Abstract
Introduction
In the absence of an HIV vaccine or cure, antiretroviral (ARV) based prevention strategies are being investigated to reduce HIV incidence. These prevention strategies depend on achieving effective drug concentrations at the site HIV exposure which is most commonly the mucosal tissues of the lower gastrointestinal tract and the female genital tract.
Areas covered
This article collates all known data regarding drug exposure in these vulnerable mucosal tissues, and reviews important mechanisms of ARV drug distribution. Research papers and abstracts describing antiretroviral pharmacokinetics in the female genital tract and lower gastrointestinal mucosal tissues available in MEDLINE® or presented at scientific conferences prior to December 2014 are reviewed in detail. Important influences on ARV mucosal tissue distribution, including protein binding, active drug transport, and endogenous hormones, are also reviewed.
Expert opinion
ARVs exhibit highly variable pharmacokinetics in mucosal tissues. In general, antiretroviral exposure is higher in the lower gastrointestinal tract compared to the female genital tract, but concentrations required for protective efficacy are largely unknown. The expected site of HIV exposure represents an important consideration when designing and optimizing antiretroviral based prevention strategies.
Keywords: antiretroviral, mucosal tissue, HIV prevention, pharmacokinetics
1. Introduction
As of the end of 2013, 35 million people worldwide were living with HIV/AIDS.(1) Highly active antiretroviral treatment (HAART) strategies effectively control HIV's progression into AIDS, restoring life expectancy and quality of life to that of an uninfected person. Nonetheless, of the 32.6 million HIV infected persons living in lower and middle-income countries, approximately 64% (20.9 million) do not have access to HAART.(1) Therefore, prevention strategies are needed to contain the epidemic.
In the absence of a licensed vaccine, the use of antiretrovirals for HIV prevention has been investigated. The utility of one of these approaches, treatment as prevention (TasP), was recently established in the landmark HPTN052 study where it was found that consistent suppression of blood plasma HIV RNA in HIV infected subjects using early HAART reduced transmission to their uninfected partners by greater than 95%.(2) This study demonstrated the highest degree of protection of any HIV prevention trial. In the United States, less than 50% of HIV infected patients on HAART exhibit a suppressed viral load.(3) This is an important limitation of the TasP approach and warrants further exploration into alternative HIV prevention strategies in uninfected individuals.
In 2012, Truvada®, a fixed dose combination tablet of two nucleoside reverse transcriptase inhibitors tenofovir disoproxil fumarate and emtricitabine, received FDA approval to be used as part of an HIV prevention package in high risk individuals. Truvada® received this licensing on the basis of a 44-75% decrease in HIV incidence in 3 clinical trials studying its use in diverse study populations including: serodiscordant heterosexual couples (Partner's PrEP) and other high risk heterosexual individuals (TDF2), and men who have sex with men (MSM; iPrEX).(4-6) Subsequently, a study investigating Truvada® pre exposure prophylaxis (PrEP) in IV drug users demonstrated 49% protection.(7) Truvada® is the first relatively discreet, user-controlled HIV prevention option available to women. Yet, clinical trials in women have exhibited mixed results: two large phase III trials, FEM-PrEP and VOICE, failed to show HIV prevention efficacy for tenofovir taken with or without emtricitabine.(8, 9) These results were explained by the low degree of adherence to the daily regimens by study subjects. Subsequently, observational analyses have directly linked the effectiveness of Truvada® for PrEP with adherence.(10, 11) However, the degree of adherence required for protection may be contingent on the site of HIV exposure as evidenced by recent data demonstrating only two doses per week of Truvada® reduced HIV incidence by up to 90% in the MSM population of iPrEX (10): a level of adherence that was also seen in FEM PrEP and VOICE.(8, 9)
The most common mode of HIV transmission is through sexual activity, whereby mucosal tissues are the primary sites of HIV exposure. For TasP approaches to be successful, drug concentrations in infected individuals must be sufficient to suppress viral replication and shedding in the local anatomical sites associated with transmission, and may be necessary at high concentrations in the mucosal fluid serving as the viral source of transmission.(12) Conversely, PrEP approaches rely on drug concentrations in an uninfected individual being sufficient to prevent viral entry, integration or replication in HIV target cells in mucosal tissue and/or regional lymph nodes at the time of exposure.(13) Consequently, when considering the utility of TasP and PrEP for global reduction in HIV incidence, a thorough understanding of antiretroviral pharmacology in mucosal tissues becomes exceedingly important to ensure that optimal drugs, doses, and dosing schedules have been selected. For this purpose, the present review will examine antiretroviral pharmacokinetics in the mucosal tissue types most commonly involved in HIV transmission –the lower gastrointestinal tract and the female genital tract.
2. Methods
Research papers and abstracts describing antiretroviral pharmacokinetics in the female genital tract and lower gastrointestinal mucosal tissues published in the MEDLINE® database from January 2000 to December 2014 were identified using the following search terms: HIV, antiretroviral, pharmacokinetics, cervicovaginal fluid, cervical tissue, vaginal tissue, cervix, vagina, rectal fluid, rectal tissue, and rectum. Individual drug names were also included in the search terms. All original articles and relevant reviews discussing antiretroviral concentrations in the mucosal fluid or tissue of the gastrointestinal and female genital tract for any commercially available antiretrovirals or antiretroviral currently under investigation were reviewed in detail. Abstracts presented at scientific conferences prior to December 2014 were also included in this review.
3. Mucosal Tissue Pharmacokinetic Considerations
Mucosal tissues represent a particularly dynamic microenvironment which is subject to a number of regulatory pathways including paracrine, autocrine and endocrine signaling. Understanding the pharmacologic factors guiding drug distribution and how these are regulated within the tissue compartment is important for interpreting mucosal tissue pharmacokinetic data. In the subsections below, we describe three of these factors: protein binding, membrane drug transporter activity, and endogenous hormones.
3.1 Protein Binding
Protein binding is an important consideration in the pharmacokinetic profile of antiretrovirals, because only the unbound fraction (ie free drug) is available for pharmacologic activity. The extent of protein binding may affect a compound's ability to exit the vasculature and penetrate into the interstitial tissue space.(14) The primary proteins responsible for binding antiretrovirals are albumin and α1 acid glycoprotein (AAG).(15) The extent of binding to these proteins is highly variable amongst the antiretroviral classes.(16) For instance, most protease inhibitors are more than 90% bound to AAG; while the non-nucleoside reverse transcriptase inhibitor (NNRTI), efavirenz is more than 99% bound to albumin.(16) Importantly, the concentration of albumin and AAG are much lower in mucosal fluid relative to blood plasma. In one investigation of cervical mucus, the mean (SD) concentration of albumin and AAG was 73 (45) and 6.6 (3.6) mg/L, respectively. Both of these values are less than 1% of the concentration found in blood plasma.(17) For one antiretroviral, maraviroc, protein binding is reduced 10-fold in the female genital tract: from approximately 76% in blood plasma to approximately 7.6% in cervicovaginal secretions.(18, 19) These findings imply that a higher degree of pharmacologic activity might be achieved in mucosal fluid relative to plasma given the same total drug concentration in the two matrices.
Alteration in protein binding can affect pharmacodynamic markers such as the 50% inhibitory concentration (IC50), and confound pharmacokinetic (PK)/pharmacodynamic (PD) analyses that extrapolate inhibitory quotients (the ratio between drug concentration at the end of the dosing interval and level of drug resistance of the viral isolate), to clinical trial data.(16) For example, the protease inhibitor SC-52151 failed to show a clinical effect despite achieving an adequate concentration in the plasma.(20) This was later shown to be due to AAG binding, which reduced the activity of the drug by 17-fold in MT-2 cells.(21) Kempf et al. found that by including just 10% fetal calf serum in cell culture media, lopinavir was 96% protein bound, thus providing proof of concept for in vitro manipulation to produce better pharmacodynamic estimates.(22) A recent in vitro investigation in CD4+ T cells isolated from peripheral blood mononuclear cells (PBMCs) found that increasing the extracellular concentration of human serum albumin (HSA) from 1 to 80 mg/mL resulted in reduced intracellular concentrations of approximately 8-fold for efavirenz (at a concentration of 5ng/mL), 1.7-fold for raltegravir (at a concentration of 5ng/mL), and 2.3-fold for etravirine (at a concentration of 2ng/mL).(23) Thus careful consideration of the extent to which a drug is protein bound is critical for translating in vitro data into the in vivo system.
3.2 Drug Transporters
Transporter proteins are located on cell membranes of tissues such as the colorectal and genital tract mucosa, and are highly involved in the uptake and efflux of drugs. Several antiretrovirals are substrates of drug transporters within the ATP binding cassette (ABC) and solute carrier (SLC) families.(24) Recent efforts have been directed at elucidating the role of mucosal tissue transporters in the distribution of drugs to the female genital and lower gastrointestinal tracts. Zhou et al. investigated the mRNA expression of several ABC efflux transporters as well as SLC and SLCO uptake transporters in human vaginal and ectocervical tissue.(25) Liver tissue was used as a reference standard due to abundant expression of the drug transporters investigated.(26) The efflux transporters MRP1 and MRP4 were highly expressed (50-100% of reference tissue) in cervicovaginal tissue, while MRP2 and the uptake transporters, OAT1 and OAT3, were less than 2% of the reference tissue. This analysis didn't include information regarding location of these transporters within the epithelium, which is important to interpret the data correctly. For instance, abundant expression of efflux transporters in the squamous epithelium adjacent to the lumen would increase elimination of drug substrates from the tissue. However efflux transporters located within the basal lamina (the most interior layer of the epithelium), would increase transport into the squamous epithelial layer and lead to accumulation of drug substrates.
Nicol et al. addressed this by quantifying the mRNA and protein expression of 3 efflux (MDR1, MPR2, MRP4) and 3 uptake (OAT1, OAT3 and OATP1B1) transporters in cervical, vaginal, and colorectal tissue using Real-Time PCR and immunohistochemistry techniques.(27) Zhou's findings agree with Nicol's analysis which revealed consistent expression of the ABC transporters at different levels in each tissue type. MDR1 and MRP2 mRNA expression in female genital mucosal tissue was found to be 3 to 4-fold higher than colorectal tissue (p<0.001). Conversely, MRP4 mRNA in colorectal tissue was 2-fold higher than female genital mucosal tissue (p<0.05). While diffuse MDR1 protein expression was seen throughout the vaginal and ectocervical tissue, expression appeared to be localized to the luminal surface in endocervical and colorectal tissue. Diffuse MRP2 protein expression was observed in the epithelia of all tissue types. Finally, MRP4 protein expression appeared diffuse throughout the vaginal squamous epithelium and colorectal columnar epithelium. However, positively stained lymphocytes and monocytes appeared to be localized to the luminal surface of the colorectal epithelium. The influx transporters OAT1, OAT3, and OAT1B1, exhibited a lower degree of expression in all tissues compared with efflux transporters: <25% of all mucosal tissue samples exhibited quantifiable expression and all detectable tissue samples were <0.1% of expression compared to kidney tissue (used as a reference due to its abundant expression of uptake transporters).(26, 27) These data suggest that efflux transporters (MDR1, MRP2, and MRP4) play a role in drug distribution in female genital and colorectal tissues.
Table 1 summarizes the drug transporter affinity for available antiretrovirals. Recent modeling work has determined that being a substrate of MRP1 positively correlates with female genital tract tissue concentrations; whereas being a substrate for MRP4 negatively correlates with tissue concentration.(28) The negative correlation between tissue penetration and MRP4 substrate status is in support of the findings that MRP4 expression appears diffuse throughout the female genital tract thus decreasing accumulation in the tissue.
Table 1. Antiretroviral Drugs Are Substrates for Drug Transporters (24, 68).
| Class | Antiretroviral | Drug Transporter |
|---|---|---|
| NRTIs | Abacavir | P-gp BCRP MRP4 |
| Emtricitabine | MRP1 | |
| Lamivudine | BCRP OCT1 OCT2 | |
| Tenofovir DF | P-gp | |
| Zidovudine | MRP4 BCRP OAT1 OAT2 OAT3 OAT4 CNT1 CNT3 ENT2 | |
| INSTIs | Dolutegravir | P-gp, BCRP |
| Raltegravir | P-gp | |
| PIs | Atazanavir | P-gp MRP1 MRP2 |
| Darunavir | P-gp OATP1A2 OATP1B1 | |
| Lopinavir | P-gp MRP1 MRP2 OATP1A2 OATP1B1 | |
| EIs | Maraviroc | P-gp |
BCRP=breast cancer resistance protein, DF=disoproxil fumarate, EIs=Entry inhibitors, INSTIs=integrase strand transfer inhibitors, NRTIs=nucleoside reverse transcriptase inhibitors, PIs=protease inhibitors
Finally, the expression of efflux transporters appears to be regulated by inflammatory cytokines released in response to infection or injury.(29, 30) Pro-inflammatory mediators like IL-6, IL-1β and INF-γ have been shown to decrease the expression of BCRP and MDR1(30-32). Since mucosal inflammation may be consequence of HIV infection(33), it is possible that stage of HIV infection may influence antiretroviral drug distribution. Investigation with the antibiotic linezolid (an MDR1 substrate) has shown altered exposure in soft tissues correlated with degree of inflammation.(34) However, the effect of local inflammation on antiretroviral drug distribution to mucosal tissues is yet to be determined.
3.3 Endogenous Hormones
Another important consideration is the effect of endogenous hormones on drug distribution to mucosal tissues. Hormonal and physiological variations occurring during the follicular, ovulatory and luteal phases of the menstrual cycle may alter certain pharmacokinetic parameters such as protein binding and oxidative hepatic metabolism.(35) In cultured epithelial cells of the female genital tract, estradiol and progesterone were found to regulate the intracellular concentration of tenofovir diphosphate.(36) Additionally, MDR1 expression in the female genital mucosa appears to be subject to hormonal regulation with highest expression correlating with the luteal phase of the menstrual cycle(37) and lowest expression in postmenopausal women.(27) However, the literature describing the clinical implications of these physiological alterations for antiretrovirals is extremely limited. Sheth et al. investigated trough cervicovaginal concentrations of tenofovir, emtricitabine, and atazanavir in 20 HIV infected women and found no correlation between cervicovaginal fluid concentration and menstrual cycle.(38) Likewise, cervical raltegravir trough concentrations in 9 healthy volunteers demonstrated no correlation with phase of menstrual cycle.(39) However, the extensive between-subject variability in raltegravir cervical tissue concentrations (coefficient of variation=195.7%) makes it difficult to conclude that menstrual cycle does not impact drug distribution to the female genital tract. No investigation to date has explored the impact of hormonal changes on drug distribution within the colorectal tissue.
4. Mucosal Tissue Pharmacokinetics
The distribution of antiretroviral agents has been widely investigated in the female genital tract by measuring drug concentrations in cervicovaginal fluid. Cervicovaginal fluid allows for more intensive pharmacokinetic sampling within individuals and overcomes some of the challenges posed by tissue biopsies. Distribution of antiretrovirals in the mucosal fluid of the lower gastrointestinal tract has not been widely studied. One study that measured drug concentration of dolutegravir in rectal fluid found that self-collected rectal fluid swabs were an unreliable surrogate for drug exposure in the rectal tissue because of large within- and between-subject variability.(40)
When evaluating mucosal tissue pharmacokinetics, a ratio between drug exposure in the compartment relative to drug exposure in the blood plasma is commonly calculated to correct for plasma drug exposure with different drug combinations and dosing strategies. The ratio can be calculated with either single sampling times or by area under the concentration time curve (AUC). Utilizing the AUC approach minimizes spurious results due to lag times in distribution to peripheral compartments. Figure 1 reports the penetration ratios for each antiretroviral in the female genital or lower gastrointestinal tissue and includes ratios derived with AUCs (when available) or concentrations at the end of the dosing interval (Ctau). A summary table of the methods and results of antiretroviral pharmacokinetic studies in the female genital mucosal tissues up to 2011 can be found in a review by Else and colleagues.(41)
Figure 1. Antiretroviral Female Genital and Lower Gastrointestinal Tract Penetration Ratios.
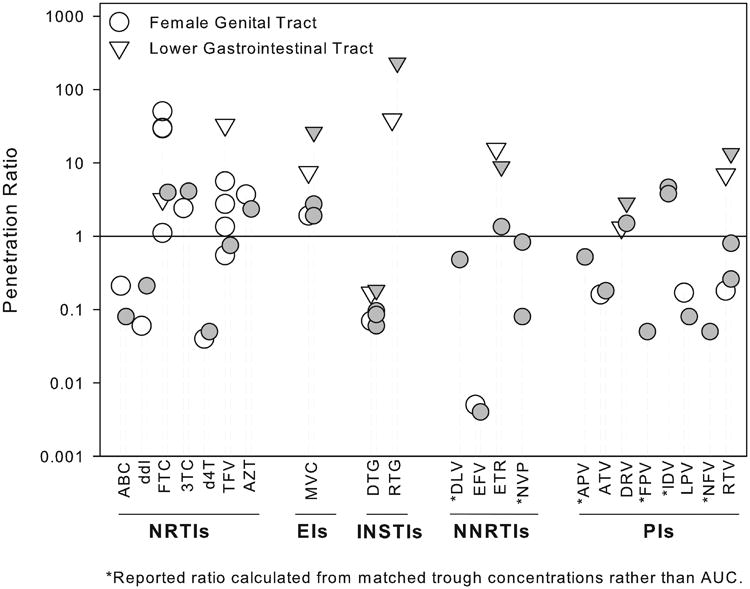
This figure reports the mucosal tissue or fluid:blood plasma area under the concentration time curve (AUC) ratios for 22 antiretrovirals stratified by class. Both single dose (open symbols) and steady state (closed symbols) are reported when available. The line of unity (mucosal tissue/fluid AUC=blood plasma AUC) is denoted by the solid reference line. In general penetration ratios tend to be higher in the lower gastrointestinal tract for most antiretrovirals. As a class nucleoside reverse transcriptase inhibitors (NRTIs) and the entry inhibitor (EI), maraviroc(MVC), tend to demonstrate a high degree of penetration (>1) into the female genital and lower gastrointestinal tracts whereas non nucleoside reverse transcriptase inhibitors (NNRTIS) and protease inhibitors (PIs) tend to exhibit low penetration (<1). ABC=abacavir, ddI=didanosine, FTC=emtricitabine, 3TC=lamivudine, d4T=stavudine, TFV=tenofovir, AZT=Zidovudine, DTG=dolutegravir, RTG=raltegravir, DLV=delavirdine, EFV=efavirenz, ETR=etravirine, NVP=nevirapine, APV=amprenavir, ATV=atazanavir, DRV=darunavir, FPV=fosamprenavir, IDV=indinavir, LPV=lopinavir, NFV=nelfinavir, RTV=ritonavir, INSTIs=integrase strand transfer inhibitors.
4.1 Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
Combination NRTIs form the backbone of most HIV treatment regimens. These agents require intracellular phosphorylation to their active metabolites, which compete with the host's endogenous nucleotides for incorporation into the elongating proviral DNA strand. In general, agents within this class exhibit a low degree of protein binding (<50%) and hepatic biotransformation.(42) Upon phosphorylation the intracellular active moieties are trapped within the cell membrane, and only parent compounds are readily detectable in extracellular fluid spaces such as blood plasma, cervicovaginal fluid, and seminal plasma. Currently one of the most commonly used NRTIs, tenofovir, is being investigated for PrEP in a 1% gel formulation. This section will review the mucosal tissue pharmacokinetics of orally and topically administered NRTIs.
Dumond et al first described the median (IQR) cervicovaginal fluid to blood plasma AUC0-tau ratios following single and multiple doses of 7 NRTIs in HIV infected patients initiating or changing antiretrovirals for HIV treatment. These are listed by rank order for the single dose in Table 2. Following the single dose, all agents were detected in cervicovaginal fluid within 4 hours and reached peak concentrations within 6 hours following a single dose.(43) Of the NRTIs only the older agents, didanosine and stavudine, exhibited cervicovaginal fluid to blood plasma ratios of less than 20%. Subsequently, tenofovir and emtricitabine were shown to exhibit 2-26 times higher cervicovaginal fluid exposure compared to blood plasma exposure when measured after a single dose over 14 days. The terminal half-life in plasma was estimated to be 47 hours for tenofovir and 49 hours for emtricitabine, and in cervicovaginal fluid, it was estimated to be 71 hours for tenofovir and 40 hours for emtricitabine.(44) Since these half-lives are similar to previously published emtricitabine triphosphate (FTCtp) and tenofovir diphosphate (TFVdp) data(45), this extended elimination phase was thought to reflect clearance and dephosphorylation of intracellular FTCtp and TFVdp.
Table 2. Distribution in Cervicovaginal Fluid after Single and Multiple Doses.
| Class | Antiretroviral | Number | CVF:BP AUC0-tau Median (Interquartile Range) | Reference | |
|---|---|---|---|---|---|
| Single Dose | Multiple Dose | ||||
| NRTIs | Zidovudine | 3 | 3.71 (1.13, 6.04) | 2.35 (1.21-21.2) | (43) |
| Lamivudine | 13 | 2.41 (1.09, 15.48) | 4.11 (2.3-5.94) | (43) | |
| Tenofovir | 10 | 1.35 (0.0023, 4.47) | 0.75 (0.37-6.45) | (43) | |
| Emtricitabine | 3 | 1.11 (0.91, 11.00) | 3.95 (1.87-6.71) | (43) | |
| Abacavir | 5 | 0.21 (0.08, 0.70) | 0.08 (0.08-0.13) | (43) | |
| Didanosine | 2 | 0.06 (0.06, 1.31) | 0.21 (0.01, 0.4) | (43) | |
| Stavudine | 3 | 0.04 (0.007, 0.35) | 0.05 (0-0.12) | (43) | |
| NNRTIs | Efavirenz | 6 | 0.005 (0.001, 0.008) | 0.004 (0.001, 0.006) | (43) |
| Nevirapine | 7 | Not available | 0.83 (0.43, 1.58) | (52) | |
| Etravirine | 12 | Not available | 1.2 (0.5, 4.8) | (56) | |
| INSTIs | Raltegravir | 6 | Not available | 4.4 (1.5, 1.7) | (64) |
| Dolutegravir | 8 | 0.07 (0.05, 0.18) | 0.06 (0.04, 0.11) | (67)a | |
| PIs | Darunavir | 8 | Not Available | 1.5 (1.0-1.6) | (79) |
| Ritonavir | 12 | 0.18 (0.005, 0.42) | 0.26 (0.11, 1.69) | (43) | |
| Lopinavir | 4 | 0.17 (0.003, 0.3) | 0.08 (0.03, 1.28) | (43) | |
| Atazanavir | 8 | 0.16 (0.1, 0.79) | 0.18 (0.05, 0.66) | (43) | |
Concentration data from HIV negative subjects
AUC=area under the concentration time curve, BP=blood plasma, CVF=cervicovaginal fluid, INSTIs=integrase strand transfer inhibitor, NRTI=nucleoside reverse transcriptase inhibitor, NNRTI=nonnucleoside reverse transcriptase inhibitor, PK=pharmacokinetics, PIs=protease inhibitor
The formation of TFVdp and FTCtp have been well characterized in the female genital tract following a single dose.(44, 46, 47) The most recent of these studies measured parent and intracellular concentrations at multiple time points over 48 hours across a 4-fold dosing range (150 to 600mg for tenofovir disoproxil fumarate and for 100 to 400mg emtricitabine).(46) While the time to peak concentrations (Tmax) of tenofovir and emtricitabine is achieved within 6 hours in cervical and vaginal tissues, the formation of the active metabolites are delayed, with a median Tmax of 24 hours for TFVdp and 12 hours for FTCtp.(46) Although TFVdp was detected in vaginal tissue for up to 14 days following a single fixed dose combination tablet of tenofovir disoproxil fumarate 300mg and emtricitabine 200mg, FTCtp was only detected for up to 2 days.(44) Louissaint et al. confirmed the persistence of TFVdp in vaginal tissue after a single dose, and calculated a terminal half-life of 53 hours.(47) This tissue half-life is consistent with TFVdp's 49.7 hour in vitro half-life in resting PBMC cultures.(45)
Multiple pharmacokinetic investigations suggest differential distribution of tenofovir and emtricitabine between mucosal tissues types, which has important implications for antiretroviral based prevention strategies. Pharmacokinetic analysis investigating exposure at 6 to 48 hours following a single dose found 10-fold higher TFVdp exposure in colorectal tissue compared to female genital mucosal tissue.(46) However, FTCtp was 170 times higher in the female genital mucosal tissue compared to the rectal tissue. When these compounds were measured over 14 days following a single dose, tenofovir and TFVdp rectal tissue AUC0-14days remained were 60-100 times higher than female genital tract tissue.(44, 47) The difference in the concentration gradient between these two studies is likely due to the delayed accumulation of TFVdp in rectal tissue (median Tmax≥48 hours) compared to female genital tract tissue (median Tmax=24 hours).(46) Emtricitabine and FTCtp AUC0-14days were approximately 10 times higher in female genital tract tissue than colorectal tissue.(44)
Topical tenofovir 1% gel has been investigated for application in both the female genital tract and the colorectum. Once instilled, TFVdp can be detected in tissues within 0.5 hours.(48, 49) A randomized cross-over PK study found that a 40mg dose of topical tenofovir in the vagina achieved more than a 100-fold higher tenofovir and TFVdp concentrations in vaginal and cervical tissue compared to the standard 300mg oral dose.(50) Maximum blood plasma concentrations following daily vaginal application of the gel was approximately 1% of the maximum concentration (Cmax) following daily oral tenofovir 300mg (median Cmax=3.9 vs 332ng/mL, respectively). Thirty minutes after application, a separate study found that the gel yielded TFVdp concentrations in colorectal tissue 6 times higher than concentrations achieved after an oral dose(49) Maximum blood plasma concentrations following 7 daily rectal administrations of the gel were ∼3% of the exposure following a single dose of oral tenofovir 300mg (median Cmax=8.61 vs 252ng/mL). Rectal administration also led to detectable tenofovir concentrations in vaginal fluid: vaginal fluid Cmax was approximately 2.5 logs lower than colorectal fluid but approximately 2-fold higher compared to vaginal fluid Cmax following oral dosing.(51)
4.2 Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
The NNRTIs constitute several first line antiretroviral therapies in combination with NRTIs. By binding non-competitively to the reverse transcriptase enzyme, they inhibit transcription of viral RNA. Delavirdine, efavirenz and nevirapine are first generation NNRTIs while rilpivirine and etravirine are second generation. The penetration of most NNRTIs into the female genital tract is limited.(14) In a study by Min et al. cervicovaginal fluid:blood plasma ratios were 0.83 for nevirapine, 0.48 for delavirdine, and < 0.08 for efavirenz.(52) These penetration ratios demonstrate an inverse relationship with the degree of protein binding exhibited by each agent (nevirapine=60% bound, delavirdine=98% bound, and efavirenz≥99.5%).(53-55) The penetration of these agents into the colorectal tissue has not been studied.
In the DIVA02 study of 12 HIV-1 infected women, the etravirine median (IQR) cervicovaginal fluid:blood plasma ratio was 1.2 (0.5-4.8).(56) Despite high (99%) protein binding, etravirine's low molecular mass and lipophilic structure may be responsible for the increased mucosal tissue penetration. A separate study in 12 HIV-negative men determined that after a single dose of etravirine, rectal tissue exposure was ∼15-fold higher than blood plasma: with repeated dosing, rectal tissue exposure was ∼9-fold higher.(57)
The pharmacokinetics of oral rilpivirine have not been investigated in mucosal tissues. However, interest has grown for the long acting parenteral formulation of the drug, both for PrEP and to increase adherence in HIV treatment. Mucosal distribution with this formulation has been investigated in HIV-negative men and women at doses of 150mg, 300mg, 600mg and 1200mg given intramuscularly (IM).(58) In men, at the 600mg dose the geometric mean (90% CI) rectal tissue:plasma ratio was 0.98(0.85-1.11) on day 7 while in women, the geometric mean (90% CI) vaginal tissue:plasma ratio was 0.72(0.4-1.05) on day 7. The authors concluded that the compartmental rilpivirine concentrations were proportional to the dose administered. A planned phase II study of the long acting injectable formulation of rilpivirine TMC278LA will evaluate the 1200 mg dose for chemoprophylaxis.(59)
Dapivirine is also being developed as a topical microbicide formulated in intravaginal rings (IVRs), gels and films. In 24 HIV-negative women, concentrations attained in the lower female genital tract 28 days after placement of an IVR were 5 log units higher than maximum concentrations achieved in the plasma over the 28 day period.(60) Further, there was a linear relationship (p=0.0157) between tissue concentration and protective effect.(61) The FAME-02 study in 60 HIV-negative women that compared the PK/PD and safety of gel (0.05%) and film (1.25mg) formulations of dapivirine found that both formulations achieved low and comparable plasma concentrations (302.47 pg/mL for gel compared to 227.13 pg/mL for film). However tissue concentrations of dapivirine from the film were four times lower than concentrations from the gel, and this correlated with greater infectivity in the ex-vivo challenge experiments with vaginal and cervical biopsies.(62) These novel formulations may be used to help achieve protective mucosal drug exposure with decreased systemic exposure and reduced dosing frequency.
4.3 Integrase Inhibitors (INSTIs)
INSTIs represent the newest class of antiretrovirals with the first agent in class, raltegravir, approved in 2007. This was followed by elvitegravir in 2012 and dolutegravir in 2013. As a class, these agents are potent, well tolerated, and exhibit lower drug interaction potential than protease inhibitors (PIs) and NNRTIs. Glucuronidation is the primary route of metabolism for raltegravir and dolutegravir, while elvitegravir is primarily metabolized by the CYP450 enzyme system. For this reason some have been proposed as second generation PrEP strategies. To date, raltegravir and dolutegravir have data available on drug penetration into mucosal tissues.
Raltegravir exhibits the lowest degree of protein binding (83%) within this class.(63) Data in HIV-negative and HIV-infected premenopausal women have shown median (IQR) raltegravir cervicovaginal fluid:blood plasma steady state AUC0-12hr ratios of 0.93 (0.4, 1.7) and 4.4 (1.5, 7.2), respectively.(64) The DIVA trial investigated raltegravir trough concentrations in the blood plasma and cervicovaginal fluid of 14 HIV infected women.(65) The median (IQR) cervicovaginal fluid:blood plasma ratio was 2.3 (1.4-4.1). Of the trial's three study subjects with bacterial vaginosis, two exhibited cervicovaginal fluid:blood plasma ratios that were 4 to 16-fold greater than the median, suggesting that inflammation may increase raltegravir exposure in the female genital tract. Raltegravir is a substrate for the MDR1 efflux transporter (66) which is highly expressed in the female genital tract and generally attenuated in the presence of inflammatory mediators, therefore accumulation of raltegravir in inflamed female genital mucosal tissue is biologically plausible.
Conversely, dolutegravir exposure in female genital tract tissues and cervicovaginal fluid was found to be <10% of blood plasma.(67) Dolutegravir is much more extensively protein bound (≥98%) than raltegravir which could potentially explain this low degree of distribution.(68) Despite this, 94% of samples collected from the female genital tract were above dolutegravir's protein adjusted IC90 of 64ng/mL(67). Since cervicovaginal fluid concentrations of AAG (the major serum protein responsible for binding dolutegravir) are approximately 1% of blood plasma(17), dolutegravir exposure in the female genital tract may be sufficient despite the compound's poor penetrative capacity.
In the lower gastrointestinal tract, raltegravir was found to rapidly distribute after a single dose, reaching concentrations in the terminal ileum which exceeded plasma concentrations within 2 hours and peak concentrations within 4 hours.(69) After a single 400mg dose, the highest concentrations were achieved in the terminal ileum followed by the splenic flexure, and the rectal tissue (AUCcomposite = 594, 258, and 143ng*hr*mL−1, respectively). Under steady-state conditions with 400mg dosed twice daily, the splenic flexure exhibited the highest exposure with an AUCcomposite of 2240ng*hr*mL−1 where the AUCcomposite was 788ng*hr*mL−1 in both the terminal ileum and the rectum. A single dose, dose ranging pharmacokinetic study investigating 200 to 800mg or raltegravir in healthy women found exposure in the lower gastrointestinal tract is approximately 25-fold greater than in the female genital tract, and colorectal exposure is similar between women and men.(70) Dolutegravir was found to poorly distribute to the rectal tissue of healthy males, with a median AUCcomposite penetration ratio of 0.17.(40) Despite low rectal exposure relative to blood plasma, 100% of concentrations from the lower gastrointestinal mucosa were above the protein adjusted IC90.(40)
Cabotegravir is an investigational INSTI, which has a similar chemical structure to dolutegravir.(71) It is formulated as a long acting injectable nanoparticle suspension for intramuscular administration in HIV treatment and prevention.(71) Cabotegravir exhibits plasma pharmacokinetics which support 4 and 8 week dosing intervals. Both dosing intervals are currently under investigation in phase IIb studies for HIV treatment.(72) Similar to dolutegravir, a 400mg IM dose of cabotegravir was found to exhibit cervical/vaginal and colorectal penetration ratios of 0.16-0.19 and ≤0.08, respectively, 2-8 weeks after a single injection.(73) While median rectal tissue concentrations at 4 and 12 weeks following a 400mg split IM injection were below the protein adjusted IC90 (16.6ng/mL), the median concentration in cervical and vaginal tissue was above this value at the week 4 and 12 visit, respectively.(73) Recently, Radzio et al. demonstrated 100% protection in macaques vaginally challenged with biweekly SHIV for up to 20 weeks after the last of 3 monthly 50mg/kg injections.(74) This study found low penetration to the mucosal compartment similar to humans with cervicovaginal and rectal fluid:plasma AUC ratios of 0.16 and 0.38, respectively. Andrews et al. also demonstrated 100% protection in macaques that were rectally challenged with weekly SHIV for up to 20 weeks following two monthly 50mg/kg injections and 5 weeks following a single injection.(75) In the single injection study, plasma concentration ≥1× PAIC90 correlated with 97% protection whereas plasma concentrations <PAIC90 resulted in 75% protection. Following plasma pharmacokinetic modeling and simulation, the group determined that an 800mg split IM dose administered every 3 months would be carried forward into clinical PrEP trials.(76)
4.4 Protease Inhibitors (PIs)
PIs inhibit the protease enzyme responsible for post translational cleavage of viral proteins. These compounds generally have large molecular weights and exhibit extensive protein binding and hepatic biotransformation via the CYP450 system.(77) These pharmacologic complexities, coupled with their post integration mechanism of action, have resulted in this class being generally overlooked for HIV prevention strategies. Therefore the literature regarding protease inhibitors' mucosal tissue pharmacokinetics is limited. Investigation of the cervicovaginal secretions of HIV infected women receiving stable HAART revealed that indinavir and darunavir exhibit the highest degree of female genital tract penetration among the protease inhibitors. Median (range) trough cervicovaginal fluid:blood plasma ratios of 3.8 (0.99-10) and 4.64(0.8-26.93) have been reported for indinavir.(52, 78) For darunavir the median (IQR) AUC cervicovaginal:blood plasma ratio is 1.5 (1.0-1.6).(79) Amprenavir, ritonavir, atazanavir, lopinavir, and saquinavir (listed in order of rank from highest to lowest) all exhibit ratios of <1.(43, 52, 78) Of all the protease inhibitors indinavir exhibits the lowest degree of protein binding (54-70%), which possibly accounts for its high penetrative capacity.(80)
Only darunavir and ritonavir have been studied in the lower gastrointestinal mucosal tissues of HIV-negative men. Following a single dose, darunavir and ritonavir were rapidly detected (within 1 hour), reaching peak concentrations by 5 hours.(57) Under steady state conditions, the rectal tissue:blood plasma AUC0-12hour ratio was ∼2.7 for darunavir and 27 for ritonavir.(57)
The potency and high genetic barrier to resistance exhibited by protease inhibitors, make them favorable HIV treatment options. It has been previously shown that viral replication can persist in lymphoid tissue despite effective HAART therapy and that this persistence may correlate with decreased antiretroviral exposure.(81, 82) Since gut associated lymphoid tissue may serve as a potential reservoir of persistent viral replication in patients, tissue penetration could be a theoretical consideration in designing ARV regimens, similar to the CNS Penetration-Effectiveness (CPE) score proposed by the CHARTER Group.(83) However, unlike the CPE score, increased mucosal tissue penetration have not yet been linked to more favorable outcomes.
4.5 Entry inhibitors
Maraviroc is a chemokine receptor antagonist that prevents the virus from binding to the CCR-5 co-receptor on CD4+ T cells. Currently, it is the only clinically approved agent in its class. Maraviroc concentrates in the female genital tract.(19) In a study of 12 healthy volunteer women, AUC0-12h in cervicovaginal fluid was 3645 (1785–7505) ng*h*mL−1 after a single 300 mg dose. After twice daily dosing for seven days, cervicovaginal fluid and vaginal tissue AUC0-12h was 7500 (3078–9090) ng*h*mL−1 and 4857 ng*h*mL−1, respectively.(19) High concentrations of maraviroc were found in the rectal tissue of HIV-negative men both after single and multiple dosing at 300 mg doses.(84) Composite AUC ratios in the rectal tissue were 7.5 (single dose) to 26-fold (multiple dose) higher than blood plasma. The maraviroc accumulation ratio from single to multiple dosing in plasma and rectal tissue was 1.4 and 4.9, respectively, which suggests an additional mechanism of increased drug exposure in colorectal tissue. Since maraviroc's bioavailability is 23%(85), it is likely that local, luminal concentrations of maraviroc passing through the gastrointestinal tract are contributing to increased rectal tissue exposure. A recent phase 1, single-dose pharmacokinetic study also found a 10-fold higher exposure of maraviroc in rectal tissue as compared to cervicovaginal tissue.(70) Since maraviroc is a substrate for the efflux transporter MDR1, and this transporter demonstrates 5.5-fold higher mRNA expression in vaginal tissue (27), it may be responsible for the differential penetration between female genital tract tissue and colorectal tissue.
Maraviroc has also been incorporated into silicone elastomer vaginal rings for use as a topical microbicide in combination with dapivirine. This system that contains 100 mg maraviroc and 25 mg dapivirine was recently studied as part of a phase I clinical trial in 24 HIV-negative women who wore the ring for 28 days. While dapivirine was detected in all 24 cervical tissue samples (mean concentration of 1.6μg/mL), only 4 of 24 (16.67%) samples exhibited detectable concentrations of maraviroc.(61) This low release of maraviroc has resulted in re-formulation efforts, which are ongoing.(86)
5. Expert Opinion
Antiretroviral based prevention has previously demonstrated a high level of effectiveness in the context of mother-to-child transmission with nevirapine and zidovudine.(87) These prevention approaches have been explored for their potential to offer efficacious, relatively discreet, user-controlled HIV prevention options to adults at high risk of HIV acquisition. Because HIV is primarily transmitted through mucosal fluids, and early infection occurs within mucosal tissues, characterizing pharmacokinetic behavior within these peripheral compartments is important for optimizing PrEP and TasP initiatives.
Given that the PrEP end user population is healthy adults, the ideal PrEP agent would have minimal side effects, a low drug interaction potential, and allow for infrequent or as-needed dosing. Oral dosing formulations for two NRTIs (tenofovir and emtricitabine), raltegravir and maraviroc all exhibit excellent capacity to penetrate into mucosal tissue with concentrations that either match or exceed blood plasma concentrations with single and multiple doses. These agents' penetrative ability coupled with relatively uncomplicated drug interaction and side effect profiles make them good candidates for PrEP. Also, alternative antiretroviral formulations, such as 1% tenofovir gel and the dapivirine ring, are designed to achieve high local tissue concentrations and low plasma exposure. These formulations offer intermittent or reduced frequency dosing options.
In general NNRTIs and PIs exhibit a low degree of penetration into tissue. While complicated drug interaction and adverse events profiles impede their use in PrEP, NNRTIs and PIs are widely used in HIV treatment. Drugs within these classes that demonstrate extensive penetration, like darunavir and etravirine, may provide some theoretical benefit to TasP approaches.
Lastly, the pharmacokinetic data demonstrating preferential distribution of certain antiretrovirals, like tenofovir, into the colorectal mucosa relative to the female genital mucosa strongly support the notion that the site of HIV exposure determines the degree of protection achieved by a PrEP regimen. This knowledge can be used to tailor PrEP strategies to the target end user. For instance future PrEP trials or marketing initiatives directed at women are more likely to benefit from rigorous adherence counseling and intervention given that the female genital tract exhibits a smaller pharmacokinetic buffer for missed doses. In contrast, investigations of intermittent Truvada® PrEP regimens for MSM is strongly supported by pharmacokinetic data. This concept was recently confirmed with the success of the IPERGAY trial, which investigated an intermittent dosing regimen before and after sex in MSM.(88)
Understanding the pharmacokinetics of antiretrovirals in the mucosal tissue represents only one aspect of optimizing antiretroviral use for HIV prevention. A thorough characterization of target concentrations required for protection and subsequently well-defined PK/PD relationships represents another important aspect of defining dosing strategies.(89) However, this has been inconsistently defined for antiretroviral prevention. One reason is the lack of consensus on appropriate preclinical models of infection for defining concentration response relationships. The limitations of in vitro and ex vivo models have been reviewed in detail elsewhere (90, 91), but include a lack of tissue architecture and relevant microenvironment, and the use of clinically appropriate virus and viral inoculum.
Using clinical trial data from the CAPRISA004 study Karim et al. determined that women with cervicovaginal fluid tenofovir concentrations of >1000ng/mL exhibited a higher degree of protection (74%) than those with <1000ng/mL (14%).(92) Whether this target tenofovir concentration is also required for oral dosing strategies remains to be determined. Using data generated from the iPrEx study, Anderson et al. have proposed an EC90 concentration of 16 fmol TFVdp/million PBMCs with oral Truvada® dosing in MSM.(93) However the concentrations used in this approach were derived from cryopreserved PBMCs which can lose up to 60% of TFVdp compared to traditional methods of cell isolation.(93, 94) Therefore, this estimate likely underestimates the true effective concentration of TFVdp in PBMCs that results in colorectal tissue protection. Given the pharmacokinetic differences between the female genital and lower gastrointestinal tracts, it's also unlikely that these results can be extrapolated to a female population.
One recent preclinical and clinical approach to determine the concentration vs response relationship for tenofovir and emtricitabine measured intracellular metabolites (TFVdp and FTCtp) relative to competitive natural substrates of reverse transcriptase (deoxyadenosine triphosphate; dATP and deoxycytidine triphosphate; dCTP).(95, 96) When combining these data into a mathematical model to simulate mucosal tissue exposure achieved by various doing strategies, 80% protection from HIV transmission was predicted for the female genital tract with daily Truvada® dosing and 90% protection was predicted for the lower gastrointestinal tract with 2 doses per week. These predictions are in close agreement with observational data from clinical trials.(10, 11)
In the absence of well-defined efficacy target concentrations, important inferences can still be made from the available mucosal tissue pharmacokinetic data. Although it's not clear which anatomical locations should be preferentially targeted to reduce HIV transmission (eg mucosal tissues, regional lymph nodes), antiretrovirals with mucosal tissue concentrations above the viral IC90 may be considered preferable for both PrEP and TasP. Since the extent of antiretroviral mucosal tissue penetration is unpredictable, tissue pharmacokinetic data are essential for optimizing dosing strategies for HIV prevention.
Highlights.
Antiretroviral distribution to mucosal tissues is governed by a number of complex factors including lipophilicity, protein binding, and active transport.
The degree of antiretroviral mucosal tissue distribution is highly variable between and within antiretroviral therapeutic drug classes (Figure 1).
As a drug class, nucleoside reverse transcriptase inhibitors (NRTIs) achieve higher exposure in the mucosal tissues compared to non-nucleoside reverse transcriptase (NNRTIs) and protease inhibitors (PIs) (Figure 1).
In general antiretrovirals exhibit higher exposure in the lower gastrointestinal mucosal tissue compared to the female genital mucosal tissue.
This article elucidates some important knowledge gaps in the field of mucosal tissue pharmacokinetics which warrant further study to better characterize, including: 1) drug transporters and their regulation in mucosal tissues 2) the effect of hormonal regulation and inflammation on mucosal tissue distribution and 3) mucosal pharmacodynamic targets for HIV prevention.
Acknowledgments
This work was supported by the Centers for AIDS Research [grant number CFAR P30 AI50410], the National Institute for Allergy and Infectious Diseases [grant number R01 AI111891 and U19 AI09611] and the National Institute of General Medical Sciences [grant number T32 GM086330]. N Srinivas is supported by the Royster Society of Fellows. The content is solely the responsibility of the authors and does not necessarily represent the official views of the supporting agencies listed above.
Footnotes
Declaration of Competing Interests and Financial Disclosure: Angela Kashuba and her laboratory are part of the study teams for CAPRISA 004 and 008, FACTS 001, MTN 006, HPTN 066, FEM-PrEP, and CONRAD 113, 114, 117, and 118
References
- 1.WHO | HIV/AIDS [Internet] [cited 11/5/2014]; Available from: http://www.who.int/mediacentre/factsheets/fs360/en/
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Key graphics from CDC analysis showing proportion of people engaged in each of the five main stages of HIV care [Internet] 2013 Available from: http://www.cdc.gov/nchhstp/newsroom/2012/Continuum-of-Care-Graphics.html.
- 4.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in botswana. N Engl J Med. 2012 Aug 2;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 7.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in bangkok, thailand (the bangkok tenofovir study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013 Jun 15;381(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 8.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among african women. N Engl J Med. 2012 Aug 2;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrazzo J, Ramjee G, Nair G, et al. Pre-exposure prophylaxis for HIV in women: Daily oral tenofovir, oral tenofovir/emtricitabine or vaginal tenofovir gel in the VOICE study (MTN 003). 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. March 3-6.2013. abstract 26LB. [Google Scholar]
- 10.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. Lancet Infect Dis. 2014 Jul 22; doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014 Jul 1;66(3):340–8. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trezza CR, Kashuba AD. Pharmacokinetics of antiretrovirals in genital secretions and anatomic sites of HIV transmission: Implications for HIV prevention. Clin Pharmacokinet. 2014 Jul;53(7):611–24. doi: 10.1007/s40262-014-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood. 2011 Jul 28;118(4):839–46. doi: 10.1182/blood-2010-12-325860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson CG, Cohen MS, Kashuba AD. Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr. 2013 Jul;63(Suppl 2):S240–7. doi: 10.1097/QAI.0b013e3182986ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt S, Gonzalez D, Derendorf H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010 Mar;99(3):1107–22. doi: 10.1002/jps.21916. [DOI] [PubMed] [Google Scholar]
- 16.Boffito M, Back DJ, Blaschke TF, et al. Protein binding in antiretroviral therapies. AIDS Res Hum Retroviruses. 2003 Sep;19(9):825–35. doi: 10.1089/088922203769232629. [DOI] [PubMed] [Google Scholar]
- 17.Salas Herrera IG, Pearson RM, Turner P. Quantitation of albumin and alpha-1-acid glycoprotein in human cervical mucus. Hum Exp Toxicol. 1991 Mar;10(2):137–9. doi: 10.1177/096032719101000209. [DOI] [PubMed] [Google Scholar]
- 18.Selzentry full prescribing information. Research Triangle Park, NC 27709: ViiV Healthcare; 2014. [Google Scholar]
- 19.Dumond JB, Patterson KB, Pecha AL, et al. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009 Aug 15;51(5):546–53. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischl MA, Richman DD, Flexner C, et al. Phase I/II study of the toxicity, pharmacokinetics, and activity of the HIV protease inhibitor SC-52151. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 May 1;15(1):28–34. doi: 10.1097/00042560-199705010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Lazdins JK, Mestan J, Goutte G, et al. In vitro effect of alpha1-acid glycoprotein on the anti-human immunodeficiency virus (HIV) activity of the protease inhibitor CGP 61755: A comparative study with other relevant HIV protease inhibitors. J Infect Dis. 1997 May;175(5):1063–70. doi: 10.1086/520352. [DOI] [PubMed] [Google Scholar]
- 22.Kempf D, Hickman D, Vasavanonda S, et al. Identification of the serum-free IC50 for lopinavir and ritonavir: A useful measure for the estimation of inhibitory quotients into plasma and sanctuary sites. 3rd International Workshop on Clinical Pharmacology of HIV Therapy; Washington DC. 2002. abstract 3.3. [Google Scholar]
- 23.Avery LB, Zarr MA, Bakshi RP, et al. Increasing extracellular protein concentration reduces intracellular antiretroviral drug concentration and antiviral effect. AIDS Res Hum Retroviruses. 2013 Nov;29(11):1434–42. doi: 10.1089/aid.2013.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kis O, Robillard K, Chan GN, Bendayan R. The complexities of antiretroviral drug-drug interactions: Role of ABC and SLC transporters. Trends Pharmacol Sci. 2010 Jan;31(1):22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Zhou T, Hu M, Cost M, et al. Short communication: Expression of transporters and metabolizing enzymes in the female lower genital tract: Implications for microbicide research. AIDS Res Hum Retroviruses. 2013 Nov;29(11):1496–503. doi: 10.1089/aid.2013.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilgendorf C, Ahlin G, Seithel A, et al. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007 Aug;35(8):1333–40. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- 27.Nicol MR, Fedoriw Y, Mathews M, et al. Expression of six drug transporters in vaginal, cervical, and colorectal tissues: Implications for drug disposition in HIV prevention. J Clin Pharmacol. 2013 Dec 17; doi: 10.1002/jcph.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson CG, Sedykh A, Nicol MR, et al. Short communication: Cheminformatics analysis to identify predictors of antiviral drug penetration into the female genital tract. AIDS Res Hum Retroviruses. 2014 Nov;30(11):1058–64. doi: 10.1089/aid.2013.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Ven R, Oerlemans R, van der Heijden JW, et al. ABC drug transporters and immunity: Novel therapeutic targets in autoimmunity and cancer. J Leukoc Biol. 2009 Nov;86(5):1075–87. doi: 10.1189/jlb.0309147. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto-Furusho JK, Villeda-Ramirez MA, Fonseca-Camarillo G, et al. High gene expression of MDR1 (ABCB1) is associated with medical treatment response and long-term remission in patients with ulcerative colitis. Inflamm Bowel Dis. 2010 Apr;16(4):541–2. doi: 10.1002/ibd.21016. [DOI] [PubMed] [Google Scholar]
- 31.Poller B, Drewe J, Krahenbuhl S, et al. Regulation of BCRP (ABCG2) and Pglycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell Mol Neurobiol. 2010 Jan;30(1):63–70. doi: 10.1007/s10571-009-9431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blokzijl H, Vander Borght S, Bok LI, Libbrecht L, et al. Decreased P-glycoprotein (P-gp/MDR1) expression in inflamed human intestinal epithelium is independent of PXR protein levels. Inflamm Bowel Dis. 2007 Jun;13(6):710–20. doi: 10.1002/ibd.20088. [DOI] [PubMed] [Google Scholar]
- 33.Herold BC, Keller MJ, Shi Q, et al. Plasma and mucosal HIV viral loads are associated with genital tract inflammation in HIV-infected women. J Acquir Immune Defic Syndr. 2013 Aug 1;63(4):485–93. doi: 10.1097/QAI.0b013e3182961cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majcher-Peszynska J, Haase G, Sass M, et al. Pharmacokinetics and penetration of linezolid into inflamed soft tissue in diabetic foot infections. Eur J Clin Pharmacol. 2008 Nov;64(11):1093–100. doi: 10.1007/s00228-008-0531-5. [DOI] [PubMed] [Google Scholar]
- 35.Kashuba AD, Nafziger AN. Physiological changes during the menstrual cycle and their effects on the pharmacokinetics and pharmacodynamics of drugs. Clin Pharmacokinet. 1998 Mar;34(3):203–18. doi: 10.2165/00003088-199834030-00003. [DOI] [PubMed] [Google Scholar]
- 36.Shen Z, Fahey JV, Rodriguez-Garcia M, et al. Female sex hormone regulation of tenofovir-diphosphate in human female reproductive tract (FRT) cells in culture. AIDS Res Hum Retroviruses. 2014 Oct;30(Suppl 1):A149–50. [Google Scholar]
- 37.Axiotis CA, Guarch R, Merino MJ, et al. P-glycoprotein expression is increased in human secretory and gestational endometrium. Lab Invest. 1991 Nov;65(5):577–81. [PubMed] [Google Scholar]
- 38.Sheth AN, Evans-Strickfaden T, Haaland R, et al. HIV-1 genital shedding is suppressed in the setting of high genital antiretroviral drug concentrations throughout the menstrual cycle. J Infect Dis. 2014 Sep 1;210(5):736–44. doi: 10.1093/infdis/jiu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell C, Roemer E, Nkwopara E, et al. Correlation between plasma, intracellular, and cervical tissue levels of raltegravir at steady-state dosing in healthy women. Antimicrob Agents Chemother. 2014 Jun;58(6):3360–5. doi: 10.1128/AAC.02757-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greener BN, Patterson KB, Prince HM, et al. Dolutegravir pharmacokinetics in the genital tract and colorectum of HIV-negative men after single and multiple dosing. J Acquir Immune Defic Syndr. 2013 Sep 1;64(1):39–44. doi: 10.1097/QAI.0b013e31829ed7a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: The fetal compartment (placenta and amniotic fluid) Antivir Ther. 2011;16(8):1139–47. doi: 10.3851/IMP1918. [DOI] [PubMed] [Google Scholar]
- 42.Sharma PL, Nurpeisov V, Hernandez-Santiago B, et al. Nucleoside inhibitors of human immunodeficiency virus type 1 reverse transcriptase. Curr Top Med Chem. 2004;4(9):895–919. doi: 10.2174/1568026043388484. [DOI] [PubMed] [Google Scholar]
- 43**.Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: Implications for oral pre- and post-exposure prophylaxis. AIDS. 2007 Sep 12;21(14):1899–907. doi: 10.1097/QAD.0b013e328270385a. This original article was the first to report concentration data for 11 antiretrovirals in the genital tract of HIV infected women following the first dose and at steady state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: Implications for prevention of HIV-1 transmission. Sci Transl Med. 2011 Dec 7;3(112):112re4. doi: 10.1126/scitranslmed.3003174. This original article was the first to report concentration data for tenofovir and emtricitabine in mucosal tissues and fluids of HIV negative healthy volunteers after a single dose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robbins BL, Srinivas RV, Kim C, et al. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998 Mar;42(3):612–7. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cottrell ML, Prince HMA, Sykes C, et al. Mucosal tissues in women exhibit different concentrations of endogenous nucleotides and intracellular and extracellular antiretroviral (ARV) concentrations: Implications for preexposure prophylaxis (PrEP) development. 20th International AIDS Conference; Melbourne, Australia. July 20-25.2014. Abstract TUPE011. [Google Scholar]
- 47*.Louissaint NA, Cao YJ, Skipper PL, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013 Nov;29(11):1443–50. doi: 10.1089/aid.2013.0044. This original article reports concentration data for tenofovir in mucosal tissues of HIV negative healthy volunteers after a single dose and confirms previous reports of differential distribution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz JL, Rountree W, Kashuba AD, et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One. 2011;6(10):e25974. doi: 10.1371/journal.pone.0025974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anton PA, Cranston RD, Kashuba A, et al. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2012 Nov;28(11):1412–21. doi: 10.1089/aid.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hendrix CW, Chen BA, Guddera V, et al. MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013;8(1):e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang KH, Hendrix C, Bumpus N, et al. A multi-compartment single and multiple dose pharmacokinetic comparison of rectally applied tenofovir 1% gel and oral tenofovir disoproxil fumarate. PLoS One. 2014 Oct 28;9(10):e106196. doi: 10.1371/journal.pone.0106196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Min SS, Corbett AH, Rezk N, et al. Protease inhibitor and nonnucleoside reverse transcriptase inhibitor concentrations in the genital tract of HIV-1-infected women. J Acquir Immune Defic Syndr. 2004 Dec 15;37(5):1577–80. doi: 10.1097/00126334-200412150-00008. This original article was the first to report concentration data for 8 antiretrovirals in the genital tract of HIV infected women at steady state. [DOI] [PubMed] [Google Scholar]
- 53.Sustiva Full Prescribing Information. Princeton, NJ 08543 USA: Bristol-Myers Squibb Company; 2014. [Google Scholar]
- 54.Viramune Full Prescribing Information. Ridgefield, CT 06877 USA: Boehringer Ingelheim Pharmaceuticals, Inc.; 2014. [Google Scholar]
- 55.Rescriptor Full Prescribing Information. Research Triangle Park, NC 27709: ViiV Healthcare; 2012. [Google Scholar]
- 56.Clavel C, Peytavin G, Tubiana R, et al. Etravirine concentrations in the cervicovaginal compartment in HIV-1-infected women receiving etravirine-containing antiretroviral therapy: DIVA 02 study. Antimicrob Agents Chemother. 2012 Jul;56(7):4018–20. doi: 10.1128/AAC.06474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown KC, Patterson KB, Jennings SH, et al. Single- and multiple-dose pharmacokinetics of darunavir plus ritonavir and etravirine in semen and rectal tissue of HIV-negative men. J Acquir Immune Defic Syndr. 2012 Oct 1;61(2):138–44. doi: 10.1097/QAI.0b013e31825cb645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jackson AG, Else LJ, Mesquita PM, et al. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin Pharmacol Ther. 2014 Sep;96(3):314–23. doi: 10.1038/clpt.2014.118. [DOI] [PubMed] [Google Scholar]
- 59.Phase II safety and acceptability of an investigational injectable product, TMC278LA, for pre-exposure prophylaxis - full text view - ClinicalTrials.gov [Internet] [cited 12/4/2014]; Available from: http://clinicaltrials.gov/ct2/show/NCT02165202?term=rilpivirine+AND+long+acting&ra nk=5.
- 60.MTN-013/IPM 026 | microbicide trials network [Internet] [cited 11/21/2014]; Available from: http://www.mtnstopshiv.org/news/studies/mtn013.
- 61.Chen BA, Panther L, Hoesley C, et al. Safety and pharmacokinetics/pharmacodynamics of dapivirine and maraviroc vaginal rings. 21st Conference on Retroviruses and Opportunistic Infections; Boston, MA. March 3-6.2014. abstract 41. [Google Scholar]
- 62.Bunge K, Dezzutti CS, Macio I, et al. FAME-02: A phase I trial to assess safety, PK, and PD of gel and film formulations of dapivirine. 21st Conference on Retroviruses and Opportunistic Infections; Boston, MA. March 3-6.2014. Abstract 42LB. [Google Scholar]
- 63.Isentress full prescribing information. Whitehouse Station, NJ 08889, USA: Merck & Co., Inc.; 2014. [Google Scholar]
- 64.Patterson KB, Prince HMA, White N, et al. Pharmacokinetics (PK) of raltegravir (RAL) in the blood plasma (BP) and genital tract (GT) in HIV+ and HIV- women. 5th Reviews in Antiviral Therapy and Infectious Diseases. 2010;7(16) abstract O17. [Google Scholar]
- 65.Clavel C, Peytavin G, Tubiana R, et al. Raltegravir concentrations in the genital tract of HIV-1-infected women treated with a raltegravir-containing regimen (DIVA 01 study) Antimicrob Agents Chemother. 2011 Jun;55(6):3018–21. doi: 10.1128/AAC.01460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hashiguchi Y, Hamada A, Shinohara T, et al. Role of P-glycoprotein in the efflux of raltegravir from human intestinal cells and CD4+ T-cells as an interaction target for anti-HIV agents. Biochem Biophys Res Commun. 2013 Sep 20;439(2):221–7. doi: 10.1016/j.bbrc.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 67.Adams JL, Patterson KB, Prince HM, et al. Single and multiple dose pharmacokinetics of dolutegravir in the genital tract of HIV negative women. Antivir Ther. 2013 Jul 31; doi: 10.3851/IMP2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tivicay full prescribing information. Research Triangle Park, NC 27709: ViiV Healthcare; 2014. [Google Scholar]
- 69.Patterson KB, Prince HA, Stevens T, et al. Differential penetration of raltegravir throughout gastrointestinal tissue: Implications for eradication and cure. AIDS. 2013 Jun 1;27(9):1413–9. doi: 10.1097/QAD.0b013e32835f2b49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cottrell ML, Prince HMA, Sykes C, et al. Mucosal tissue pharmacokinetics of maraviroc and raltegravir in women: Implications for chemoprophylaxis. 15th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy; Washington DC, USA. May 19-21.2014. Abstract O_08. [Google Scholar]
- 71.Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013 Nov;8(6):565–71. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.AIDS info drug database-cabotegravir [Internet] 2014 Available from: http://aidsinfo.nih.gov/drugs/513/cabotegravir/0/professional.
- 73.Spreen W, Ford SL, Chen S, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014 Dec 15;67(5):481–6. doi: 10.1097/QAI.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 74.Radzio J, Spreen WR, Yueh YL, et al. Monthly GSK744 long-acting injections protect macaques against repeated vaginal SHIV exposures. 21st Conference on Retrovirus and Opportunistic Infections; Boston, MA. March 3-6.2014. oral abstract 40LB. [Google Scholar]
- 75.Andrews CD, Spreen WR, Mohri H, et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science. 2014 Mar 7;343(6175):1151–4. doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spreen B, Rinehart A, Smith K, et al. HIV PrEP dose rationale for cabotegravir (GSK1265744) long-acting injectable nanosuspension. AIDS Res Hum Retroviruses. 2014 Oct;30(Suppl 1):A12. [Google Scholar]
- 77.Griffin L, Annaert P, Brouwer KL. Influence of drug transport proteins on the pharmacokinetics and drug interactions of HIV protease inhibitors. J Pharm Sci. 2011 Sep;100(9):3636–54. doi: 10.1002/jps.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Launay O, Tod M, Louchahi K, et al. Differential diffusions of indinavir and lopinavir in genital secretions of human immunodeficiency virus-infected women. Antimicrob Agents Chemother. 2004 Feb;48(2):632–4. doi: 10.1128/AAC.48.2.632-634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patterson K, Jennings S, Falcon R, et al. Darunavir, ritonavir, and etravirine pharmacokinetics in the cervicovaginal fluid and blood plasma of HIV-infected women. Antimicrob Agents Chemother. 2011 Mar;55(3):1120–2. doi: 10.1128/AAC.00889-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson PL, Brundage RC, Bushman L, et al. Indinavir plasma protein binding in HIV-1-infected adults. AIDS. 2000 Oct 20;14(15):2293–7. doi: 10.1097/00002030-200010200-00010. [DOI] [PubMed] [Google Scholar]
- 81.Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014 Feb 11;111(6):2307–12. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kline C, Ndjomou J, Franks T, et al. Persistence of viral reservoirs in multiple tissues after antiretroviral therapy suppression in a macaque RT-SHIV model. PLoS One. 2013 Dec 18;8(12):e84275. doi: 10.1371/journal.pone.0084275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008 Jan;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown KC, Patterson KB, Malone SA, et al. Single and multiple dose pharmacokinetics of maraviroc in saliva, semen, and rectal tissue of healthy HIV-negative men. J Infect Dis. 2011 May 15;203(10):1484–90. doi: 10.1093/infdis/jir059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abel S, Russell D, Whitlock LA, et al. Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects. Br J Clin Pharmacol. 2008 Apr;65(Suppl 1):60–7. doi: 10.1111/j.1365-2125.2008.03137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen BA, Panther L, Hoesley C, et al. Safety and pharmacokinetics/pharmacodynamics of dapivirine and maraviroc vaginal rings. 21st Conference on Retroviruses and Opportunistic Infections; Boston, MA. March 3-6.2014. Abstract 41. [Google Scholar]
- 87.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in kampala, uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003 Sep 13;362(9387):859–68. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 88.Second european PrEP study closes placebo arm early due to high effectiveness [Internet] 2014 Available from: http://www.aidsmap.com/Second-European-PrEP-study-closes-placebo-arm-early-due-to-high-effectiveness/page/2917367/
- 89.Rajman I. PK/PD modelling and simulations: Utility in drug development. Drug Discov Today. 2008 Apr;13(7-8):341–6. doi: 10.1016/j.drudis.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 90.Dezzutti CS, Hladik F. Use of human mucosal tissue to study HIV-1 pathogenesis and evaluate HIV-1 prevention modalities. Curr HIV/AIDS Rep. 2013 Mar;10(1):12–20. doi: 10.1007/s11904-012-0148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arien KK, Kyongo JK, Vanham G. Ex vivo models of HIV sexual transmission and microbicide development. Curr HIV Res. 2012 Jan 1;10(1):73–8. doi: 10.2174/157016212799304661. [DOI] [PubMed] [Google Scholar]
- 92.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012 Sep 12;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baheti G, Kiser JJ, Havens PL, Fletcher CV. Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob Agents Chemother. 2011 Nov;55(11):5294–9. doi: 10.1128/AAC.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cottrell M, Yang K, Prince H, et al. HIV Research for Prevention: AIDS Vaccine, Microbicide and ARV-based Prevention Science (HIV R4P) Cape Town South Africa: 2014. Oct 28-31, Predicting effective truvada® PrEP dosing strategies with a novel PK-PD model incorporating tissue active metabolites and endogenous nucleotides (EN) Abstract OA22.06 LB. [Google Scholar]
- 96.Garcia-Lerma JG, Aung W, Cong ME, et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol. 2011 Jul;85(13):6610–7. doi: 10.1128/JVI.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


