Abstract
Background
Pregnancy increases the risk of malaria and this is associated with poor health outcomes for both the mother and the infant, especially during the first or second pregnancy. To reduce these effects, the World Health Organization recommends that pregnant women living in malaria endemic areas sleep under insecticide‐treated bednets, are treated for malaria illness and anaemia, and receive chemoprevention with an effective antimalarial drug during the second and third trimesters.
Objectives
To assess the effects of malaria chemoprevention given to pregnant women living in malaria endemic areas on substantive maternal and infant health outcomes. We also summarised the effects of intermittent preventive treatment with sulfadoxine‐pyrimethamine (SP) alone, and preventive regimens for Plasmodium vivax.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL, MEDLINE, EMBASE, LILACS, and reference lists up to 1 June 2014.
Selection criteria
Randomized controlled trials (RCTs) and quasi‐RCTs of any antimalarial drug regimen for preventing malaria in pregnant women living in malaria‐endemic areas compared to placebo or no intervention. In the mother, we sought outcomes that included mortality, severe anaemia, and severe malaria; anaemia, haemoglobin values, and malaria episodes; indicators of malaria infection, and adverse events. In the baby, we sought foetal loss, perinatal, neonatal and infant mortality; preterm birth and birthweight measures; and indicators of malaria infection. We included regimens that were known to be effective against the malaria parasite at the time but may no longer be used because of parasite drug resistance.
Data collection and analysis
Two review authors applied inclusion criteria, assessed risk of bias and extracted data. Dichotomous outcomes were compared using risk ratios (RR), and continuous outcomes using mean differences (MD); both are presented with 95% confidence intervals (CI). We assessed the quality of evidence using the GRADE approach.
Main results
Seventeen trials enrolling 14,481 pregnant women met our inclusion criteria. These trials were conducted between 1957 and 2008, in Nigeria (three trials), The Gambia (three trials), Kenya (three trials), Mozambique (two trials), Uganda (two trials), Cameroon (one trial), Burkina Faso (one trial), and Thailand (two trials). Six different antimalarials were evaluated against placebo or no intervention; chloroquine (given weekly), pyrimethamine (weekly or monthly), proguanil (daily), pyrimethamine‐dapsone (weekly or fortnightly), and mefloquine (weekly), or intermittent preventive therapy with SP (given twice, three times or monthly). Trials recruited women in their first or second pregnancy (eight trials); only multigravid women (one trial); or all women (eight trials). Only six trials had adequate allocation concealment.
For women in their first or second pregnancy, malaria chemoprevention reduces the risk of moderate to severe anaemia by around 40% (RR 0.60, 95% CI 0.47 to 0.75; three trials, 2503 participants, high quality evidence), and the risk of any anaemia by around 17% (RR 0.83, 95% CI 0.74 to 0.93; five trials,, 3662 participants, high quality evidence). Malaria chemoprevention reduces the risk of antenatal parasitaemia by around 61% (RR 0.39, 95% CI 0.26 to 0.58; seven trials, 3663 participants, high quality evidence), and two trials reported a reduction in febrile illness (low quality evidence). There were only 16 maternal deaths and these trials were underpowered to detect an effect on maternal mortality (very low quality evidence).
For infants of women in their first and second pregnancies, malaria chemoprevention probably increases mean birthweight by around 93 g (MD 92.72 g, 95% CI 62.05 to 123.39; nine trials, 3936 participants, moderate quality evidence), reduces low birthweight by around 27% (RR 0.73, 95% CI 0.61 to 0.87; eight trials, 3619 participants, moderate quality evidence), and reduces placental parasitaemia by around 46% (RR 0.54, 95% CI 0.43 to 0.69; seven trials, 2830 participants, high quality evidence). Fewer trials evaluated spontaneous abortions, still births, perinatal deaths, or neonatal deaths, and these analyses were underpowered to detect clinically important differences.
In multigravid women, chemoprevention has similar effects on antenatal parasitaemia (RR 0.38, 95% CI 0.28 to 0.50; three trials, 977 participants, high quality evidence)but there are too few trials to evaluate effects on other outcomes.
In trials giving chemoprevention to all pregnant women irrespective of parity, the average effects of chemoprevention measured in all women indicated it may prevent severe anaemia (defined by authors, but at least < 8 g/L: RR 0.19, 95% CI 0.05 to 0.75; two trials, 1327 participants, low quality evidence), but consistent benefits have not been shown for other outcomes.
In an analysis confined only to intermittent preventive therapy with SP, the estimates of effect and the quality of the evidence were similar.
A summary of a single trial in Thailand of prophylaxis against P. vivax showed chloroquine prevented vivax infection (RR 0.01, 95% CI 0.00 to 0.20; one trial, 942 participants).
Authors' conclusions
Routine chemoprevention to prevent malaria and its consequences has been extensively tested in RCTs, with clinically important benefits on anaemia and parasitaemia in the mother, and on birthweight in infants.
8 May 2019
No update planned
Review superseded
The intervention is clearly effective. The questions now are around head‐to‐head comparisons not included in this review.
Plain language summary
The effect of taking antimalarial drugs routinely to prevent malaria in pregnancy
Pregnancy increases the risk of malaria and this is associated with poor health outcomes for both the mother and the infant, especially during the first or second pregnancy. For this reason, women are encouraged to try and prevent malaria infection during pregnancy by sleeping under mosquito bed‐nets, and by taking drugs effective against malaria throughout pregnancy as chemoprevention.
This Cochrane Review looked at all drug regimens compared to placebo. The review authors sought to summarise and quantify the overall effects of chemoprevention. Seventeen trials were included, all conducted between 1957 and 2008, and all but two in countries of Africa.
For women in their first or second pregnancy, malaria chemoprevention prevents moderate to severe anaemia (high quality evidence); and prevents malaria parasites being detected in the blood (high quality evidence). It may also prevent malaria illness. We don't know if it prevents maternal deaths, as this would require very large studies to detect an effect.
In their infants, malaria chemoprevention improves the average birthweight (moderate quality evidence), and reduces the number of low birthweight infants (moderate quality evidence). We are not sure if chemoprevention reduces mortality of babies in the first week, month and year, as again studies would need to be very large to show these effects.
Summary of findings
Summary of findings for the main comparison. Summary of findings table 1.
| Malaria chemoprevention for pregnant women (parity 0‐1) living in endemic areas: maternal outcomes | |||||
| Patient or population: Pregnant women (parity 0‐1) Settings: Malaria‐endemic areas Intervention: Malaria chemoprevention (any regimen) Control: Placebo or no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Chemoprevention | ||||
| Mortality All‐cause death | 7 per 1000 | 8 per 1000 (3 to 20) | RR 1.15 (0.44 to 3.06) | 2097 (3 trials) | ⊕⊝⊝⊝ very low1,2 |
| Severe anaemia During the third trimester | 145 per 1000 | 87 per 1000 (68 to 108) | RR 0.60 (0.47 to 0.75) | 2503 (3 trials) | ⊕⊕⊕⊕ high3,4,5,6 |
| Anaemia | 649 per 1000 | 539 per 1000 (480 to 604) | RR 0.83 (0.74 to 0.93) | 3662 (5 trials) | ⊕⊕⊕⊕ high3,6,7,8 |
| Uncomplicated clinical malaria | 173 per 1000 | 64 per 1000 (31 to 128) | RR 0.37 (0.18 to 0.74) | 307 (2 trials) | ⊕⊕⊝⊝ low4,9,10 |
| Antenatal parasitaemia | 286 per 1000 | 111 per 1000 (74 to 165) | RR 0.39 (0.26 to 0.58) | 3663 (8 trials) | ⊕⊕⊕⊕ high3,6,7,11 |
| Severe adverse effects12 | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (eg, the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 for risk of bias: Only one of these trials adequately described allocation concealment to be considered at low risk of selection bias. 2 Downgraded by 2 for imprecision: These trials were not adequately powered to detect a difference in mortality. Only 15 deaths occurred in these three trials.To confidently detect a 25% reduction in maternal mortality in a setting of 350 deaths/100,000 would require a sample size of over 100,000. 3 No serious risk of bias: Exclusion of the trials at high risk of bias did not change the statistical significance or clinical importance of the result. 4 No serious inconsistency: This finding was consistent across all trials and statistical heterogeneity was low. 5 No serious indirectness: These trials were conducted in Kenya and Mozambique between 1996 and 2005, all three trials administered IPT with SP. The definition of severe anaemia was variable; Hb < 8 g/dL, Hb < 7 g/dL, or PCV < 21%. 6 No serious imprecision: This result is statistically significant and the meta‐analysis is adequately powered to detect this effect. 7 No serious inconsistency: Although statistical heterogeneity was high, all trials favoured chemoprevention but there was variability in the size of the effect. 8 No serious indirectness: These trials were conducted in Nigeria, Kenya and Uganda between 1978 and 1999. Three trials administered IPT as SP, one gave weekly chloroquine, and one gave daily proguanil. The definition of anaemia was variable: Hb < 12 g/dL, Hb < 11 g/dL, Hb < 10 g/dL, PCV < 33% and PCV < 30%. 9 Downgraded by 1 for risk of bias. Both trials had high or unclear risk of selection bias and an attrition rate above 20%. 10 Downgraded by 1 for indirectness: Both these trials, from Cameroon 1993 and Mozambique 2002, measured fever history only as proxy for malaria illness. 11 Not downgraded for inconsistency. Despite substantive quantitative heterogeneity (I2 69% across six trials), all show at least a reduction of 23%, often more 11 No serious indirectness: These trials were conducted in The Gambia, Nigeria, Kenya and Mozambique between 1978 and 2005. Five trials gave IPT as SP, one gave pyrimethamine‐dapsone, one pyrimethamine, and one proguanil. 12 Reporting of adverse events was generally poor. No severe adverse events were reported.
Summary of findings 2. Summary of findings table 2.
| Malaria chemoprevention for pregnant women (parity 0‐1) living in endemic areas: infant outcomes | |||||
| Patient or population: Pregnant women (parity 0‐1) Settings: Malaria‐endemic areas Intervention: Malaria chemoprevention (any regimen) Control: Placebo or no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Chemoprevention | ||||
| Spontaneous abortion | 33 per 1000 | 21 per 1000 (13 to 33) | RR 0.65 (0.41 to 1.02) | 2876 (5 trials) | ⊕⊕⊝⊝ low1,2,3,4 |
| Stillbirth | 33 per 1000 | 32 per 1000 (21 to 49) | RR 0.97 (0.64 to 1.49) | 2703 (3 trials) | ⊕⊕⊝⊝ low2,4,5,6, |
| Perinatal mortality | 104 per 1000 | 76 per 1000 (56 to 104) | RR 0.73 (0.54 to 1.00) | 1620 (2 trials) | ⊕⊕⊝⊝ low2,4,5,7, |
| Neonatal mortality | 37 per 1000 | 23 per 1000 (14 to 39) | RR 0.62 (0.37 to 1.05) | 2156 (2 trials) | ⊕⊕⊝⊝ low2,4,5,7, |
| Preterm birth | 164 per 1000 | 140 per 1000 (108 to 181) | RR 0.85 (0.66 to 1.10) | 1493 (2 trials) | ⊕⊕⊝⊝ low1,2,4 |
| Low birthweight | 152 per 1000 | 110 per 1000 (92.7 to 132.2) | RR 0.73 (0.61 to 0.87) | 3619 (8 trials) | ⊕⊕⊕⊝ moderate9,10 |
| Mean birthweight | The mean birthweight in the control groups ranged from 2723 g to 3079 g |
The mean birthweight in the intervention groups was 92.72 g higher (62.05 higher to 123.39 higher) | ‐ | 3936 (9 trials) | ⊕⊕⊕⊝ moderate5,10 |
| Placental parasitaemia | 307 per 1000 | 160 per 1000 (132 to 211) | RR 0.54 (0.43 to 0.69) | 2830 (7 trials) | ⊕⊕⊕⊕ high3,11,12 |
| Cord blood haemoglobin | The mean haemoglobin in the control group was 15.8 g/dL | The mean haemoglobin in the intervention groups was 1.8 g/dL lower (3.46 lower to 0.14 lower) | ‐ | 64 (1 trial) | ⊕⊝⊝⊝ very low1,13,14 |
| *The basis for the assumed risk (eg the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 for serious risk of bias: None of the trials described adequate measures to prevent selection bias. 2 No serious inconsistency: The effect is consistent across trials and statistical heterogeneity is low. 3 No serious indirectness: These trials were conducted in The Gambia, Cameroon, Kenya and Mozambique between 1990 and 2002. One gave chemoprevention as weekly chloroquine and four trials gave IPT with SP. 4 Downgraded by 1 for serious imprecision: The 95% CI is wide and sample remains underpowered to detect or rule out an effect. 5 Downgraded by 1 for serious risk of bias: Only one trial adequately described methods to prevent selection bias. 6 No serious indirectness: Trials were conducted in Cameroon and Kenya between 1993 and 1997. One trial gave weekly chloroquine and the others gave IPT as SP. 7 No serious indirectness: The trials were conducted in The Gambia and Kenya between 1984 and 1997. One trial used IPT with SP and one gave pyrimethamine‐dapsone which is no longer in use. 8 No serious indirectness: Both trials were conducted in Kenya and used IPT with SP. 9 Downgraded by 1 for serious risk of bias: Only two of these trials were at low risk of selection bias. 10 No serious indirectness: These trials were conducted in The Gambia, Cameroon, Kenya, Uganda and Mozambique between 1986 and 2005. The majority of trials used IPT with SP. 11 No serious inconsistency: Although statistical heterogeneity was high, all trials favoured chemoprevention but there was variability in the size of the effect. 12 No serious indirectness: These trials were conducted in The Gambia, Cameroon, Kenya, Uganda and Mozambique between 1990 and 2002. The majority of trials used IPT with SP. 13 Downgraded by 1 for serious indirectness: This single trial used a regimen that is no longer in use (proguanil). 14 Downgraded by 1 for serious imprecision: Only a single small trial has evaluated this comparison.
Summary of findings 3. Summary of findings table 3.
| Malaria chemoprevention for pregnant women (parity 2+) living in endemic areas: maternal outcomes | |||||
| Patient or population: Pregnant women (parity 2+) Settings: Malaria‐endemic areas Intervention: Malaria chemoprevention (any regimen) Control: Placebo or no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Chemoprevention | ||||
| Mortality All‐cause death | 5 per 1000 | 7 per 1000 (2 to 26 | RR 1.47 (0.42 to 5.21) | 2239 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 |
| Severe anaemia During the third trimester | 68 per 1000 | 65 per 1000 (28 to 153) | RR 0.96 (0.41 to 2.25) | 2682 (2 trials) | ⊕⊕⊝⊝ low1,4,5 |
| Anaemia | The mean PCV in the control group was 30.4 % | The mean PCV in the intervention group was 0.3 % higher (0.7 lower to 1.3 higher) | ‐ | 244 (1 trial) | ⊕⊝⊝⊝ very low6,7,8 |
| Uncomplicated clinical malaria | ‐ | ‐ | ‐ | ‐ (0 trials) | ‐ |
| Antenatal parasitaemia | 108 per 1000 | 41 per 1000 (30 to 54) | RR 0.38 (0.28 to 0.50) | 3022 (4 trials) | ⊕⊕⊕⊕ high9,10 |
| Severe adverse events11 | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (eg the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: These trials are at low risk of bias. 2 Downgraded by 1 for serious indirectness: This single trial was conducted in The Gambia between 2002 and 2004 and administered IPT as monthly SP. The findings may not be easily generalised to elsewhere. 3 Downgraded by 2 for very serious imprecision: Only ten deaths occurred in this trial. Much larger trials would be needed to detect or exclude effects on maternal mortality. 4 No serious indirectness: These two trials were conducted in The Gambia in 2002‐2004 and Mozambique between 2003 and 2005. 5 Downgraded by 2 for very serious imprecision: The 95% CI are very wide and include the possibility of both clinically important benefits and harms. 6 Downgraded by 1 for serious risk of bias: This single trial is at unclear risk of selection bias. 7 Downgraded by 1 for serious indirectness: This trial administered chemoprevention as pyrimethamine‐dapsone which is no longer in use. 8 Downgraded by 1 for serious imprecision: A much larger sample size is required to confidently detect or exclude an effect. 9 No serious risk of bias: Two of the four trials were at low risk of selection bias and exclusion of the other two trials did not change the size of the effect. 10 No serious indirectness: These three trials were conducted in The Gambia, Nigeria and Mozambique between 1986 and 2005. The biggest and most recent trial administered IPT with SP (two doses)
11 Reporting of adverse events was generally poor. No severe adverse events were reported.
Summary of findings 4. Summary of findings table 4.
| Malaria chemoprevention for pregnant women (parity 2+) living in endemic areas: infant outcomes | |||||
| Patient or population: Pregnant women (parity 2+) Settings: Malaria‐endemic areas Intervention: Malaria chemoprevention (any regimen) Control: Placebo or no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Chemoprevention | ||||
| Spontaneous abortion | ‐ | ‐ | ‐ | ‐ (0 trials) |
‐ |
| Stillbirth | ‐ | ‐ | ‐ | ‐ (0 trials) |
‐ |
| Perinatal deaths | ‐ | ‐ | ‐ | ‐ (0 trials) |
‐ |
| Neonatal mortality | 26 per 1000 | 38 per 1000 (23 to 62) |
RR 1.46 (0.90 to 2.38) |
2017 (1 trial) |
⊕⊝⊝⊝ very low1,2,3 |
| Preterm birth | ‐ | ‐ | ‐ | ‐ (0 trials) |
‐ |
| Low birthweight | 60 per 1000 | 63 per 1000 (46 to 85) | RR 0.86 (0.63 to 1.17) | 2743 (3 trials) | ⊕⊕⊝⊝ low3,4,5 |
| Mean birthweight | ‐ | ‐ | ‐ | ‐ (0 trials) |
‐ |
| Placental parasitaemia | ‐ | ‐ | ‐ | ‐ (0 trials) |
‐ |
| Cord blood haemoglobin | ‐ | ‐ | ‐ | ‐ (0 trials) |
‐ |
| *The basis for the assumed risk (eg the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: This single trial was at low risk of selection bias. 2 Downgraded by 1 for serious indirectness: This single trial was conducted in The Gambia between 2002 and 2004 and administered IPT as monthly SP. The findings may not be easily generalised to elsewhere. 3 Downgraded by 2 for serious imprecision: The 95% CI is very wide and includes clinically important effects and no effect. A much larger sample size is required to confidently detect or exclude an effect. 4 No serious risk of bias: These trials are at low risk of selection bias. 5 No serious indirectness: These trials were conducted in The Gambia, Mozambique, and Uganda between 2002 and 2008.
Summary of findings 5. Summary of findings table 5.
| Malaria chemoprevention for all pregnant women (all parities) living in endemic areas: maternal outcomes | |||||
| Patient or population: Pregnant women (all parities) Settings: Malaria‐endemic areas Intervention: Malaria chemoprevention (any regimen) Control: Placebo or no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Chemoprevention | ||||
| Mortality All‐cause death | 1 per 1000 | 1 per 1000 (0 to 3) | RR 0.84 (0.25 to 2.74) | 6026 (4 trials) | ⊕⊕⊝⊝ low1,2,3 |
| Severe anaemia During the third trimester | 26 per 1000 | 5 per 1000 (1 to 19) | RR 0.19 (0.05 to 0.75) | 1327 (2 trials) | ⊕⊕⊝⊝ low2,4,5,6 |
| Anaemia | 206 per 1000 | 212 per 1000 (179 to 253) | RR 1.03 (0.87 to 1.23) | 3027 (3 trials) | ⊕⊕⊕⊝ moderate1,2,7,8 |
| Uncomplicated clinical malaria | 114 per 1000 | 42 per 1000 (13 to 140) | RR 0.37 (0.11 to 1.23) | 3455 (4 trials) | ⊕⊕⊝⊝ low1,9,10 |
| Antenatal parasitaemia | 152 per 1000 | 106 per 1000 (67 to 172) | RR 0.70 (0.44 to 1.13) | 3455 (4 trials) | ⊕⊕⊝⊝ low1,8,11 |
| Severe adverse effects12 | ‐ | ‐ | ‐ | ‐ (0 trials) |
‐ |
| *The basis for the assumed risk (eg the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: The two most recent trials adequately described allocation concealment to be considered at low risk of selection bias. 2 No serious inconsistency: This finding was consistent across all trials and statistical heterogeneity was low. 3 Downgraded by 2 for very serious imprecision: These trials were not adequately powered to detect a difference in mortality. Only nine deaths occurred in these four trials. To confidently detect a 25% reduction in maternal mortality in a setting of 350 deaths/100,000 would require a sample size of over 100,000. 4 No serious risk of bias: One of these two trials adequately described allocation concealment to be at low risk of bias. 5 Downgraded by 1 for serious indirectness: Only a single trial from Mozambique provides data on the currently used regimen of IPT as two doses of SP. The definition of severe anaemia was PCV <21%. 6 Downgraded by 1 for serious imprecision: The number of events is very low and the trials underpowered to be confident in these results. 7 No serious indirectness: These trials were conducted in Thailand, Mozambique and Uganda between 1988 and 2008. The two recent trials administered IPT as two doses of SP. The definition of anaemia was variable; Hb < 11 g/dL, PCV < 33% and PCV <30%. 8 Downgraded by 1 for serious imprecision: Although the finding is of no effect. The 95% CI includes what may be clinically important differences. 9 Downgraded by 1 for serious inconsistency: The two old trials from 1957 and 1988 suggest clinically important benefits with chemoprophylaxis ‐ however, the two recent trials providing two doses of SP find no evidence of an effect. 10 Downgraded by 1 for serious indirectness: The finding of no effect in the two recent trials may be due to the declining efficacy of two doses of SP. 11 Downgraded for by 1 for serious inconsistency. There is substantive heterogeneity between trials (I2 = 79%), and this finding of no effect is in contrast to findings of benefit in both women of low parity and multigravidae. The finding of no effect in two of the recent trials may reflect declining efficacy in the regimens used. 12 Reporting of adverse events was generally poor. No severe adverse events were reported.
Summary of findings 6. Summary of findings table 6.
| Malaria chemoprevention for pregnant women (all parities) living in endemic areas: infant outcomes | |||||
| Patient or population: Pregnant women (all parities) Settings: Malaria‐endemic areas Intervention: Malaria chemoprevention (any regimen) Control: Placebo or no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Chemoprevention | ||||
| Spontaneous abortion | 12 per 1000 | 11 per 1000 (7 to 16) | RR 0.89 (0.58 to 1.36) | 5767 (3 trials) | ⊕⊕⊝⊝ low1,2,3,4 |
| Stillbirth | 22 per 1000 | 22 per 1000 (17 to 30) | RR 1.02 (0.76 to 1.36) | 7130 (5 trials) | ⊕⊕⊕⊝ moderate1,2,5 |
| Perinatal mortality | 33 per 1000 | 41 per 1000 (31 to 54) | RR 1.24 (0.94 to 1.63) | 5216 (4 trials) | ⊕⊕⊕⊝ moderate1,2,5 |
| Neonatal mortality | 62 per 1000 | 56 per 1000 (44 to 72) | RR 0.91 (0.71 to 1.16) | 6313 (5 trials) | ⊕⊕⊕⊝ moderate1,2,5 |
| Preterm birth | 85 per 1000 | 81 per 1000 (55 to 117) | RR 0.95 (0.65 to 1.38) | 1174 (2 trials) | ⊕⊕⊝⊝ low2,5,6,10 |
| Low birthweight | 119 per 1000 | 126 per 1000 (106 to 151) | RR 1.06 (0.89 to 1.27) | 3644 (4 trials) | ⊕⊕⊝⊝ low1,2,5,10 |
| Mean birthweight | The mean birthweight in the control groups ranged from 2797 g to 3161 g |
The mean birthweight in the intervention groups was 0.54 g lower (24.6 g lower to 23.6 g higher) | ‐ | 6007 (5 trials) | ⊕⊕⊕⊝ moderate1,7,8,10 |
| Placental parasitaemia | 181 per 1000 | 80 per 1000 (27 to 233) | RR 0.44 (0.15 to 1.29) | 3200 (4 trials) | ⊕⊕⊝⊝ low1,9,10 |
| *The basis for the assumed risk (eg the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: The two most recent trials adequately described allocation concealment to be considered at low risk of selection bias. 2 No serious inconsistency: The finding of no difference is consistent across trials and statistical heterogeneity is low 3 No serious indirectness: These trials were conducted in the Burkina Faso, Mozambique and Uganda between 1988 and 2008. One gave chemoprevention as weekly chloroquine and two trials gave IPT with SP. 4 Downgraded by 2 for very serious imprecision: The 95% CI is wide and sample remains underpowered to detect or rule out an effect. 5 Downgraded by 1 for serious imprecision: The 95% CI is wide and sample remains underpowered to detect or rule out an effect. 6 No serious risk of bias: The most recent trial adequately described allocation concealment to be considered at low risk of selection bias. 7 No serious inconsistency: Although substantial statistical heterogeneity is present (I2 = 72%), this relates to the oldest trial which found a benefit with chemoprevention. The subsequent four trials have consistently found no clinically important difference. 8 No serious imprecision: The 95% CI probably excludes clinically important benefits. 9 Downgraded by 1 for serious inconsistency: The two old trials from 1957 and 1988 suggest clinically important benefits with chemoprophylaxis ‐ however, the two recent trials providing two doses of SP find no evidence of an effect. 10 Downgraded by 1 for serious indirectness: The finding of no effect in the recent trials may be due to the declining efficacy of two doses of SP which is no longer recommended.
Summary of findings 7. Summary of findings table 7.
| Intermittent preventive treatment with SP for pregnant women (parity 0‐1) living in malaria endemic areas: maternal outcomes | |||||
| Patient or population: Pregnant women (parity 0‐1) Settings: Malaria‐endemic areas Intervention: Intermittent preventive treatment with SP (2 doses, 3 doses, or monthly dosing) Control: Placebo or no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | IPT (SP) | ||||
| Mortality All‐cause death | 7 per 1000 | 8 per 1000 (3 to 20) | RR 1.15 (0.44 to 3.06) | 2097 (2 trials) | ⊕⊝⊝⊝ very low1,2 |
| Severe anaemia During the third trimester | 145 per 1000 | 87 per 1000 (68 to 108) | RR 0.60 (0.47 to 0.75) | 2503 (3 trials) | ⊕⊕⊕⊕ high3,4,5,6 |
| Anaemia | 617 per 1000 | 543 per 1000 (480 to 604) | RR 0.88 (0.81 to 0.96) | 3291 (4 trials) | ⊕⊕⊕⊝ moderate1,6,7,8 |
| Uncomplicated clinical malaria | 9 per 100 | 2 per 100 (0 to 10) | RR 0.24 (0.05 to 1.12) | 174 (1 trial) | ⊕⊝⊝⊝ very low9,10,11 |
| Antenatal parasitaemia | 286 per 1000 | 108 per 1000 (69 to 169) | RR 0.38 (0.24 to 0.59) | 2832 (4 trials) | ⊕⊕⊕⊕ high3,6,7,12 |
| Severe adverse effects13 | ‐ | ‐ | ‐ | ‐ (0 trials) |
‐ |
| *The basis for the assumed risk (eg the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 for risk of bias: Only one of these trials adequately described allocation concealment to be considered at low risk of selection bias. 2 Downgraded by 2 for imprecision: These trials were not adequately powered to detect a difference in mortality. Only 15 deaths occurred in these two trials. To confidently detect a 50% reduction in maternal mortality in a setting of 350 deaths/100,000 would require a sample size of over 100,000. 3 No serious risk of bias: Exclusion of the trials at high risk of bias did not change the statistical significance or clinical importance of the result. 4 No serious inconsistency: This finding was consistent across all trials and statistical heterogeneity was low. 5 No serious indirectness: These trials were conducted in Kenya and Mozambique between 1996 and 2005, all three trials administered IPT with SP. The definition of severe anaemia was variable; Hb < 8 g/dL, Hb < 7g/dL, or PCV < 21%. 6 No serious imprecision: This result is statistically significant and the meta‐analysis is adequately powered to detect this effect. 7 No serious inconsistency: Although statistical heterogeneity was high, all trials favoured IPT with SP but there was variability in the size of the effect. 8 No serious indirectness: These trials were conducted Kenya between 1996 and 1999. The definition of anaemia was variable; Hb < 11 g/dL, Hb < 10 g/dL. 9 Downgraded by 1 for risk of bias: This trial is at unclear risk of selection bias. 10 Downgraded by 1 for indirectness: This trial from Mozambique 2002, measured fever history only as proxy for malaria illness. 11 Downgraded by 1 for serious imprecision: The 95% CI is wide and includes clinically important benefits and no effect. 12 No serious indirectness: These trials were conducted in the Kenya and Mozambique between 1996 and 2005. 13Reporting of adverse events was generally poor. No severe adverse events were reported.
Summary of findings 8. Summary of findings table 8.
| Intermittent preventive treatment with SP for pregnant women (parity 0‐1) living in malaria endemic areas: infant outcomes | |||||
| Patient or population: Pregnant women (parity 0‐1) Settings: Malaria‐endemic areas Intervention: Intermittent preventive treatment with SP (2 doses, 3 doses, or monthly dosing) Control: Placebo or no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | IPT (SP) | ||||
| Spontaneous abortion | 34 per 1000 | 21 per 1000 (13 to 33) | RR 0.61 (0.38 to 0.99) | 2567 (3 trials) | ⊕⊕⊝⊝ low1,2,3,4 |
| Stillbirth | 33 per 1000 | 32 per 1000 (21 to 49) | RR 0.97 (0.64 to 1.47) | 2703 (3 trials) | ⊕⊕⊝⊝ low2,4,5,6 |
| Perinatal mortality | 80 per 1000 | 62 per 1000 (42 to 94) | RR 0.78 (0.52 to 1.17) | 1237 (1 trial) | ⊕⊕⊝⊝ low7 |
| Neonatal mortality | 37 per 1000 | 23 per 1000 (14 to 39) | RR 0.62 (0.37 to 1.05) | 2156 (2 trials) | ⊕⊕⊝⊝ low2,4,5,6 |
| Preterm birth | 164 per 1000 | 140 per 1000 (108 to 181) | RR 0.85 (0.66 to 1.10) | 1493 (2 trials) | ⊕⊕⊝⊝ low1,2,4 |
| Low birthweight | 128 per 1000 | 104 per 1000 (86 to 127) | RR 0.81 (0.67 to 0.99) | 3043 (4 trials) | ⊕⊕⊕⊝ moderate8,9 |
| Mean birthweight | The mean birthweight in the control groups ranged from 2908 g to 3079 g |
The mean birthweight in the intervention groups was 84.18 g higher (40.1 to 128.3 higher) | ‐ | 2127 (3 trials) | ⊕⊕⊕⊝ moderate5,9 |
| Placental parasitaemia | 225 per 1000 | 101 per 1000 (74 to 137) | RR 0.45 (0.33 to 0.61) | 1633 (3 trials) | ⊕⊕⊕⊝ moderate5,10 |
| Cord blood haemoglobin | ‐ | ‐ | ‐ | ‐ (0 trials) |
‐ |
| *The basis for the assumed risk (eg the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 for serious risk of bias: None of the trials described adequate measures to prevent selection bias. 2 No serious inconsistency: The effect is consistent across trials and statistical heterogeneity is low 3 No serious indirectness: These trials were conducted in the Kenya and Mozambique between 1996 and 2002. 4 Downgraded by 1 for serious imprecision: The 95% CI is wide and sample remains underpowered to detect or rule out an effect. 5 Downgraded by 1 for serious risk of bias: Only one trial adequately described methods to prevent selection bias. 6 No serious indirectness: Trials were conducted in Kenya between 1996 and 1997. 7 Downgraded by 2 for serious imprecision: The 95% CI is wide and sample remains underpowered to detect or rule out an effect. 8 Downgraded by 1 for serious risk of bias: Only two of these trials were at low risk of selection bias. 9 No serious indirectness: These trials were conducted in the Kenya, Uganda and Mozambique between 1996 and 2008. 10 No serious inconsistency: Although statistical heterogeneity was high, all trials favoured chemoprevention but there was variability in the size of the effect.
Background
Description of the condition
Approximately 125 million women living in malaria‐endemic areas become pregnant each year (Dellicour 2010), and pregnancy is known to increase the risk of malaria infection and the severity of the illness compared to non‐pregnant women in the same age group (Desai 2007). Studies have also shown a strong association between malaria infection in pregnancy and consequent maternal anaemia, and low birthweight in infants, particularly in women in their first or second pregnancy (Desai 2007; Steketee 2001).
To reduce the burden and consequences of malaria in pregnancy, the World Health Organization (WHO) recommends that all pregnant women living in malaria‐endemic areas: i) sleep under a long lasting insecticide‐treated bednet (ITN; Gamble 2006; WHO 2012); ii) are treated when anaemic or when ill with malaria; and iii) receive some form of malaria chemoprevention. Currently the WHO recommends 'intermittent‐preventive therapy' with sulfadoxine‐pyrimethamine (SP) during the second and third trimesters in Africa (WHO 2013).
Description of the intervention
Over the years a variety of drugs have been evaluated for malaria chemoprevention in pregnancy, including amodiaquine, chloroquine, dapsone‐pyrimethamine, mefloquine, proguanil, pyrimethamine as monotherapy and as the fixed dose combination SP, and others. All have specific toxic and adverse effects, which are outlined in standard texts (WHO 2010), and these may be important factors influencing maternal adherence. For example, proguanil can cause mouth ulcers, chloroquine can cause itch, and mefloquine can cause dizziness and headaches.
How the intervention might work
Chemoprevention encompasses malaria chemoprophylaxis, and also the use of treatment courses given regularly to women. This is termed intermittent preventive treatment (IPT), defined as a full therapeutic course of antimalarial medicine given to pregnant women at routine prenatal visits, regardless of whether the recipient is infected with malaria. combines elements of a treatment effect through clearance or suppression of existing malaria infections in the placental and peripheral blood of mother, and a post‐treatment prophylactic effect by preventing new infections for several weeks after each dose (White 2005). Daily, weekly, or bi‐weekly malaria chemoprophylaxis is thought to work primarily through the prevention of new malaria infections. However, a reduction in malaria infections per se may be insufficient to justify the use of chemoprevention for widespread use without subsequent benefits on clinically important outcomes in the mother and her baby. These may include a reduction in clinical malaria episodes, a reduced risk of anaemia, improved birthweight, or more substantive outcomes such as a reduction in severe maternal illness, or fewer deaths in the mother and infant (see Figure 1).
1.
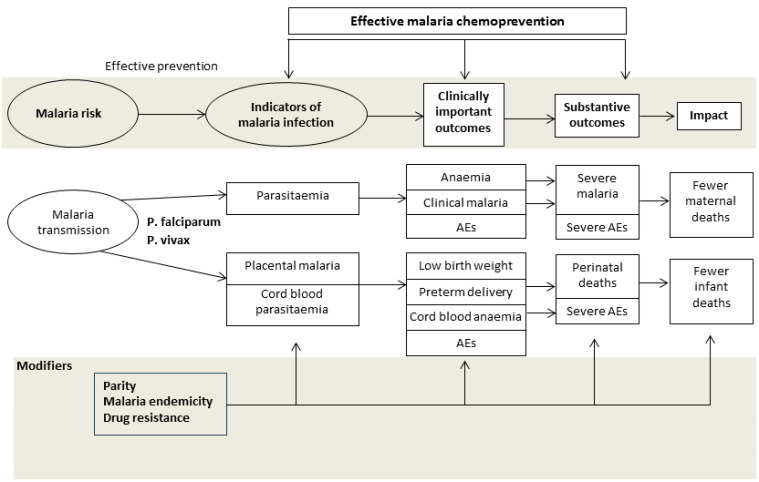
Drugs for preventing malaria in pregnancy: conceptual framework.
The effects of malaria chemoprevention may differ between settings dependent on the local malaria epidemiology. In highly endemic areas with stable transmission, mothers may have partial immunity to malaria, and chronic subclinical placental infection are common leading to maternal anaemia and low birthweight, especially in primi‐ and secundigravidae. In contrast, where malaria transmission is low or unstable, the degree of life‐long acquired and pregnancy‐specific protective immunity may be lower and malaria infections are more likely to result in clinical episodes or severe illness, leading to low birthweight due to a preterm birth, foetal loss or maternal death.
Another potential effect modifier is HIV status. Many malaria‐endemic areas, especially in east and southern Africa, also have a high prevalence of HIV infection among pregnant women. Compared to HIV negative women, HIV positive women are more likely to carry malaria parasites in their blood, have higher parasite densities, and are more likely to have placental parasitaemia, anaemia, and malaria symptoms and deliver low birthweight babies (Nkhoma 2012a; Nkhoma 2012b; ter Kuile 2004).
Why it is important to do this review
This Cochrane Review aims to address the following questions:
Does chemoprevention reduce mortality and substantive outcomes in the mother and infant?
What is the potential reduction in the burden of malaria in pregnancy that can be achieved by successful malaria chemoprevention in pregnancy?
Are the effects consistent in low parity and high parity women?
This review summarises the underpinning evidence of the protective efficacy achieved with antimalarial chemoprevention regimens on the effects on malaria and its consequences on the mother and baby when compared against placebo or no chemoprevention (case‐management strategies only). It does not compare different regimens. These were included in earlier editions of this Cochrane Review (Garner 2006); a more recent review has examined the effects of different IPT regimens in pregnant women (Kayentao 2013).
Objectives
In malaria‐endemic areas, to assess the effects in pregnant women of:
Malaria chemoprevention versus no chemoprevention irrespective of the regimen;
Malaria chemoprevention with SP (called intermittent preventive treatment) with no chemoprevention;
Preventive regimens for Plasmodium vivax.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Pregnant women of any gravidity living in malaria‐endemic areas, defined as regions where transmission occurs and malaria is a characteristic of the region.
Types of interventions
Interventions
Any antimalarial drug chemoprevention regimen given to pregnant women.
Controls
Placebo or no intervention,
Types of outcome measures
For the conceptual framework, see Figure 1.
Maternal outcomes
Impact: maternal deaths (number of maternal deaths reported: death of a pregnant woman during pregnancy or within 42 days of termination of pregnancy).
Substantive outcomes: severe malaria, which includes severe anaemia (defined as Hb < 8 g/dL, < 7 g/dL, < 6 g/dL); severe adverse events.
Clinically important outcomes: anaemia (anaemia defined as Hb < between 10 and 12 g/dL); mean haemoglobin (g/dL) or mean PCV (%); clinical malaria (history of fever episodes prior to delivery); adverse events.
Indicators of malaria infection: parasitaemia (defined as the presence of asexual stage parasites in thick smears in peripheral, placental, or cord blood).
Infant outcomes
Impact: neonatal and Infant mortality.
Substantive outcomes: foetal loss (including spontaneous abortion (spontaneous expulsion of a fetus before it is able to survive independently); stillbirth (birth of a foetus with no vital signs, born after the 28th week of pregnancy); perinatal mortality; severe adverse events, including congenital anomalies (a defect that is present at birth).
Clinically important outcomes: preterm birth (delivery at < 37 weeks gestation); low birthweight (< 2500 g); mean birthweight; cord blood anaemia; adverse events.
Indicators of malaria infection: placental malaria; haemoglobin levels (infant), cord blood haemoglobin (g/dL), and cord blood PCV; cord blood parasitaemia.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register (1 June 2014); Central Register of Controlled Trials (CENTRAL); MEDLINE (1966 to 1 June 2014); EMBASE (1974 to February 2012); and LILACS (1982 to February 2012).
Researchers
We contacted researchers working in the field for unpublished data, confidential reports, and raw data of published trials.
Reference lists
We also checked the citations of literature reviews, and of all trials identified by the above methods, and asked the referees to check the search strategy.
Data collection and analysis
Selection of studies
We applied inclusion criteria to all trials, including those in the previous edition of this Cochrane Review. DR‐P and PG independently screened all trials identified by the search strategy (Appendix 1). Using a form based on the inclusion criteria, DR‐P and PG assessed eligibility independently. FK checked the completeness of the included trials. We retrieved full text articles for all potentially relevant trials, applied the inclusion criteria, and then compared decisions. We resolved any differences by discussion and, when necessary, consulted with co‐authors.Trials identified in the initial abstract screening which did not meet the inclusion criteria are listed in the 'Characteristics of excluded studies'.
Data extraction and management
DR‐P and PG independently extracted data using a data extraction form. We extracted data on trial characteristics, including trial site, year, local malaria transmission and resistance, trial methods, participants, interventions, doses and outcomes and entered this data into Review Manager 5.1. The number of participants randomized and the number analysed in the experimental and control arms were extracted in each group for each outcome. For dichotomous outcomes, we recorded the number of participants experiencing the event and the number assessed in each treatment group. For continuous outcomes, we extracted the arithmetic means, standard deviations for each treatment group and the number of participants assessed in each group. We calculated and reported the loss to follow‐up in each group.
Assessment of risk of bias in included studies
We independently assessed the trials' methodological quality (risk of bias) of each trial, using the Cochrane Collaboration's tool for assessing the risk of bias (Higgins 2011). The following six components were assessed for each trial: generation of allocation sequence, allocation concealment, blinding (of participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, and other sources of bias. Each component was classified by 'yes' (low risk of bias), 'no' (high risk of bias), or 'unclear' to indicate level of bias. Where our judgement was 'unclear', we attempted to contact the trial authors for clarification.
Measures of treatment effect
We used the risk ratio (RR) to summarise dichotomous outcomes, reported the mean difference for continuous outcomes, and used the rate ratio for count outcomes. We presented all measures of effect with 95% confidence intervals (CI). One trial had four arms: one a comparison of IPT with nets, and a second comparison with no nets, and these were treated as separate comparisons (Njagi 2003i KEN; Njagi 2003ii KEN); a second trial had two intervention comparisons, so in meta‐analysis we split the control group in half for dichotomous outcomes. For continuous outcomes, we split the denominator of the control in half, but applied no correction to the standard deviation.
Unit of analysis issues
If the original trial analyses had not adjusted for clustering, we planned to adjust the results for clustering by multiplying the standard errors of the treatment effect by the square root of the design effect. The design effect would be calculated as 1+(m‐1)*ICC where m was the average cluster size and ICC was the intra‐cluster correlation coefficient. We planned to estimate the ICC from other trials included in the review or by contacting trial investigators. We also planned to include trials with multiple treatment arms if relevant to any of the comparisons. One trial randomized by compound in The Gambia (Greenwood 1989 GMB). However, we know that compounds are quite small, are grouped around families, and that, even if two women were pregnant at the same time in one family, this would not be quantitatively important in terms of overestimating the precision of the effect estimate.
Dealing with missing data
We planned to use intention‐to‐treat (ITT) data from the original trials, but it was more practical to use a complete‐case analysis, such that we excluded participants for whom no outcome was reported from the analysis. This analysis assumes that the participants for whom an outcome is available are representative of the original randomized patients. If data from the trial reports were insufficient, unclear, or missing, we attempted to contact the trial authors for additional information. In one trial with no standard deviation for birthweight, we used the average of the standard deviation for the other included trials.
Assessment of heterogeneity
We inspected the forest plots to detect overlapping CIs, applying the Chi2 test and a P value of 0.10 as the cut‐off value to determine statistical significance. We also estimated the I2‐statistic and categorized the degree of heterogeneity using standard cut‐offs (Higgins 2011).
Data synthesis
We used Review Manager 5.1 for the analysis.
Our primary analysis is stratified by parity, with results grouped into women of low parity (0‐1) and multigravidae (1+).
We included a category called 'all women'. This included trials that recruited women irrespective of parity. This analysis included the trials which had stratified the analysis by parity (and were therefore included in the primary analysis), and a second set of trials, which had not. This analysis provides information on the population effects of a policy of providing chemoprevention to all pregnant women.
We used RRs for dichotomous variables and mean differences (MD) for continuous variables; all results are presented with 95% CIs. In the absence of heterogeneity, we used a fixed‐effect model for the meta‐analysis, and where we detected heterogeneity we used a random‐effects model. Weighted averages were calculated where required. We converted Packed Cell Volume (PCV) values to haemoglobin values by dividing by three.
Subgroup analysis and investigation of heterogeneity
We grouped the analysis by parity. Although we intended to investigate heterogeneity by a variety of factors (including HIV status, risk of bias, geographical region, malaria transmission pattern, antimalarial resistance, ITN use, drug regimen), there were insufficient data to do this.
Results
Description of studies
Results of the search
The search was conducted up to 01 June 2014 for the time period 1964 to 2014, and identified 181 references of which two were duplicate trial reports. Out of 179, we retrieved 53 full‐text articles for eligibility screening (Figure 2).
2.
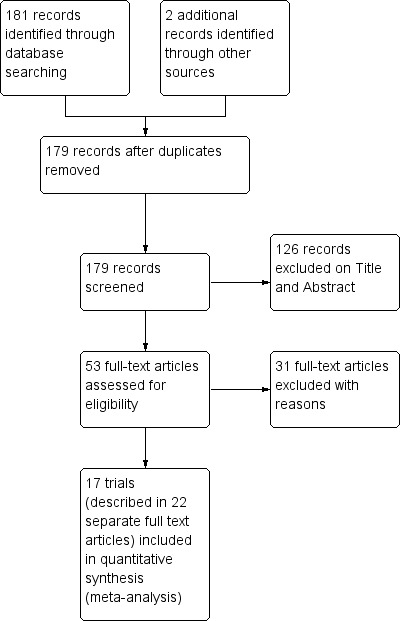
Study flow diagram.
Included studies
Seventeen chemoprevention trials, enrolling 20,256 pregnant women, met our inclusion criteria (see 'Characteristics of included studies'). These trials were conducted between 1957 and 2008, in Nigeria (three trials), The Gambia (three trials), Kenya (three trials), Mozambique (two trials), Uganda (two trials), Cameroon (one trial), Burkina Faso (one trial), and Thailand (two trials).
Six different antimalarials were evaluated against placebo or no preventive intervention (ie passive case detection and treatment of clinical cases only); chloroquine (given weekly), pyrimethamine (weekly or monthly), proguanil (daily), pyrimethamine‐dapsone (weekly or fortnightly), SP (given twice, monthly or intermittently for up to four doses at least one month apart), and mefloquine (weekly) (see Appendix 2). Fifteen trials reported that drug administration was supervised, and in two trials it was unsupervised (Fleming 1986 NGA; Ndyomugyenyi 2000 UGA).
Eight trials recruited women in all parity groups; four reported aggregate results, and four disaggregated by parity. The rest only recruited low parity women: six were parity 0, and two were women of parity 0‐1. One trial only recruited multigravidae (see Appendix 3).
In four trials, all women in both intervention and control groups received a long‐lasting ITNs at recruitment (Menendez 2008 MOZ; Ndyomugyenyi 2000 UGA; Ndyomugyenyi 2011 UGA; Njagi 2003i KEN). One additional trial mentioned that ITNs were in use in the area, with a use of 26% (Shulman 1999 KEN; ter Kuile 2007). In six trials iron and folic acid were routinely administered to all pregnant women (Fleming 1986 NGA; Mbaye 2006 GMB; Nahlen 1989 NGA; Njagi 2003i KEN; Njagi 2003ii KEN; Parise 1998i KEN; Parise 1998ii KEN; Villegas 2007 THA), in one trial only iron was administered (Shulman 1999 KEN), and in one trial both iron and folic acid were given to anaemic women (Nosten 1994 THA). The remaining trials did not comment on use of iron or folic acid.
One trial was randomized by compound, but for the analysis we assumed that it was individually randomized (Greenwood 1989 GMB). Two trials with multiple intervention arms were presented by individual arms, and the placebo patients split between the two arms where the treatment arms were both included in the meta‐analysis; Parise 1998i KEN compared two doses of SP versus no intervention while Parise 1998ii KEN compared monthly SP versus no intervention; Njagi 2003i KEN compared SP + ITNs versus placebo + ITNs; and Njagi 2003ii KEN compared SP alone versus placebo.
Excluded studies
We excluded 32 trials for the reasons given in the 'Characteristics of excluded studies' table. Also in this review update, we excluded one previously included trial (Hamilton 1972 UGA) as iron was administered to one of the control groups and folic acid to the other, but nothing was mentioned of iron and folates being administered to women in the intervention group (chloroquine).
Risk of bias in included studies
See Figure 3 for a summary of the risk of bias assessments. We have presented further details in the 'Characteristics of included studies' tables.
3.
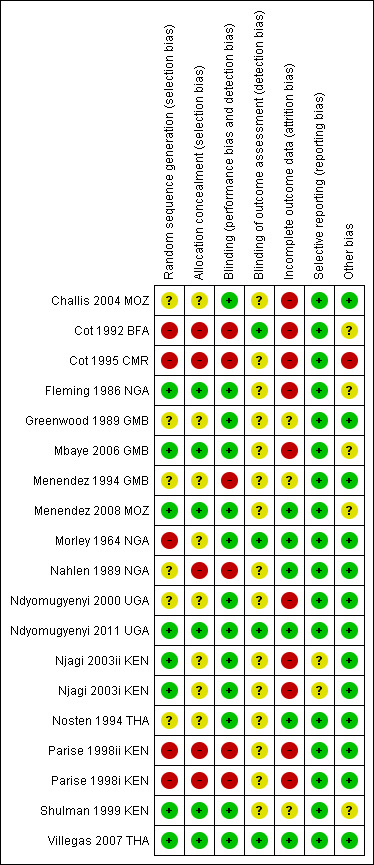
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Six trials adequately described methods of sequence generation and allocation concealment to be considered at low risk of selection bias (Fleming 1986 NGA; Mbaye 2006 GMB; Menendez 2008 MOZ; Ndyomugyenyi 2011 UGA; Shulman 1999 KEN; Villegas 2007 THA). Four trials were quasi‐RCT and so at high risk of selection bias (Cot 1992 BFA; Cot 1995 CMR; Morley 1964 NGA; Parise 1998i KEN; Parise 1998ii KEN), and in the remaining seven trials the risk was unclear.
Blinding
Eleven trials used placebo tablets, identical in taste and appearance to the active drug, and were assessed as having low risk of performance bias.
Four trials explicitly stated that outcome assessors were blinded and were assessed as having low risk of detection bias (Cot 1992 BFA; Morley 1964 NGA; Ndyomugyenyi 2011 UGA; Villegas 2007 THA). In the remaining included trials the risk was unclear.
Incomplete outcome data
Six trials had an attrition rate lower than 10% in both the intervention and control arm (Menendez 2008 MOZ; Morley 1964 NGA; Nahlen 1989 NGA; Ndyomugyenyi 2011 UGA; Nosten 1994 THA; Villegas 2007 THA). The remaining 11 trials were at high or unclear risk of attrition bias.
Selective reporting
Birthweight data were not available in one trial, but we obtained this data from a subsequent review (Njagi 2003i KEN; Njagi 2003ii KEN; ter Kuile 2007).
Other potential sources of bias
In one trial, 18 participants were replaced by others after randomization (Fleming 1986 NGA). We sought differences in baseline values with haemoglobin (Analysis 1.4) and detected no obvious difference.
1.4. Analysis.
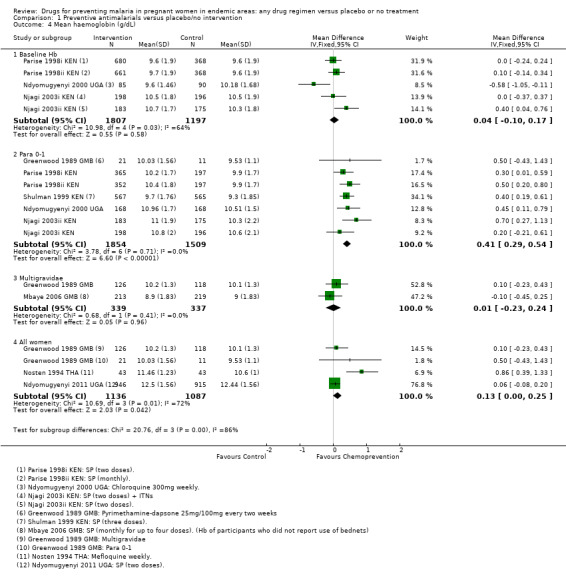
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 4 Mean haemoglobin (g/dL).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
Comparison 1: Chemoprevention (any drug regimen) versus placebo/no chemoprevention
Chemoprevention for women in their first or second pregnancy
Maternal outcomes (see Table 1)
Only 15 maternal deaths were reported across all trials with no difference between groups (three trials, 2097 participants, Analysis 1.1, very low quality evidence). Maternal death, even in these settings, is a relatively rare event occurring in less than five women per 1000 pregnancies. Consequently trials would need to enrol over 125,000 women to be adequately powered to detect or exclude effects as large as a 25% relative reduction (see Table 9).
1.1. Analysis.
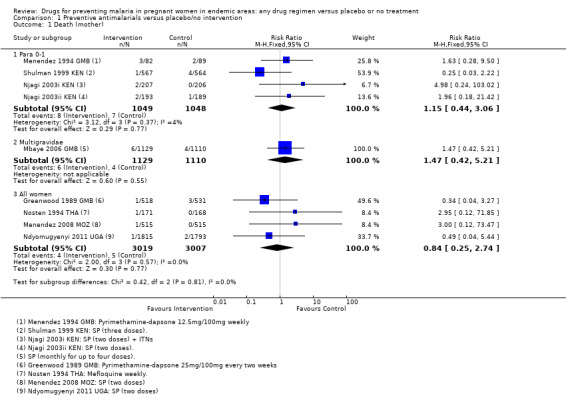
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 1 Death (mother).
1. Optimal information size calculations: Chemoprevention versus placebo.
| Outcome | Assumed risk | Source | Clinically important relative reduction | Sample size required1,2 |
| Maternal mortality | 350/100,000 | Analysis 1.1 | 25% | 125228 |
| Severe anaemia | 150/1000 | Analysis 1.2 | 25% | 2540 |
| Anaemia | 650/1000 | Analysis 1.3 | 25% | 284 |
| Malaria | 170/1000 | Analysis 1.5 | 25% | 2194 |
| Parasitaemia | 290/1000 | Analysis 1.6 | 25% | 1124 |
| Spontaneous abortions | 32/1000 | Analysis 1.9 | 25% | 13348 |
| Still births | 33/1000 | Analysis 1.10 | 25% | 12932 |
| Neonatal deaths | 37/1000 | Analysis 1.12 | 25% | 11492 |
| Preterm birth | 160/1000 | Analysis 1.13 | 25% | 2356 |
| Low birthweight | 150/1000 | Analysis 1.14 | 25% | 2540 |
| Placental parasitaemia | 300/1000 | Analysis 1.18 | 25% | 1074 |
1 All calculations are based on: 2‐sided tests, with a ratio of 1:1, power of 0.8, and confidence level of 0.05. 2 All calculations were performed using: http://www.sealedenvelope.com/power/binary‐superiority
No trials reported on episodes of severe malaria, but three trials reported moderate to severe anaemia (defined as Hb < 7/8 g/dL or PCV < 21%). Overall, chemoprevention was associated with a 40% reduction in the risk of moderate to severe anaemia in the third trimester (RR 0.60, 95% CI 0.47 to 0.75; three trials, 2503 participants, Analysis 1.2, high quality evidence). This effect was consistent despite variation in doses, and differences in the definition and timing of assessment for severe anaemia (I2 =0); Parise 1998ii KEN recorded severe anaemia at delivery (after three doses of SP); Shulman 1999 KEN at 34 weeks (after three doses of SP); Menendez 2008 MOZ at delivery (after two doses of SP), and Parise 1998i KEN at the beginning of the third trimester clinic visit (when the second dose of SP was due, and these women had only had one SP dose).
1.2. Analysis.
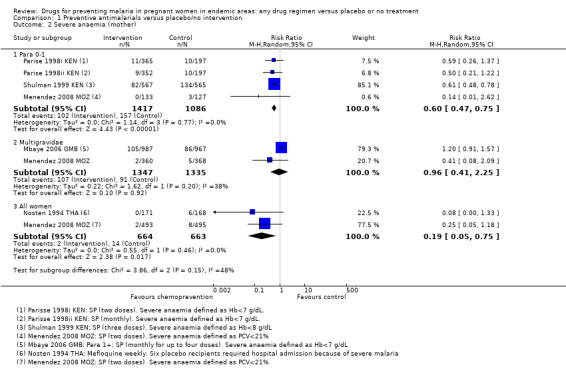
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 2 Severe anaemia (mother).
Chemoprevention was also associated with a reduction in the risk of any anaemia (defined as Hb < 10/11/12 g/dL or PCV < 33%/30%), although this reduction was generally of smaller magnitude (RR 0.83, 95% CI 0.74 to 0.93; five trials, 3662 participants, Analysis 1.3, high quality evidence). In addition, measures of mean haemoglobin in the third trimester were higher in those receiving chemoprevention (MD 0.41 g/dL, 95% CI 0.29 to 0.54; five trials, 3363 participants, Analysis 1.4).
1.3. Analysis.
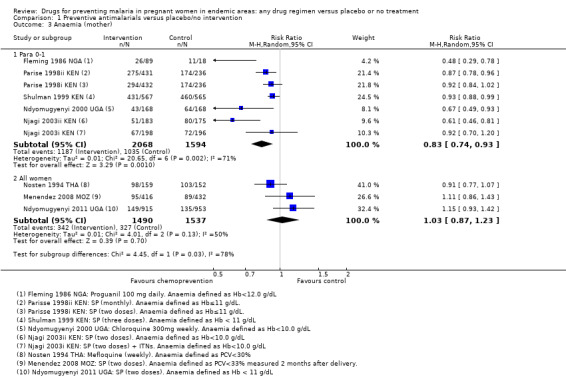
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 3 Anaemia (mother).
Chemoprevention was associated with fewer episodes of presumed clinical malaria (history of fever), but this outcome was only reported in two small trials (RR 0.37, 95% CI 0.18 to 0.74; two trials, 307 participants, Analysis 1.5, low quality evidence). Instead most trials reported antenatal parasitaemia, defined as either parasitaemia at delivery or parasitaemia at 34 to 36 weeks, with most trials showing benefits but wide variation in the size of the reduction (RR 0.39, 95% CI 0.26 to 0.58; eight trials, 3663 participants, I2 = 82; Analysis 1.6, high quality evidence) This heterogeneity is probably not unexpected given the differences in chemoprevention regimens and malaria endemicity.
1.5. Analysis.
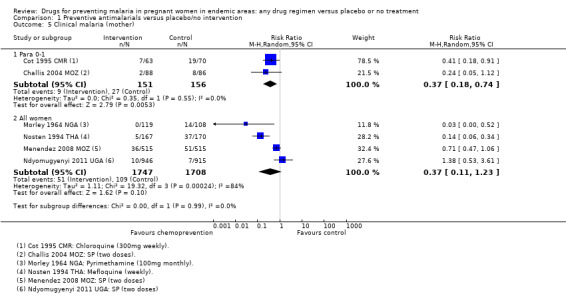
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 5 Clinical malaria (mother).
1.6. Analysis.
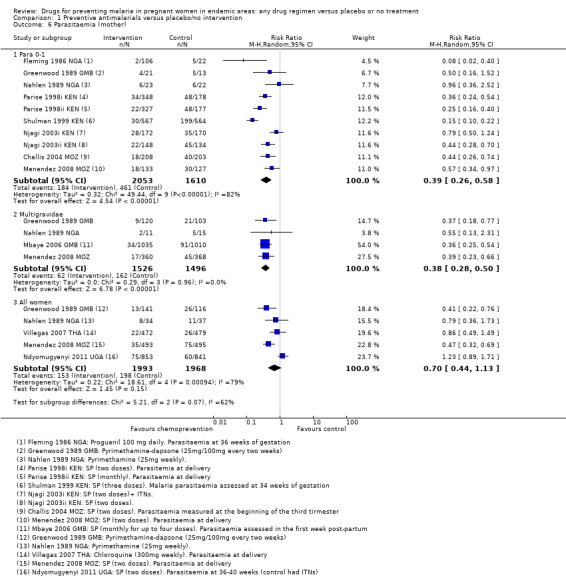
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 6 Parasitaemia (mother).
Infant outcomes (see Table 2).
The trials and the meta‐analyses are underpowered to confidently detect or exclude effects on spontaneous abortion, perinatal deaths, or neonatal deaths (see Table 9). The CIs range from important benefits to no evidence of any harm in four outcomes: spontaneous abortions (RR 0.65, 95% CI 0.41 to 1.02; five trials, 2876 participants, Analysis 1.9, low quality evidence); perinatal deaths (RR 0.73, 95% CI 0.54 to 1.00; two trials, 1620 participants, Analysis 1.11, low quality evidence); neonatal deaths (RR 0.62, 95% CI 0.37 to 1.05; two trials, 2156 participants, Analysis 1.12, low quality evidence). The preterm births analysis was (RR 0.85, 95% CI 0.66 to 1.10; two trials, 1493 participants, Analysis 1.13, low quality evidence).
1.9. Analysis.
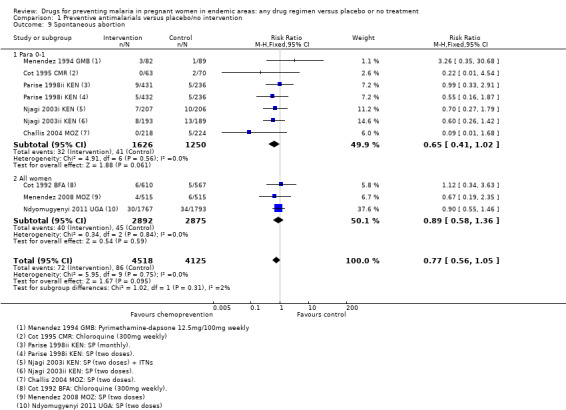
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 9 Spontaneous abortion.
1.11. Analysis.
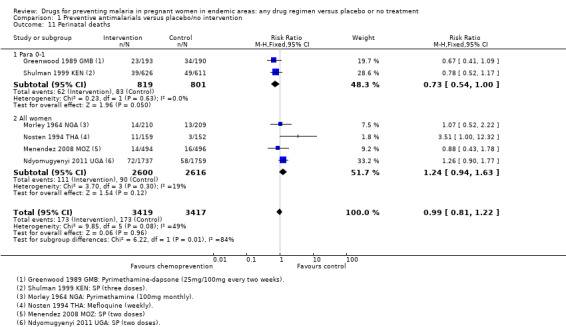
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 11 Perinatal deaths.
1.12. Analysis.
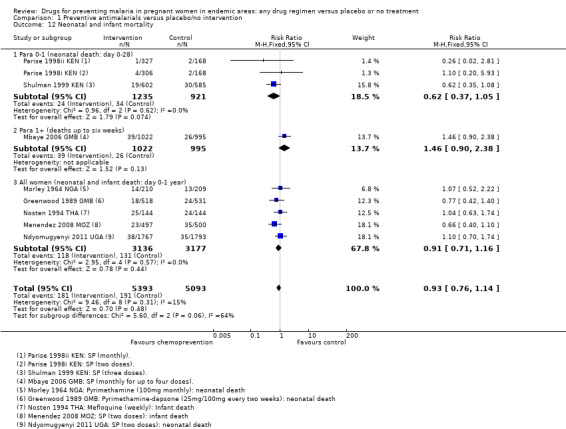
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 12 Neonatal and infant mortality.
1.13. Analysis.
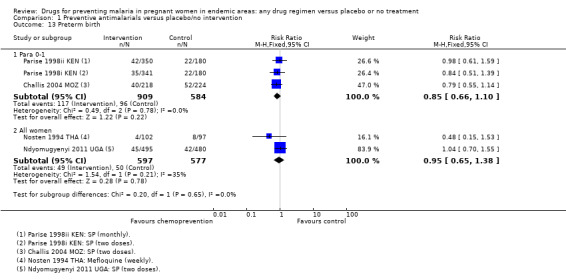
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 13 Preterm birth.
Chemoprevention was associated with fewer low birthweight infants (RR 0.73, 95% CI 0.61 to 0.87; eight trials, 3619 participants, Analysis 1.14, moderate quality evidence). and mean birthweight was higher with chemoprevention (MD 92.72 g, 95% CI 62.05 to 123.39; nine trials, 3936 participants, Analysis 1.15, moderate quality evidence).
1.14. Analysis.
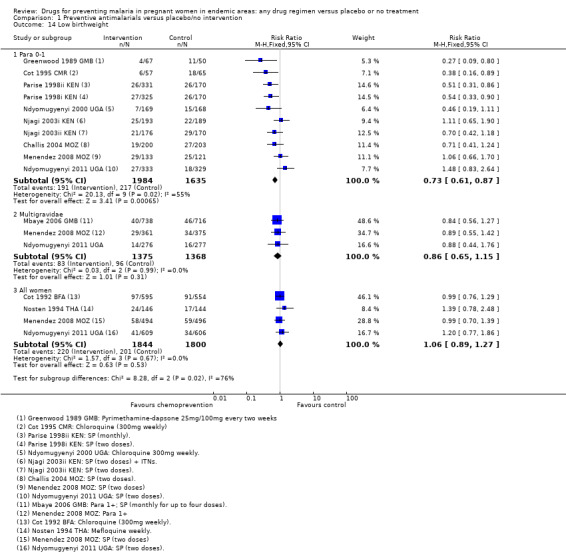
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 14 Low birthweight.
1.15. Analysis.
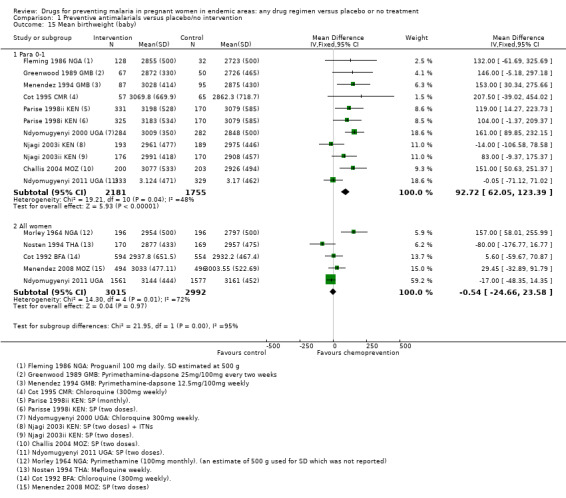
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 15 Mean birthweight (baby).
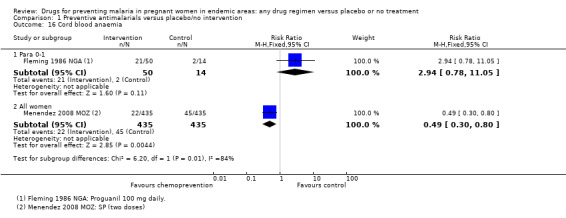
One very small trial reported no difference in the prevalence of cord blood anaemia (64 participants, Analysis 1.16), and a lower cord blood haemoglobin in babies born to women receiving chemoprevention (MD ‐1.80 g/dL, 95% CI ‐3.46 to ‐0.14; one trial, 64 participants, Analysis 1.17, very low quality evidence).
1.16. Analysis.
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 16 Cord blood anaemia.
1.17. Analysis.
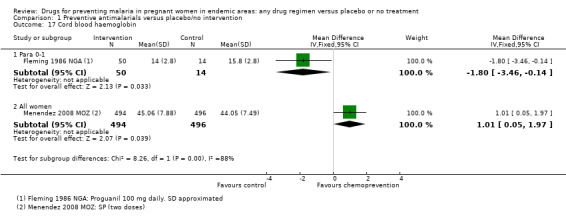
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 17 Cord blood haemoglobin.
Chemoprevention resulted in fewer cases of placental parasitaemia (RR 0.54, 95% CI 0.43 to 0.69; seven trials, 2830 participants, Analysis 1.17, high quality evidence). Only one trial examined cord blood parasitaemia, but there were too few events to be confident of the result (RR 0.47, 95% CI 0.22 to 1.01; one trial, 1335 participants, Analysis 1.19). The children born to mothers receiving monthly SP had reduced cord parasitaemia, whereas those born to mothers receiving two doses of SP did not (Parise 1998i KEN).
1.19. Analysis.
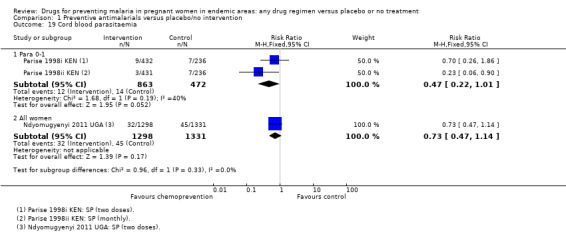
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 19 Cord blood parasitaemia.
Chemoprevention for multigravidae
Maternal outcomes (see Table 3).
Four trials provided data on multigravidae women. Only one trial assessed mortality with six deaths in the chemoprevention group and four in the control group (RR 1.47, 95% CI 0.42 to 5.21; one trial, 2239 participants, Analysis 1.1, very low quality evidence).
No trials reported episodes of severe malaria, but two reported severe anaemia. In one trial more women had severe anaemia in the chemoprevention group (RR 1.20, 95% CI 0.91 to 1.57; one trial, 1954 participants), and the second trial had few events and consequently very wide CIs (RR 0.41, 95% CI 0.08 to 2.09; one trial, 728 participants). The 95% CIs of the overall meta‐analysis does not exclude effects as large as those seen in women in their first or second pregnancy but this is probably unlikely (RR 0.96, 95% CI 0.41 to 2.25; two trials, 2682 participants, Analysis 1.2).
No trials reported the risk of mild anaemia, but two trials reported mean haemoglobin at delivery without clinically important differences between groups (MD 0.01 g/dL, 95% CI ‐0.23 to 0.24; two trials, 676 participants, Analysis 1.4).
No trial measured malaria or febrile episodes in the mother. Four trials reported antenatal parasitaemia, and all four trials report large effects of a similar magnitude to those seen in women in their first or second pregnancy (RR 0.38, 95% CI 0.28 to 0.50; four trials, 3022 participants, Analysis 1.6, high quality evidence).
Infant outcomes (see Table 4).
Two trials included information on infant outcomes after chemoprevention given to multigravid women.
Spontaneous abortions, stillbirths and perinatal deaths were not reported. One trial reported deaths in the first six weeks of life with slightly higher deaths following chemoprevention, but with wide CIs including the possibility of no difference between groups (RR 1.46, 95% CI 0.90 to 2.38; one trial, 2017 participants, Analysis 1.12).
No trials reported mean birthweight in infants born to multigravid women, but three reported the risk of low birthweight. The trend is in favour of chemoprevention but neither the trials, or the meta‐analysis reached standard levels of statistical significance (RR 0.86, 95% CI 0.64 to 1.17; three trials, 2743 participants, Analysis 1.14, very low quality evidence).
No trials reported measures of placental parasitaemia, cord blood parasitaemia, or cord blood haemoglobin.
Chemoprevention for all women
To evaluate the population effects of a policy of chemoprevention for all pregnant women, regardless of parity, this third analysis includes all trials which recruited women of any parity. Some of these presented results stratified by parity and were included in the analyses above, but a few additional trials did not provide their outcome data stratified by parity.
Maternal outcomes (see Table 5).
For maternal mortality, only nine maternal deaths were recorded in trials recruiting women of all parities; 4/3019 with chemoprevention and 5/3007 without (four trials, 6026 participants, Analysis 1.1, low quality evidence).
For severe anaemia in the mother, there were very few events recorded in the two trials but the risk was lower with chemoprevention (RR 0.19, 95% CI 0.05 to 0.75; two trials, 1327 participants, Analysis 1.2, low quality evidence). For any anaemia, no population differences were demonstrated (RR 1.03, 95% CI 0.87 to 1.23; three trials, 3027 participants, Analysis 1.3, moderate quality evidence). Three trials reported mean haemoglobin, with only one very small trial from the early 1990s finding benefit with chemoprevention (three trials, 2223 participants, Analysis 1.4).
Clinical malaria (or history of fever) was reported in four of the trials across all parity groups. The older, and smaller trials, suggested a population benefit on clinical malaria but this was not seen in the two recent and much larger trials using two doses of SP (four trials, 3455 participants, Analysis 1.5, low quality evidence).
For parasitaemia at delivery, there was considerable heterogeneity between trials (I2 = 79%). Of the two most recent trials, both large, and both administering two doses of SP, one trial from Mozambique demonstrated a benefit with chemoprevention and one from Uganda did not (five trials, 3961 participants, Analysis 1.6, low quality evidence).
Infant outcomes (see Table 6).
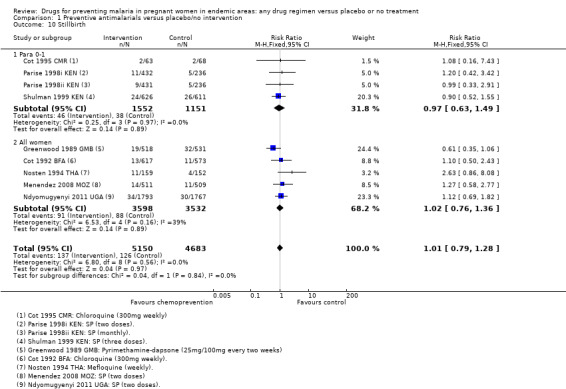
In trials recruiting women of all parities, no differences were demonstrated for spontaneous abortions (three trials, 5767 participants, Analysis 1.9, low quality evidence), stillbirths (five trials, 7130 participants, Analysis 1.10, moderate quality evidence), perinatal deaths (four trials, 5216 participants, Analysis 1.11, moderate quality evidence), or neonatal and infant deaths (five trials, 6313 participants, Analysis 1.12, moderate quality evidence). We also pooled across all trials for these outcomes (including those which only recruited women in their first or second pregnancies), and no differences were demonstrated.
1.10. Analysis.
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 10 Stillbirth.
Population benefits for the infants were not demonstrated for pre‐term birth (two trials, 1174 participants, Analysis 1.13, low quality evidence), low birthweight (four trials, 3644 participants, Analysis 1.14, low quality evidence), or mean birthweight (five trials, 6007 participants, Analysis 1.15, moderate quality evidence).
The effects of chemoprevention on placental parasitaemia were mixed (I2 = 94%), with large effects in two older trials administering monthly pyrimethamine or weekly chloroquine, and no effect demonstrated in the two more recent trials administering two doses of SP (four trials, 3200 participants, Analysis 1.18, low quality evidence).
1.18. Analysis.
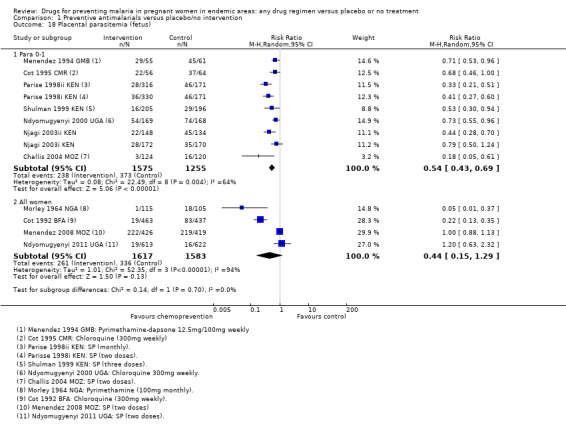
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 18 Placental parasitemia (fetus).
One trial in Mozambique found a large effect in reducing the risk of cord blood anaemia (RR 0.49, 95% CI 0.30 to 0.80; one trial, 870 participants, Analysis 1.16), and increase in mean cord PCV (MD 1.01%, 95% CI 0.05 to 1.97; one trial, 990 participants, Analysis 1.17).
Adverse effects
We aggregated adverse effects across all parity groups. Reporting of adverse effects was generally poor. Only five trials specifically stated that no adverse effects attributable to the drugs were observed in the mothers, and the rest either did not report adverse effects or the information was unclear. Four trials reported adverse events following SP (Analysis 1.7), and one trial following mefloquine (Analysis 1.8). No differences were seen between the treatment and control groups.
1.7. Analysis.
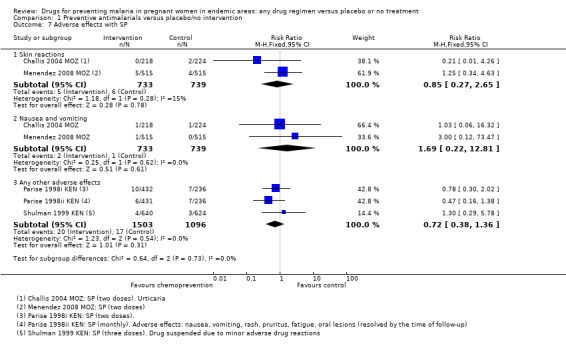
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 7 Adverse effects with SP.
1.8. Analysis.
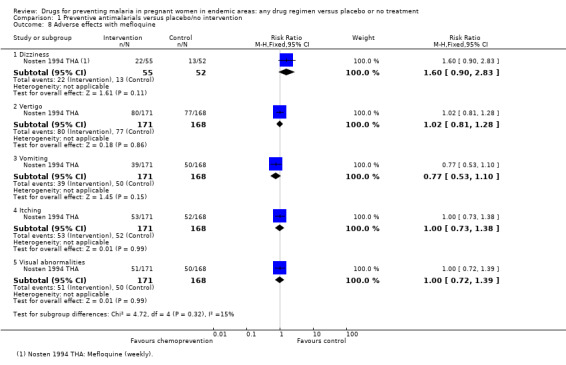
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 8 Adverse effects with mefloquine.
Again, reporting of adverse events in the neonate was generally poor. Episodes of neonatal kernicterus were reported in two trials, and congenital anomalies in two trials, with no differences detected (Analysis 1.20).
1.20. Analysis.
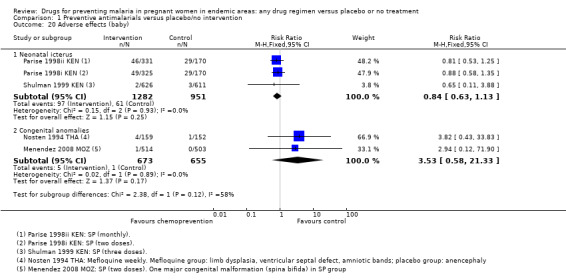
Comparison 1 Preventive antimalarials versus placebo/no intervention, Outcome 20 Adverse effects (baby).
Comparison 2. SP IPT chemoprevention for women in their first or second pregnancy
The above analysis examines the effects of drugs known to be effective in preventing malaria at the particular time the trials were carried out. As the WHO currently recommends intermittent dosing with SP, we performed an additional analysis to provide the effect estimates for SP compared to no drug or placebo. The analysis is exactly the same as comparison one, but we included only the six SP trials. These trials administered SP in two doses (Parise 1998i KEN; Njagi 2003i KEN; Njagi 2003ii KEN; Challis 2004 MOZ; Menendez 2008 MOZ; Ndyomugyenyi 2011 UGA), three doses (Shulman 1999 KEN), or monthly (Parise 1998ii KEN).
Maternal outcomes (see Table 7).
For maternal death, no effect was demonstrated but the analysis is underpowered (Analysis 2.1).
2.1. Analysis.
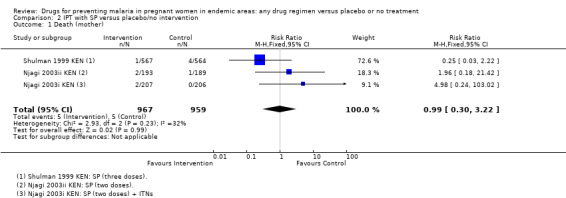
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 1 Death (mother).
For women of low parity, restricting the analysis to trials of SP did not substantially change the estimates of benefit on severe anaemia (RR 0.60, 95% CI 0.47 to 0.75; three trials, 2503 participants, Analysis 2.2, high quality evidence), mild anaemia (RR 0.88, 95% CI 0.88 to 0.96; three trials, 3219 participants, Analysis 2.3, moderate quality evidence), or mean haemoglobin (MD 0.41 g higher, 95% CI 0.27 to 0.54; three trials, 2995 participants, Analysis 2.4).
2.2. Analysis.
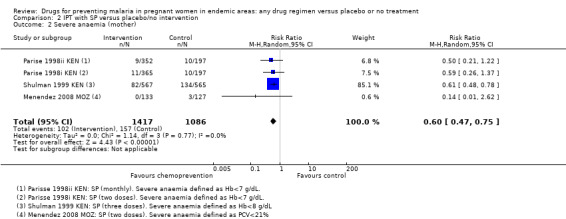
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 2 Severe anaemia (mother).
2.3. Analysis.
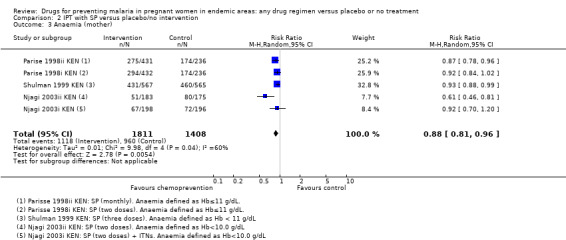
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 3 Anaemia (mother).
2.4. Analysis.
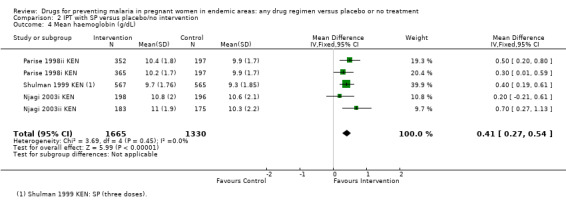
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 4 Mean haemoglobin (g/dL).
Similarly, the reduction in antenatal parasitaemia is consistent with the overall effect from trials of any chemoprevention (RR 0.38, 95% 0.24 to 0.59; four trials, 2832 participants, Analysis 2.5, high quality evidence), but there is insufficient data to draw conclusions on clinical malaria (RR 0.24, 95% CI 0.05 to 1.12; one trial, 174 participants, very low quality evidence (Analysis 2.6).
2.5. Analysis.
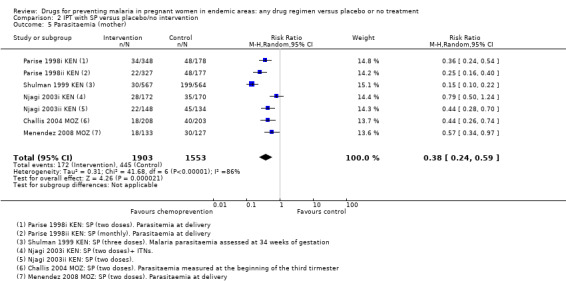
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 5 Parasitaemia (mother).
2.6. Analysis.
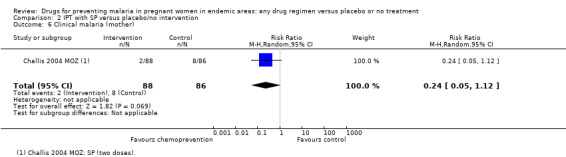
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 6 Clinical malaria (mother).
Infant outcomes (see Table 8).
The trials and the meta‐analyses are underpowered to confidently detect or exclude effects on spontaneous abortion, stillbirth, perinatal deaths, or neonatal deaths, but restricting the analysis to trials of SP did not substantially change the estimates of effect (see Analysis 2.7; Analysis 2.8; Analysis 2.9; Analysis 2.10; low quality evidence). The trend is towards a reduction in pre‐term birth but the 95% CI is wide and includes the possibility of no effect (RR 0.85, 95% CI 0.66 to 1.10; two trials, 1493 participants, Analysis 2.11, low quality evidence).
2.7. Analysis.
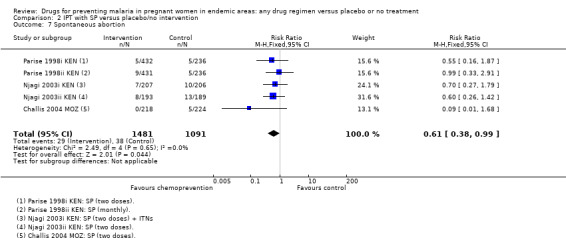
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 7 Spontaneous abortion.
2.8. Analysis.
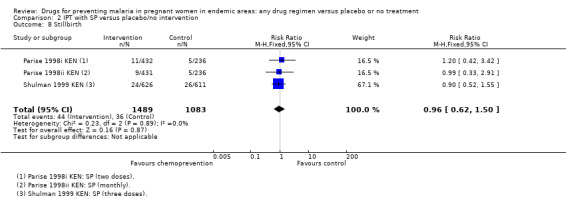
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 8 Stillbirth.
2.9. Analysis.
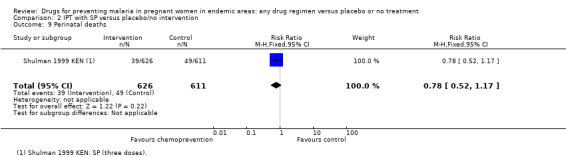
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 9 Perinatal deaths.
2.10. Analysis.
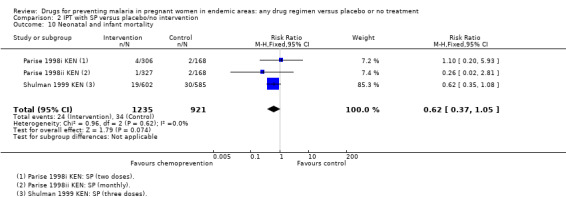
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 10 Neonatal and infant mortality.
2.11. Analysis.
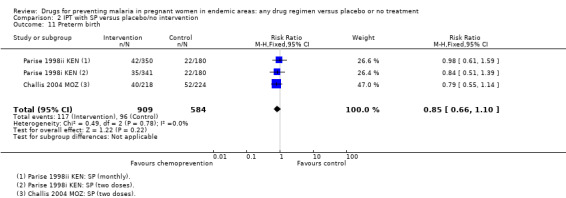
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 11 Preterm birth.
Overall, chemoprevention with SP reduced the incidence of low birthweight but this effect seems to be reducing over time, with large effects in the older trials and no effect seen in the more recent trials using two doses of SP (four trials, 3043 participants, Analysis 2.12, moderate quality evidence). However, mean birthweight was higher with SP, and this effect was still present in the most recent trials (MD 105.5 g, 95% CI 68.02 to 142.9, four trials, 2693 participants, Analysis 2.13, moderate quality evidence).
2.12. Analysis.
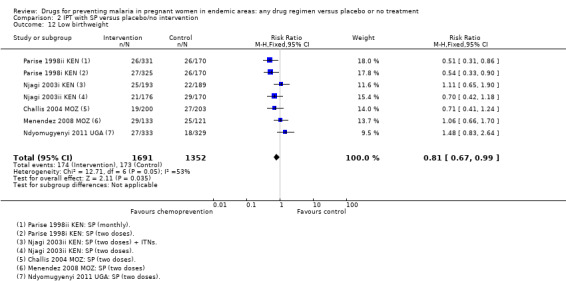
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 12 Low birthweight.
2.13. Analysis.
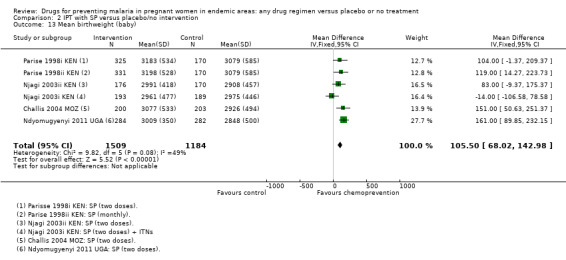
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 13 Mean birthweight (baby).
Chemoprevention with SP reduced placental parasitaemia (RR 0.45, 95% CI 0.33 to 0.61; three trials, 1633 participants, Analysis 2.14, high quality evidence) but only one trial of SP reported cord parasitaemia (RR 0.47, 95% CI 0.22 to 1.01; one trial, 1335 participants, Analysis 2.15).
2.14. Analysis.
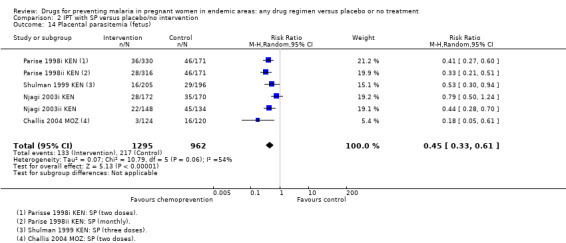
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 14 Placental parasitemia (fetus).
2.15. Analysis.
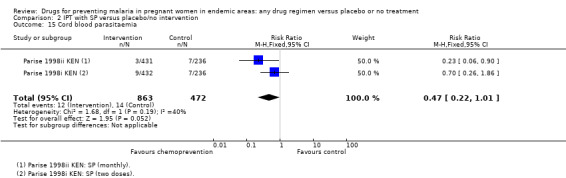
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 15 Cord blood parasitaemia.
Adverse effects
No effects were detected with icterus (two trials, 2233 participants, Analysis 2.16) or congenital abnormalities (one trial, 1017 participants, Analysis 2.16).
2.16. Analysis.
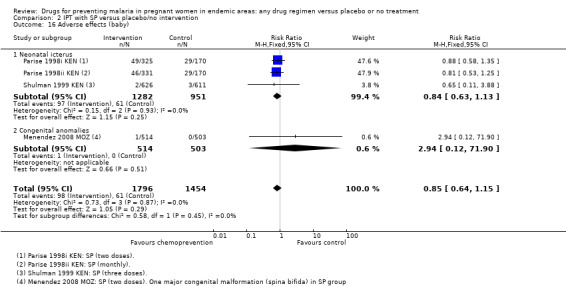
Comparison 2 IPT with SP versus placebo/no intervention, Outcome 16 Adverse effects (baby).
Comparison 3. Chemoprevention for P. vivax
Only one trial reported on chemoprevention for P. vivax, conducted in Thailand with weekly prophylaxis with chloroquine. It was rated at low risk of bias on all criteria. It seemed to prevent completely all episodes of P. vivax malaria (RR 0.01, 95% CI 0.00 to 0.20; 942 participants, see Table 10), but had no effect on maternal anaemia, low birthweight, or mean birthweight. It was underpowered to assess effects on mortality.
2. Chloroquine versus placebo (effect on P. vivax malaria).
| Outcomes | Trials | Participants | Effect estimate | Comment |
| Death (mother) | 1 | 951 | Risk ratio 0.34 (0.01, 8.28) | ‐ |
| Severe anaemia | 1 | ‐ | ‐ | Not reported |
| Anaemia | 1 | 951 | Risk ratio 1.00 (0.92, 1.08) | Defined as PCV < 30% |
| Clinical malaria | 1 | ‐ | ‐ | Not reported |
| P. vivax parasitaemia | 1 | 942 | Risk ratio 0.01 (0.00, 0.20) | History of antenatal parasitaemia. Nine women censored (they had P. falciparum infection prior to their first P. vivax episode) |
| Adverse effects with chloroquine | 1 | 951 | Risk ratio 2.03 (0.18, 22.31) | The 5 most commonly reported adverse events were headache, anorexia, sleep disorder, dizziness and weakness. CQ group: drug suspended in two cases (1 ‐ constipation,1‐ nausea) One woman in the placebo group was complaining of visual problems |
| Spontaneous abortion | 1 | 951 | Risk ratio 0.71 (0.36, 1.39) | ‐ |
| Stillbirth | 1 | 865 | Risk ratio 0.24 (0.03, 2.17) | ‐ |
| Perinatal deaths | 1 | ‐ | ‐ | Not reported |
| Neonatal and infant mortality | 1 | ‐ | ‐ | Not reported |
| Preterm birth (All) | 1 | 733 | Risk ratio 0.93 (0.46, 1.85) | ‐ |
| Preterm birth (Para 0) | 1 | 141 | Risk ratio 2.41 (0.63, 9.24) | ‐ |
| Preterm birth (Para 2+) | 1 | 592 | Risk ratio 0.62 (0.26, 1.46) | ‐ |
| Low birthweight (All) | 1 | 733 | Risk ratio 1.02 (0.71, 1.46) | ‐ |
| Low birthweight (Para 0) | 1 | 141 | Risk ratio 1.20 (0.65, 2.21) | ‐ |
| Low birthweight (Para 2+) | 1 | 592 | Risk ratio 0.94 (0.60, 1.47) | ‐ |
| Mean birthweight (All) | 1 | 733 | Mean difference ‐8.20 (‐73.41, 57.02) | ‐ |
| Mean birthweight (Para 0) | 1 | 141 | Mean difference ‐36.00 (‐188.73, 116.73) | Mean (SD) 2741 ± 481 versus 2777 ± 435 in the CQ versus placebo group |
| Mean birthweight (Para 2+) | 1 | 592 | Mean difference ‐2.00 (‐74.12, 70.12) | Mean (SD) 2954 ± 423 versus 2956 ± 471 in the CQ versus placebo group |
| Placental malaria | 1 | ‐ | ‐ | Not reported |
| Cord blood haemoglobin | 1 | ‐ | ‐ | Not reported |
| Cord blood parasitaemia | 1 | ‐ | ‐ | Not reported |
| Adverse effects (baby) | 1 | 864 | Risk ratio 1.22 (0.33, 4.50) | Congenital anomalies: Amniotic banding, brachydactyly; anophthalmia, Down's syndrome,; amniotic banding, absent digit toes; two cleft lip, one cleft palate in the placebo group. |
Discussion
Summary of main results
We included 17 trials, enrolling 14,481 pregnant women, in this Cochrane Review.
For women in their first or second pregnancy, malaria chemoprevention reduces the risk of moderate to severe anaemia by around 40% (high quality evidence), and the risk of any anaemia by around 17% (high quality evidence). Malaria chemoprevention reduces the risk of antenatal parasitaemia by around 61% (high quality evidence), and two trials reported a reduction in febrile illness (low quality evidence). There were only 16 maternal deaths and these trials were underpowered to detect an effect on maternal mortality (very low quality evidence).
For infants of women in their first and second pregnancies, malaria chemoprevention probably increases mean birthweight by around 93 g (moderate quality evidence), reduces low birthweight by around 27% (moderate quality evidence), and reduces placental parasitaemia by around 46% (high quality evidence). Fewer trials evaluated spontaneous abortions, still births, perinatal deaths, or neonatal deaths, and these analyses were underpowered to detect clinically important differences.
In multigravid women, chemoprevention has similar effects on antenatal parasitaemia (high quality evidence) but there are too few trials to evaluate effects on other outcomes.
In trials giving chemoprevention to all pregnant women irrespective of parity, the average effects of chemoprevention measured in all women indicated it may prevent severe anaemia (low quality evidence), but consistent benefits have not been shown for other outcomes.
In an analysis confined only to intermittent preventive therapy with SP, the estimates of effect and the quality of the evidence were similar.
A summary of a single trial in Thailand of prophylaxis against vivax showed chloroquine prevented vivax infection (RR 0.01, 95% CI 0.00 to 0.20; 942 participants).
Overall completeness and applicability of evidence
Trials were almost exclusively from Africa and published between 1964 and 2011. These trials, from a variety of settings and using varied chemoprevention regimens, found fairly consistent clinically important benefits for low parity women and their infants.
However, it is possible that with the introduction of ACTs, declining malaria transmission in some areas of Africa, and increasing quality of antenatal services, that the attributable fraction of malaria towards maternal anaemia and low birthweight has been reduced and the large effects seen in these trials may be attenuated by less malaria and better individualized care of women during pregnancy.
Quality of the evidence
The evidence for effects on maternal, foetal and neonatal mortality is generally considered of low or very low quality because the trials and the meta‐analysis remain significantly underpowered to confidently prove or exclude clinically important effects.
For women of low parity, we considered the evidence of clinically important effects on anaemia and antenatal parasitaemia to be of high quality, meaning we can have confidence in these results. For the infants of women of low parity, we considered the effects on birthweight to only be of moderate quality because of the high risk of bias of most of the older trials. This means we can have only moderate confidence in the magnitude of these effects.
Trials did not describe the routine health services available to detect and treat malaria infection in both intervention and control arms, but many trials were done some years ago in areas with very basic curative health services available. However, in the future with declining levels of malaria the individual management of illness and malaria at clinic may become an important option to control malaria in pregnancy.
Potential biases in the review process
It seems unlikely that we have missed any trials. As trials did not systematically document adverse effects, it is likely that these have been underestimated in this review.
Agreements and disagreements with other studies or reviews
The findings of this Cochrane Review are consistent with previous editions (Garner 2006; ter Kuile 2007). The findings are also consistent with the findings of a review comparing observational and randomized evidence (McClure 2013). McClure 2013 points out that the fairly modest effects seen in RCTs, where delivery of care is often strengthened and adherence assured, were attenuated in the observational studies where, the authors surmise, delivery of the intervention and adherence to it may be attenuated. However, this contrasts with a study estimating the effects of IPT with SP on low birthweight and neonatal mortality from survey data: the trial estimates are remarkably similar to the results observed with IPT with SP from the trial data reported in this and previous analysis (Eisele 2012; ter Kuile 2007).
Authors' conclusions
Implications for practice.
Routine chemoprevention to prevent malaria and its consequences has been extensively tested in RCTs, with clinically important benefits on anaemia and parasitaemia in the mother, and on birthweight in infants.
The data also assists in showing the potential attribution of malaria towards key endpoints, and what can be achieved by successful prevention to assist in modelling studies examining the impact of malaria on pregnancy.
Implications for research.
Identifying current effective chemoprevention regimens remains a challenge, especially with the spread of drug‐resistant malaria, in particular against SP which is the only antimalarial currently recommended for IPT in pregnant women. There is justification for assessing the safety and efficacy of effects of alternative drugs that can replace SP in areas with high SP resistance, or alternative strategies that could replace IPT during pregnancy, such as intermittent screen and treat (IST) approaches that focus on prompt accessible treatment for anaemia and asymptomatic parasitaemia (Tagbor 2010).
All new trials should systematically and carefully collect adverse effects of regimens.
The data on the longer term impact on infants is poor and needs further study: currently the evidence mainly relates to effects on clinically important outcomes, such as preterm birth and birthweight.
There is a dearth of data from endemic areas outside of Africa, such as Asia and Latin America.
What's new
| Date | Event | Description |
|---|---|---|
| 29 September 2014 | New citation required but conclusions have not changed | We repeated all searches. Trial inclusion criteria, data extraction, risk of bias assessment, and data entry were all done afresh. We additionally carried out GRADE analysis and a sensitivity analysis of IPT. Contributions of individuals are outlined in section 'Contributions of authors'. |
| 29 September 2014 | New search has been performed | Review updated. |
History
Protocol first published: Issue 1, 1995 Review first published: Issue 1, 1995
| Date | Event | Description |
|---|---|---|
| 16 September 2008 | Amended | Converted to new review format with minor editing. |
| 20 August 2006 | Amended | 2006, Issue 4: added Challis 2004 MOZ and Kayentao 2005 MLIa; meta‐analysis stratified by prophylaxis and intermittent preventive treatment; review title shortened. |
| 20 November 2002 | Amended | 2003, Issue 1: Review overhauled to reflect current methods; title was altered to "Drugs for preventing malaria‐related illness in pregnant women and death in the newborn" (from "Prevention versus treatment for malaria in pregnant women"); we excluded mosquito nets as these are now covered by Gamble 2006; primary outcome measures were adjusted following feedback from readers; methodological quality of trials reassessed; Martin 1982 trial previously included, but now excluded because it is not randomized. |
| 28 February 2001 | Amended | Primary outcome measures defined; Parise 1998 trial added. |
Acknowledgements
We thank Metin Gulmezoglu, an author on a previous version of this Cochrane Review. Others contributed to earlier published versions of this review and are acknowledged there. In this version, we thank Mari Luntamo and her team for contributing unpublished data and stratified analysis by HIV status of the mother, not included in their original published paper.
This document is an output from a project funded by the UK Department for International Development (DFID) for the benefit of developing countries. The views expressed are not necessarily those of DFID.
The editorial base for the Cochrane Infectious Diseases Group is funded by UK Aid from the UK Government for the benefit of developing countries.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | malaria | MALARIA | MALARIA | MALARIA | malaria |
| 2 | pregnan* | malaria | malaria | malaria | pregnan* |
| 3 | 1 and 2 | 1 or 2 | 1 or 2 | 1 or 2 | 1 and 2 |
| 4 | — | PREGNANCY | PREGNANCY | PREGNANCY | — |
| 5 | — | pregnan* | pregnan* | pregnan$ | — |
| 6 | — | 4 or 5 | 4 or 5 | 4 or 5 | — |
| 7 | — | 3 and 6 | 3 and 6 | 3 and 6 | — |
| 8 | — | — | Limit 7 to human | Limit 7 to human | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2005); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 2. Chemoprophylaxis regimens evaluated in the trials
| Chemoprevention regimen | Trials | ||
| Drug | Dose | Frequency | |
| Chloroquine | 300 mg | Weekly | Cot 1992 BFA; Cot 1995 CMR; Ndyomugyenyi 2000 UGA; Villegas 2007 THA |
| Pyrimethamine | 100 mg | Monthly | Morley 1964 NGA |
| 25 mg | Weekly | Nahlen 1989 NGA | |
| Proguanil | 100 mg | Daily | Fleming 1986 NGA |
| Pyrimethamine‐dapsone | 25 mg/100 mg | Every two weeks | Greenwood 1989 GMB |
| 12.5 mg/100 mg | Weekly | Menendez 1994 GMB | |
| Sulfadoxine‐pyrimethamine | 1500 mg/75 mg | One to two doses | Shulman 1999 KEN |
| Two doses | Challis 2004 MOZ; Menendez 2008 MOZ; Njagi 2003i KEN; Parise 1998i KEN | ||
| Up to four doses | Mbaye 2006 GMB | ||
| Monthly | Parise 1998ii KEN | ||
| Mefloquine | 500 mg loading dose, 250 mg weekly for 4 weeks, 125 mg weekly until delivery | Weekly | Nosten 1994 THA |
Appendix 3. Trial participants: number of previous pregnancies
| No. of pregnancies | Trials | number of trials |
| All women | Morley 1964 NGA; Nahlen 1989 NGA; Cot 1992 BFA; Nosten 1994 THA; Greenwood 1989 GMB; Villegas 2007 THA; Menendez 2008 MOZ; Ndyomugyenyi 2011 UGA; | 8 |
| First pregnancy | Fleming 1986 NGA; Menendez 1994 GMB; Cot 1995 CMR; Shulman 1999 KEN; Ndyomugyenyi 2000 UGA; Challis 2004 MOZ | 6 |
| First or second pregnancy | Parise 1998i KEN; Njagi 2003ii KEN | 2 |
| Only multiparous women | Mbaye 2006 GMB | 1 |
Nahlen 1989 NGA; Greenwood 1989 GMB; Menendez 2008 MOZ all provided data disaggregated by parity.
Appendix 4. Percentage of randomized participants included in the analyses
| Trial | Women | Newborns | ||||
| Outcome | n/Na | % in analysis | Outcome | n/Na | % in analysis | |
| Challis 2004 MOZ | Parasitaemia | 411/600 | 69 | Low birthweight | 403/600 | 67 |
| Cot 1992 BFA | Placental malaria | 904/1464 | 62 | Birthweight | 1148/1148 | 100 |
| Cot 1995 CMR | Placental malaria | 120/266 | 57 | Birthweight | 209/266 | 79 |
| Fleming 1986 NGA | Haemoglobin | 107/200 | 45 | Perinatal death | 152/200 | 76 |
| Greenwood 1989 GMB | Parasitaemia | 257/1049 | 24 | Birthweight | 877/1034 | 85 |
| Menendez 1994 GMB | Placental malaria | 116/230 | 50 | Birthweight | 182/203 | 90 |
| Morley 1964 NGA | Antenatal parasitaemia | 227/429 | 53 | Birthweight | 429/429 | 100 |
| Nahlen 1989 NGA | Parasitaemia | 71/71 | 100 | — | — | — |
| Ndyomugyenyi 2000 UGA | Anaemia | 510/860 | 59 | Congenital malaria | 337/510 | 66 |
| Nosten 1994 THA | Parasitaemia | 399/399 | 100 | Birthweight | 290/290 | 100 |
| Parise 1998i KEN, Parise 1998ii KEN | Haemoglobin | 1378/2077 | 66 | — | — | — |
| Shulman 1999 KEN | Severe anaemia | 1132/1264 | 90 | — | — | — |
aNumber analysed/number randomized.
Data and analyses
Comparison 1. Preventive antimalarials versus placebo/no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (mother) | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Para 0‐1 | 4 | 2097 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.44, 3.06] |
| 1.2 Multigravidae | 1 | 2239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.42, 5.21] |
| 1.3 All women | 4 | 6026 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.25, 2.74] |
| 2 Severe anaemia (mother) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Para 0‐1 | 4 | 2503 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.47, 0.75] |
| 2.2 Multigravidae | 2 | 2682 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.41, 2.25] |
| 2.3 All women | 2 | 1327 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.05, 0.75] |
| 3 Anaemia (mother) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Para 0‐1 | 7 | 3662 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.74, 0.93] |
| 3.2 All women | 3 | 3027 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.87, 1.23] |
| 4 Mean haemoglobin (g/dL) | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Baseline Hb | 5 | 3004 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.10, 0.17] |
| 4.2 Para 0‐1 | 7 | 3363 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.29, 0.54] |
| 4.3 Multigravidae | 2 | 676 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.23, 0.24] |
| 4.4 All women | 3 | 2223 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.00, 0.25] |
| 5 Clinical malaria (mother) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Para 0‐1 | 2 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.18, 0.74] |
| 5.2 All women | 4 | 3455 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.11, 1.23] |
| 6 Parasitaemia (mother) | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Para 0‐1 | 10 | 3663 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.26, 0.58] |
| 6.2 Multigravidae | 4 | 3022 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.28, 0.50] |
| 6.3 All women | 5 | 3961 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.44, 1.13] |
| 7 Adverse effects with SP | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Skin reactions | 2 | 1472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.65] |
| 7.2 Nausea and vomiting | 2 | 1472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.22, 12.81] |
| 7.3 Any other adverse effects | 3 | 2599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.38, 1.36] |
| 8 Adverse effects with mefloquine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Dizziness | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.90, 2.83] |
| 8.2 Vertigo | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.81, 1.28] |
| 8.3 Vomiting | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.10] |
| 8.4 Itching | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.38] |
| 8.5 Visual abnormalities | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 9 Spontaneous abortion | 10 | 8643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.05] |
| 9.1 Para 0‐1 | 7 | 2876 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.41, 1.02] |
| 9.2 All women | 3 | 5767 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.36] |
| 10 Stillbirth | 9 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.28] |
| 10.1 Para 0‐1 | 4 | 2703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.63, 1.49] |
| 10.2 All women | 5 | 7130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.36] |
| 11 Perinatal deaths | 6 | 6836 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.22] |
| 11.1 Para 0‐1 | 2 | 1620 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.54, 1.00] |
| 11.2 All women | 4 | 5216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.94, 1.63] |
| 12 Neonatal and infant mortality | 9 | 10486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.76, 1.14] |
| 12.1 Para 0‐1 (neonatal death: day 0‐28) | 3 | 2156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.37, 1.05] |
| 12.2 Para 1+ (deaths up to six weeks) | 1 | 2017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.90, 2.38] |
| 12.3 All women (neonatal and infant death: day 0‐1 year) | 5 | 6313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.71, 1.16] |
| 13 Preterm birth | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Para 0‐1 | 3 | 1493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.10] |
| 13.2 All women | 2 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.65, 1.38] |
| 14 Low birthweight | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Para 0‐1 | 10 | 3619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.87] |
| 14.2 Multigravidae | 3 | 2743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.65, 1.15] |
| 14.3 All women | 4 | 3644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.89, 1.27] |
| 15 Mean birthweight (baby) | 15 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 Para 0‐1 | 11 | 3936 | Mean Difference (IV, Fixed, 95% CI) | 92.72 [62.05, 123.39] |
| 15.2 All women | 5 | 6007 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐24.66, 23.58] |
| 16 Cord blood anaemia | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Para 0‐1 | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.78, 11.05] |
| 16.2 All women | 1 | 870 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.80] |
| 17 Cord blood haemoglobin | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 Para 0‐1 | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐3.46, ‐0.14] |
| 17.2 All women | 1 | 990 | Mean Difference (IV, Fixed, 95% CI) | 1.01 [0.05, 1.97] |
| 18 Placental parasitemia (fetus) | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Para 0‐1 | 9 | 2830 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.43, 0.69] |
| 18.2 All women | 4 | 3200 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.15, 1.29] |
| 19 Cord blood parasitaemia | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Para 0‐1 | 2 | 1335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.22, 1.01] |
| 19.2 All women | 1 | 2629 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.14] |
| 20 Adverse effects (baby) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Neonatal icterus | 3 | 2233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.13] |
| 20.2 Congenital anomalies | 2 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.53 [0.58, 21.33] |
Comparison 2. IPT with SP versus placebo/no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (mother) | 3 | 1926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.30, 3.22] |
| 2 Severe anaemia (mother) | 4 | 2503 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.47, 0.75] |
| 3 Anaemia (mother) | 5 | 3219 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.96] |
| 4 Mean haemoglobin (g/dL) | 5 | 2995 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.27, 0.54] |
| 5 Parasitaemia (mother) | 7 | 3456 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.24, 0.59] |
| 6 Clinical malaria (mother) | 1 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.05, 1.12] |
| 7 Spontaneous abortion | 5 | 2572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.38, 0.99] |
| 8 Stillbirth | 3 | 2572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.50] |
| 9 Perinatal deaths | 1 | 1237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.17] |
| 10 Neonatal and infant mortality | 3 | 2156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.37, 1.05] |
| 11 Preterm birth | 3 | 1493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.10] |
| 12 Low birthweight | 7 | 3043 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.67, 0.99] |
| 13 Mean birthweight (baby) | 6 | 2693 | Mean Difference (IV, Fixed, 95% CI) | 105.50 [68.02, 142.98] |
| 14 Placental parasitemia (fetus) | 6 | 2257 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.33, 0.61] |
| 15 Cord blood parasitaemia | 2 | 1335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.22, 1.01] |
| 16 Adverse effects (baby) | 4 | 3250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.15] |
| 16.1 Neonatal icterus | 3 | 2233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.13] |
| 16.2 Congenital anomalies | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.12, 71.90] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Challis 2004 MOZ.
| Methods | Trial design: RCT Data collected: 2001 to 2002 Length of follow‐up: from first antenatal visit to first week after delivery Frequency of follow‐up: monthly |
|
| Participants | Parity: 0‐1 Number: 600 Inclusion criteria: nulliparous and primiparous women under 21 years Excluded: none stated |
|
| Interventions |
Other: clinical malaria symptoms treated with CQ, SP or quinine and tetracycline irrespective of allotment Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: Mozambique Urban/rural: both (women from Matola ‐ town and Boane ‐ village) Malaria transmission: 20% prevalence Drug resistance: chloroquine resistance present HIV prevalence: 10% Funding: Department of Research Co‐operation with Developing countries (SAREC) at the Swedish International Development Authority (Sida) and from Mid Sweden Research and Development Centre (FoU) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The data were analysed on an ITT basis. ITT includes a random allocation procedure producing comparable groups and an analysis of the data according to the way we intended to treat the subjects". Women were "randomly assigned" to receive SP or placebo. No sufficient information provided how the allocation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Packages of SP or placebo tablets. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Three tablets (SP or placebo) were given in a double‐blind manner: either SP/SP – an initial treatment dose of SP at enrolment with a second dose at the beginning of the third trimester; or placebo/placebo…The placebo dose was three similar tablets in shape and colour as SP tablets." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided, except that all slides were analysed and double checked at the malaria laboratory at the Ministry of Health. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At second dose: 189/600 = 31.5% lost to follow‐up. At delivery: 309/600 women = 51.5% lost to the follow‐up peripheral blood analyses (153/300 = 51% from the placebo group and 156/300 = 52% from the SP group). |
| Selective reporting (reporting bias) | Low risk | No selective reporting observed. |
| Other bias | Low risk | None identified. |
Cot 1992 BFA.
| Methods | Trial design: Quasi‐RCT Data collected: 1987 to 1988 Length of follow‐up: approximately five months (from the first visit to the clinic which was for most women before the 5th month of pregnancy, until delivery) Frequency of follow‐up: twice a week |
|
| Participants | Parity: all women Number: 1464 Inclusion criteria: every pregnant woman attending urban maternal and child health centre Excluded: none stated |
|
| Interventions |
Other: no information Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: Burkina Faso Urban/rural: urban (the city of Banfora) Malaria transmission: hyperendemic, with seasonal transmission Drug resistance: chloroquine resistance may be present 19% parasitaemia in trial population Funding: INSERM (Institut National de Ia Santé et de Ia Recherche Médicale): Reseau Nord‐Sud no. 486 NS2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "For the sake of simplicity, an alternate allocation of treatment was performed, in which the women were divided into two groups (treated and control)." No specific procedure used to generate allocation sequence. |
| Allocation concealment (selection bias) | High risk | Allocation not concealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | "For technical reasons, it was not possible to give a placebo to women in the control group." Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Laboratory technicians had no information on the status of the individuals from whom the samples had been taken, as did the midwives who weighed the newborn babies" Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rate: 263/1464 (17.96%). There were 20.3 % (151/745 women) with no outcome in the experimental arm (chloroquine): 29 excluded after randomization (stillbirths, abortions, multiple pregnancies). The other 122/745 women (16.4%) delivered outside of the hospital. There were 22.9% (165/719 women) with no outcome in the control arm: 24 excluded, 141/719 (19.6%) delivered outside of the hospital. |
| Selective reporting (reporting bias) | Low risk | No selective reporting observed. |
| Other bias | Unclear risk | Approximately 20 women were allocated to the control group at the beginning of the trial and reclassified in the treated group a few days later. "These subjects were not clearly identified, and it was impossible to exclude them afterwards." |
Cot 1995 CMR.
| Methods | Trial design: Quasi‐RCT Data collected: 1991 to 1993 Length of follow‐up: from first prenatal visit until delivery (two to five months) Frequency of follow‐up: weekly |
|
| Participants | Parity: para 0 Number: 266 Inclusion criteria: primigravidae antenatal clinic attendees Excluded: none stated |
|
| Interventions |
Other: no information Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: Cameroon Urban/rural: urban (town of Ebolowa) Malaria transmission: hyperendemic area with high transmission all year round Drug resistance: moderate chloroquine resistance Funding: Ministère Français de la Coopération (FAC paludisme) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "After being examined by the hospital physician, any primigravida living in the study area and attending the clinic for a first prenatal visit… was introduced to an investigator who obtained their informed consent and allocated them alternately to a chloroquine treatment (CQ) group or a control (CT) group." Trial described as "randomized, double‐blind", but participants were "alternately allocated". |
| Allocation concealment (selection bias) | High risk | Allocation not concealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Women in the control group followed the usual hospital procedures; placebos were not used". Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded. No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was 21.4% (28/131) in the experimental arm (chloroquine) and 21.5% (29/135) in the control arm for the duration of the pregnancy. |
| Selective reporting (reporting bias) | Low risk | No selective reporting observed. Antenatal parasitaemia not clearly reported. |
| Other bias | High risk | "Of the CT group women, 39 (56%) declared that on their own initiative, they had taken one or more short treatments of either chloroquine or amodiaquine during the course of their pregnancy because they thought they had contracted malaria." Possible protocol violation. |
Fleming 1986 NGA.
| Methods | Trial design: RCT Data collected: unclear (before 1985). First attendance to the clinic: 1977 to 1978 Length of follow‐up: from first prenatal visit until 6 weeks after delivery Frequency of follow‐up: at least once every two weeks up to the 36th week of gestation and subsequently, weekly until delivery Haematological observations were performed at first attendance, 28 weeks and 36 weeks of gestation, at delivery and 6 weeks postpartum |
|
| Participants | Parity: para 0 Number: 200 Inclusion criteria: primigravidae under 16 years attending antenatal clinic; Hausa tribe Excluded: severe anaemia |
|
| Interventions |
Other: all received single dose chloroquine on entry; folic acid and iron supplements included in randomized design Administration supervised: no |
|
| Outcomes |
|
|
| Notes | Location: Nigeria Urban/rural: urban (Zaria) Malaria transmission: unstable area with seasonal transmission Drug resistance: none Funding: WHO, Ahmadu Bello University, Smith Kline and French Laboratories Ltd (UK) and Imperial Chemical Industries |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants "randomly allocated" to one of five treatment groups, using random numbers table. |
| Allocation concealment (selection bias) | Low risk | "Neither the researchers nor the patients were aware of the treatment allocated until after the completion of the study." Treatment allocation code was used. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The manufacturers supplied active tablets or spansules and the placebos, which could not be distinguished by sight." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded. No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Only 89 women out of 200 delivered in the hospital... 12/200 (6%) did not attend again (the clinic) after the first or second visits; a further 72/200 (36%) did not continue until the postnatal visit." Inadequate details but there is evidence to suggest that the attrition rate was quite high. |
| Selective reporting (reporting bias) | Low risk | No selective reporting observed. |
| Other bias | Unclear risk | "18 patients were replaced in the trial by others; this was arranged by a moderator (Dr. B. M. Greenwood), who was not otherwise involved in the research, but had access to the treatment allocation code for this purpose…Eighteen patients were replaced in the trial by others." |
Greenwood 1989 GMB.
| Methods | Trial design: Trial randomized by compound Data collected: 1984 to 1987 Length of follow‐up: from first prenatal visit until one week after delivery Frequency of follow‐up: unclear but administration was on weekly basis |
|
| Participants | Parity: all women Number: 1049 Inclusion criteria: all women in trial villages who became pregnant; some sub‐studies only followed up primigravidae Excluded: none stated |
|
| Interventions |
Given by village people employed by the project Other: no information Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: The Gambia Urban/rural: urban Malaria transmission: seasonal Drug resistance: none reported Funding: Unclear For the analysis we assumed that it is individually RCT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Once a woman had reported to a traditional birth attendant that she was pregnant, she was allocated to receive one tablet of Maloprim fortnightly or placebo and issued with a record card by an MRC field worker. Randomization was by compound." No details provided of a specific procedure used to generate allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | "Treatment was indicated on the record card by a pictorial representation of a coloured tablet (white for Maloprim, pink for placebo)". Insufficient details provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo tablets used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "1208 pregnancies which progressed beyond the 28th week were recorded during the 3 years of the survey. During 1049 (87%) of these pregnancies women reported to the TBA resident and received one or more doses of Maloprim or placebo." Unclear risk. Assumption is that attrition rate was 13.2% (159/1208, where 159 = 1208‐1049). |
| Selective reporting (reporting bias) | Low risk | No apparent risk. |
| Other bias | Low risk | None identified. |
Mbaye 2006 GMB.
| Methods | Trial design: RCT Data collected: 2002 to 2004 Length of follow‐up: From the 1st antenatal visit to 1 year after delivery Frequency of follow‐up: twice per week before delivery; 6 weeks and 1 year after delivery |
|
| Participants | Parity: multigravidae only Number: 2688 Inclusion criteria: pregnancy of more than 15 weeks duration Excluded: Hb concentration of < 7 g/dL; allergy to sulphonamides; severe or chronic disease |
|
| Interventions |
Other: iron and folic acid for all Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: The Gambia Urban/rural: urban (around the town Farafenni) Malaria transmission: seasonal Drug resistance: unknown HIV: HIV negative women; prevalence of HIV infection among antenatal clinic attenders < 1% Funding: The Medical Research Council and the Gates Malaria Partnership, funded by the Bill and Melinda Gates foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were individually randomized in blocks of 12". |
| Allocation concealment (selection bias) | Low risk | "Tablets were pre‐packed in envelopes…pre‐labelled with the same packet number and placed in a wallet bearing the subject’s number and packet number." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical SP and placebo tablets used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate quite high: 459/2688 (17.1 %): Loss to follow‐up in SP group 223/1346 (16.6%) and in the placebo group 236/1342 (17.6%). |
| Selective reporting (reporting bias) | Low risk | No apparent risk. |
| Other bias | Unclear risk | Limited information obtained on bednet use (an important variable in determining the efficacy of IPT). Actual birthweights obtained from only 5% of women (87% of the newborn babies were weighed between 3 and 5 days after birth). |
Menendez 1994 GMB.
| Methods | Trial design: Cluster‐RCT Data collected: 1987 to 1990 Length of follow‐up: from first antenatal visit to third day after delivery Frequency of follow‐up: unclear but administration by traditional birth attendants was on weekly basis |
|
| Participants | Parity: 0 Number: 230 Inclusion criteria: primigravidae resident in trial area Excluded: none stated |
|
| Interventions |
Given by village people employed by the project Other: no information Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: The Gambia Urban/rural: rural (trial area: 15 villages and 3 hamlets, 12 to 35 km from the town of Farafenni) Malaria transmission: seasonal HIV: no information provided Drug resistance: none reported Funding: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "a randomized, double‐blind, placebo‐controlled community based trial" but no details of the way allocation sequence was generated are provided. |
| Allocation concealment (selection bias) | Unclear risk | "After consent had been obtained, women were randomized by compound of residence to receive weekly either one tablet of Maloprim or placebo." Comment: insufficient detail. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. "Two hundred and thirty women were recruited into the study over a 3‐year period…" Afterwards, only 82 women are mentioned as participants in the maloprim group and 89 women in the placebo group. Overall attrition rate 59/230 (25.7%) The total number of women with incomplete outcome data 28/230 (12.2%). Four women had an abortion, 17 had stillbirths, five women died, and 2 other women (0.9%) were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No selective reporting observed. |
| Other bias | Low risk | No other source of bias identified. |
Menendez 2008 MOZ.
| Methods | Trial design: RCT Data collected: August 2003 to April 2005 Length of follow‐up: from recruitment until 8 weeks postpartum Frequency of follow‐up: unclear. Mean number of outpatient visits during pregnancy 1.64 in the SP and 1.83 in the placebo group. Mean number of visits post‐partum 0.69 in the SP group and 0.68 in the placebo group |
|
| Participants | Parity: all Number: 1030 Inclusion criteria: permanent residents of the CISM trial area with gestational age ≤ 28 weeks Excluded: allergic to sulpha drugs |
|
| Interventions |
Other: ITNs Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: Mozambique Urban/rural: urban Malaria transmission: perennial malaria transmission with some seasonality Drug resistance: evidence suggests that SP was highly effective in the area during the trial HIV: In the SP group, 26.5% (117/441 women), and in the placebo group, 21.2% (91/429 women). Overall: 23.9% Funding: Fondo de Investigaciones Sanitarias del Instituto de Salud Carlos III (grant number CM03/00125); Banco de Bilbao, Vizcaya, Argentaria Foundation (grant number BBVA 02‐0); Spanish Agency for International Cooperation (AECI) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated sequential list contained the study numbers linked to treatment identification letters, randomly ordered in blocks of 10". |
| Allocation concealment (selection bias) | Low risk | "Tablets of SP or placebo... were stored in 10 bottles labelled only with a single treatment identification letter." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | SP and placebo tablets "identical in shape and colour". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the SP group 35/515 (6.8%) did not receive 2 doses and birthweight was not measured for 7/501 (1.4%) live births. In the placebo group 29/515 (5.6%) did not receive 2 doses and birthweight was not measured for 7/503 (1.4%) live births. |
| Selective reporting (reporting bias) | Low risk | None identified (trial protocol available). |
| Other bias | Unclear risk | Data were analysed by ITT analysis whereby all randomized women were included regardless of whether or not they had received the intervention and the number of doses. Women with a multiple delivery (twins or triplets) as well as those who did not receive all three doses were also included in the analysis. |
Morley 1964 NGA.
| Methods | Trial design: Quasi‐RCT Data collected: 1957 Length of follow‐up: from first antenatal visit to delivery Frequency of follow‐up: insufficient detail (drugs given monthly) |
|
| Participants | All women Number: 429 Inclusion criteria: all pregnant women registered at dispensary Excluded: none stated |
|
| Interventions |
Other: fever treated with chloroquine sulphate in both groups Administration supervised: women were given drugs during antenatal visits |
|
| Outcomes |
|
|
| Notes | Location: Nigeria Urban/rural: rural (the village of Imesi) Malaria transmission: holoendemic area Drug resistance: none Funding: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "As the pregnant women were registered at the dispensary, they were given consecutive numbers and allotted to one or other of two groups. All women with even numbers were given 2 tablets (50 mg) of pyrimethamine once a month… The control group (the odd numbers) were given two tablets of placebo". Comment: not randomized. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pyrimethamine and "similar tablets" placebo were used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blood films were examined in the hospital laboratory… The technicians did not know to which group a mother belonged." Assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Birthweight: data available for 93.7% (402/429 women). Incomplete data outcome for 6.3% (27/429) women: 17 stillbirths and 10 twin deliveries were excluded. |
| Selective reporting (reporting bias) | Low risk | No selective reporting observed. |
| Other bias | Low risk | None identified. |
Nahlen 1989 NGA.
| Methods | Trial design: RCT Data collected: from January to June 1988 Length of follow‐up: 77 days (mean interval from day 7 post‐chloroquine treatment to documentation of parasitaemia was 74 days for pyrimethamine group) Frequency of follow‐up: weekly. Follow‐up examinations and blood smears were obtained on days 2, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, and 77 |
|
| Participants | Parity: all Number: 71 Inclusion criteria: antenatal and attending hospital and health centre; < 34 weeks gestation; no recent chloroquine taken; parasitaemic > 500 parasites/µL blood Excluded: history of antimalarial drug ingestion during the previous week |
|
| Interventions |
Other: treated with two doses of chloroquine at recruitment; folic acid and iron given to all women Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: Nigeria Urban/rural: urban (Ilorin, the capital of Kwara State) Malaria transmission: endemic area Drug resistance: possible pyrimethamine resistance present Funding: US Agency for International Development, Africa Child Survival‐Initiative‐Combatting Childhood Communicable Diseases Project, 698‐0421 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Women in group 2 were assigned randomly to a pyrimethamine treatment or a control group." The statement that women were randomly assigned is insufficient to be confident that the allocation sequence was genuinely randomized. |
| Allocation concealment (selection bias) | High risk | "The treated group was observed to take 25 mg of pyrimethamine weekly and was instructed to take folic acid and iron supplements daily, while the control group took only folic acid and iron daily." Allocation not concealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "In vivo tests were completed successfully in all 71 women enrolled." Comment: There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No apparent risk. |
| Other bias | Low risk | None identified. |
Ndyomugyenyi 2000 UGA.
| Methods | Trial design: RCT Data collected: 1996 to 1998 Length of follow‐up: from first antenatal visit to first week postpartum Frequency of follow‐up: monthly |
|
| Participants | Parity: 0 Number: 860 Inclusion criteria: primigravidae Excluded: severe anaemia (< 8 g) |
|
| Interventions | 1. Chloroquine
2. Placebo
3. Iron + folate (not included in the analysis) Other: clinical malaria symptoms treated with 25 mg/kg of chloroquine for three days, ITNs Administration supervised: no |
|
| Outcomes | 1. Haemoglobin 2. Birthweight | |
| Notes | Location: Uganda Urban/rural: rural (Hoima District) Malaria transmission: hyperendemic area Drug resistance: unknown Funding: The Danish Bilharziasis Laboratory, Denmark |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After clinical and laboratory examination, women were randomly assigned to 1 of the 3 intervention group". Insufficient details. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo and active tablets of the same colour and shape. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided to make a judgement whether or not the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A high attrition rate of 32.6% (268 out of 823 women were lost to follow‐up). |
| Selective reporting (reporting bias) | Low risk | No apparent risk. |
| Other bias | Low risk | None identified. |
Ndyomugyenyi 2011 UGA.
| Methods | Trial design: individually RCT Data collected: 2004 to 2008 Length of follow‐up: from first antenatal visit to 28 days after delivery Frequency of follow‐up: regularly through ANC clinics, and every seven days postnatally |
|
| Participants | Parity: all parities Number: 5775 randomized; 4715 singleton births followed up Inclusion criteria: pregnant women < 27 weeks at first clinic visit Excluded: > 26 weeks pregnant, non‐residents and temporary residents |
|
| Interventions |
Drugs given under direct observation. Two doses of SP. |
|
| Outcomes | Prevalence of maternal anaemia (Hb < 11.0 g/L) mean Hb at 36 to 40 weeks Clinical malaria Peripheral and placental parasitaemia Abortions, preterm births, stillbirths, perinatal deaths, neonatal deaths Low birthweight Mean birthweight |
|
| Notes | Location: Kabale Highlands, Uganda Urban/rural: rural Malaria transmission: low/unstable area Drug resistance: SP thought to be effective HIV: low Funding: Gates Partnership |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number list". |
| Allocation concealment (selection bias) | Low risk | "individual sealed envelopes". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Tablets of SP or placebo, identical in shape and colour". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All study participants, health staff and researchers were blind to drug assignment (SP or placebo)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Delivery follow‐up: 92%, 92%, and 93% to one month. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None identified. |
Njagi 2003i KEN.
| Methods | RCT | |
| Participants | Low parity (0‐1) Number: 963 Inclusion criteria: gestational age of between 12 and 24 weeks Exclusion criteria: HIV/AIDS, severe systemic diseases |
|
| Interventions |
Other: Folic acid and iron given to all women Administration supervised: yes |
|
| Outcomes |
Length of follow‐up: From 1st antenatal visit to 1 week after delivery Frequency of follow‐up: monthly antenatal clinic visits |
|
| Notes | Location: Western Kenya Malaria transmission: intense Drug resistance: unknown |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number sequences in blocks of 12. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Placebo and active drug tablets were of equal size, colour and shape. The investigators had no knowledge of the assigned groups until after data collection, editing and data analysis were completed." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate 17.4% (168/963): 114 lost due to migration, 35 – home delivery, 19 – refused to continue. Attrition rate in ITN and SP group 35/242 (14.5%), in ITN and placebo group 32/238 (13.4%), in SP group 52/245 (21.2%), in placebo group 49/238 (20.6%). Together with the exclusions, 211/963 (21.9%) women with no treatment outcome. |
| Selective reporting (reporting bias) | Unclear risk | Mentioned that mode of delivery, birthweight and baby’s Hb were recorded but they were never reported. The trial report fails to include results for a key outcome that would be expected to have been reported for such a trial. |
| Other bias | Low risk | None identified. |
Njagi 2003ii KEN.
| Methods | As for Njagi 2003i KEN | |
| Participants | As for Njagi 2003i KEN | |
| Interventions |
Other: Folic acid and iron given to all women |
|
| Outcomes | As for Njagi 2003i KEN | |
| Notes | As for Njagi 2003i KEN | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number sequences in blocks of 12. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Placebo and active drug tablets were of equal size, colour and shape. The investigators had no knowledge of the assigned groups until after data collection, editing and data analysis were completed." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate 17.4% (168/963): 114 lost due to migration, 35 – home delivery, 19 – refused to continue. Attrition rate in ITN and SP group 35/242 (14.5%), in ITN and placebo group 32/238 (13.4%), in SP group 52/245 (21.2%), in placebo group 49/238 (20.6%). Together with the exclusions, 211/963 (21.9%) women with no treatment outcome. |
| Selective reporting (reporting bias) | Unclear risk | Mentioned that mode of delivery, birthweight and baby's Hb were recorded but they were never reported. The trial report fails to include results for a key outcome that would be expected to have been reported for such a trial. |
| Other bias | Low risk | None identified. |
Nosten 1994 THA.
| Methods | Trial design: RCT Data collected: 1987 to 1990 Length of follow‐up: from first antenatal visit at > 20 weeks of estimated gestation to 2 years after delivery Frequency of follow‐up: weekly |
|
| Participants | Parity: all Number: 339 Inclusion criteria: antenatal attendees > 20 weeks of gestation Excluded: none stated |
|
| Interventions |
Other: treated antenatally if parasitaemic; given folic acid and iron if anaemic Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: Thailand Urban/rural: rural (camps Wangka, Shoklo, Bonoko) Malaria transmission: unstable malarious area (mesoendemic) Drug resistance: multiple drug resistance present Funding: United Nations Development Programme/World Bank/WHO Special Programme for Research and Training in Tropical Diseases; Wellcome Trust of Great Britain; Prevention Foundation, The Hague |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial described as "a double‐blind, placebo‐controlled trial". No details provided of the sequence generation method used. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo tablets identical with treatments were used. "The investigators were unaware of the randomization". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate 8% (10/119) in Phase 1 and 8% (18/220) in Phase 2. Across groups: 7.1% (12/170) were excluded from the mefloquine group and 9.5% (16/169) were excluded from the placebo group. Explanation provided. |
| Selective reporting (reporting bias) | Low risk | No apparent risk. |
| Other bias | Low risk | None identified. |
Parise 1998i KEN.
| Methods | Trial design: Quasi‐RCT Data collected:1994 to 1996 Length of follow‐up: from first antenatal visit to delivery; for infants: follow‐up at 3‐7 days of life and at 6 weeks of age Frequency of follow‐up: at two and four weeks after enrolment and then monthly until delivery |
|
| Participants | Parity: para 0‐1 Number: 2077 Inclusion criteria: antenatal clinic attendees; first or second pregnancy Excluded: prior ADRs to sulfa‐containing or other antimalarial medications |
|
| Interventions |
Other: 200 mg ferrous sulphate and 5 mg folic acid daily Administration supervised: Yes |
|
| Outcomes |
|
|
| Notes | Location: Kenya Urban/rural: urban Malaria transmission: hyperendemic area Drug resistance: chloroquine HIV seroprevalence : 2SP ‐ 26.9% (53/196); Case management ‐ 26.9% (57/212) Funding: UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (ID No. 940060); the US Agency for International Development through the Health and Human Resources Analysis for Africa (HHRAA) Project through a Participating Agency Service Agreement (PASA number AOT‐0483‐P‐HI‐2171) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Women were systematically assigned to receive one of three regimens using a rotating assignment based on day of clinic visit." Comment: allocation was not random. |
| Allocation concealment (selection bias) | High risk | Allocation schedule not concealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. Women were systematically assigned to receive either two‐dose SP with treatment doses at enrolment and again early in the third trimester, or case management (CM). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Six hundred ninety‐nine women (34%) were lost to follow‐up during pregnancy because they moved out of the study area or failed to return for follow‐up and the study team was unable to locate their houses." Data was not available for 36.5% (248/680) women in the 2 SP and 35.9% (264/736) women in the case management group. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was available. No selective reporting observed. |
| Other bias | Low risk | No apparent risk. |
Parise 1998ii KEN.
| Methods | As for Parise 1998i KEN | |
| Participants | As for Parise 1998i KEN | |
| Interventions |
|
|
| Outcomes | As for Parise 1998i KEN | |
| Notes | As for Parise 1998i KEN HIV seroprevalence: Monthly SP ‐ 23.7% (40/169); Case management ‐ 26.9% (57/212) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Women were systematically assigned to receive one of three regimens using a rotating assignment based on day of clinic visit." Comment: allocation was not random. |
| Allocation concealment (selection bias) | High risk | Allocation schedule not concealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided as to whether the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Six hundred ninety‐nine women (34%) were lost to follow‐up. Data was not available for 34.8% (230/661) in the monthly SP and 35.9% (264/736) in the case management group. |
| Selective reporting (reporting bias) | Low risk | No selective reporting observed. |
| Other bias | Low risk | None identified. |
Shulman 1999 KEN.
| Methods | Trial design: RCT Data collected: 1996 to 1997 Length of follow‐up: from first antenatal visit to one month post delivery (neonatal period) Frequency of follow‐up: unclear (drug administered as follows: three doses for women recruited at 16 to 19 weeks of gestation; two for those recruited at 20 to 26 weeks; and one for those recruited at 27 to 30 weeks, followed by a visit at 34 weeks and a visit 4 weeks after delivery). |
|
| Participants | Parity: 0 Number: 1264 Inclusion criteria: primigravidae attending antenatal clinics at a health centre (1) or hospital (1); singleton pregnancy; 16 to 30 weeks gestation Excluded: severely anaemic and sick patients excluded |
|
| Interventions |
Other: ferrous sulphate; impregnated bed nets in use in the area Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: Kenya Urban/rural: rural (Kilifi) Malaria transmission: hyperendemic and mesoendemic areas Drug resistance: present Funding: UK Department for International Development and KEMRI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were assigned unique identification numbers sequentially… identification numbers had been randomly allocated to a number between zero and nine, in blocks of ten." Comment: randomization method, using permuted blocks |
| Allocation concealment (selection bias) | Low risk | Drugs supplied in bottles. "Questionnaires were premarked with this unique identification number and the bottle number. The code relating bottle numbers to their contents was retained by a statistician and clinician, not involved in the study." Comment: allocation concealed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | SP and placebo tablets, "identical in appearance and taste". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate 11.41% (73/640) in the SP group and 9.5 % (59/624) in the placebo group, signifying the number of women with no blood test during third trimester. |
| Selective reporting (reporting bias) | Low risk | Trial protocol available; no apparent risk of selective reporting identified. |
| Other bias | Unclear risk | Protocol violation: 6 women from SP group and 8 from placebo group reported taking extra doses of SP (unclear whether women from the placebo group took placebo tablets, or real SP). 69 women from SP group reported taking chloroquine. 61 women from placebo group reported taking chloroquine. |
Villegas 2007 THA.
| Methods | Trial design: RCT Data collected: November 1998 to January 2000 (infant follow‐up completed in December 2001) Length of follow‐up: Mother: from the first antenatal visit to delivery; infant follow‐up completed 1 year after delivery Frequency of follow‐up: weekly |
|
| Participants | Parity: all Number: 951 Inclusion criteria: pregnant women of all parities, of any gestational age, with a negative malaria smear and able to comply with the trial protocol Excluded: allergy to chloroquine, inability to tolerate oral drugs, severe renal or hepatic impairment, tuberculosis treatment, a history of epilepsy or diabetes mellitus or both, or signs of labour |
|
| Interventions |
Other: ferrous sulphate + folic acid Administration supervised: yes |
|
| Outcomes |
|
|
| Notes | Location: Thailand Urban/rural: rural (Maela Refugee Camp and the vicinity of Maw Ker Tai village) Malaria transmission: low, seasonal transmission Drug resistance: possible chloroquine resistance HIV prevalence: no information Funding: Wellcome Trust of Great Britain, Ministerio de Salud de Venezuela (Proyecto Control de Enfermedades), the UNDP/World Bank/WHO Special Programme for Research training in Tropical Diseases (Research Training Grant) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were assigned unique identification numbers sequentially. All identification numbers were allocated randomly by computer to a number between one and ten, in blocks of ten (five randomly allocated to CQ and five to placebo in each block)". Randomization method, using permuted blocks. |
| Allocation concealment (selection bias) | Low risk | "Each unique identification number was linked to a brown paper envelope which contained the study drugs in weekly allotments, sealed into zippered plastic bags… labelled with week number of the study. The preparation of the study drugs was done in Mae Sot by the SMRU pharmacist who was not involved with any other aspect of the study. The study codes and randomization list was retained by a clinician at SMRU..." Allocation was concealed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo and active tablets, "identical in appearance and taste". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigators and staff participating in the trial were unaware of the study codes until data collection was completed." Outcome assessors were probably blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 49/1000 pregnant women (4.9%), out of which 28/500 (5.6%) in the chloroquine group and 21/500 (4.2%) in the placebo group were excluded from the final analysis of efficacy against P. vivax. Reasons for exclusion were provided. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | No apparent risk. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asa 2008 NGA | No placebo/no intervention group. Compares chloroquine with SP. |
| Briand 2009 BEN | No placebo/no intervention group. Compares SP with mefloquine. |
| Clerk 2008 GHA | No placebo/no intervention group. Compares SP with amodiaquine or amodiaquine plus SP. |
| Deen 2001 | The study is a part of a double‐blinded, placebo‐controlled, village‐randomized malaria transmission‐reduction trial, comparing the efficacy of a single dose of artesunate and SP against placebo. However, target group is the general population (14,017 villagers). Women who were "thought that they might be pregnant", were advised not to take the study drugs. Some of them unknowingly took the drugs and their outcomes are reported. There is no specific method of randomization of the pregnant women who "accidentally" took the drugs, to ensure similarity of the groups. Also, distribution is uneven: N = 287 in the intervention group versus N =40 in the control group. |
| Diakite 2011 MLI | No placebo/no intervention group. Compares intermittent SP: 2 doses versus 3 doses. |
| Diallo 2007 MLI | No placebo/no intervention group. Compares weekly chloroquine with intermittent SP. |
| Dolan 1993 | Trial of impregnated mosquito nets. |
| Filler 2006 MWI | No placebo/no intervention group. Compares intermittent SP: 2 doses versus 3 doses. |
| Gies 2009 | Described as "a health centre randomized trial". This study evaluated the IPT‐SP uptake in a community‐based trial where health centres were randomized to one of three arms: IPT‐SP with health promotion, IPT‐SP without promotion and weekly CQ. The purpose was to assess the impact of a village‐based promotional campaign to enhance antenatal clinic (ANC) attendance. |
| Hamer 2007 ZMB | No placebo/no intervention group. Compares intermittent SP: 2 doses versus 3 doses |
| Hamilton 1972 UGA | This previously included trial was excluded in the updated version because Hamilton and his team administered iron to one of the control groups and folic acid to the other, but nothing was mentioned of iron and folates being administered to women in the intervention group (chloroquine). |
| Helitzer 1994 | 4 clinics trying different methods to achieve adherence; not randomized. |
| Kayentao 2005 MLI | No placebo/no intervention group. Compares weekly chloroquine with intermittent SP. |
| Luntamo 2010 MWI | No placebo/no intervention group. Compares intermittent SP: 2 doses versus 3 doses. |
| Martin 1982 | Reported as randomized 100 women, but analysis is by whether women complied, and those that did not comply (37 participants) analysed as a separate group. |
| McDermott 1988 | Started as a RCT, but discontinued when reports elsewhere noted an association between amodiaquine and agranulocytosis; trial then became an observational study with the 2 arms of the trial combined. |
| McGready 2001 | Trial of repellent. |
| Menéndez 2011 | Study done in the context of a randomized, double‐blind, placebo‐controlled trial of IPT‐ SP for malaria prevention (already included, Menendez 2008 MOZ). |
| Mutabingwa 1993 TZA | No placebo/no intervention group. Compares weekly chloroquine with daily proguanil. |
| Naniche 2008 | Study done in the context of a randomized, double‐blind, placebo‐controlled trial of IPT‐ SP for malaria prevention (already included, Menendez 2008 MOZ). |
| Ouedraogo 2008 BFA | No placebo/no intervention group. Compares weekly chloroquine with intermittent SP. |
| Pertet 1994 | Possible RCT; wrote to the authors in 1998; no response. |
| Randriam. 2011 MDG | No placebo/no intervention group. Compares weekly chloroquine with intermittent SP. |
| Schultz 1994 MWI | No placebo/no intervention group. Compares weekly chloroquine with intermittent SP. |
| Serra‐Casas 2010 | Study is done in the context of a randomized, double‐blind, placebo‐controlled trial of IPT‐ SP for malaria prevention during pregnancy (already included, Menendez 2008 MOZ), investigating the effect of IPT‐SP on maternal and cord Immunoglobulin G (IgG) levels and comparing antibody levels between intervention groups. The study is mostly about the association between antibody levels and morbidity outcomes, and not focused on the specific outcomes included in the protocol for the review. |
| Shulman 1998 | Study of impregnated mosquito nets. |
| Steketee 1996 | Comparison between mefloquine and chloroquine. |
| Tagbor 2010 | A randomized controlled non‐inferiority trial conducted in Ghana, comparing the safety and efficacy of intermittent screening and treatment (IST), a new strategy for malaria control, and treatment with SP. There were two intervention groups: SP and IST; IST and treatment with amodiaquine+artesunate (AQ+AS), versus the control group ‐ standard IPT‐SP. We excluded this study because a different strategy (not chemoprevention but early screening and treatment) was used in the intervention arm. |
| Thaler 2006 | Study, comparing riboflavin (not an active antimalarial drug) to placebo. |
| Tukur 2007 NGA | No placebo/no intervention group. Compares chloroquine once only followed by weekly pyrimethamine with intermittent SP. |
| Valea 2010 BFA | No placebo/no intervention group. Compares intermittent SP: 2 doses versus 3 doses. |
Contributions of authors
DR‐P re‐ran the searches, re‐extracted data with PG, updated the risk of bias tables, created GRADE tables, and rewrote the results. PG assisted with the update, provided advice on the structure and analysis, completed the conceptual framework, checked the GRADE assessments and revised the results, and wrote the discussion. DS contributed to the GRADE assessment, rewriting the results, and restructuring the review. KK and FK helped with conceptualising the questions and interpreting the results in context. KK and FK carefully considered all the included trials and checked for accuracy and completeness. All authors contributed to the final agreed version of the review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
HRP‐UNDP/UNFPA/WHO/World Bank Special Programme in Human Reproduction, Geneva, Switzerland.
External sources
Department for International Development (DFID), UK.
Declarations of interest
PG is Director of Evidence Building and Synthesis Research Consortium that receives money to increase the number of evidence‐informed decisions by intermediary organizations, including WHO and national decision‐makers that benefit the poor in middle‐ and low‐income countries. DS is employed as part of this Consortium. PG is the coordinator of a WHO Collaborating Centre for Evidence Synthesis for Infectious and Tropical Diseases (http://apps.who.int/whocc/Detail.aspx?cc_ref=UNK‐234&cc_code=unk&cc_contact=garner&): one of the Centre's aims is to help WHO in its role as an infomediary in communicating reliable summaries of research evidence to policy makers, clinicians, teachers, and the public in developing countries.
Feiko ter Kuile is Chief Executive Officer of the Malaria in Pregnancy Consortium, a network of 47 research institutions worldwide conducting research on the treatment and prevention of malaria in pregnancy, funded by the Bill and Melinda Gates Foundation. He is principal investigator on several trials investigating intermittent preventive treatment and intermittent screening and treatment in pregnancy.
Unchanged
References
References to studies included in this review
Challis 2004 MOZ {published data only}
- Challis K, Osman NB, Cotiro M, Nordahl G, Dgedge M, Bergström S. Impact of a double dose of sulphadoxine‐pyrimethamine to reduce prevalence of pregnancy malaria in southern Mozambique. Tropical Medicine and International Health 2004;9(10):1066‐73. [DOI] [PubMed] [Google Scholar]
Cot 1992 BFA {published and unpublished data}
- Cot M, Roisin A, Barro D, Yada A, Verhave J, Carnevale P, et al. Effect of chloroquine chemoprophylaxis during pregnancy on birth weight: results of a randomized trial. American Journal of Tropical Medicine and Hygiene 1992;46(1):21‐7. [DOI] [PubMed] [Google Scholar]
Cot 1995 CMR {published and unpublished data}
- Cot M, Hesran JY, Miailhes P, Esveld M, Etya'ale D, Breart G. Increase of birth weight following chloroquine chemoprophylaxis during the first pregnancy: results of a randomized trial in Cameroon. American Journal of Tropical Medicine and Hygiene 1995;53(6):581‐5. [DOI] [PubMed] [Google Scholar]
Fleming 1986 NGA {published data only}
- Fleming AF, Briggs ND, Rossiter CE. Growth during pregnancy in Nigerian teenage primigravidae. British Journal of Obstetrics and Gynaecology 1985;92(Suppl 5):32‐9. [Google Scholar]
- Fleming AF, Ghatoura GB, Harrison KA, Briggs ND, Dunn DT. The prevention of anaemia in pregnancy in primigravidae in the guinea savanna of Nigeria. Annals of Tropical Medicine and Parasitology 1986;80(2):211‐33. [DOI] [PubMed] [Google Scholar]
Greenwood 1989 GMB {published and unpublished data}
- Greenwood AM, Menendez C, Todd J, Greenwood BM. The distribution of birthweights in Gambian women who received malaria chemoprophylaxis during their first pregnancy and in control women. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(3):311‐2. [DOI] [PubMed] [Google Scholar]
- Greenwood BM, Greenwood AM, Snow RW, Byass P, Bennett S, Hatib‐N'Jie AB. The effects of malaria chemoprophylaxis given by traditional birth attendants on the course and outcome of pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 1989;83(5):589‐94. [DOI] [PubMed] [Google Scholar]
- Menendez C, Todd J, Alonso P, Lulat S, Francis N, Greenwood BM. Malaria chemoprophylaxis, infection of the placenta and birth weight in Gambian primigravidae. Journal of Tropical Medicine and Hygiene 1994;97(4):244‐8. [PubMed] [Google Scholar]
Mbaye 2006 GMB {published data only}
- Mbaye A, Richardson K, Balajo B, Dunyo S, Shulman C, Milligan P, et al. A randomized, placebo‐controlled trial of intermittent preventive treatment with sulphadoxine–pyrimethamine in Gambian multigravidae. Tropical Medicine and International Health 2006;11(7):992‐1002. [DOI] [PubMed] [Google Scholar]
Menendez 1994 GMB {published data only}
- Menendez C, Todd J, Alonso PL, Lulat S, Francis N, Greenwood BM. Malaria chemoprophylaxis, infection of the placenta and birth weight in Gambian primigravidae. Journal of Tropical Medicine and Hygiene 1994;97(4):244‐8. [PubMed] [Google Scholar]
Menendez 2008 MOZ {published data only}
- Menéndez C, Bardají A, Sigauque B, Romagosa C, Sanz S, Serra‐Casas E, et al. A randomized placebo‐controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS One 2008;3(4):e1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menéndez C, Bardají A, Sigauque B, Sanz S, Aponte JJ, Mabunda S, Alonso PL. Malaria prevention with IPTp during pregnancy reduces neonatal mortality. PLoS ONE 2010;5(2):e9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morley 1964 NGA {published data only}
- Morley D, Woodland M, Cuthbertson WF. Controlled trial of pyrimethamine in pregnant women in an African village. British Medical Journal 1964;1(5384):667‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nahlen 1989 NGA {published data only}
- Nahlen BL, Akintunde A, Alakija T, Nguyen‐Dinh P, Ogunbode O, Edungbola LD, et al. Lack of efficacy of pyrimethamine prophylaxis in pregnant Nigerian women. Lancet 1989;2(8667):830‐4. [DOI] [PubMed] [Google Scholar]
Ndyomugyenyi 2000 UGA {published data only}
- Ndyomugyenyi R, Magnussen P. Chloroquine prophylaxis, iron‐folic acid supplementation or case management of malaria attacks in primigravidae in western Uganda: effects on maternal parasitaemia and haemoglobin levels and on birthweight. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(4):413‐8. [DOI] [PubMed] [Google Scholar]
- Ndyomugyenyi R, Magnussen P. Chloroquine prophylaxis, iron/folic‐acid supplementation or case management of malaria attacks in primigravidae in western Uganda: effects on congenital malaria and infant haemoglobin concentrations. Annals of Tropical Medicine and Parasitology 2000;94(8):759‐70. [DOI] [PubMed] [Google Scholar]
Ndyomugyenyi 2011 UGA {published data only}
- Ndyomugyenyi R, Clarke SE, Hutchison CL, Hansen KS, Magnussen P. Efficacy of malaria prevention during pregnancy in an area of low and unstable transmission: an individually‐randomised placebo‐controlled trial using intermittent preventive treatment and insecticide‐treated nets in the Kabale Highlands, southwestern Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene 2011;105(11):607‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Njagi 2003ii KEN {published and unpublished data}
- Njagi JK, Magnussen P, Estambale B, Ouma J, Mugo B. Prevention of anaemia in pregnancy using insecticide‐treated bednets and sulfadoxine‐pyrimethamine in a highly malarious area of Kenya: a randomized controlled trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(3):277‐82. [DOI] [PubMed] [Google Scholar]
Njagi 2003i KEN {published data only}
- Njagi JK, Magnussen P, Estambale B, Ouma J, Mugo B. Prevention of anaemia in pregnancy using insecticide‐treated bednets and sulfadoxine‐pyrimethamine in a highly malarious area of Kenya: a randomized controlled trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(3):277‐82. [DOI] [PubMed] [Google Scholar]
Nosten 1994 THA {published and unpublished data}
- Nosten F, ter Kuile F, Maelankiri L, Chongsuphajaisiddhi T, Nopdonrattakoon L, Tangkitchot S, et al. Mefloquine prophylaxis in pregnancy: a double‐blind placebo‐controlled trial. Journal of Infectious Diseases 1994;169(3):595‐603. [DOI] [PubMed] [Google Scholar]
Parise 1998ii KEN {published and unpublished data}
- Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, et al. Efficacy of sulfadoxine‐pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. American Journal of Tropical Medicine and Hygiene 1998;59(5):813‐22. [DOI] [PubMed] [Google Scholar]
Parise 1998i KEN {published and unpublished data}
- Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, et al. Efficacy of sulfadoxine‐pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. American Journal of Tropical Medicine and Hygiene 1998;59(5):813‐22. [DOI] [PubMed] [Google Scholar]
Shulman 1999 KEN {published and unpublished data}
- Shulman CE, Dorman EK, Cutts F, Kawuondo K, Bulmer JN, Peshu N, et al. Intermittent sulphadoxine‐pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo‐controlled trial. Lancet 1999;353(9153):632‐6. [DOI] [PubMed] [Google Scholar]
Villegas 2007 THA {published data only}
- Villegas L, McGready R, Htway M, Paw MK, Pimanpanarak M, Arunjerdja R, et al. Chloroquine prophylaxis against vivax malaria in pregnancy: a randomized, double‐blind, placebo‐controlled trial. Tropical Medicine and International Health 2007;12(2):209‐18. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asa 2008 NGA {published data only}
- Asa OO, Onayade AA, Fatusi AO, Ijadunola KT, Abiona TC. Efficacy of intermittent preventive treatment of malaria with sulphadoxine‐pyrimethamine in preventing anaemia in pregnancy among Nigerian women. Maternal and Child Health Journal 2008;12(6):692‐8. [DOI] [PubMed] [Google Scholar]
Briand 2009 BEN {published data only}
- Briand V, Bottero J, Noël H, Masse V, Cordel H, Guerra J, et al. Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open‐label equivalence trial comparing sulfadoxine‐pyrimethamine with mefloquine. Journal of Infectious Diseases 2009;200(6):991‐1001. [DOI] [PubMed] [Google Scholar]
Clerk 2008 GHA {published data only}
- Clerk CA, Bruce J, Affipunguh PK, Mensah N, Hodgson A, Greenwood B, et al. A randomized, controlled trial of intermittent preventive treatment with sulfadoxine‐pyrimethamine, amodiaquine, or the combination in pregnant women in Ghana. Journal of Infectious Diseases 2008;198(8):1202‐11. [DOI] [PubMed] [Google Scholar]
Deen 2001 {published data only}
- Deen JL, Seidlein L, Pinder M, Walraven GEL, Greenwood BM. The safety of the combination artesunate and pyrimethamine‐sulfadoxine given during pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(4):424‐8. [DOI] [PubMed] [Google Scholar]
Diakite 2011 MLI {published data only}
- Diakite OS, Kayentao K, Traoré BT, Djimde A, Traoré B, Diallo M, et al. Superiority of 3 over 2 doses of intermittent preventive treatment with sulfadoxine‐pyrimethamine for the prevention of malaria during pregnancy in Mali: a randomized controlled trial. Clinical Infectious Diseases 2011;53(3):215‐23. [DOI] [PubMed] [Google Scholar]
Diallo 2007 MLI {published data only}
- Diallo M, Dabo CAT, Saye R, Yattara O, Diarra MA, Kayentao K, et al. Randomized clinical trial of two malaria prophylaxis regimens for pregnant women in Faladie, Mali [Essai clinique randomisé de deux schémas de préventioncontre le paludisme au cours de la grossesse à Faladiè (Mali)]. Médecine Tropicale 2007;67(5):477‐80. [PubMed] [Google Scholar]
Dolan 1993 {published data only}
- Dolan G, ter Kuile FO, Jacoutot V, White NJ, Luxemburger C, Malankirii L, et al. Bed nets for the prevention of malaria and anaemia in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(6):620‐6. [DOI] [PubMed] [Google Scholar]
Filler 2006 MWI {published data only}
- Filler SJ, Kazembe P, Thigpen M, Macheso A, Parise ME, Newman RD, et al. Randomized trial of 2‐dose versus monthly sulfadoxine‐pyrimethamine intermittent preventive treatment for malaria in HIV‐positive and HIV‐negative pregnant women in Malawi. Journal of Infectious Diseases 2006;194(3):286–93. [DOI] [PubMed] [Google Scholar]
Gies 2009 {published data only}
- Gies S, Coulibaly SO, Ouattara FT, D'Alessandro U. Individual efficacy of intermittent preventive treatment with sulfadoxine‐pyrimethamine in primi‐ and secundigravidae in rural Burkina Faso: Impact on parasitaemia, anaemia and birth weight. Tropical Medicine and International Health 2009;14(2):174‐82. [DOI] [PubMed] [Google Scholar]
Hamer 2007 ZMB {published data only}
- Hamer DH, Mwanakasale V, Macleod WB, Chalwe V, Mukwamataba D, Champo D, et al. Two‐dose versus monthly intermittent preventive treatment of malaria with sulfadoxine‐pyrimethamine in HIV‐seropositive pregnant Zambian women. Journal of Infectious Diseases 2007;196(11):1585‐94. [DOI] [PubMed] [Google Scholar]
Hamilton 1972 UGA {published data only}
- Hamilton PJ, Gebbie DA, Wilks NE, Lothe F. The role of malaria, folic acid deficiency and haemoglobin AS in pregnancy at Mulago hospital. Transactions of the Royal Society of Tropical Medicine and Hygiene 1972;66(4):594‐602. [DOI] [PubMed] [Google Scholar]
Helitzer 1994 {published data only}
- Helitzer‐Allen DL, Macheso A, Wirima J, Kendall C. Testing strategies to increase use of chloroquine chemoprophylaxis during pregnancy in Malawi. Acta Tropica 1994;58(3‐4):255‐66. [DOI] [PubMed] [Google Scholar]
Kayentao 2005 MLI {published and unpublished data}
- Kayentao K, Kodio M, Newman RD, Maiga H, Doumtabe D, Ongoiba A, et al. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. Journal of Infectious Diseases 2005;191(1):109‐16. [DOI] [PubMed] [Google Scholar]
Luntamo 2010 MWI {published data only}
- Luntamo M, Kulmala T, Cheung YB, Maleta K, Ashorn P. The effect of antenatal monthly sulphadoxine‐pyrimethamine, alone or with azithromycin, on foetal and neonatal growth faltering in Malawi: a randomised controlled trial. Tropical Medicine and International Health 2013;18(4):386‐97. [DOI] [PubMed] [Google Scholar]
- Luntamo M, Kulmala T, Mbewe B, Cheung YB, Maleta K, Ashorn P. Effect of repeated treatment of pregnant women with sulfadoxine‐pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. American Journal of Tropical Medicine and Hygiene 2010;83(6):1212‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntamo M, Rantala A‐M, Meshnick SR, Cheung YB, Kulmala T, Maleta K, et al. The effect of monthly sulfadoxine‐pyrimethamine, alone or with azithromycin, on PCR‐diagnosed malaria at delivery: a randomized controlled trial. PLoS One 2012;7(7):e41123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 1982 {published data only}
- Martin GE, Nkwate CC. Administration of a single monthly dose of 600 mg chloroquine base in the control of malaria in pregnant women [Administration de la dose unique mensuelle de 600 mg de chloroquine base dans le controle du paludisme chez les femmes enceintes]. CEAC Bulletin 1982;53:41‐7. [Google Scholar]
McDermott 1988 {published data only}
- McDermott JM, Heymann DL, Wirima JJ, Macheso AP, Wahl RD, Steketee RW, et al. Efficacy of chemoprophylaxis in preventing Plasmodium falciparum parasitaemia and placental infection in pregnant women in Malawi. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988;82(4):520‐3. [DOI] [PubMed] [Google Scholar]
McGready 2001 {published data only}
- McGready R, Hamilton KA, Simpson JA, Cho T, Luxemburger C, Edwards R, et al. Safety of the insect repellent N,N‐diethyl‐M‐toluamide (DEET) in pregnancy. American Journal of Tropical Medicine and Hygiene 2001;65(4):285‐9. [DOI] [PubMed] [Google Scholar]
Menéndez 2011 {published data only}
- Menéndez C, Serra‐Casas E, Scahill MD, Sanz S, Nhabomba A, Bardají A, et al. HIV and placental infection modulate the appearance of drug‐resistant Plasmodium falciparum in pregnant women who receive intermittent preventive treatment. Clinical Infectious Diseases 2011;52(1):41‐8. [DOI] [PubMed] [Google Scholar]
Mutabingwa 1993 TZA {published and unpublished data}
- Mutabingwa T, Malle L, Geus A, Oosting J. Malaria chemosuppression in pregnancy. I. The effect of chemosuppressive drugs on maternal parasitaemia. Tropical and Geographical Medicine 1991;45(1):6‐14. [PubMed] [Google Scholar]
Naniche 2008 {published data only}
- Naniche D, Lahuerta M, Bardaji A, Sigauque B, Romagosa C, Berenguera A, et al. Mother‐to‐child transmission of HIV‐1: association with malaria prevention, anaemia and placental malaria. HIV Medicine 2008;9(9):757‐64. [DOI] [PubMed] [Google Scholar]
Ouedraogo 2008 BFA {published data only}
- Ouédraogo A, Bougouma EC, Diarra A, Konaté AT, Nébié I, Tiono AB, et al. Comparative impact of three malaria preventive regimens during pregnancy on maternal anemia due to malaria in Burkina Faso [Impact comparatif de trois schémas de prévention du paludismependant la grossesse sur l’anémie maternelle, associéeà l’infection palustre au Burkina Faso]. Médecine et maladies infectieuses 2008;38(4):180‐6. [DOI] [PubMed] [Google Scholar]
- Tiono AB, Ouedraogo A, Bougouma EC, Diarra A, Konaté AT, Nébié I, et al. Placental malaria and low birth weight in pregnant women living in a rural area of Burkina Faso following the use of three preventive treatment regimens. Malaria Journal 2009;8:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pertet 1994 {unpublished data only}
- Pertet AM. Marital status and history of a previous child are risk factors in infant mortality. Social Science & Medicine 1994;38(11):1589. [DOI] [PubMed] [Google Scholar]
Randriam. 2011 MDG {published data only}
- Randriambelomanana JA, Rakotoarisoa H, Herinirina SA, Zafindravola BA, Andrianampanalinarivo HR. Comparison of efficacy of chloroquine versus sulfadoxine–pyrimethamine in malaria prevention in pregnant women in the Toamasina region (Madagascar) [Comparaison de l’efficacité de la chloroquine versus sulfadoxine–pyriméthamine dans la prévention du paludisme chez la femme enceintedans la région de Toamasina (Madagascar)]. Bulletin de la Société de Pathologie Exotique 2011;104(4):243‐9. [DOI] [PubMed] [Google Scholar]
Schultz 1994 MWI {published data only}
- Schultz LJ, Steketee RW, Macheso A, Kazembe P, Chitsulo L, Wirima JJ. The efficacy of antimalarial regimens containing sulfadoxine‐pyrimethamine and/or chloroquine in preventing peripheral and placental Plasmodium falciparum infection among pregnant women in Malawi. American Journal of Tropical Medicine and Hygiene 1994;51(5):515‐22. [DOI] [PubMed] [Google Scholar]
Serra‐Casas 2010 {published data only}
- Serra‐Casas E, Menéndez C, Bardají A, Quintó L, Dobaño C, Sigauque B, et al. The effect of intermittent preventive treatment during pregnancy on malarial antibodies depends on HIV status and is not associated with poor delivery outcomes. Journal of Infectious Diseases 2010;201(1):123‐31. [DOI] [PubMed] [Google Scholar]
Shulman 1998 {published data only}
- Shulman CE, Dorman EK, Talisuna AO, Lowe BS, Nevill C, Snow RW, et al. A community randomized controlled trial of insecticide‐treated bednets for the prevention of malaria and anaemia among primigravid women on Kenyan coast. Tropical Medicine & International Health 1998;3(3):197‐204. [DOI] [PubMed] [Google Scholar]
Steketee 1996 {published data only}
- Bloland P, Slutsker L, Steketee RW, Wirima JJ, Heymann DL, Breman JG. Rates and risk factors for mortality during the first two years of life in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):82‐6. [DOI] [PubMed] [Google Scholar]
- Mangochi Malaria Research Project. Malaria prevention in pregnancy: the effects of treatment and chemoprophylaxis on placental malaria infection, low birth weight, and fetal, infant, and child survival. U.S. Agency for International Development in conjunction with Centers for Disease Control and Prevention, Atlanta, Ga; Africa Regional Project (698‐0421) 1996.
- McDermott JM, Slutsker L, Steketee RW, Wirima JJ, Breman JG, Heymann DL. Prospective assessment of mortality among a cohort of pregnant women in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):66‐70. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Wirima JJ, Steketee RW, Breman JG, Heymann DL. The effect of placental malaria infection on perinatal mortality in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):61‐5. [DOI] [PubMed] [Google Scholar]
- Redd SC, Wirima JJ, Steketee RW, Breman JG, Heymann DL. Transplacental transmission of Plasmodium falciparum in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):57‐60. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Bloland PB, Chilima B, Mermin JH, Chitsulo L, et al. Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type‐1. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):42‐9. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Campbell CC. Developing effective strategies for malaria prevention programs for pregnant African women. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):95‐100. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):33‐41. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Slutsker L, Breman JG, Heymann DL. Comparability of treatment groups and risk factors for parasitemia at the first antenatal clinic visit in a study of malaria treatment and prevention in pregnancy in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):17‐23. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Slutsker L, Khoromana CO, Heymann DL, Breman JG. Malaria treatment and prevention in pregnancy: indications for use and adverse events associated with use of chloroquine or mefloquine. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):50‐6. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Slutsker L, Roberts JM, Khoromana CO, Heymann DL, et al. Malaria parasite infection during pregnancy and at delivery in mother, placenta, and newborn: efficacy of chloroquine and mefloquine in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):24‐32. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Slutsker WL, Khoromana CO, Breman JG, Heymann DL. Objectives and methodology in a study of malaria treatment and prevention in pregnancy in rural Malawi: The Mangochi Malaria Research Project. American Journal of Tropical Medicine and Hygiene 1996;55(Suppl 1):8‐16. [DOI] [PubMed] [Google Scholar]
Tagbor 2010 {published data only}
- Tagbor H, Bruce J, Agbo M, Greenwood B, Chandramohan D. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: a randomised controlled non‐inferiority trial. PLoS One 2010;5(12):e14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thaler 2006 {published data only}
- Thaler J, Neugebauer J, Wolf J, Dakouré‐Ouedraogo M, Köhler H, Wessel L, et al. Effects of riboflavin given to pregnant women on the incidence of malaria: Results of a prospective randomized double blind study [Auswirkungen einer prophylaktischen Gabe von Riboflavin an Schwangere auf die Häufigkeit der Malaria: Ergebnisse einer prospektiven randomisierten Studie]. Geburtshilfe und Frauenheilkunde 2006;66:383‐90. [Google Scholar]
Tukur 2007 NGA {published data only}
- Tukur IU, Thacher TD, Sagay AS, Madaki JK. A comparison of sulfadoxine‐pyrimethamine with chloroquine and pyrimethamine for prevention of malaria in pregnant Nigerian women. American Journal of Tropical Medicine and Hygiene 2007;76(6):1019‐23. [PubMed] [Google Scholar]
Valea 2010 BFA {published data only}
- Valea I, Tinto H, Drabo MK, Huybregts L, Henry MC, Roberfroid D, et al. Intermittent preventive treatment of malaria with sulphadoxine‐pyrimethamine during pregnancy in Burkina Faso: effect of adding a third dose to the standard two‐dose regimen on low birth weight, anaemia and pregnancy outcomes. Malaria Journal 2010;9:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Dellicour 2010
- Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile, FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Medicine 2010;7(1):e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Desai 2007
- Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. The Lancet Infectious Diseases 2007;7(2):93‐104. [DOI] [PubMed] [Google Scholar]
Eisele 2012
- Eisele TP, Larsen DA, Anglewicz PA, Keating J, Yukich J, Bennett A, et al. Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta‐analysis of 32 national cross‐sectional datasets in Africa. The Lancet Infectious Diseases 2012;12(12):942‐9. [DOI] [PubMed] [Google Scholar]
Gamble 2006
- Gamble C, Ekwaru JP, ter Kuile FO. Insecticide‐reated nets for preventing malaria in pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003755.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins J, Green S (editors). Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions. Version 4.2.5 [updated May 2005]; Appendix 5b. www.cochrane.org/resources/handbook/hbook.htm (accessed 1 March 2006).
Higgins 2011
- Higgins JPT Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2011. [Google Scholar]
Kayentao 2013
- Kayentao K, Garner P, Eijk AM, Naidoo I, Roper C, Mulokozi A, et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine‐pyrimethamine and risk of low birth weight in Africa: systematic review and meta‐analysis. Journal of the American Medical Association 2013;309(6):594‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
McClure 2013
- McClure EM, Goldenberg RL, Dent AE, Meshnick SR. A systematic review of the impact of malaria prevention in pregnancy on low birth weight and maternal anemia. International Journal of Gynaecology and Obstetrics 2013;121(2):103‐9. [DOI] [PubMed] [Google Scholar]
Nkhoma 2012a
- Nkhoma ET, Bowman NM, Kalilani‐Phiri L, Mwapasa V, Rogerson SJ, Meshnick SR. The effect of HIV infection on the risk, frequency, and intensity of Plasmodium falciparum parasitemia in primigravid and multigravid women in Malawi. American Journal of Tropical Medicine and Hygiene 2012;87(6):1022‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nkhoma 2012b
- Nkhoma ET, Kalilani‐Phiri L, Mwapasa V, Rogerson SJ, Meshnick SR. Effect of HIV infection and Plasmodium falciparum parasitemia on pregnancy outcomes in Malawi. American Journal of Tropical Medicine and Hygiene 2012b;87(1):29‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Steketee 2001
- Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria‐endemic areas. American Journal of Tropical Medicine and Hygiene 2001;64(1‐2 Suppl):28‐35. [DOI] [PubMed] [Google Scholar]
ter Kuile 2004
- ter Kuile FO, Parise ME, Verhoeff FH, Udhayakumar V, Newman RD, Eijk AM, et al. The burden of co‐infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub‐saharan Africa. American Journal of Tropical Medicine and Hygiene 2004;71(2 Suppl):41‐54. [PubMed] [Google Scholar]
ter Kuile 2007
- ter Kuile FO, Eijk AM, Filler SJ. Effect of sulfadoxine‐pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. Journal of the American Medical Association 2007;297(23):2603‐16. [DOI] [PubMed] [Google Scholar]
White 2005
- White NJ. Intermittent presumptive treatment for malaria. PLoS Medicine 2005;2(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Guidelines for the treatment of malaria. 2nd Edition. Geneva: World Health Organization, 2010. [Google Scholar]
WHO 2012
- World Health Organization. World Malaria Report. World Health Organization, Geneva 2012.
WHO 2013
- World Health Organization. WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine‐pyrimethamine (IPTp‐SP). http://www.who.int/malaria/publications/atoz/policy_brief_iptp_sp_policy_recommendation/en/ 2013.
References to other published versions of this review
Garner 1994
- Garner P, Brabin B. A review of randomized controlled trials of routine antimalarial drug prophylaxis during pregnancy in endemic malarious areas. Bulletin of the World Health Organization 1994;72(1):89‐99. [PMC free article] [PubMed] [Google Scholar]
Garner 1995
- Garner P. Routine antimalarial drug chemoprophylaxis during pregnancy in endemic malarious areas. Cochrane Database of Systematic Reviews 1995, Issue 1. [Google Scholar]
Garner 2000
- Garner P, Gulmezoglu AM. Prevention versus treatment for malaria in pregnant women. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000169] [DOI] [PubMed] [Google Scholar]
Garner 2003
- Garner P, Gulmezoglu AM. Drugs for preventing malaria‐related illness in pregnant women and death in the newborn. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD000169] [DOI] [PubMed] [Google Scholar]
Garner 2006
- Garner P, Gulmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD000169.pub2] [DOI] [PubMed] [Google Scholar]


