broader group of settings.
Authors' conclusions
Implications for practice
The results support the use of smoking cessation counselling interventions that begin during the hospitalisation period and include at least one month of follow-up supportive contact after discharge. There is no evidence that less intensive counselling interventions, particularly those that do not continue after hospital discharge, are effective in promoting smoking cessation. The efficacy of the counselling intervention does not clearly vary by a smoker's admitting diagnosis, and it is appropriate to offer the intervention to hospitalised smokers regardless of their admitting diagnosis. Adding nicotine replacement therapy to an intensive counselling intervention further increases the efficacy of hospital-initiated interventions and should be routinely offered. There is insufficient evidence regarding the benefit of adding varenicline or bupropion to counselling, although there was a trend toward statistical significance for varenicline and the results are compatible with data which show the effectiveness of varenicline in other settings. Bupropion may not be effective when added to counselling started in the hospital.
Implications for research
The impact of an intensive counselling intervention that continues after hospital discharge is well-established. The efficacy of adding NRT to a counselling intervention in hospitalised patients is now also established, with a relative risk estimate that is consistent with the established efficacy of NRT. Further studies testing the efficacy of adding varenicline to counselling are warranted in view of the results of early studies. They might generate sufficient data to produce a statistically significant result in future pooled analyses. The pooled evidence of studies with bupropion does not provide support for further studies of the drug in this context.
Research is needed to identify effective strategies for implementing and disseminating this evidence into routine practice in health care systems.
Additional research is needed to assess the cost-effectiveness of the intensive counselling intervention and to explore the impact of counselling on health and healthcare utilization outcomes.
Supplementary Material
Figure 2.
1 - Intervention v Control, by intensity of counselling intervention
Figure 3.
2 - Intervention v Control, trials with post discharge intervention
Figure 4.
3 - Intervention v Control, trials in rehabilitation centers
Figure 5.
4 - Intervention v Control, trials of pharmacotherapy (pharmacotherapy systematically varied by group)
Figure 6.
5 - Intervention v Control, by intervention intensity within diagnostic subgroups
Acknowledgements
Mike Murphy was an author on the original version of this review (2001, Issue 2), and on the first update (2003, Issue 1). The authors would like to thank Sarah Welch and Sarah Roberts of the ICRF General Practice Research Group for their assistance in extracting data for the original review, Nete Villebro, Hitomi Kobayashi and Roland Fischer for their assistance in translating and extracting data, and Corinne Husten, John Britton and Ian Campbell for helpful comments during peer review. Jamie Hartmann-Boyce completed the expanded Risk of Bias tables for studies included in the review before the 2012 update.
Declarations of interest
Dr Rigotti was the co-author of three studies included in the review. Dr. Rigotti's research is funded by the U.S. National Institutes of Health, by private nonprofit foundations, and by the pharmaceutical companies that make investigational or approved smoking cessation products. Her work on this review was funded by a Midcareer Investigator Award in Patient-Oriented Research from the U.S. National Heart Lung and Blood Institute.
Sources of support
Internal sources
Department of Primary Health Care, Oxford University, UK
External sources
NHS Research and Development Programme, UK
NIH / NHLBI Mid-career Investigator Award in Patient Oriented Research (#K24-044440), USA
2 Glossary of tobacco-specific terms
- Abstinence
A period of being quit, i.e. stopping the use of cigarettes or other tobacco products, May be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence
- Biochemical verification
Also called 'biochemical validation' or 'biochemical confirmation': A procedure for checking a tobacco user's report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine, or saliva, or by measuring levels of carbon monoxide in exhaled breath or in blood.
- Bupropion
A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant)
- Carbon monoxide (CO)
A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence.
- Cessation
Also called 'quitting' The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing the behaviour
- Continuous abstinence
Also called 'sustained abstinence' A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence
- Cold Turkey'
Quitting abruptly, and/or quitting without behavioural or pharmaceutical support.
- Craving
A very intense urge or desire [to smoke]. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599–614
- Dopamine
A neurotransmitter in the brain which regulates mood, attention, pleasure, reward, motivation and movement
- Efficacy
Also called 'treatment effect' or 'effect size': The difference in outcome between the experimental and control groups
- Harm reduction
Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco.
- Lapse/slip
Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse.
- nAChR
[neural nicotinic acetylcholine receptors]: Areas in the brain which are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine
- Nicotine
An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking.
- Nicotine Replacement Therapy (NRT)
A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco-free The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges.
- Outcome
Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial.
- Pharmacotherapy
A treatment using pharmaceutical drugs, e.g. NRT, bupropion
- Point prevalence abstinence (PPA)
A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long-term quitters. cf. prolonged abstinence, continuous abstinence
- Prolonged abstinence
A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. See: Hughes et al 'Measures of abstinence in clinical trials: issues and recommendations'; Nicotine & Tobacco Research, 2003: 5 (1); 13–25
- Relapse
A return to regular smoking after a period of abstinence
- Secondhand smoke
Also called passive smoking or environmental tobacco smoke [ETS] A mixture of smoke exhaled by smokers and smoke released from smouldering cigarettes, cigars, pipes, bidis, etc. The smoke mixture contains gases and particulates, including nicotine, carcinogens and toxins.
- Self-efficacy
The belief that one will be able to change one's behaviour, e.g. to quit smoking
- SPC [Summary of Product Characteristics]
Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively.
- Tapering
A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment
- Titration
A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects.
- Withdrawal
A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599–614
Appendices
1 Details of search strategies for Tobacco Addiction register and CINAHL
Search strategy for the Tobacco Addiction specialised register: (hospital and patient*) or hospitali* or inpatient* or admission* or admitted
Search strategy for CINAHL (EBSCO):
| S14 | S4 and S5 and S13 |
| S13 | S6 or S7 or S8 or S9 or S10 or S11 or S12 |
| S12 | MH Placebos |
| S11 | TX RCT |
| S10 | MH (Random assignment OR Clinical Trials+ OR Quantitative Studies) |
| S9 | TX "control group*" |
| S8 | TX "treatment arm" |
| S7 | TX (trial and (control* OR comparative)) |
| S6 | TX (random* OR factorial* OR placebo* OR assign* OR allocat* |
| S5 | MJ (smok* OR tobacco OR nicotine) |
| S4 | S1 or S2 or S3 |
| S3 | MJ (hospitali* OR inpatient*) |
| S2 | TI (hospitali* OR inpatient* OR admission* OR admitted) or AB (hospitali* OR inpatient* OR admission* OR admitted) |
| S1 | TI (hospital with patient*) or AB (hospital with patient*) |
Footnotes
Contributions of authors
NR and CC extracted data for the 2012 update, with input from LS. NR and CC wrote the update, with input from MM and LS. All authors except CC were involved in the conception, data extraction and writing of the original review.
References to studies
Included studies
- *.Bolman C, De Vries H, van Breukelen G. A minimal-contact intervention for cardiac inpatients: long-term effects on smoking cessation. Preventive Medicine. 2002;35:181–192. doi: 10.1006/pmed.2002.1036. [DOI] [PubMed] [Google Scholar]
- Bolman C, de Vries H, Van Breukelen G. Evaluation of a nurse-managed minimal-contact smoking cessation intervention for cardiac inpatients. Health Education Research. 2002;17:99–116. doi: 10.1093/her/17.1.99. [DOI] [PubMed] [Google Scholar]
- Spencer L, Anderson DRE. A minimal-contact intervention for cardiac inpatients: Long-term effects on smoking cessation. American Journal of Health Promotion. 2004;18:399–400. [Google Scholar]
- Borglykke A, Pisinger C, Jorgensen T, Ibsen H. The effectiveness of smoking cessation groups offered to hospitalised patients with symptoms of exacerbations of chronic obstructive pulmonary disease (COPD) The Clinical Respiratory Journal. 2008;2(3):158–165. doi: 10.1111/j.1752-699X.2008.00055.x. [DOI] [PubMed] [Google Scholar]
- Campbell IA, Prescott RJ, Tjeder-Burton SM. Smoking cessation in hospital patients given repeated advice plus nicotine or placebo chewing gum. Respiratory Medicine. 1991;85:155–157. doi: 10.1016/s0954-6111(06)80295-7. [DOI] [PubMed] [Google Scholar]
- Campbell IA, Prescott RJ, Tjeder-Burton SM. Transdermal nicotine plus support in patients attending hospital with smoking related diseases - a placebo controlled study. Respiratory Medicine. 1996;90(1):47–51. doi: 10.1016/s0954-6111(96)90244-9. [DOI] [PubMed] [Google Scholar]
- Caruthers D, Perkins K, Sereika S, Albrecht S, Dunbar-Jacob J. Enhancing tobacco abstinence following hospitalization (POS1-59). Society for Research on Nicotine and Tobacco 12th Annual Meeting; February 15–18; Orlando, Florida. 2006. [Google Scholar]
- *.Ockene JK, Kristeller J, Goldberg R, Ockene IS, Merriam P, Barrett S, et al. Smoking cessation and severity of disease: The coronary artery smoking intervention study. Health Psychology. 1992;11:119–126. doi: 10.1037//0278-6133.11.2.119. [DOI] [PubMed] [Google Scholar]
- Rosal MC, Ockene JK, Ma YS, Hebert JR, Ockene IS, Merriam P, et al. Coronary Artery Smoking Intervention Study (CASIS): 5-year Follow-up. Health Psychology. 1998;17(5):476–478. doi: 10.1037//0278-6133.17.5.476. [DOI] [PubMed] [Google Scholar]
- Chouinard MC, Robichaud-Ekstrand S. Predictive value of the transtheoretical model to smoking cessation in hospitalized patients with cardiovascular disease. European Journal of Cardiovascular Prevention & Rehabilitation. 2007;14(1):51–58. doi: 10.1097/HJR.0b013e328014027b. [DOI] [PubMed] [Google Scholar]
- Chouinard MC, Robichaud-Ekstrand S. The effectiveness of a nursing inpatient smoking cessation program in individuals with cardiovascular disease. Nursing Research. 2005;54(4):243–254. doi: 10.1097/00006199-200507000-00006. [DOI] [PubMed] [Google Scholar]
- Cossette S, Frasure-Smith N, Robert M, Chouinard MC, Juneau M, Guertin MCal. A pre assessment for nursing intervention to support tobacco cessation in patients hospitalized for cardiac problems: a pilot study (So-Live). [French] Recherche En Soins Infirmiers. 2011;(105):60–75. [PubMed] [Google Scholar]
- Croghan GA, Croghan IT, Frost MH, Sloan JA, Novotny PJ, Nelson MA, et al. Smoking cessation interventions and postoperative outcomes in esophageal and lung cancer patients [POS3-101]. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 20–23 March; Prague, Czech Republic. 2005. p. 139. [Google Scholar]
- de Azevedo RCS, Mauro MLF, Lima DD, Gaspar KC, da Silva VF, Botega NJ. General hospital admission as an opportunity for smoking-cessation strategies: A clinical trial in Brazil. General Hospital Psychiatry. 2010;32(6):599–606. doi: 10.1016/j.genhosppsych.2010.09.013. [DOI] [PubMed] [Google Scholar]
- DeBusk RF, Houston-Miller N, Superko HR, Dennis CA, Thomas RJ, Lew HT, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Annals of Internal Medicine. 1994;120(9):721–729. doi: 10.7326/0003-4819-120-9-199405010-00001. [DOI] [PubMed] [Google Scholar]
- Dornelas EA, Sampson RA, Gray JF, Waters D, Thompson PD. A randomized controlled trial of smoking cessation counseling after myocardial infarction. Preventive Medicine. 2000;30:261–268. doi: 10.1006/pmed.2000.0644. [DOI] [PubMed] [Google Scholar]
- Feeney GF, McPherson A, Connor JP, McAlister A, Young MR, Garrahy P. Randomized controlled trial of two cigarette quit programmes in coronary care patients after acute myocardial infarction. Internal Medicine Journal. 2001;31(8):470–475. doi: 10.1046/j.1445-5994.2001.00110.x. [DOI] [PubMed] [Google Scholar]
- Floter S, Kroger C. Effectiveness of telephone aftercare following a smoking cessation program for women on inpatient rehabilitation. [German] Deutsche Medizinische Wochenschrift. 2009;134(47):2382–2387. doi: 10.1055/s-0029-1242698. [DOI] [PubMed] [Google Scholar]
- Froelicher ES, Christopherson DJ, Miller NH, Martin K. Women's initiative for nonsmoking (WINS) IV: description of 277 women smokers hospitalized with cardiovascular disease. Heart & Lung. 2002;31:3–14. doi: 10.1067/mhl.2002.121247. [DOI] [PubMed] [Google Scholar]
- Froelicher ES, Christopherson DJ. Women's Initiative for Nonsmoking (WINS) I: Design and methods. Heart & Lung. 2000;29(6):429–437. doi: 10.1067/mhl.2000.110323. [DOI] [PubMed] [Google Scholar]
- Froelicher ES, Li WW, Mahrer-Imhof R, Christopherson D, Stewart AL. Women's Initiative for Non-Smoking (WINS) VI: Reliability and validity of health and psychosocial measures in women smokers with cardiovascular disease. Heart & Lung. 2004;33(3):162–175. doi: 10.1016/j.hrtlng.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Li WW, Froelicher ES. Predictors of smoking relapse in women with cardiovascular disease in a 30-month study: Extended analysis. Heart and Lung: Journal of Acute and Critical Care. 2008;37(6):455–465. doi: 10.1016/j.hrtlng.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Mahrer-Imhof R, Froelicher ES, Li WW, Parker KM, Benowitz N. Women's Initiative for Nonsmoking (WINS)V: under-use of nicotine replacement therapy. Heart & Lung. 2002;31:368–373. doi: 10.1067/mhl.2002.126539. [DOI] [PubMed] [Google Scholar]
- Martin K, Froelicher ES, Miller NH. Women's Initiative for Nonsmoking (WINS) II: The intervention. Heart & Lung. 2000;29(6):438–445. doi: 10.1067/mhl.2000.110576. [DOI] [PubMed] [Google Scholar]
- Oka RK, Katapodi MC, Lim JW, Bacchetti P, Froelicher ES. Quantifying smoking cessation outcomes: from The Women's Initiative for Nonsmoking Study (X): methodological implications. Nursing Research. 2006;55(4):292–297. doi: 10.1097/00006199-200607000-00010. [DOI] [PubMed] [Google Scholar]
- *.Sivarajan Froelicher ES, Miller NH, Christopherson DJ, Martin K, Parker KM, Amonetti M, et al. High rates of sustained smoking cessation in women hospitalized with cardiovascular disease: the Women's Initiative for Nonsmoking (WINS) Circulation. 2004;109(5):587–593. doi: 10.1161/01.CIR.0000115310.36419.9E. [DOI] [PubMed] [Google Scholar]
- Hajek P, Taylor TZ, Mills P. Brief intervention during hospital admission to help patients to give up smoking after myocardial infarction and bypass surgery: Randomised controlled trial. BMJ. 2002;324:87–89. doi: 10.1136/bmj.324.7329.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasuo S, Tanaka H, Oshima A. Efficacy of a smoking relapse prevention program by postdischarge telephone contacts: a randomized trial (Japanese) Nippon Koshu Eisei Zasshi [Japanese Journal of Public Health] 2004;51(6):403–412. [PubMed] [Google Scholar]
- Haug S, Meyer C, John U. Efficacy of an internet program for smoking cessation during and after inpatient rehabilitation treatment: A quasi-randomized controlled trial. Addictive Behaviors. 2011;36(12):1369–1372. doi: 10.1016/j.addbeh.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Hennrikus D, Lando HA, McCarty MC, Vessey JT. The effectiveness of a systems approach to smoking cessation in hospital inpatients. Society for Research on Nicotine and Tobacco 7th Annual Meeting; March 23-23; Seattle Washington. 2001. p. 47. [Google Scholar]
- *.Hennrikus DJ, Lando HA, McCarty MC, Klevan D, Holtan N, Huebsch JA, et al. The TEAM project: the effectiveness of smoking cessation interventions with hospital patients. Preventive Medicine. 2005;40:249–258. doi: 10.1016/j.ypmed.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Lando H, Hennrikus D, McCarty M, Vessey J. Predictors of quitting in hospitalized smokers. Nicotine & Tobacco Research. 2003;5(2):215–222. doi: 10.1080/0955300031000083436. [DOI] [PubMed] [Google Scholar]
- Lacasse Y, Lamontagne R, Martin S, Arsenault M. Randomized trial of a smoking cessation intervention in patients hospitalized in a cardio-respiratory institute. Chest. 2005;128(4) Suppl:204S. [Google Scholar]
- Lacasse Y, Lamontagne R, Martin S, Simard S, Arsenault M. Randomized trial of a smoking cessation intervention in hospitalized patients. Nicotine & Tobacco Research. 2008;10(7):1215–1221. doi: 10.1080/14622200801979142. [DOI] [PubMed] [Google Scholar]
- Lewis SF, Piasecki TM, Fiore MC, Anderson JE, Baker TB. Transdermal nicotine replacement for hospitalized patients: A randomized clinical trial. Preventive Medicine. 1998;27(2):296–303. doi: 10.1006/pmed.1998.0266. [DOI] [PubMed] [Google Scholar]
- Floeter S, Metz K, Kroeger C, Donath C, Piontek D, Gradi S. Evaluation of telephone booster sessions after intensive inpatient treatment - a randomized treatment control trial (POS1-78). Society for Research on Nicotine and Tobacco 12th Annual Meeting; February 15–18; Orlando, Florida. 2006. [Google Scholar]
- Kroeger C, Metz K, Buhler A. Smoking cessation in patients treated in rehabilitation hospitals. Suchtmedizin in Forschung und Praxis. 2004;6(1):61–66. [Google Scholar]
- *.Metz K, Floter S, Kroger C, Donath C, Piontek D, Gradl S. Telephone booster sessions for optimizing smoking cessation for patients in rehabilitation centers. Nicotine & Tobacco Research. 2007;9(8):853–863. doi: 10.1080/14622200701485000. [DOI] [PubMed] [Google Scholar]
- Meysman M, Boudrez H, Nackaerts K, Dieriks B, Indemans R, Vermeire P. Smoking cessation rates after a nurse-led inpatient smoking cessation intervention. Journal of Smoking Cessation. 2010;5(1):69–76. [Google Scholar]
- *.Miller NH, Smith PM, DeBusk RF, Sobel DS, Taylor CB. Smoking cessation in hospitalized patients - Results of a randomized trial. Archives of Internal Medicine. 1997;157:409–415. [PubMed] [Google Scholar]
- Taylor CB, Miller NH, Herman S, Smith PM, Sobel D, Fisher L, et al. A nurse-managed smoking cessation program for hospitalized smokers. American Journal of Public Health. 1996;86:1557–1560. doi: 10.2105/ajph.86.11.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrack R, Hilleman DE, Maciejewski SR, Grollmes TL, Cloutier DA, Mohiuddin SM. Cost analysis of an intensive smoking cessation intervention in cardiac patients. Journal of the American College of Cardiology. 2008;51(10):A257. [Google Scholar]
- Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hilleman DE. Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131:446–452. doi: 10.1378/chest.06-1587. [DOI] [PubMed] [Google Scholar]
- Mohjuddin SM, Mooss AN, Hunter CB, Grollmes T, Cloutier DA, Hilleman DE. Intensive multicomponent smoking cessation intervention decreases mortality in hospitalized high-risk cardiac smokers. Journal of the American College of Cardiology. 2006;47(4):375A. [Google Scholar]
- Molyneux A, Lewis S, Leivers U, Anderton A, Antoniak M, Brackenridge A, et al. Clinical trial comparing nicotine replacement therapy (NRT) plus brief counselling, brief counselling alone, and minimal intervention on smoking cessation in hospital inpatients. Thorax. 2003;58(6):484–488. doi: 10.1136/thorax.58.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley MJ, Nagle AL, Hensley MJ, Schofield MJ, Koschel A. Efficacy of a brief nurse provided nicotine management intervention for hospitalised smokers. Respirology. 2002;7:A12. [Google Scholar]
- *.Nagle AL, Hensley MJ, Schofield MJ, Koschel AJ. A randomised controlled trial to evaluate the efficacy of a nurse-provided intervention for hospitalised smokers. Australian and New Zealand Journal of Public Health. 2005;29(3):285–291. doi: 10.1111/j.1467-842x.2005.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Ortega F, Vellisco A, Marquez E, Lopez-Campos JL, Rodriguez A, de los Angeles SM, et al. Effectiveness of a cognitive orientation program with and without nicotine replacement therapy in stopping smoking in hospitalised patients [Efectividad de un programa de orientacion cognitiva con y sin tratamiento sustitutivo con nicotina en la cesacion tabaquica en pacientes hospitalizados] Archivos de Bronconeumologia. 2011;47(1):3–9. doi: 10.1016/j.arbres.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Ortigosa AM, Gomez FJO, Ramalle-Gomara E, Reta IS, Esteban MVF, Diaz MQ. Efficacy of an intervention in smoking cessation in patients with myocardial infarction [Eficacia de una intervencion para dejar de fumar en pacientes con infarto de miocardio] Medicina Clinica (Barc) 2000;114:209–210. doi: 10.1016/s0025-7753(00)71246-2. [DOI] [PubMed] [Google Scholar]
- Pedersen L, Johansen S, Eksten L. Smoking cessation among patients with acute heart disease. A randomised intervention project] (Danish) Ugeskrift for Laeger. 2005;167(33):3044–3047. [PubMed] [Google Scholar]
- Pederson LL, Wanklin JM, Lefcoe NM. The effects of counseling on smoking cessation among patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. International Journal of the Addictions. 1991;26(1):107–119. doi: 10.3109/10826089109056242. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Moisan JT. Smoking cessation among hospitalized patients: A quasi-experimental study in Quebec. Canadian Journal of Public Health. 1998;89:264–269. doi: 10.1007/BF03403933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planer D, Lev I, Elitzur Y, Sharon N, Ouzan E, Pugatsch T, et al. Bupropion for smoking cessation in patients with acute coronary syndrome. Archives of Internal Medicine. 2011;171(12):1055–1060. doi: 10.1001/archinternmed.2011.72. [DOI] [PubMed] [Google Scholar]
- Brown DW. Nurse-led intervention increases smoking cessation among people with coronary heart disease. Evidence Based Healthcare. 2004;8:128–130. [Google Scholar]
- Quist-Paulsen P, Bakke PS, Gallefoss F. Does smoking cessation improve quality of life in patients with coronary heart disease? Scandinavian Cardiovascular Journal. 2006;40(1):11–16. doi: 10.1080/14017430500384855. [DOI] [PubMed] [Google Scholar]
- Quist-Paulsen P, Bakke PS, Gallefoss F. Predictors of smoking cessation in patients admitted for acute coronary heart disease. European Journal of Cardiovascular Prevention & Rehabilitation. 2005;12(5):472–477. doi: 10.1097/01.hjr.0000183914.90236.01. [DOI] [PubMed] [Google Scholar]
- *.Quist-Paulsen P, Gallefoss F. Randomised controlled trial of smoking cessation intervention after admission for coronary heart disease. BMJ. 2003;327(7426):1254–1257. doi: 10.1136/bmj.327.7426.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist-Paulsen P, Lydersen S, Bakke PS, Gallefoss F. Cost effectiveness of a smoking cessation program in patients admitted for coronary heart disease. European Journal of Cardiovascular Prevention & Rehabilitation. 2006;13(2):274–280. doi: 10.1097/01.hjr.0000192742.81231.91. [DOI] [PubMed] [Google Scholar]
- Reid R, Pipe A, Higginson L, Johnson K, D'Angelo MS, Cooke D, et al. Stepped care approach to smoking cessation in patients hospitalized for coronary artery disease. Journal of Cardiopulmonary Rehabilitation. 2003;23(3):176–182. doi: 10.1097/00008483-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Reid RD, Pipe AL, Quinlan B, Oda J. Interactive voice response telephony to promote smoking cessation in patients with heart disease: A pilot study. Patient Education & Counseling. 2007;66(3):319–326. doi: 10.1016/j.pec.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, McKool KM, Shiffman S. Predictors of smoking cessation after coronary artery bypass graft surgery. Results of a randomized trial with 5-year follow-up. Annals of Internal Medicine. 1994;120(4):287–293. doi: 10.7326/0003-4819-120-4-199402150-00005. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Arnsten JH, McKool KM, Wood Reid KM, Pasternak RC, Singer DE. Efficacy of a smoking cessation program for hospital patients. Archives of Internal Medicine. 1997;157(22):2653–2660. [PubMed] [Google Scholar]
- *.Rigotti NA, Thorndike AN, Regan S, McKool K, Pasternak RC, Chang Y, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. American Journal of Medicine. 2006;119(12):1080–1087. doi: 10.1016/j.amjmed.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Thorndike A, Rigotti N, Regan S, Pasternak R, McKool K, Emmons K, Singer D. Depression and relapse to smoking in patients hospitalized with acute cardiovascular disease [POS3-094]. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 20–23 March; Prague, Czech Republic. 2005. p. 137. [Google Scholar]
- Thorndike AN, Regan S, McKool K, Pasternak RC, Swartz S, Torres-Finnerty N, et al. Depressive symptoms and smoking cessation after hospitalization for cardiovascular disease. Archives of Internal Medicine. 2008;168(2):186–191. doi: 10.1001/archinternmed.2007.60. [DOI] [PubMed] [Google Scholar]
- Simon JA, Solkowitz SN, Carmody TP, Browner WS. Smoking cessation after surgery - A randomized trial. Archives of Internal Medicine. 1997;157:1371–1376. [PubMed] [Google Scholar]
- Simon JA, Carmody TP, Hudes ES, Snyder E, Murray J. Intensive smoking cessation counseling versus minimal counseling among hospitalized smokers treated with transdermal nicotine replacement: a randomized trial. American Journal of Medicine. 2003;114(7):555–562. doi: 10.1016/s0002-9343(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Simon JA, Duncan C, Huggins J, Solkowitz S, Carmody TP. Sustained-release bupropion for hospital-based smoking cessation: a randomized trial. Nicotine & Tobacco Research. 2009;11(6):663–669. doi: 10.1093/ntr/ntp047. [DOI] [PubMed] [Google Scholar]
- Bitton A. Intensive smoking cessation programs for hospitalized coronary patients: A proven intervention in need of implementation. Journal of Clinical Outcomes Management. 2009;16(9):398–399. [Google Scholar]
- Rigotti NA. Helping smokers with cardiac disease to abstain from tobacco after a stay in hospital. CMAJ Canadian Medical Association Journal. 2009;180:1283–1284. doi: 10.1503/cmaj.090729. [Comment]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Smith PM, Burgess E. Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. CMAJ Canadian Medical Association Journal. 2009;180(13):1297–1303. doi: 10.1503/cmaj.080862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson KV, Brinn MP, Smith BJ, Garside CG, Goldsworthy SJ, Fitridge RA. Varenicline tartrate and counselling versus counselling alone in a randomised controlled trial for inpatient smoking cessation: 3 month interim results [Abstract] Respirology. 2010;15(Suppl 1):A30. [Google Scholar]
- Smith BJ, Peters MJ, Fitridge RA, Esterman AJ, Litt JC, Horowitz JD. Varenicline Tartrate And Counselling Versus Counselling Alone In A Randomised Controlled Trial For Inpatient Smoking Cessation: 6 Month Interim Results [Abstract] American Journal of Respiratory and Critical Care Medicine. 2011;183:A5445. (Meeting Abstracts) [Google Scholar]
- Smith PM, Corso L, Brown KS, Cameron R. Nurse case-managed tobacco cessation interventions for general hospital patients: Results of a randomized clinical trial. Canadian Journal of Nursing Research. 2011;43:98–117. [PubMed] [Google Scholar]
- Steinberg MB, Randall J, Greenhaus S, Schmelzer AC, Richardson DL, Carson JL. Tobacco dependence treatment for hospitalized smokers: A randomized, controlled, pilot trial using varenicline. Addictive Behaviors. 2011;36(12):1127–1132. doi: 10.1016/j.addbeh.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Glasgow RE, Hollis JF, Lichtenstein E, Vogt TM. A smoking-cessation intervention for hospital patients. Medical Care. 1993;31(1):65–72. doi: 10.1097/00005650-199301000-00005. [DOI] [PubMed] [Google Scholar]
- Allaway L, Stevens VJ. Respiratory care practitioners can provide effective smoking-cessation counseling to hospitalized smokers. Respiratory Care. 1996;41(11):1026–1029. [Google Scholar]
- *.Stevens VJ, Glasgow RE, Hollis JF, Mount K. Implementation and effectiveness of a brief smoking-cessation intervention for hospital patients. Medical Care. 2000;38(5):451–459. doi: 10.1097/00005650-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Houston-Miller NH, Killen JD, DeBusk RF. Smoking cessation after acute myocardial infarction: Effects of a nurse-managed intervention. Annals of Internal Medicine. 1990;113:118–123. doi: 10.7326/0003-4819-113-2-118. [DOI] [PubMed] [Google Scholar]
- Vial RJ, Jones TE, Ruffin RE, Gilbert AL. Smoking cessation program using nicotine patches linking hospital to the community. Journal of Pharmacy Practice and Research. 2002;32(1):57–62. [Google Scholar]
Excluded studies
- Agewall S, Fagerberg B, Berglund G, Schmidt C, Wendelhag I, Wikstrand J. Multiple risk intervention trial in high risk hypertensive men: comparison of ultrasound intima-media thickness and clinical outcome during 6 years of follow-up. Journal of Internal Medicine. 2001;249(4):305–314. doi: 10.1046/j.1365-2796.2001.00818.x. [DOI] [PubMed] [Google Scholar]
- Allen B, Pederson LL, Leonard EH. Effectiveness of physicians-in-training counseling for smoking cessation in African Americans. Journal of the National Medical Association. 1998;90:597–604. [PMC free article] [PubMed] [Google Scholar]
- Asfar T, Ward KD, Vander Weg MW, Hammal F, Eissenberg T, Maziak W. Characteristics of completers and dropouts in Syria's first smoking cessation trial [POS2-132]. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 20–23 March 2005; Prague, Czech Republic. 2005. p. 110. [Google Scholar]
- Avanzini F, Di GP, Amodeo R, Baldo S, Bergna ML, Busi Gal. Effectiveness of a nurse-led educational intervention for patients admitted for acute coronary syndrome. [Italian] Assistenza Infermieristica e Ricerca:Air. 2011;30(1):16–23. [PubMed] [Google Scholar]
- Becker BM, Bock BC, Partridge R. Smoking cessation interventions in the emergency department for smokers with chest pain. Academic Emergency Medicine. 2003;10(5):507. [Google Scholar]
- Bize R, Stoianov R, Ruffieux C, Ghali W, Paccaud F, Cornuz J. Effectiveness of a low-intensity smoking cessation intervention for hospitalized patients. European Journal of Cancer Prevention. 2006;15(5):464–470. doi: 10.1097/00008469-200610000-00013. [DOI] [PubMed] [Google Scholar]
- Blom RA, Mulder M, van Spiegel PI, Christenhusz LCA. Determinants of successful quitting in patients with COPD [POS3-088]. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 20–23 March; Prague, Czech Republic. 2005. p. 135. [Google Scholar]
- *.British Thoracic Society. Comparison of four methods of smoking withdrawal in patients with smoking related diseases. British Medical Journal. 1983;286:595–597. doi: 10.1136/bmj.286.6365.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Thoracic Society. Smoking withdrawal in hospital patients: factors associated with outcome. Thorax. 1984;39:651–656. doi: 10.1136/thx.39.9.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt A, Illingworth D, Shaw T, Thornley P, White P, Turner R. Stopping smoking after myocardial infarction. Lancet. 1974;1(7582):304–306. doi: 10.1016/s0140-6736(74)92607-5. [DOI] [PubMed] [Google Scholar]
- Chan SSC, Chan SC, Lam TH, Lau C-P, et al. The effectiveness of a stage-matched smoking cessation intervention to cardiac patients: Preliminary results of a randomized controlled trial (POS3-97). Society for Research on Nicotine and Tobacco 9th Annual Meeting; February 19–22; New Orleans, Louisiana. 2003. Rapid Communications Posters. [Google Scholar]
- Chan SS, Leung DY, Lau C, Wong V, Lam T. Cost-effectiveness analysis of a low intensity nurse-led stage-matched smoking cessation intervention to cardiac patients in Hong Kong. Circulation. 2010;122(2):E87. [Google Scholar]
- Choo YM, Ong KC, Lee WK. Effectiveness of a smoking cessation program among hospitalized patients in Singapore. Chest. 2004;126(4):713S. [Google Scholar]
- Colby SM, Monti PM, Barnett NP, Rohsenow DJ, Weissman K, Spirito A, et al. Brief motivational interviewing in a hospital setting for adolescent smoking: a preliminary study. Journal of Consulting and Clinical Psychology. 1998;66(3):574–578. doi: 10.1037//0022-006x.66.3.574. [DOI] [PubMed] [Google Scholar]
- Cole TK. Smoking cessation in the hospitalized patient using the transtheoretical model of behavior change. Heart & Lung. 2001;30(2):148–158. doi: 10.1067/mhl.2001.111249. [DOI] [PubMed] [Google Scholar]
- Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR. High-dose nicotine patch therapy - percentage of replacement and smoking cessation. JAMA. 1995;274(17):1353–1358. [PubMed] [Google Scholar]
- Davies SL, Kohler CL, Fish L, Taylor BE, Foster GE, Annang L. Evaluation of an intervention for hospitalized African American smokers. American Journal of Health Behavior. 2005;29(3):228–239. doi: 10.5993/ajhb.29.3.4. [DOI] [PubMed] [Google Scholar]
- Elsony A, Salieh M, Elhaj H, Adam K. Brief smoking cessation intervention with tuberculosis patients in Sudan [PA2-5]. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 20–23 March 2005; Prague, Czech Republic. 2005. p. 22. [Google Scholar]
- Emmons KM, Goldstein MG, Roberts M, Cargill B, Sherman CB, Millman R, et al. The use of nicotine replacement therapy during hospitalization. Annals of Behavioral Medicine. 2000;22(4):325–329. doi: 10.1007/BF02895669. [DOI] [PubMed] [Google Scholar]
- Freund M, Campbell E, Paul C, Sakrouge R, Lecathelinais C, Knight J, et al. Increasing hospital-wide delivery of smoking cessation care for nicotine-dependent in-patients: A multi-strategic intervention trial. Addiction. 2009;104(5):839–849. doi: 10.1111/j.1360-0443.2009.02520.x. [DOI] [PubMed] [Google Scholar]
- Fung PR, Snape-Jenkinson SL, Godfrey MT, Love KW, Zimmerman PV, Yang IA, et al. Effectiveness of hospital-based smoking cessation. Chest. 2005;128(1):216–223. doi: 10.1378/chest.128.1.216. [DOI] [PubMed] [Google Scholar]
- Gadomski AM, Gavett J, Krupa N, Tallman N, Jenkins P. Effectiveness of an inpatient smoking cessation program. Journal of Hospital Medicine (Online) 2011;6(1):E1–E8. doi: 10.1002/jhm.641. [DOI] [PubMed] [Google Scholar]
- Galvin K, Webb C, Hillier V. Assessing the impact of a nurse-led health education intervention for people with peripheral vascular disease who smoke: the use of physiological markers, nicotine dependence and withdrawal. International Journal of Nursing Studies. 2001;38(1):91–105. doi: 10.1016/s0020-7489(00)00048-1. [DOI] [PubMed] [Google Scholar]
- Gariti P, Alterman A, Mulvaney F, Mechanic K, Dhopesh V, Yu E, et al. Nicotine intervention during detoxification and treatment for other substance use. American Journal of Drug and Alcohol Abuse. 2002;28(4):673–681. doi: 10.1081/ada-120015875. [DOI] [PubMed] [Google Scholar]
- Gies CE, Buchman D, Robinson J, Smolen D. Effect of an inpatient nurse-directed smoking cessation program. Western Journal of Nursing Research. 2008;30(1):6–19. doi: 10.1177/0193945907302729. [DOI] [PubMed] [Google Scholar]
- *.Gritz ER, Carr CR, Rapkin D, Abemayor E, Chang LJ, Wong WK, et al. Predictors of long-term smoking cessation in head and neck cancer patients. Cancer Epidemiology, Biomarkers & Prevention. 1993;2(3):261–270. [PubMed] [Google Scholar]
- Gritz ER, Ward PH, Beumer J, Carr CR, Rapkin DA. Increasing adherence to a provider-implemented smoking cessation intervention for head and neck cancer patients. In: Association of Community Cancer Centers, editor. Advances in Cancer Control: Screening and Prevention. New York: Wiley-Liss; 1990. pp. 83–93. [PubMed] [Google Scholar]
- Hand S, Edwards S, Campbell IA, Cannings R. Controlled trial of three weeks nicotine replacement treatment in hospital patients also given advice and support. Thorax. 2002;57(8):715–718. doi: 10.1136/thorax.57.8.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen TA, Nordrehaug JE, Eide GE, Hanestad BR. Does a telephone follow-up intervention for patients discharged with acute myocardial infarction have long-term effects on health-related quality of life? A randomised controlled trial. Journal of Clinical Nursing. 2009;18(9):1334–1345. doi: 10.1111/j.1365-2702.2008.02654.x. [DOI] [PubMed] [Google Scholar]
- Hanssen TA, Nordrehaug JE, Eide GE, Hanestad BR. Improving outcomes after myocardial infarction: a randomized controlled trial evaluating effects of a telephone follow-up intervention. European Journal of Cardiovascular Prevention & Rehabilitation. 2007;14(3):429–437. doi: 10.1097/HJR.0b013e32801da123. [DOI] [PubMed] [Google Scholar]
- Hasan FM, Pischke K, Saiyed S, Macys D, McCleary N. Hypnotherapy as an aid to smoking cessation of hospitalized patients: Preliminary results. Chest. 2007;132:527S. [Google Scholar]
- Hasan FM, Saiyed S, Pischke K, Macys D, Bettencourt AM, Beal L. Non-pharmacologic therapy for smoking cessation of hospitalized patients: a role for hypnotherapy [Abstract]. American Thoracic Society International Conference; May 15–20 2009; San Diego. 2009. p. A3959. [Google Scholar]
- Hilleman DE, Mohiuddin SM, Packard KA. Comparison of conservative and aggressive smoking cessation treatment strategies following coronary artery bypass graft surgery. Chest. 2004;125(2):435–438. doi: 10.1378/chest.125.2.435. [DOI] [PubMed] [Google Scholar]
- Holmes-Rovner M, Stommel M, Corser WD, Olomu A, Holtrop JS, Siddiqi A, et al. Does outpatient telephone coaching add to hospital quality improvement following hospitalization for acute coronary syndrome? Journal of General Internal Medicine. 2008;23(9):1464–1470. doi: 10.1007/s11606-008-0710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtrop JS, Corser W, Jones G, Brooks G, Holmes-Rovner M, Stommel M. Health behavior goals of cardiac patients after hospitalization. American Journal of Health Behavior. 2006;30(4):387–399. doi: 10.5555/ajhb.2006.30.4.387. [DOI] [PubMed] [Google Scholar]
- Holtrop JS, Corser W, Stommel M, Holmes-Rovner M. Predictors of smoking cessation and relapse after hospitalization for acute coronary syndrome (POS1-111). Society for Research on Nicotine and Tobacco 13th Annual Meeting; February 21–24; Austin, Texas. 2007. [DOI] [PubMed] [Google Scholar]
- Horn K, Dino G, Hamilton C, Noerachmanto N, Zhang J. Feasibility of a smoking cessation intervention for teens in the emergency department: reach, implementation fidelity, and acceptability. American Journal of Critical Care. 2008;17(3):205–216. [PubMed] [Google Scholar]
- Jeong HS, Chae JS, Moon JS, Yoo YS. An individualized teaching program for atherosclerotic risk factor reduction in patients with myocardial infarction. Yonsei Medical Journal. 2002;43(1):93–100. doi: 10.3349/ymj.2002.43.1.93. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Budz B, Mackay M, Miller C. Evaluation of a nurse-delivered smoking cessation intervention for hospitalized patients with cardiac disease. Heart & Lung. 1999;28(1):55–64. doi: 10.1016/s0147-9563(99)70043-9. [DOI] [PubMed] [Google Scholar]
- Jones C, Griffiths RD, Skirrow P, Humphris G. Smoking cessation through comprehensive critical care. Intensive Care Medicine. 2001;27(9):1547–1549. doi: 10.1007/s001340101051. [DOI] [PubMed] [Google Scholar]
- Joseph A, Lexau B, Willenbring M, Nugent S, Nelson D. Factors associated with readiness to stop smoking among patients in treatment for alcohol use disorder. American Journal on Addictions. 2004;13(4):405–417. doi: 10.1080/10550490490483116. [DOI] [PubMed] [Google Scholar]
- Joseph A, Hecht S, Murphy S, Gross M, Lando H, Bliss R, et al. A randomized controlled trial of smoking reduction in heart disease patients [PA2-4]. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 20–23 March 2005; Prague, Czech Republic. 2005. p. 22. [Google Scholar]
- *.Joseph A, Hecht S, Murphy S, Gross M, Lando H, Bliss R, et al. A randomized controlled trial of smoking reduction in heart disease patients. Nicotine & Tobacco Research. 2005;7(4):687. [Google Scholar]
- Kalman D, Hayes K, Colby SM, Eaton CA, Rohsenow DJ, Monti PM. Concurrent versus delayed smoking cessation treatment for persons in early alcohol recovery - A pilot study. Journal of Substance Abuse Treatment. 2001;20(3):233–238. doi: 10.1016/s0740-5472(00)00174-4. [DOI] [PubMed] [Google Scholar]
- Lewis KE, Dixon H, Edwards VM, Whitehead C, Durgan L, Sykes R. Can smokers pass seamlessly from a hospital based to a community based smoking cessation service? A randomised controlled trial [Abstract] Thorax. 2006;61(Suppl 2):ii22. [Google Scholar]
- *.Lewis KE, Durgan L, Edwards VM, Dixon H, Whitehead C, Sykes RN. Can smokers switch from a hospital-based to a community-based stop smoking service? An open-label, randomized trial comparing three referral schemes. Nicotine & Tobacco Research. 2009;11(6):756–764. doi: 10.1093/ntr/ntp061. [DOI] [PubMed] [Google Scholar]
- Lisspers J, Sundin O, HofmanBang C, Nordlander R, Nygren A, Ryden L, et al. Behavioral effects of a comprehensive, multifactorial program for lifestyle change after percutaneous transluminal coronary angioplasty: a prospective, randomized, controlled study. Journal of Psychosomatic Research. 1999;46:143–154. doi: 10.1016/s0022-3999(98)00074-9. [DOI] [PubMed] [Google Scholar]
- McHugh F, Lindsay GM, Hanlon P, Hutton I, Brown MR, Morrison C, et al. Nurse-led shared care for patients on the waiting list for coronary artery bypass surgery: a randomised controlled trial. Heart. 2001;86:317–323. doi: 10.1136/heart.86.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Meenan RT, Stevens VJ, Hornbrook MC, La Chance PA, Glasgow RE, Hollis JF, et al. Cost-effectiveness of a hospital-based smoking cessation intervention. Medical Care. 1998;36(5):670–678. doi: 10.1097/00005650-199805000-00007. [MEDLINE: 1998255497] [DOI] [PubMed] [Google Scholar]
- Moller AM, Villebro N, Pedersen T, Tonnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114–117. doi: 10.1016/S0140-6736(02)07369-5. [DOI] [PubMed] [Google Scholar]
- Mosca L, Christian AH, Mochari-Greenberger H, Kligfield P, Smith SCJ. A randomized clinical trial of secondary prevention among women hospitalized with coronary heart disease. Journal of Women's Health. 2010;19(2):195–202. doi: 10.1089/jwh.2009.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackaerts K, Salhi B, Devolder A, Meysman M, Boudrez H, Van-Dyck L. A randomised, double-blind, placebo-controlled phase 3 study investigating the effficacy of nicotine substitution (NS) on nicotine withdrawal symptoms (NWS) in hospitalised smokers (HS) [Abstract]. European Respiratory Society Annual Congress; September 12–16; Vienna, Austria. 2009. p. 4632. [Google Scholar]
- Nasell H, Adami J, Samnegard E, Tonnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. Journal of Bone & Joint Surgery - American Volume. 2010;92:1335–1342. doi: 10.2106/JBJS.I.00627. [DOI] [PubMed] [Google Scholar]
- Ong KC, Cheong GN, Prabhakaran GL, Earnest A. Predictors of success in smoking cessation among hospitalized patients. Respirology. 2005;10(1):63–69. doi: 10.1111/j.1440-1843.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- Ranote P, Smith M, Smith S, Ballance K, Miles J. Effects of an immediate smoking cessation programme on long-term quit rates in patients admitted to acute medical wards of a UK general hospital [abstract]; American Thoracic Society 99th International Conference; 2003. p. A027. [Google Scholar]
- Bottorff JL, Johnson JL, Moffat B, Fofonoff D, Budz B, Groening M. Synchronizing clinician engagement and client motivation in telephone counseling. Qualitative Health Research. 2004;14(4):462–477. doi: 10.1177/1049732303262602. [DOI] [PubMed] [Google Scholar]
- Ratner PA, Johnson JL, Richardson CG, Bottorff JL, Moffat B, Mackay M, et al. Efficacy of a smoking-cessation intervention for elective-surgical patients. Research in Nursing and Health. 2004;27(3):148–161. doi: 10.1002/nur.20017. [DOI] [PubMed] [Google Scholar]
- Regan S, Reyen M, Lockhart AC, Richards AE, Rigotti NA. An interactive voice response system to continue a hospital-based smoking cessation intervention after discharge. Nicotine & Tobacco Research. 2011;13(4):255–260. doi: 10.1093/ntr/ntq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RD, Pipe AL, Quinlan B. Promoting smoking cessation during hospitalization for coronary artery disease. Canadian Journal of Cardiology. 2006;22:775–780. doi: 10.1016/s0828-282x(06)70294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman PB, Dinowitz S, Nashed AH, Eskin B, Sylvan E, Allegra C, et al. The emergency department as a potential site for smoking cessation intervention: A randomized, controlled trial. Academic Emergency Medicine. 2000;7(4):348–353. doi: 10.1111/j.1553-2712.2000.tb02235.x. [DOI] [PubMed] [Google Scholar]
- Rissel C, Salmon A, Hughes AM. Evaluation of a (pilot) stage-tailored brief smoking cessation intervention among hospital patients presenting to a hospital pre-admission clinic. Australian Health Review. 2000;23(3):83–93. doi: 10.1071/ah000083. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Spiga R, Rhoades HM, Fuentes F, Grabowski J. Smoking cessation in women with cardiac risk: a comparative study of two theoretically based therapies. Nicotine & Tobacco Research. 1999;1:87–94. doi: 10.1080/14622299050011191. [DOI] [PubMed] [Google Scholar]
- Smith PM, Reilly KR, Houston-Miller N, DeBusk RF, Taylor CB. Application of a nurse-managed inpatient smoking cessation program. Nicotine & Tobacco Research. 2002;4(2):211–222. doi: 10.1080/14622200210123590. [DOI] [PubMed] [Google Scholar]
- Strecher VJ, Becker MH, Kirscht JP, Eraker SA, Graham-Tomasi RP. Evaluation of a minimal-contact smoking cessation program in a health care setting. Patient Education and Counselling. 1985;7:395–407. doi: 10.1016/0738-3991(85)90049-7. [DOI] [PubMed] [Google Scholar]
- Sundblad BM, Larsson K, Nathell L. High rate of smoking abstinence in COPD patients: Smoking cessation by hospitalization. Nicotine & Tobacco Research. 2008;10(5):883–890. doi: 10.1080/14622200802023890. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Sako H, Sasa H, Sako H, Iwata M, Hashimoto I, et al. A pilot study on inducement of smoking cessation by a simple 5A (asking, advice, assess, assist, and arrange) approach at outpatient clinics. Asian Pacific Journal of Cancer Prevention. 2006;7(1):131–135. [PubMed] [Google Scholar]
- Targhetta R, Bernhard L, Sorokaty JM, Balmes JL, Nalpas B, Perney P. Intervention study to improve smoking cessation during hospitalization. Public Health. 2011;125(7):457–463. doi: 10.1016/j.puhe.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Miller NH, Cameron RP, Fagans EW, Das S. Dissemination of an effective inpatient tobacco use cessation program. Nicotine & Tobacco Research. 2005;7:129–137. doi: 10.1080/14622200412331328420. [DOI] [PubMed] [Google Scholar]
- Thomsen T, Tonnesen H, Okholm M, Kroman N, Maibom A, Sauerberg ML, et al. Brief smoking cessation intervention in relation to breast cancer surgery: a randomized controlled trial. Nicotine & Tobacco Research. 2010;12(11):1118–1124. doi: 10.1093/ntr/ntq158. [DOI] [PubMed] [Google Scholar]
- Wakefield M, Olver I, Whitford H, Rosenfeld E. Motivational interviewing as a smoking cessation intervention for patients with cancer: randomized controlled trial. Nursing Research. 2004;53(6):396–405. doi: 10.1097/00006199-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Warner DO, Patten CA, Ames SC, Offord KP, Schroeder DR. Effect of nicotine replacement therapy on stress and smoking behavior in surgical patients. Anesthesiology. 2005;102(6):1138–1146. doi: 10.1097/00000542-200506000-00013. [DOI] [PubMed] [Google Scholar]
- Wewers ME, Bowen JM, Stanislaw AE, Desimone VB. A nurse-delivered smoking cessation intervention among hospitalized postoperative patients--influence of a smoking- related diagnosis: a pilot study. Heart & Lung. 1994;23:151–156. [MEDLINE: 94266626] [PubMed] [Google Scholar]
- Winickoff JP, Healey EA, Regan S, Park ER, Cole C, Friebely J, et al. Using the postpartum hospital stay to address mothers' and fathers' smoking: the NEWS study. Pediatrics. 2010;125(3):518–525. doi: 10.1542/peds.2009-0356. [DOI] [PubMed] [Google Scholar]
- Wolfenden L, Wiggers J, Knight J, Campbell E, Rissel C, Kerridge R, et al. A programme for reducing smoking in pre-operative surgical patients: randomised controlled trial. Anaesthesia. 2005;60(2):172–179. doi: 10.1111/j.1365-2044.2004.04070.x. [DOI] [PubMed] [Google Scholar]
- Wolfenden L, Wiggers J, Campbell E, Knight J. Pilot of a preoperative smoking cessation intervention incorporating post-discharge support from a Quitline. Health Promotion Journal of Australia. 2008;19(2):158–160. doi: 10.1071/he08158. [DOI] [PubMed] [Google Scholar]
Studies awaiting classification
- Brunner-Frandsen NS, Bak S. Smoking cessation intervention after stroke or transient ischemic attack. A randomised controlled trial. Cerebrovascular Diseases. 2010;29(323) doi: 10.1093/ntr/ntr233. [DOI] [PubMed] [Google Scholar]
- Jimenez G, Lopez J, Martinez M, Romero A, Gil B, de Dios LJ, et al. Effectiveness of a specialised intervention for smoking cessation in hospitalised patients [Abstract] European Respiratory Journal. 2007;30(Suppl 51):502s. [Google Scholar]
Ongoing studies
Other references
Additional references
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews. 2012 doi: 10.1002/14651858.CD006103.pub2. Art. No.: CD006103. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;7414:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [updated March 2011]. available from www.cochrane-handbook.org. [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews. 2007;(1) doi: 10.1002/14651858.CD000031.pub3. Art. No.: CD000031. [DOI] [PubMed] [Google Scholar]
- Ladapo JA, Jaffer FA, Weinstein MC, Froelicher ES. Projected cost-effectiveness of smoking cessation interventions in patients hospitalized with myocardial infarction. Archives of Internal Medicine. 2011;171(1):39–45. doi: 10.1001/archinternmed.2010.479. [DOI] [PubMed] [Google Scholar]
- Liu SK, Prior E, Warren C, Brown T, Snide J, Butterly JR. Improving the quality of care for the hospitalized tobacco user--one institution's transformational journey. Journal of Cancer Education. 2010;25(3):297–301. doi: 10.1007/s13187-010-0135-5. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Kristeller J, Goldberg R, Ockene IS, Merriam P, Barrett S, et al. Smoking cessation and severity of disease: The coronary artery smoking intervention study. Health Psychology. 1992;11:119–126. doi: 10.1037//0278-6133.11.2.119. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(2) doi: 10.1002/14651858.CD000165.pub3. Art. No.: CD000165. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(1) doi: 10.1002/14651858.CD000146.pub3. Art. No.: CD000146. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: An overview of the randomized trials. Progress in Cardiovascular Diseases. 1985;27(5):335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
Other published versions of this review
- Munafo M, Rigotti N, Lancaster T, Stead L, Murphy M. Interventions for smoking cessation in hospitalised patients: a systematic review. Thorax. 2001;56:656–663. doi: 10.1136/thorax.56.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Munafo MR, Murphy MF, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews. 2001;(2) doi: 10.1002/14651858.CD001837. Art. No.: CD001837. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Munafo MR, Murphy MF, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews. 2003;(1) doi: 10.1002/14651858.CD001837. Art. No.: CD001837. [DOI] [PubMed] [Google Scholar]
- Rigotti N, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD001837.pub2. Art. No.: CD001837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.