Abstract
Although the mammillary bodies were one of the first neural structures to be implicated in memory, it has long been assumed that their main function was to act primarily as a hippocampal relay, passing information on to the anterior thalamic nuclei and from there to the cingulate cortex. This view not only afforded the mammillary bodies no independent role in memory, it also neglected the potential significance of other, nonhippocampal, inputs to the mammillary bodies. Recent advances have transformed the picture, revealing that projections from the tegmental nuclei of Gudden, and not the hippocampal formation, are critical for sustaining mammillary body function. By uncovering a role for the mammillary bodies that is independent of its subicular inputs, this work signals the need to consider a wider network of structures that form the neural bases of episodic memory.
Keywords: Anterograde amnesia, Fornix, Mammillothalamic tract, Medial diencephalon, Papez circuit
1 INTRODUCTION
The hippocampal-mammillary body projections hold a noteworthy position in history; they were the arguably the first hippocampal projections to undergo experimental analysis and, until the mid-twentieth century, were the principal focus of attention in terms of hippocampal outputs (Gudden, 1881; MacLean, 1990). Both the medial temporal lobe and medial diencephalon, comprising the hippocampus and mammillary bodies, respectively, have been implicated in event memory for over a hundred years, but there remains much uncertainty about how these brain regions interact to support this function. Since Papez proposed his model of emotion in 1937, most accounts of mammillary body function have emphasized the importance of hippocampal inputs to this region, effectively relegating the mammillary bodies to the status of a relay within an “extended hippocampal system” (Aggleton and Brown, 1999; Papez, 1937). However, recent advances in our understanding challenge the prevalent hippocampal-centric view of mammillary body function. In contrast to traditional models, it appears that independent ascending projections from the mammillary bodies are key for some aspects of hippocampal function.
2 THE PAPEZ CIRCUIT: ANATOMY
In his proposed mechanism of emotion, Papez described a circuit that originated in the cortex, then “built up in the hippocampal formation and …transferred to the mammillary body and thence through the anterior thalamic nuclei to the cortex of the gyrus cinguli” (Papez, 1937, p. 728). In terms of hippocampal outputs, the projections to the mammillary bodies were seen as key. The mammillary bodies are remarkable for a number of reasons. They are clearly discernable as two spherical structures on the underside of the brain. Originally referred to as the “testicles of the brain,” they have subsequently come to be known as the “breasts of the brain” (Jones, 2011). They comprise just two major nuclei groups, the lateral and medial nuclei, with a narrow array of cell types in each (Vann and Aggleton, 2004). The mammillary bodies have major connections with a limited number of regions, and most of these pathways can be readily seen in a dissected brain (Fig. 1). The pattern of connectivity is strikingly similar between rodents and primates (e.g., Allen and Hopkins, 1989; Saunders et al., 2012; Vann et al., 2007). The mammillary bodies receive a dense input from the hippocampus via the fornix (Gudden, 1881). It was originally thought that these projections arose from the hippocampus proper (CA1-4), but Swanson and Cowan (1975) showed that the subicular complex was, in fact, the source of projections to the mammillary bodies.
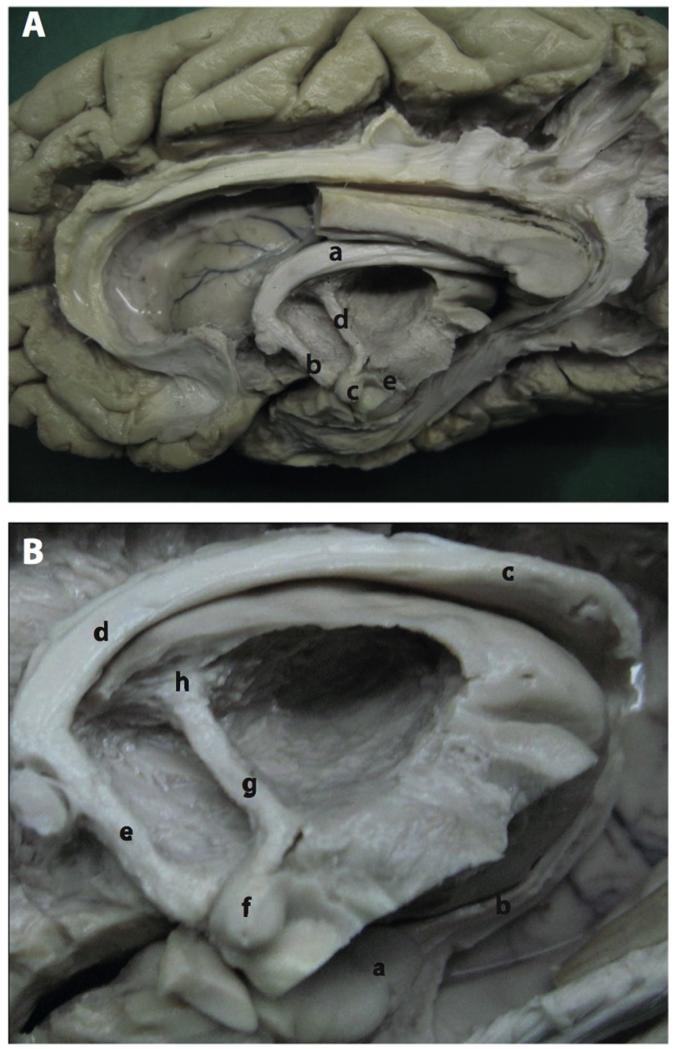
FIGURE 1.
Dissection of the Papez circuit. (A) Photograph of a dissection of the medial aspect of the hemisphere: a, body of fornix; b, descending postcommissural fornix; c, mammillary bodies; d, mammillothalamic tract; e, mammillotegmental tract. (B) Photograph showing the mammillary body and surrounding structures after removal of the dentate gyrus: a, hippocampus; b, fimbria; c, crus of the fornix; d, body of the fornix; e, descending postcommissural fornix; f, mammillary body; g, mammillothalamic tract; h, anterior thalamic nuclei.
Figure reproduced with permission from Shah et al. (2012).
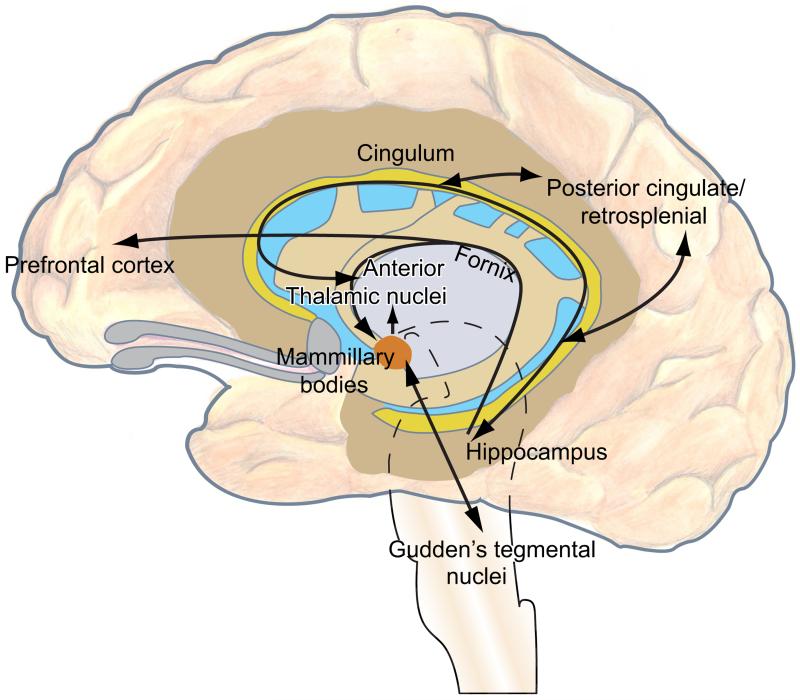
Although Papez only included hippocampal-mammillary body projections in his circuit, more recent variants also include the direct hippocampal-anterior thalamic projections (Fig. 3). As with the mammillary body projections, the anterior thalamic projections also arise from the subiculum but from a different cell population (Aggleton et al., 2005; Ishizuka, 2001; Wright et al., 2010; Fig. 2). The hippocampal-mammillary body projections are purely fornical (see Fig. 2); however, the projections to the anterior thalamic nuclei also have a nonfornical component (Dillingham et al., in press; Saunders et al., 2005).
FIGURE 3.
Updated Papez circuit. The original circuit has been modified to include the direct hippocampal-anterior thalamic nuclei connections. The mammillary body connections with Gudden’s tegmental nuclei have also been included.
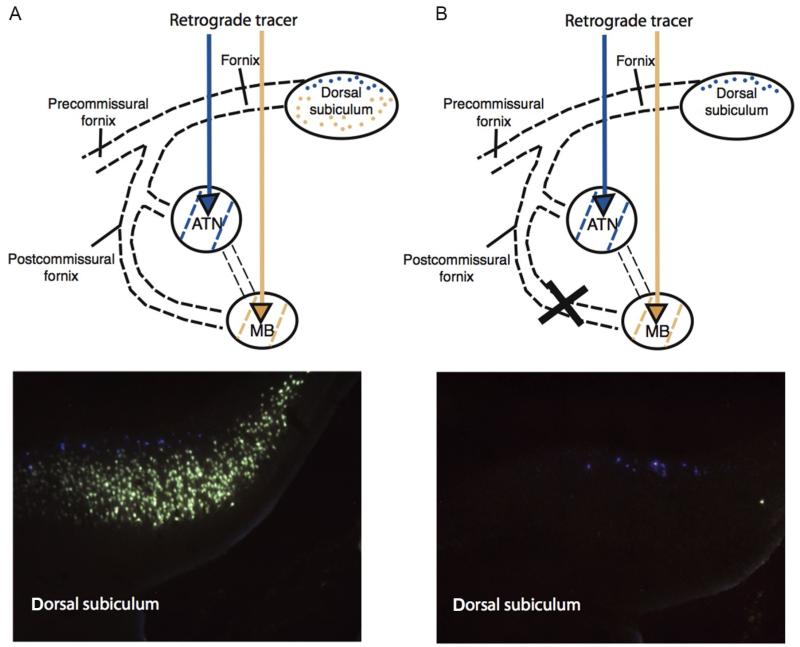
FIGURE 2.
Disconnection of the descending postcommissural fornix in rats. Rats underwent a control surgery (A) or a radiofrequency lesion of the descending postcommissural fornix (B). Fluorescent retrograde tracers were then injected into the mammillary bodies and anterior thalamic nuclei. In an intact rat (left), neurons containing the two retrograde tracers can clearly be seen. The deep subicular layers (Fast Blue (gray in the print version)) project to the anterior thalamic nuclei whereas the more superficial layers (Fluorogold (light gray in the print version)) project to the mammillary bodies. Following postcommissural fornix lesions, there is a complete disconnection of subicular-mammillary projections as shown by the loss of Fluorogold (light gray in the print version) label. This demonstrates that the hippocampal formation projects to the mammillary bodies solely by way of the fornix. Rats with these disconnection lesions are only very mildly impaired on spatial memory tasks (Vann, 2013; Vann et al., 2011).
The next stage in the circuit is the projection from the mammillary bodies to the anterior thalamic nuclei by way of the mammillothalamic tract (Cruce, 1975; Seki and Zyo, 1984; Vann et al., 2007). Despite some initial confusion (Clark, 1938), this pathway is now known to be unidirectional. The lateral mammillary nucleus projects bilaterally to the anterodorsal thalamic nuclei, while the larger medial mammillary nucleus projects unilaterally to the anteroventral and anteromedial thalamic nuclei (Cruce, 1975; Seki and Zyo, 1984; Vann et al., 2007).
The final stage of the original Papez circuit is the projection from the anterior thalamic nuclei to the cingulate gyrus (Papez, 1937). The retrosplenial cortex is typically viewed as the principal output within the cingulate gyrus. The anterior thalamic nuclei are reciprocally connected with the retrosplenial cortex (Shibata, 1993a; Sripanidkulchai and Wyss, 1986), and these connections are carried within the cingulum bundle (Domesick, 1970; Shibata, 1993b). Although not included in the original Papez circuit, there are also direct projections from the anterior thalamic nuclei to the hippocampal formation (Shibata, 1993a), anterior cingulate (Shibata, 1993b), and frontal cortex (Shibata and Kato, 1993). As such, the Papez circuit is well positioned to influence an extended network of regions and feed back into itself, both directly and indirectly.
3 THE PAPEZ CIRCUIT: FUNCTION
While we now know that structures within the Papez circuit are important for memory, it was not until the mid-twentieth century that this view became widely accepted. Up to this point, the hippocampus had been linked to a number of different functions including sensation, olfaction, and attention. Papez originally made the link with emotion as a result of his work on the rabies virus. The rabies virus principally affects the hippocampus, and Papez, therefore, attributed the emotive changes seen following this infection to the hippocampal pathology. Earlier authors had implicated the diencephalic region in emotive processes (Cannon, 1931; Penfield, 1934); Papez used his knowledge of the connectivity between the hippocampus, diencephalon, and associated cortical structures, to develop the neural bases of emotion. Despite Papez ascribing a role for the mammillary bodies and hippocampus in emotion, both these structures had previously been implicated in memory. Gudden had identified mammillary body atrophy in cases of Korsakoff’s syndrome (Gudden, 1896), a key feature of which is amnesia; this finding was subsequently followed up by Gamper (1928). Brown and Schafer first reported a memory disturbance following medial temporal lobe lesions in a rhesus monkey (Brown and Schäfer, 1888) and in 1900, Bechterew described a patient who became amnesic following a stroke; a postmortem revealed hippocampal pathology (Bechterew, 1900). Given the apparent importance of these structures for mnemonic function, particularly following Scoville and Milner’s seminal paper on H.M. (1957), the Papez circuit was adopted as a memory circuit (Aggleton and Brown, 1999; Barbizet, 1963; Benedek and Juba, 1941; Delay and Brion, 1969).
Support for the Papez “memory” circuit comes from evidence that each of its constituent neural structures appear to contribute to memory. As Barbizet described it “…bilateral lesions of the…circuit of Papez, will disturb the organization and recall of memory without interfering with immediate memory, the recognition of verbal and visual data, or motor abilities.…” (Barbizet, 1970, p. 63). Damage to the hippocampus, mammillary bodies, anterior thalamic nuclei, and cingulate gyrus (retrosplenial cortex) can result in anterograde amnesia in patients, i.e., an inability to lay down new episodic memories (e.g., Aggleton et al., 2005; Clarke et al., 1994; Dusoir et al., 1990; Harding et al., 2000; Hildebrandt et al., 2001; Kahn and Crosby, 1972; McDonald et al., 2001; Valenstein et al., 1987). Similarly, rats with lesions to the different components of this system are impaired on tests of spatial memory (Aggleton et al., 1986, 1995a; Harker and Whishaw, 2004; Parker and Gaffan, 1997a,b; Vann and Aggleton, 2002, 2003). Thus, evidence from studies into the functional properties of the various gray matter structures that constitute Papez circuit is consistent with the proposal that this is indeed a memory circuit. But what about the connections between these structures? If these structures work together as a functional unit, it would be expected that the tracts between these brain regions are as critical as the brain regions themselves. The fornix, mammillothalamic tract, and cingulum bundle are the principal white matter tracts within the Papez circuit and they will be considered in turn, starting with the cingulum bundle and working back to the fornix.
3.1 CINGULUM BUNDLE
The cingulum bundle was originally considered to be the principal association fiber pathway of cingulate gyrus (Kappers et al., 1936). In terms of the Papez circuit, the cingulum bundle is the route by which the Papez circuit was thought to influence cortical structures, carrying the fibers from the anterior thalamic nuclei to the cingulate gyrus. Cingulum bundle lesions in rats generally result in rather mild impairments on spatial tasks such as T-maze alternation (Aggleton et al., 1995b) and water maze tasks (Harker and Whishaw, 2004; Warburton et al., 1998), while sparing object location memory (Ennaceur et al., 1997). There are very few reported cases of patients with damage to the more posterior section of the cingulum bundle, i.e., that part most likely to disrupt the Papez circuit. One such reported case did have anterograde amnesia but, in addition to the cingulum bundle pathology, had damage to the adjacent retrosplenial cortex making it difficult to attribute the cause of the memory impairment (Valenstein et al., 1987).
The limited findings there are regarding the cingulum bundle generally appear to be consistent with the Papez circuit supporting memory, albeit, the deficits in rats are perhaps milder than would be expected. However, there are a number of other efferent and afferent fibers carried within the cingulum bundle (Schmahmann and Pandya, 2006) which make interpretation of the lesion effects very difficult. Furthermore, the cingulum bundle contains many short association fibers and very few fibers transverse the length of the cingulum bundle (Jones et al., 2013); the effects of cingulum bundle lesions would likely depend very much on the location and extent of the lesion. It is, therefore, possible that the cingulum lesions in rats did not completely disconnect the anterior thalamic afferents and may have underestimated the importance of this pathway for memory. In any case, the importance of anterior thalamic projections for normal retrosplenial functioning has been repeatedly shown. Anterior thalamic lesions can result in retrosplenial dysfunction such as reduction in immediate-early gene expression (Dumont et al., 2012; Jenkins et al., 2002a,b), decrease in spine density (Harland et al., 2014), and disruption of long-term depression (Garden et al., 2009).
3.2 MAMMILLOTHALAMIC TRACT
Within the Papez circuit, the mammillary bodies are considered to be relay nuclei, passing information from the hippocampal formation to the anterior thalamic nuclei, by way of the mammillothalamic tract. All neurons within the mammillary bodies are thought to project to the anterior thalamic nuclei (Guillery, 1955; Vann et al., 2007) consistent with the mammillary bodies acting as a relay. Furthermore, the reported lack of interneurons in the rodent mammillary bodies would again reinforce the notion of a relay structure (Veazey et al., 1982). However, there are interneurons in human and nonhuman primate mammillary bodies (Bernstein et al., 2007; Dixon et al., 2004). The medial mammillary bodies reach their developmental peak in primates (Clark, 1938), so it is possible that the interneurons are species specific; alternatively, interneurons in rodents may have been missed due to methodological reasons (Dixon et al., 2004).
If the mammillary nuclei are relay structures and their principal role is to transfer information to the anterior thalamic nuclei, mammillothalamic tract lesions should be functionally equivalent to mammillary body lesions. We tested this prediction in a cohort of rats with either neurotoxic mammillary body lesions or radiofrequency mammillothalamic tract lesions (Vann and Aggleton, 2003). Across several spatial tasks, the performance of these lesion groups was remarkably similar and both groups were impaired relative to surgical control rats (Vann and Aggleton, 2003). It does, therefore, appear that the mammillary bodies contribute to mnemonic function by way of the mammillothalamic tract. This conclusion is consistent with findings from patients where damage to the mammillothalamic tract is the key predictor as to whether patients with thalamic infarcts suffer memory problems (Carlesimo et al., 2007; Van der Werf et al., 2000, 2003; von Cramon et al., 1985).
The mammillothalamic tract inputs seem to be critical for normal anterior thalamic functioning. Anterior thalamic lesions and mammillothalamic tract lesions both disrupt performance on spatial memory tasks (e.g., Aggleton et al., 1995a, 1996; Byatt and Dalrymple-Alford, 1996; Mair et al., 2003; Nelson and Vann, 2014; Vann, 2013; Vann and Aggleton, 2003). Moreover, both lesions result in a remarkably similar pattern of hypoactivity, as measured by the immediate-early gene c-fos, in an array of distal brain regions (e.g., Jenkins et al., 2002b; Vann and Albasser, 2009). Similarly, in a group of patients with Wernicke’s encephalopathy, the strength of functional connectivity between the mammillary bodies and anterior thalamic nuclei correlated with memory performance (Kim et al., 2009). These data are consistent with the notion that mammillary bodies contribute to memory via their projections to the anterior thalamic nuclei. A further implication is that anterior thalamic lesion effects are to some extent driven by the loss of their inputs from the mammillary bodies.
3.3 POSTCOMMISSURAL FORNIX
Central to the current “extended hippocampal” memory models is the notion that the direct projections from the hippocampal formation to the mammillary bodies, via the fornix, are critical for memory (Aggleton and Brown, 1999). Fornix lesions can result in memory impairments in both patients and animals (e.g., Aggleton et al., 2000; Cassel et al., 1997, 1998; McMackin et al., 1995; Park et al., 2000; Vann et al., 2008), and using tractography, it has been shown that fornix integrity is linked to memory and scene perception (Metzler-Baddeley et al., 2012; Postans et al., 2014; Rudebeck et al., 2009). Not only do these findings implicate the Papez circuit in memory, they have also been taken as indirect evidence that, in terms of memory, the hippocampus drives the medial diencephalon. This assumption could only be made if the fornix carried projections solely to the medial diencephalon, which is not the case. At the level of the anterior commissure, half the fibers in the fornix continue forward to form the precommissural fornix (Nauta, 1956; Poletti and Creswell, 1977; Powell et al., 1957; Raisman et al., 1966; Simpson, 1952) and innervate areas including the basal forebrain (including septum), ventral striatum, and prefrontal cortex (Nauta, 1956; Poletti and Creswell, 1977). The precommissural fornix also comprises the substantial projections from the septum to the hippocampus (Votaw and Lauer, 1963). Thus, only half the fibers in the fornix are in fact relevant to the functions of the Papez circuit. Even within the postcommissural fornix, about one half to two-thirds of the fibers do not reach the mammillary bodies (e.g., Powell et al., 1957). A large component directly innervates the anterior thalamic nuclei and the remaining fibers then descend to innervate the mammillary bodies (Guillery, 1956) among other regions (Kishi et al., 2000; Sprague and Meyer, 1950). Therefore, in terms of hippocampal-mammillary projections, approximately one quarter of fornical fibers are actually relevant. In order to assess the importance of hippocampal-mammillary projections for memory, this subcomponent of fornix fibers become crucial. While the cornerstone of all of standard memory models is the notion that the direct projections from the hippocampus to the mammillary bodies, via the fornix, are critical for memory, remarkably this hypothesis had never been tested directly until recently.
With three separate cohorts of rats, we have now shown that lesions of the descending postcommissural fornix, i.e., that part of the fornix that innervates the mammillary bodies, have little, if no effect on tests of spatial memory (Vann, 2013; Vann et al., 2011). These are the same tests that are sensitive to mammillary body, mammillothalamic tract, anterior thalamic, and hippocampal lesions (e.g., Aggleton et al., 1995a, 1996; Vann and Aggleton, 2003). This result is most surprising given this pathway is historically seen as the backbone of the Papez circuit. Why might these results have come about? Perhaps the lesions were incomplete leaving a residual pathway intact, which was sufficient to support these functions? The use of retrograde tracers confirmed the completeness of the disconnection (Fig. 2). Furthermore, loss of fornical fibers results in mammillary body shrinkage (reflecting a loss of fibers and not neurons), and the mammillary body shrinkage following descending postcommissural fornix lesions was comparable to that found after complete fornix lesions (Vann, 2013; Vann et al., 2011). A second possibility is that the direct projection to the anterior thalamic nuclei from the hippocampal formation makes the indirect projection, via the mammillary bodies, redundant. There are a number of reasons why this explanation is unlikely. As previously mentioned, the projections to the mammillary bodies and anterior thalamic nuclei arise from different subiculum populations with no overlap in terms of their origin (Ishizuka, 2001; Wright et al., 2010; Yoder and Taube, 2011; Fig. 2). Moreover, the electrophysiological properties of the anterior thalamic nuclei inputs from the mammillary bodies and fornix are antagonistic (Tsanov et al., 2011), suggesting complementary rather than duplicate functions. If the anterior thalamic nuclei received the same information from both routes, and it was this information that was needed to support memory, then mammillothalamic tract lesions would be expected to have comparable effects to postcommissural fornix lesion—i.e., mild or no effect. Yet, as already noted, mammillary body and mammillothalamic tract lesions produce consistent spatial memory impairments that are markedly more severe than the effects of descending postcommissural fornix lesions. This leaves us with the possibility that for certain classes of memory, at least, the mammillary bodies’ contribution to memory is independent of their subicular complex inputs. A further implication is that the hippocampal-mammillary body projections are not required for at least some classes of memory.
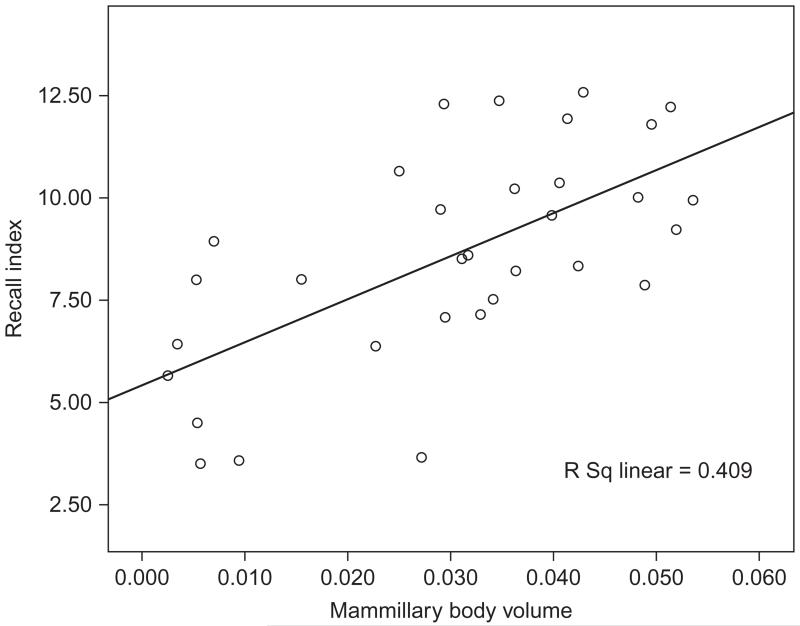
While we are able to selectively disconnect those hippocampal-mammillary body projections in rats, it is much harder to assess this specific component of the system in humans. However, there is some evidence from human studies that mammillary body function might reflect more than their hippocampal input. Disconnecting the fornix results in a maximum 50% reduction in mammillary body volume, principally reflecting a loss of fibers (Loftus et al., 2000; Vann, 2013; Vann et al., 2011; Zola-Morgan et al., 1989). It is, therefore, of particular interest to look at those patients whose mammillary bodies appear to be reduced by more than 50%, as these cases may reveal a contribution beyond the loss of fornical inputs. We were able to address this issue by testing a large group of patients who had undergone surgery for the removal of colloid cysts in the third ventricle (Tsivilis et al., 2008). Patients were selected for the study purely on their etiology and not on the basis of any particular neuropsychological profile. Moreover, neuropsychological testing was carried out without prior knowledge of neuropathology. Volumetric estimates were derived for 13 regions of interest including the mammillary bodies, fornix, hippocampus, parahippocampal cortex, and prefrontal cortex (Tsivilis et al., 2008). In the group of 38 patients, the mammillary bodies were the only structure to consistently correlate with memory performance (Fig. 4). Indeed, the mammillary body volume significantly correlated with performance on 13 of the 14 tests of recollective memory. Those patients with the smallest mammillary bodies were significantly more impaired on tests of memory (Tsivilis et al., 2008; Vann et al., 2009; Fig. 4). Remarkably, at least 9 of the 11 patients with the smallest mammillary bodies had a greater than 50% reduction in mammillary body volume (Tsivilis et al., 2008), i.e., performance of the patients most impaired on tests of recall did not simply reflect the loss of fornical inputs.
FIGURE 4.
Scatterplot showing the positive relationship between mammillary body volume (intracranial normalized) and index of recollective memory. Pearson correlation was significant (p<0.001).
Data are from Tsivilis et al. (2008).
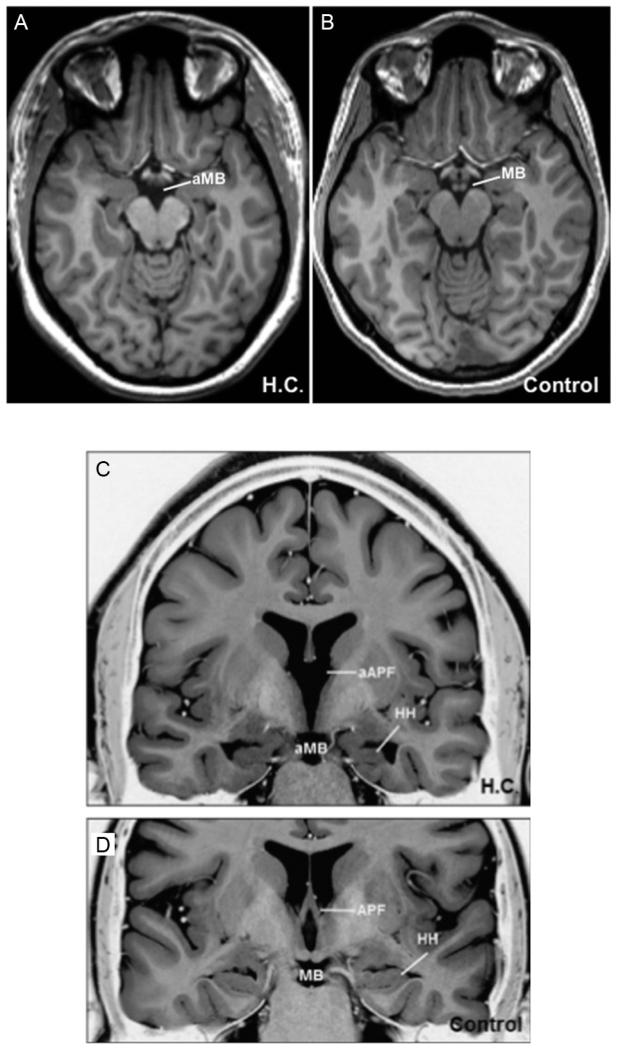
Another particularly striking case is that of patient H.C., a well-studied developmental amnesic. Rosenbaum et al. (2014) recently reported a previously unnoticed finding that H.C. has a complete absence of the mammillary bodies (Fig. 5). H.C. has atrophic and abnormally orientated hippocampi, which were originally thought to be a result of prematurity with associated respiratory difficulties. However, the absence of mammillary bodies cannot simply be a result of the reduced hippocampal volume as again, this would only explain a maximum 50% reduction in mammillary body volume. The mammillothalamic tract also appears to be absent bilaterally (Rosenbaum et al., 2014). Together, this pattern of neuropathology is consistent with a prenatal abnormality within the Papez circuit. There are some aspects of H.C.’s memory impairment that are more in keeping with findings from Korsakoff syndrome patients (Cermak et al., 1974) than typically associated with developmental amnesics (Gardiner et al., 2006; Meier et al., 2009). For example, H.C.’s recognition memory is not improved by a levels-of-processing manipulation (Rosenbaum et al., 2011); furthermore, H.C.’s cued recall performance can be benefitted by spaced (as opposed to massed) repetition (Green et al., 2014; Rosenbaum et al., 2014). It, therefore, appears that some aspects of H.C.’s memory impairments may well reflect a loss beyond what would be expected on the basis of hippocampal pathology and the loss of hippocampal-mammillary body projections alone (Rosenbaum et al., 2014).
FIGURE 5.
MR images of patient H.C., a developmental amnesic with congenital absence of the mammillary bodies. Axial T1 images showing absent mammillary bodies (aMB) in H.C. (A) compared to a control (B). Inverted T2 coronal images showing absent mammillary bodies and pillar of the fornix (aPF) in H.C. (C) compared to a control (D).
Reprinted with permission from Rosenbaum et al. (2014).
3.4 NONHIPPOCAMPAL INPUTS TO THE MAMMILLARY BODIES
If the mammillary bodies make a contribution to memory that is independent of their hippocampal inputs, this naturally raises the question as to which inputs are driving mammillary body function. The mammillary bodies do have connections with other structures including dense reciprocal connections with Gudden’s tegmental nuclei by way of the mammillary peduncle and mammillotegmental tract, as well as a sizeable input from the prefrontal cortex (Allen and Hopkins, 1989). However, until recently, very little was known about the functional significance of these nonhippocampal inputs to the mammillary bodies. We have shown that selective lesions to the ventral tegmental nucleus of Gudden produce robust deficits on the very same spatial tasks that are known to be sensitive to the effects of mammillary body, mammillothalamic, and hippocampal damage (Vann, 2009, 2013). Equally striking is the finding that these deficits are more marked and enduring than found after lesions of the descending postcommissural fornix (Vann, 2013). One earlier report had also found that descending postcommissural fornix transection has only a marginal effect on an operant task (differential reinforcement for low rats of response) that is sensitive to mammillary body damage (Tonkiss and Rawlins, 1992), again supporting a contribution from nonhippocampal inputs. The implication of these results is manifest: not only is the ventral tegmental nucleus of Gudden able to support mammillary body function in the absence of their hippocampal inputs, it is these inputs from the limbic midbrain, rather than the hippocampus, are vital to mammillary bodies’ contribution to memory. That the mammillary bodies and ventral tegmental nucleus of Gudden are functionally interrelated is also supported by the finding that ventral tegmental nucleus of Gudden lesions produce equivalent effects on functional markers in the same distal brain regions as is found after mammillothalamic tract lesions, but not descending postcommissural fornix lesions (Vann, 2013). The evidence that inputs from the ventral tegmental nucleus of Gudden are vital for medial mammillary body function mirrors findings that the lateral mammillary are functionally reliant on projections from the dorsal tegmental nucleus (e.g., Bassett and Taube, 2001; Bassett et al., 2007).
4 MEDIAL DIENCEPHALIC-TEMPORAL LOBE INTERACTIONS
Traditional models emphasize the hippocampal inputs to the mammillary bodies within an extended memory system emanating from the hippocampus. If the mammillary bodies are more than a hippocampal relay and make a contribution to memory that is dependent on their inputs from the limbic midbrain rather than the hippocampus (Dillingham et al., 2014; Vann, 2013; Vann et al., 2011), then these models require revision. Evidence from cross-lesions studies would suggest that the hippocampus and anterior thalamic nuclei are functionally interdependent (Henry et al., 2004; Warburton et al., 2001), but it is possible that the hippocampus depends on the ascending inputs from the medial diencephalon, i.e., the opposite direction to that traditionally believed. So how might projections from the medial diencephalon, and mammillary bodies in particular, contribute to hippocampal function? One suggestion is that the ventral tegmental nucleus of Gudden, via their connections with the mammillary bodies, may influence hippocampal function through the regulation of theta activity (Kocsis et al., 2001; Vertes et al., 2004). Indeed extensive mammillary body lesions attenuate both the frequency and amplitude of theta cell firing in the hippocampus (Sharp and Koester, 2008a). A further mechanism through which the mammillary bodies can modulate hippocampal activity is the head-direction system. There is now considerable evidence that interactions between the dorsal tegmental nucleus of Gudden and lateral mammillary nuclei play a critical role in both the generation and propagation of the head-direction signal (Bassett et al., 2007; Blair et al., 1999; Taube, 2007). Thus, there are at least two parallel but separate routes through the mammillary bodies that can influence mnemonic processing within the hippocampus and other medial diencephalic structures (Dillingham et al., 2014).
There is further evidence suggesting the hippocampus is dependent on inputs from the medial diencephalon: lesions of the anterior thalamic nucleus and/or mammillothalamic tract lesions have been shown to reduce the expression of the immediate-early gene c-fos in the hippocampus (Jenkins et al., 2002b; Vann and Albasser, 2009), attenuate hippocampal CREB phosphorylation (Dumont et al., 2012), decrease spinal density of cells in CA1 (Harland et al., 2014), and disrupt task-dependent increases in hippocampal acetylcholine levels (Savage et al., 2003; Vetreno et al., 2008). However, using other measures, hippocampal function seems unaffected by anterior thalamic lesions. For example, hippocampal expression of another immediate-early gene, zif268, is not disrupted by anterior thalamic lesions (Dumont et al., 2012). Hippocampal levels of cytochrome oxidase, a measure of metabolic activity, are equally unchanged by anterior thalamic nuclei damage (Mendez-Lopez et al., 2013). Moreover, lesions to the mammillothalamic tract or mammillary bodies, which reliably impair tests of spatial memory (e.g., Nelson and Vann, 2014; Vann, 2013; Vann and Aggleton, 2003) and disrupt cortical head-direction signaling (Sharp and Koester, 2008b), nonetheless leave hippocampal place cell firing intact (Sharp and Koester, 2008b). The clinical picture is equally mixed, with some reports that medial diencephalic pathology can lead to hippocampal hypoactivity (e.g.,Caulo et al., 2005; Kapur et al., 1994; Reed et al., 2003), while other neuroimaging studies suggest that the hippocampus can appear functionally intact following pathology in the medial diencephalon (e.g., Martin et al., 1992; Ozyurt et al., 2014; Paller et al., 1997). The data, therefore, are currently inconclusive. There is some evidence that the hippocampus is functionally dependent on its inputs from the medial diencephalon but also instances of apparent “normal” hippocampal function despite the presence of marked memory impairments.
The proponents of the “extended hippocampal memory system” argue that damage to the different components of the Papez circuit should result in similar impairments consistent with a unitary memory system (e.g., Aggleton and Brown, 1999; Warrington and Weiskrantz, 1982). However, others have questioned what the benefits of such a memory circuit might be and suggested that the medial diencephalon and medial temporal lobe support different aspects of memory (e.g., Parkin, 1984, 1996; Squire, 1981). Diencephalic amnesia has been proposed to specifically reflect an impairment in encoding (Butters and Cermak, 1980; Cermak et al., 1980; Huppert and Piercy, 1977; Sweeney-Reed et al., 2014; Vann and Aggleton, 2003; Wetzel and Squire, 1980); conversely, differences in forgetting rates between diencephalic and medial temporal lobe amnesics have been taken to suggest that the medial temporal lobe is particularly important for consolidation (e.g., Parkin, 1992; Squire, 1981) (but see Aggleton, 2008; Freed and Corkin, 1988; Freed et al., 1987; Kopelman, 2002; McKee and Squire, 1992; Parkin, 1992). A further dissociation between diencephalic and medial temporal lobe amnesia has been reported for temporal order memory, with diencephalic amnesics performing disproportionately worse on this aspect of memory (Hunkin and Parkin, 1993; Hunkin et al., 1994; Kopelman et al., 1997; Squire, 1982). As studies involving diencephalic amnesics typically include Korsakoff patients, the argument has been made that the poor performance on temporal memory reflects the additional frontal pathology that can be found in this patient group (Aggleton and Brown, 1999; Squire, 1982). However, patients with lesions restricted to the medial diencephalon also show impaired temporal memory (Hildebrandt et al., 2001; Parkin and Hunkin, 1993). Furthermore, anterior thalamic lesions in rats impair disrupt aspects of temporal processing (Dumont and Aggleton, 2013; Wolff et al., 2006), and unpublished findings from our laboratory indicate that these processes depend critically on the mammillary body inputs to the anterior thalamus (see also Tonkiss and Rawlins, 1992). So it appears that the temporal memory impairments cannot simply be attributed to co-occurring frontal pathology, and it may be that the medial diencephalon and medial temporal lobe make separate but interconnected contributions to memory.
5 CONCLUSIONS
Ever since Papez proposed his influential circuit, the mammillary bodies have been viewed principally in terms of their hippocampal inputs. However, for both the medial and lateral mammillary bodies, their ascending inputs from the tegmental nuclei appear to be, functionally, more important than those from the hippocampal formation (e.g., Dillingham et al., 2014; Goodridge and Taube, 1997; Stackman and Taube, 1997; Taube et al., 1996; Tonkiss and Rawlins, 1992; Vann, 2013). These findings are inconsistent with those models of memory based on the Papez circuit and raise new questions as to how the medial diencephalon and medial temporal lobe might interact to support memory. From cross-lesion studies, it is clear that the anterior thalamic nuclei and hippocampus are interdependent, for at least some aspects of memory (Henry et al., 2004; Warburton et al., 2001). However, instead of the hippocampus driving the medial diencephalon, as previously thought, the reverse might be true, i.e., the hippocampus is dependent on inputs from the medial diencephalon. Nevertheless, there is also evidence that the hippocampus is, according to some measures, functionally intact following medial diencephalic lesions, despite the presence of memory impairments. It is clear that further research is needed to consolidate these seemingly disparate findings, but it does raise the possibility that in some instances the medial diencephalon and hippocampus make distinct contributions to memory. Medial diencephalic amnesia has been known about for longer than medial temporal lobe amnesia, but due to the overwhelming interest in the hippocampus, it has become the older, less-fashionable relation of medial temporal lobe amnesia. However, if we are to truly understand the neural bases of memory, we must look beyond the hippocampus and refocus on these other interconnected structures, whose contribution to memory is often woefully overlooked.
ACKNOWLEDGMENTS
S.D.V. is funded by a Wellcome Trust Senior Research Fellowship in Basic Biomedical Science (Grant number WT090954AIA). Thanks to Lorraine Woods for help with illustrations and John Aggleton for helpful discussion.
REFERENCES
- Aggleton JP. EPS Mid-Career Award 2006. Understanding anterograde amnesia: disconnections and hidden lesions. Q. J. Exp. Psychol. (Hove) 2008;61:1441–1471. doi: 10.1080/17470210802215335. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 1999;22:425–444. discussion 444-489. [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav. Brain Res. 1986;19:133–146. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Neave N, Nagle S, Hunt PR. A comparison of the effects of anterior thalamic, mammillary body and fornix lesions on reinforced spatial alternation. Behav. Brain Res. 1995a;68:91–101. doi: 10.1016/0166-4328(94)00163-a. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Neave N, Nagle S, Sahgal A. A comparison of the effects of medial prefrontal, cingulate cortex, and cingulum bundle lesions on tests of spatial memory: evidence of a double dissociation between frontal and cingulum bundle contributions. J. Neurosci. 1995b;15:7270–7281. doi: 10.1523/JNEUROSCI.15-11-07270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Nagle S, Neave N. The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behav. Brain Res. 1996;81:189–198. doi: 10.1016/s0166-4328(96)89080-2. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, McMackin D, Carpenter K, Hornak J, Kapur N, Halpin S, Wiles CM, Kamel H, Brennan P, Carton S, Gaffan D. Differential cognitive effects of colloid cysts in the third ventricle that spare or compromise the fornix. Brain. 2000;123:800–815. doi: 10.1093/brain/123.4.800. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43:1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Saunders RC. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur. J. Neurosci. 2005;22:2519–2530. doi: 10.1111/j.1460-9568.2005.04450.x. [DOI] [PubMed] [Google Scholar]
- Allen GV, Hopkins DA. Mamillary body in the rat: topography and synaptology of projections from the subicular complex, prefrontal cortex, and midbrain tegmentum. J. Comp. Neurol. 1989;286:311–336. doi: 10.1002/cne.902860303. [DOI] [PubMed] [Google Scholar]
- Barbizet J. Defect of memorizing of hippocampal-mammillary origin: a review. J. Neurol. Neurosurg. Psychiatry. 1963;26:127–135. doi: 10.1136/jnnp.26.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbizet J. Human Memory and Its Pathology. W.H. Freeman; San Francisco: 1970. [Google Scholar]
- Bassett JP, Taube JS. Neural correlates for angular head velocity in the rat dorsal tegmental nucleus. J. Neurosci. 2001;21:5740–5751. doi: 10.1523/JNEUROSCI.21-15-05740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JP, Tullman ML, Taube JS. Lesions of the tegmentomammillary circuit in the head direction system disrupt the head direction signal in the anterior thalamus. J. Neurosci. 2007;27:7564–7577. doi: 10.1523/JNEUROSCI.0268-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechterew W. Demonstration eines Gehirns mit Zerstörung der vorderen und inneren Teile der Hirnrinde beider Schläfenlappen. Neurol. Zentralbl. 1900;19:990–991. [Google Scholar]
- Benedek L, Juba A. Uber das anomische substrat des Korsakowschen Syndromes. Schweizerisches Arch. Psychiatr. Nervenkr. 1941;46:174–184. [Google Scholar]
- Bernstein HG, Krause S, Krell D, Dobrowolny H, Wolter M, Stauch R, Ranft K, Danos P, Jirikowski GF, Bogerts B. Strongly reduced number of parvalbumin-immunoreactive projection neurons in the mammillary bodies in schizophrenia: further evidence for limbic neuropathology. Ann. N.Y. Acad. Sci. 2007;1096:120–127. doi: 10.1196/annals.1397.077. [DOI] [PubMed] [Google Scholar]
- Blair HT, Cho JW, Sharp PE. The anterior thalamic head-direction signal is abolished by bilateral but not unilateral lesions of the lateral mammillary nucleus. J. Neurosci. 1999;19:6673–6683. doi: 10.1523/JNEUROSCI.19-15-06673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Schäfer EA. An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philos. Trans. R. Soc. Lond. B. 1888;179:303–327. [Google Scholar]
- Butters N, Cermak LS. Alcoholic Korsakoff’s Syndrome: An Information-Processing Approach to Amnesia. Academic Press; New York/London: 1980. [Google Scholar]
- Byatt G, Dalrymple-Alford JC. Both anteromedial and anteroventral thalamic lesions impair radial-maze learning in rats. Behav. Neurosci. 1996;110:1335–1348. doi: 10.1037//0735-7044.110.6.1335. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Again the James-Lange and the thalamic theories of emotion. Psychol. Rev. 1931;38:281–295. [Google Scholar]
- Carlesimo GA, Serra L, Fadda L, Cherubini A, Bozzali M, Caltagirone C. Bilateral damage to the mammillothalamic tract impairs recollection but not familiarity in the recognition process: a single case investigation. Neuropsychologia. 2007;45:2467–2479. doi: 10.1016/j.neuropsychologia.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Cassel JC, Duconseille E, Jeltsch H, Will B. The fimbria-fornix/cingular bundle pathways: a review of neurochemical and behavioural approaches using lesions and transplantation techniques. Prog. Neurobiol. 1997;51:663–716. doi: 10.1016/s0301-0082(97)00009-9. [DOI] [PubMed] [Google Scholar]
- Cassel JC, Cassel S, Galani R, Kelche C, Will B, Jarrard L. Fimbria-fornix vs selective hippocampal lesions in rats: effects on locomotor activity and spatial learning and memory. Neurobiol. Learn. Mem. 1998;69:22–45. doi: 10.1006/nlme.1997.3807. [DOI] [PubMed] [Google Scholar]
- Caulo M, Van Hecke J, Toma L, Ferretti A, Tartaro A, Colosimo C, Romani GL, Uncini A. Functional MRI study of diencephalic amnesia in Wernicke-Korsakoff syndrome. Brain. 2005;128:1584–1594. doi: 10.1093/brain/awh496. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Butters N, Moreines J. Some analyses of the verbal encoding deficit of alcoholic Korsakoff patients. Brain Lang. 1974;1:141–150. [Google Scholar]
- Cermak LS, Uhly B, Reale L. Encoding specificity in the alcoholic Korsakoff patient. Brain Lang. 1980;11:119–127. doi: 10.1016/0093-934x(80)90115-7. [DOI] [PubMed] [Google Scholar]
- Clark WELG. The Hypothalamus: Morphological, Functional, Clinical and Surgical Aspects. Oliver and Boyd; Edinburgh: 1938. [Google Scholar]
- Clarke S, Assal G, Bogousslavsky J, Regli F, Townsend DW, Leenders KL, Blecic S. Pure amnesia after unilateral left polar thalamic infarct: topographic and sequential neuropsychological and metabolic (PET) correlations. J. Neurol. Neurosurg. Psychiatry. 1994;57:27–34. doi: 10.1136/jnnp.57.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruce JA. An autoradiographic study of the projections of the mammillothalamic tract in the rat. Brain Res. 1975;85:211–219. doi: 10.1016/0006-8993(75)90072-4. [DOI] [PubMed] [Google Scholar]
- Delay J, Brion S. Le Syndrome de Korsakoff. Masson; Paris: 1969. [Google Scholar]
- Dillingham CM, Frizzati A, Nelson AJ, Vann SD. How do mammillary body inputs contribute to anterior thalamic function? Neurosci. Biobehav. Rev. 2014 doi: 10.1016/j.neubiorev.2014.07.025. http://dx.doi.org/10.1016/j.neubiorev.2014.07.025, [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillingham CM, Erichsen JT, O’Mara SM, Aggleton JP, Vann SD. Fornical and non-fornical projections from the rat hippocampal formation to the anterior thalamic nuclei. Hippocampus. 2015 doi: 10.1002/hipo.22421. http://dx.doi.org/10.1002/hipo.22421, [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon G, Garrick T, Whiteman I, Sarris M, Sithamparanathan S, Harper CG. Characterization of gabaergic neurons within the human medial mamillary nucleus. Neuroscience. 2004;127:365–372. doi: 10.1016/j.neuroscience.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Domesick VB. The fasciculus cinguli in the rat. Brain Res. 1970;20:19–32. doi: 10.1016/0006-8993(70)90150-2. [DOI] [PubMed] [Google Scholar]
- Dumont JR, Aggleton JP. Dissociation of recognition and recency memory judgments after anterior thalamic nuclei lesions in rats. Behav. Neurosci. 2013;127:415–431. doi: 10.1037/a0032750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont JR, Amin E, Poirier GL, Albasser MM, Aggleton JP. Anterior thalamic nuclei lesions in rats disrupt markers of neural plasticity in distal limbic brain regions. Neuroscience. 2012;224:81–101. doi: 10.1016/j.neuroscience.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusoir H, Kapur N, Byrnes DP, McKinstry S, Hoare RD. The role of diencephalic pathology in human memory disorder. Evidence from a penetrating paranasal brain injury. Brain. 1990;113:1695–1706. doi: 10.1093/brain/113.6.1695. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp. Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Freed DM, Corkin S. Rate of forgetting in H.M.: 6-month recognition. Behav. Neurosci. 1988;102:823–827. doi: 10.1037//0735-7044.102.6.823. [DOI] [PubMed] [Google Scholar]
- Freed DM, Corkin S, Cohen NJ. Forgetting in H.M.: a second look. Neuropsychologia. 1987;25:461–471. doi: 10.1016/0028-3932(87)90071-6. [DOI] [PubMed] [Google Scholar]
- Gamper E. Zur Frage der Polioencephalitis der chronischen Alkoholiker. Anatomische Befunde beim chronischem Korsakow und ihre Beziehungen zum klinischen Bild. Deutsche Z. Nervenheilkd. 1928;102:122–129. [Google Scholar]
- Garden DL, Massey PV, Caruana DA, Johnson B, Warburton EC, Aggleton JP, Bashir ZI. Anterior thalamic lesions stop synaptic plasticity in retrosplenial cortex slices: expanding the pathology of diencephalic amnesia. Brain. 2009;132:1847–1857. doi: 10.1093/brain/awp090. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Brandt KR, Vargha-Khadem F, Baddeley A, Mishkin M. Effects of level of processing but not of task enactment on recognition memory in a case of developmental amnesia. Cogn. Neuropsychol. 2006;23:930–948. doi: 10.1080/02643290600588442. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Taube JS. Interaction between the postsubiculum and anterior thalamus in the generation of head direction cell activity. J. Neurosci. 1997;17:9315–9330. doi: 10.1523/JNEUROSCI.17-23-09315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Weston T, Wiseheart M, Rosenbaum RS. Long-term spacing effect benefits in developmental amnesia: case experiments in rehabilitation. Neuropsychology. 2014;28:685–694. doi: 10.1037/neu0000070. [DOI] [PubMed] [Google Scholar]
- Gudden B.A.v. Beitrag zur Kenntniss des Corpus mammillare und der sogenannten Schenkel des Fornix. Arch. Psychiat. Nervenkr. 1881;11:428–452. [Google Scholar]
- Gudden H. Klinische und anatomische Beitrage zur Kenntnis der multiplen Alkohol-neuritis nebst Bernerkungen uber die Regenerationsvorgange im peripheren Nervensystem. Arch. Psychiatr. 1896;28:643–741. [Google Scholar]
- Guillery RW. A quantitative study of the mamillary bodies and their connexions. J. Anat. 1955;89:19–32. [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. Degeneration in the post-commissural fornix and the mamillary peduncle of the rat. J. Anat. 1956;90:350–370. [PMC free article] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123:141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. Impaired place navigation in place and matching-to-place swimming pool tasks follows both retrosplenial cortex lesions and cingulum bundle lesions in rats. Hippocampus. 2004;14:224–231. doi: 10.1002/hipo.10159. [DOI] [PubMed] [Google Scholar]
- Harland BC, Collings DA, McNaughton N, Abraham WC, Dalrymple-Alford JC. Anterior thalamic lesions reduce spine density in both hippocampal CA1 and retrosplenial cortex, but enrichment rescues CA1 spines only. Hippocampus. 2014;24:1232–1247. doi: 10.1002/hipo.22309. [DOI] [PubMed] [Google Scholar]
- Henry J, Petrides M, St-Laurent M, Sziklas V. Spatial conditional associative learning: effects of thalamo-hippocampal disconnection in rats. Neuroreport. 2004;15:2427–2431. doi: 10.1097/00001756-200410250-00025. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Muller S, Bussmann-Mork B, Goebel S, Eilers N. Are some memory deficits unique to lesions of the mammillary bodies? J. Clin. Exp. Neuropsychol. 2001;23:490–501. doi: 10.1076/jcen.23.4.490.1234. [DOI] [PubMed] [Google Scholar]
- Hunkin NM, Parkin AJ. Recency judgements in Wernicke-Korsakoff and post-encephalitic amnesia: influences of proactive interference and retention interval. Cortex. 1993;29:485–499. doi: 10.1016/s0010-9452(13)80255-9. [DOI] [PubMed] [Google Scholar]
- Hunkin NM, Parkin AJ, Longmore BE. Aetiological variation in the amnesic syndrome: comparisons using the list discrimination task. Neuropsychologia. 1994;32:819–825. doi: 10.1016/0028-3932(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Piercy M. Recognition memory in amnesic patients: a defect of acquisition? Neuropsychologia. 1977;15:643–652. doi: 10.1016/0028-3932(77)90069-0. [DOI] [PubMed] [Google Scholar]
- Ishizuka N. Laminar organization of the pyramidal cell layer of the subiculum in the rat. J. Comp. Neurol. 2001;435:89–110. doi: 10.1002/cne.1195. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Dias R, Amin E, Aggleton JP. Changes in Fos expression in the rat brain after unilateral lesions of the anterior thalamic nuclei. Eur. J. Neurosci. 2002a;16:1425–1432. doi: 10.1046/j.1460-9568.2002.02211.x. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Dias R, Amin E, Brown MW, Aggleton JP. Fos imaging reveals that lesions of the anterior thalamic nuclei produce widespread limbic hypoactivity in rats. J. Neurosci. 2002b;22:5230–5238. doi: 10.1523/JNEUROSCI.22-12-05230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Mamillary or mammillary? What’s in an “m”? J. Hist. Neurosci. 2011;20:152–159. doi: 10.1080/0964704X.2010.533089. [DOI] [PubMed] [Google Scholar]
- Jones DK, Christiansen KF, Chapman RJ, Aggleton JP. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia. 2013;51:67–78. doi: 10.1016/j.neuropsychologia.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn EA, Crosby EC. Korsakoff’s syndrome associated with surgical lesions involving the mammillary bodies. Neurology. 1972;22:117–125. doi: 10.1212/wnl.22.2.117. [DOI] [PubMed] [Google Scholar]
- Kappers CUA, Crosby EC, Huber GC. The Comparative Anatomy of the Nervous System of Vertebrates, Including Man. Macmillan Co.; New York: 1936. [Google Scholar]
- Kapur N, Scholey K, Moore E, Barker S, Mayes A, Brice J, Fleming J. The mammillary bodies revisited: their role in human memory functioning. In: Cermak LS, editor. Neuropsychological Explorations of Memory and Cognition: Essays in Honor of Nelson Butters. Critical Issues in NeuropsychologyPlenum Press; New York: 1994. pp. 159–189. [Google Scholar]
- Kim E, Ku J, Namkoong K, Lee W, Lee KS, Park JY, Lee SY, Kim JJ, Kim SI, Jung YC. Mammillothalamic functional connectivity and memory function in Wernicke’s encephalopathy. Brain. 2009;132:369–376. doi: 10.1093/brain/awn311. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J. Comp. Neurol. 2000;419:205–222. doi: 10.1002/(sici)1096-9861(20000403)419:2<205::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Di Prisco GV, Vertes RP. Theta synchronization in the limbic system: the role of Gudden’s tegmental nuclei. Eur. J. Neurosci. 2001;13:381–388. [PubMed] [Google Scholar]
- Kopelman MD. Disorders of memory. Brain. 2002;125:2152–2190. doi: 10.1093/brain/awf229. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Stanhope N, Kingsley D. Temporal and spatial context memory in patients with focal frontal, temporal lobe, and diencephalic lesions. Neuropsychologia. 1997;35:1533–1545. doi: 10.1016/s0028-3932(97)00076-6. [DOI] [PubMed] [Google Scholar]
- Loftus M, Knight RT, Amaral DG. An analysis of atrophy in the medial mammillary nucleus following hippocampal and fornix lesions in humans and nonhuman primates. Exp. Neurol. 2000;163:180–190. doi: 10.1006/exnr.2000.7361. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The Triune Brain in Evolution: Role in Paleocerebral Functions. Plenum Press; New York: 1990. [DOI] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MC. Impairment of radial maze delayed nonmatching after lesions of anterior thalamus and parahippocampal cortex. Behav. Neurosci. 2003;117:596–605. doi: 10.1037/0735-7044.117.3.596. [DOI] [PubMed] [Google Scholar]
- Martin PR, Rio D, Adinoff B, Johnson JL, Bisserbe JC, Rawlings RR, Rohrbaugh JW, Stapleton JM, Eckardt MJ. Regional cerebral glucose utilization in chronic organic mental disorders associated with alcoholism. J. Neuropsychiatry Clin. Neurosci. 1992;4:159–167. doi: 10.1176/jnp.4.2.159. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Crosson B, Valenstein E, Bowers D. Verbal encoding deficits in a patient with a left retrosplenial lesion. Neurocase. 2001;7:407–417. doi: 10.1076/neur.7.5.407.16250. [DOI] [PubMed] [Google Scholar]
- McKee RD, Squire LR. Equivalent forgetting rates in long-term memory for diencephalic and medial temporal lobe amnesia. J. Neurosci. 1992;12:3765–3772. doi: 10.1523/JNEUROSCI.12-10-03765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMackin D, Cockburn J, Anslow P, Gaffan D. Correlation of fornix damage with memory impairment in six cases of colloid cyst removal. Acta Neurochir. 1995;135:12–18. doi: 10.1007/BF02307408. [DOI] [PubMed] [Google Scholar]
- Meier B, Theiler-Burgi M, Perrig W. Levels of processing and amnesia affect perceptual priming in fragmented picture naming. Int. J. Neurosci. 2009;119:1061–1075. doi: 10.1080/00207450802336691. [DOI] [PubMed] [Google Scholar]
- Mendez-Lopez M, Arias JL, Bontempi B, Wolff M. Reduced cytochrome oxidase activity in the retrosplenial cortex after lesions to the anterior thalamic nuclei. Behav. Brain Res. 2013;250:264–273. doi: 10.1016/j.bbr.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Hunt S, Jones DK, Leemans A, Aggleton JP, O’Sullivan MJ. Temporal association tracts and the breakdown of episodic memory in mild cognitive impairment. Neurology. 2012;79:2233–2240. doi: 10.1212/WNL.0b013e31827689e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJ. An experimental study of the fornix system in the rat. J. Comp. Neurol. 1956;104:247–271. doi: 10.1002/cne.901040205. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Vann SD. Mammilliothalamic tract lesions disrupt tests of visuo-spatial memory. Behav. Neurosci. 2014;128:494–503. doi: 10.1037/bne0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyurt J, Lorenzen A, Gebhardt U, Warmuth-Metz M, Muller HL, Thiel CM. Remote effects of hypothalamic lesions in the prefrontal cortex of craniopharygioma patients. Neurobiol. Learn. Mem. 2014;111:71–80. doi: 10.1016/j.nlm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Paller KA, Acharya A, Richardson BC, Plaisant O, Shimamura AP, Reed BR, Jagust WJ. Functional neuroimaging of cortical dysfunction in alcoholic Korsakoff’s syndrome. J. Cogn. Neurosci. 1997;9:277–293. doi: 10.1162/jocn.1997.9.2.277. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Arch. Neurol. Psychiatry. 1937;38:725–743. [Google Scholar]
- Park SA, Hahn JH, Kim JI, Na DL, Huh K. Memory deficits after bilateral anterior fornix infarction. Neurology. 2000;54:1379–1382. doi: 10.1212/wnl.54.6.1379. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. The effect of anterior thalamic and cingulate cortex lesions on object-in-place memory in monkeys. Neuropsychologia. 1997a;35:1093–1102. doi: 10.1016/s0028-3932(97)00042-0. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. Mamillary body lesions in monkeys impair object-in-place memory: functional unity of the fornix-mamillary system. J. Cogn. Neurosci. 1997b;9:512–521. doi: 10.1162/jocn.1997.9.4.512. [DOI] [PubMed] [Google Scholar]
- Parkin AJ. Amnesic syndrome: a lesion-specific disorder? Cortex. 1984;20:479–508. doi: 10.1016/s0010-9452(84)80053-2. [DOI] [PubMed] [Google Scholar]
- Parkin AJ. Functional significance of the etiological factors in human amnesia. In: Squire LR, Butters N, editors. Neuropsychology of Memory. second ed. Guildford Press; New York: 1992. [Google Scholar]
- Parkin AJ. Explorations in Cognitive Neuropsychology. Blackwell Publishers; Oxford, Oxfordshire/Cambridge, MA: 1996. [Google Scholar]
- Parkin AJ, Hunkin NM. Impaired temporal context memory on anterograde but not retrograde tests in the absence of frontal pathology. Cortex. 1993;29:267–280. doi: 10.1016/s0010-9452(13)80180-3. [DOI] [PubMed] [Google Scholar]
- Penfield W. The influence of the diencephalon and hypophysis upon general autonomic function. Can. Med. Assoc. J. 1934;30:589–598. [PMC free article] [PubMed] [Google Scholar]
- Poletti CE, Creswell G. Fornix system efferent projections in the squirrel monkey: an experimental degeneration study. J. Comp. Neurol. 1977;175:101–128. doi: 10.1002/cne.901750107. [DOI] [PubMed] [Google Scholar]
- Postans M, Hodgetts CJ, Mundy ME, Jones DK, Lawrence AD, Graham KS. Interindividual variation in fornix microstructure and macrostructure is related to visual discrimination accuracy for scenes but not faces. J. Neurosci. 2014;34:12121–12126. doi: 10.1523/JNEUROSCI.0026-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TP, Guillery RW, Cowan WM. A quantitative study of the fornixmamillothalamic system. J. Anat. 1957;91:419–437. [PMC free article] [PubMed] [Google Scholar]
- Raisman G, Cowan WM, Powell TP. An experimental analysis of the efferent projection of the hippocampus. Brain. 1966;89:83–108. doi: 10.1093/brain/89.1.83. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Lasserson D, Marsden P, Stanhope N, Stevens T, Bello F, Kingsley D, Colchester A, Kopelman MD. FDG-PET findings in the Wernicke-Korsakoff syndrome. Cortex. 2003;39:1027–1045. doi: 10.1016/s0010-9452(08)70876-1. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Carson N, Abraham N, Bowles B, Kwan D, Kohler S, Svoboda E, Levine B, Richards B. Impaired event memory and recollection in a case of developmental amnesia. Neurocase. 2011;17:394–409. doi: 10.1080/13554794.2010.532138. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gao F, Honjo K, Raybaud C, Olsen RK, Palombo DJ, Levine B, Black SE. Congenital absence of the mammillary bodies: a novel finding in a well-studied case of developmental amnesia. Neuropsychologia. 2014;65:82–87. doi: 10.1016/j.neuropsychologia.2014.09.047. [DOI] [PubMed] [Google Scholar]
- Rudebeck SR, Scholz J, Millington R, Rohenkohl G, Johansen-Berg H, Lee AC. Fornix microstructure correlates with recollection but not familiarity memory. J. Neurosci. 2009;29:14987–14992. doi: 10.1523/JNEUROSCI.4707-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RC, Mishkin M, Aggleton JP. Projections from the entorhinal cortex, perirhinal cortex, presubiculum, and parasubiculum to the medial thalamus in macaque monkeys: identifying different pathways using disconnection techniques. Exp. Brain Res. 2005;167:1–16. doi: 10.1007/s00221-005-2361-3. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Vann SD, Aggleton JP. Projections from Gudden’s tegmental nuclei to the mammillary body region in the cynomolgus monkey (Macaca fascicularis) J. Comp. Neurol. 2012;520:1128–1145. doi: 10.1002/cne.22740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Chang Q, Gold PE. Diencephalic damage decreases hippocampal acetylcholine release during spontaneous alternation testing. Learn. Mem. 2003;10:242–246. doi: 10.1101/lm.60003. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press; New York/Oxford: 2006. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Zyo K. Anterior thalamic afferents from the mamillary body and the limbic cortex in the rat. J. Comp. Neurol. 1984;229:242–256. doi: 10.1002/cne.902290209. [DOI] [PubMed] [Google Scholar]
- Shah A, Jhawar SS, Goel A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection. J. Clin. Neurosci. 2012;19:289–298. doi: 10.1016/j.jocn.2011.04.039. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Koester K. Lesions of the mammillary body region alter hippocampal movement signals and theta frequency: implications for path integration models. Hippocampus. 2008a;18:862–878. doi: 10.1002/hipo.20474. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Koester K. Lesions of the mammillary body region severely disrupt the cortical head direction, but not place cell signal. Hippocampus. 2008b;18:766–784. doi: 10.1002/hipo.20436. [DOI] [PubMed] [Google Scholar]
- Shibata H. Direct projections from the anterior thalamic nuclei to the retrohippocampal region in the rat. J. Comp. Neurol. 1993a;337:431–445. doi: 10.1002/cne.903370307. [DOI] [PubMed] [Google Scholar]
- Shibata H. Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. J. Comp. Neurol. 1993b;330:533–542. doi: 10.1002/cne.903300409. [DOI] [PubMed] [Google Scholar]
- Shibata H, Kato A. Topographic relationship between anteromedial thalamic nucleus neurons and their cortical terminal fields in the rat. Neurosci. Res. 1993;17:63–69. doi: 10.1016/0168-0102(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Simpson DA. The efferent fibres of the hippocampus in the monkey. J. Neurol. Neurosurg. Psychiatry. 1952;15:79–92. doi: 10.1136/jnnp.15.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JM, Meyer M. An experimental study of the fornix in the rabbit. J. Anat. 1950;84:354–368. [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Two forms of human amnesia: an analysis of forgetting. J. Neurosci. 1981;1:635–640. doi: 10.1523/JNEUROSCI.01-06-00635.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Comparisons between forms of amnesia: some deficits are unique to Korsakoff’s syndrome. J. Exp. Psychol. Learn. Mem. Cogn. 1982;8:560–571. doi: 10.1037//0278-7393.8.6.560. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K, Wyss JM. Thalamic projections to retrosplenial cortex in the rat. J. Comp. Neurol. 1986;254:143–165. doi: 10.1002/cne.902540202. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J. Neurosci. 1997;17:4349–4358. doi: 10.1523/JNEUROSCI.17-11-04349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. Hippocampo-hypothalamic connections: origin in subicular cortex, not Ammon’s horn. Science. 1975;189:303–304. doi: 10.1126/science.49928. [DOI] [PubMed] [Google Scholar]
- Sweeney-Reed CM, Zaehle T, Voges J, Schmitt FC, Buentjen L, Kopitzki K, Esslinger C, Hinrichs H, Heinze HJ, Knight RT, Richardson-Klavehn A. Corticothalamic phase synchrony and cross-frequency coupling predict human memory formation. eLife. 2014 doi: 10.7554/eLife.05352. http://dx.doi.org/10.7554/eLife.05352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Goodridge JP, Golob EJ, Dudchenko PA, Stackman RW. Processing the head direction cell signal: a review and commentary. Brain Res. Bull. 1996;40:477–484. doi: 10.1016/0361-9230(96)00145-1. discussion 484-486. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Rawlins JN. Mammillary body lesions and restricted subicular output lesions produce long-lasting DRL performance impairments in rats. Exp. Brain Res. 1992;90:572–582. doi: 10.1007/BF00230941. [DOI] [PubMed] [Google Scholar]
- Tsanov M, Vann SD, Erichsen JT, Wright N, Aggleton JP, O’Mara SM. Differential regulation of synaptic plasticity of the hippocampal and the hypothalamic inputs to the anterior thalamus. Hippocampus. 2011;21:1–8. doi: 10.1002/hipo.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat. Neurosci. 2008;11:834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110:1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Uylings HB, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38:613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Vann SD. Gudden’s ventral tegmental nucleus is vital for memory: re-evaluating diencephalic inputs for amnesia. Brain. 2009;132:2372–2384. doi: 10.1093/brain/awp175. [DOI] [PubMed] [Google Scholar]
- Vann SD. Dismantling the Papez circuit for memory in rats. eLife. 2013;2:e00736. doi: 10.7554/eLife.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav. Neurosci. 2002;116:85–94. [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Evidence of a spatial encoding deficit in rats with lesions of the mammillary bodies or mammillothalamic tract. J. Neurosci. 2003;23:3506–3514. doi: 10.1523/JNEUROSCI.23-08-03506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat. Rev. Neurosci. 2004;5:35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- Vann SD, Albasser MM. Hippocampal, retrosplenial, and prefrontal hypoactivity in a model of diencephalic amnesia: evidence towards an interdependent subcortical-cortical memory network. Hippocampus. 2009;19:1090–1102. doi: 10.1002/hipo.20574. [DOI] [PubMed] [Google Scholar]
- Vann SD, Saunders RC, Aggleton JP. Distinct, parallel pathways link the medial mammillary bodies to the anterior thalamus in macaque monkeys. Eur. J. Neurosci. 2007;26:1575–1586. doi: 10.1111/j.1460-9568.2007.05773.x. [DOI] [PubMed] [Google Scholar]
- Vann SD, Denby C, Love S, Montaldi D, Renowden S, Coakham HB. Memory loss resulting from fornix and septal damage: impaired supra-span recall but preserved recognition over a 24-hour delay. Neuropsychology. 2008;22:658–668. doi: 10.1037/a0012542. [DOI] [PubMed] [Google Scholar]
- Vann SD, Tsivilis D, Denby CE, Quamme JR, Yonelinas AP, Aggleton JP, Montaldi D, Mayes AR. Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc. Acad. Natl. Sci. U.S.A. 2009;106:5442–5447. doi: 10.1073/pnas.0812097106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Erichsen JT, O’Mara SM, Aggleton JP. Selective disconnection of the hippocampal formation projections to the mammillary bodies produces only mild deficits on spatial memory tasks: implications for fornix function. Hippocampus. 2011;21:945–957. doi: 10.1002/hipo.20796. [DOI] [PubMed] [Google Scholar]
- Veazey RB, Amaral DG, Cowan WM. The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). I. Cytoarchitectonic organization. J. Comp. Neurol. 1982;207:114–134. doi: 10.1002/cne.902070203. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav. Cogn. Neurosci. Rev. 2004;3:173–200. doi: 10.1177/1534582304273594. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Anzalone SJ, Savage LM. Impaired, spared, and enhanced ACh efflux across the hippocampus and striatum in diencephalic amnesia is dependent on task demands. Neurobiol. Learn. Mem. 2008;90:237–244. doi: 10.1016/j.nlm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Cramon DY, Hebel N, Schuri U. A contribution to the anatomical basis of thalamic amnesia. Brain. 1985;108:993–1008. doi: 10.1093/brain/108.4.993. [DOI] [PubMed] [Google Scholar]
- Votaw CL, Lauer EW. An afferent hippocampal fiber system in the fornix of the monkey. J. Comp. Neurol. 1963;121:195–206. doi: 10.1002/cne.901210205. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Aggleton JP, Muir JL. Comparing the effects of selective cingulate cortex lesions and cingulum bundle lesions on water maze performance by rats. Eur. J. Neurosci. 1998;10:622–634. doi: 10.1046/j.1460-9568.1998.00074.x. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Baird A, Morgan A, Muir JL, Aggleton JP. The conjoint importance of the hippocampus and anterior thalamic nuclei for allocentric spatial learning: evidence from a disconnection study in the rat. J. Neurosci. 2001;21:7323–7330. doi: 10.1523/JNEUROSCI.21-18-07323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. Amnesia: a disconnection syndrome? Neuropsychologia. 1982;20:233–248. doi: 10.1016/0028-3932(82)90099-9. [DOI] [PubMed] [Google Scholar]
- Wetzel CD, Squire LR. Encoding in anterograde amnesia. Neuropsychologia. 1980;18:177–184. doi: 10.1016/0028-3932(80)90063-9. [DOI] [PubMed] [Google Scholar]
- Wolff M, Gibb SJ, Dalrymple-Alford JC. Beyond spatial memory: the anterior thalamus and memory for the temporal order of a sequence of odor cues. J. Neurosci. 2006;26:2907–2913. doi: 10.1523/JNEUROSCI.5481-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NF, Erichsen JT, Vann SD, O’Mara SM, Aggleton JP. Parallel but separate inputs from limbic cortices to the mammillary bodies and anterior thalamic nuclei in the rat. J. Comp. Neurol. 2010;518:2334–2354. doi: 10.1002/cne.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Taube JS. Projections to the anterodorsal thalamus and lateral mammillary nuclei arise from different cell populations within the postsubiculum: implications for the control of head direction cells. Hippocampus. 2011;21:1062–1073. doi: 10.1002/hipo.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Lesions of the hippocampal formation but not lesions of the fornix or the mammillary nuclei produce long-lasting memory impairment in monkeys. J. Neurosci. 1989;9:898–913. doi: 10.1523/JNEUROSCI.09-03-00898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]