Abstract
Objective
To assess the effectiveness of L-cystine dimethyl ester (CDME), an inhibitor of cystine crystal growth, for the treatment of cystine urolithiasis in a Slc3a1 knockout mouse model of cystinuria.
Methods
CDME (200 μg per mouse) or water was delivered by gavage daily for four weeks. Higher doses by gavage or in the water supply were administered to assess organ toxicity. Urinary amino acids and cystine stones were analyzed to assess drug efficacy using several analytical methods.
Results
Treatment with CDME led to a significant decrease in stone size compared with the water group (p = 0.0002), but the number of stones was greater (p = 0.005). The change in stone size distribution between the two groups was evident by micro computed tomography. Overall, cystine excretion in urine was the same between the two groups (p = 0.23), indicating that CDME did not interfere with cystine metabolism. SEM analysis of cystine stones from the CDME group demonstrated a change in crystal habit, with numerous small crystals. L-cysteine methyl ester was detected by UPLC-MS in stones from the CDME group only, indicating that a CDME metabolite was incorporated into the crystal structure. No pathological changes were observed at the doses tested.
Conclusions
These data demonstrate that CDME promotes formation of small stones but does not prevent stone formation, consistent with the hypothesis that CDME inhibits cystine crystal growth. Combined with the lack of observed adverse effects, our findings support the use of CDME as a viable treatment for cystine urolithiasis.
Keywords: Cystinuria, L-cystine dimethyl ester, L-cystine methyl ester, Slc3a1 knockout mice, urolithiasis
INTRODUCTION
Cystinuria is a rare cause of kidney stones, accounting for only 1% of urolithiasis cases in adults, but it accounts for 6–8% of pediatric cases1–4. Stone formation secondary to cystinuria often presents in the first decade of life, and the majority of patients have their first stone by the end of their teenage years4,5. Although rare, cystine stones can lead to serious consequences for patients, because they are large and tend to recur, often resulting in multiple treatments and progressive decline in renal function in pediatric and adult patients3–5. Patients with cystine stones have a greater incidence of chronic kidney disease than patients suffering from the more common calcium oxalate stones6. Despite the morbidity associated with cystine urolithiasis, treatments for cystinuria have not substantially changed in the past 30 years7,8.
At the molecular level, cystinuria is characterized by defective transport of cystine and dibasic amino acids in the kidney and small intestine7–9. Cystinuria is an autosomal recessive disorder caused by mutations in either SLC3A1 or SLC7A9. These genes encode the rBAT and bo,+AT subunits, respectively, of the heterodimeric cystine transporter10,11. The inability to reabsorb cystine and dibasic amino acids from the glomerular filtrate results in the hyper-excretion of these amino acids in the urine (cystine excretion >400 mg/day compared with <30 mg/day in normal subjects). This can lead to the formation of cystine stones in the kidney and, to a lesser extent, in the bladder6,12,13. The dibasic amino acids have high solubility and do not precipitate in the urine.
Current therapeutic strategies for cystinuria aim to increase cystine solubility by increasing urine volume and pH. Hyperdiuresis is the most important first line treatment, requiring daily consumption of >4 liters of water and urine volumes >3 liters6. However, this intake is difficult to achieve and maintain, especially in the pediatric population. Other treatments include urine alkalinization to achieve a pH approaching 8.0 (where cystine solubility increases) and reducing animal protein and sodium intake, but these manipulations are also difficult to achieve and maintain. Pharmaceutical agents D-penicillamine (Cuprimine®) and tiopronin (Thiola®) are options for the most severely affected patients, but these thiol drugs have significant side effects limiting their widespread use4,14.
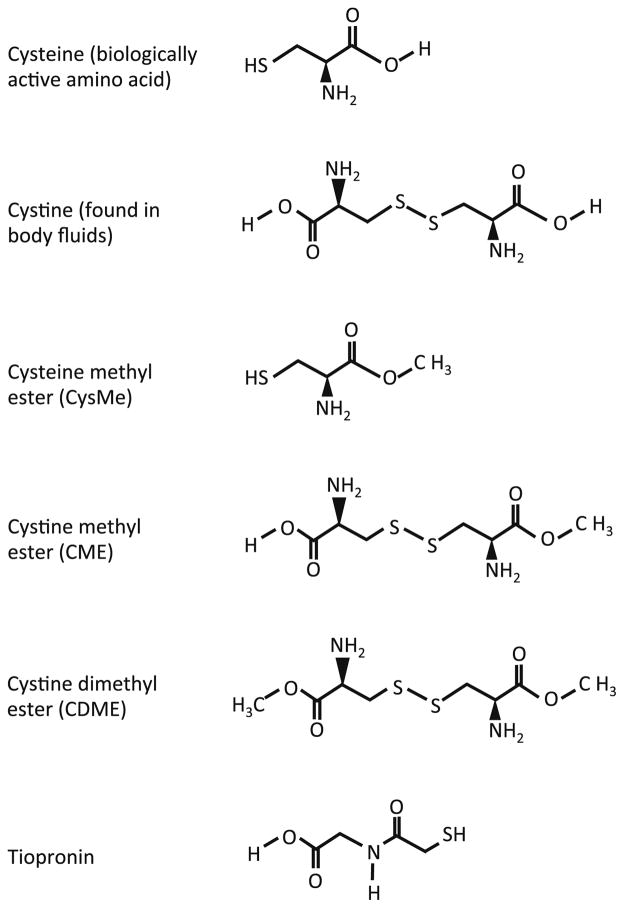
Recent efforts by our group have focused on the development of drugs with an alternative mechanism of action to treat urolithiasis secondary to cystinuria. Cystine crystal formation and aggregation are prerequisites for stone formation; cystine mimics therefore provide a rational strategy for cystine stone prophylaxis in the context of cystinuria15,16. In a previous study, we used atomic force microscopy (AFM) to demonstrate that the use of crystal growth inhibitors can effectively interfere with cystine crystal formation in vitro15. We identified L-cystine dimethyl ester (CDME) (Figure 1) as an effective inhibitor of cystine crystal growth. L-cysteine methyl ester (CysME), which is the equivalent of half CDME, was a less potent inhibitor15.
Figure 1. Molecular structures of cystine and cystine analogues.
Cysteine is the biologically active amino acid while cystine is the oxidized form and is found in body fluids. CDME was used in this study to inhibit cystine crystal growth in vivo. CysME is the reduced form of CDME. CME also inhibits cystine crystal growth in vitro15 but is not commercially available. Tiopronin is the active ingredient in Thiola®, the drug currently used for the treatment of cystinuria.
As a proof of concept, we evaluated CDME using our Slc3a1 knockout mouse model which closely mimics human cystinuria, except for the propensity for stone formation in the bladder as opposed to the kidney17. To test the hypothesis that CDME is an effective inhibitor of stone formation in vivo, we treated knockout male mice (which are more severely affected than female mice) with CDME or water alone and compared biochemical parameters and stone characteristics between the two groups. Our results provide strong support for the evaluation of CDME as a novel agent for the clinical management of cystinuria-induced urolithiasis.
MATERIALS AND METHODS
Mice
We selected 2–3 month old knockout male mice for CDME treatment. Approximately 50% of mice at this age group have bladder stones, making this the ideal age group to assess the effects of CDME treatment on mice with and without stones. Animal studies were conducted in accordance with Rutgers University IACUC policies.
CDME administration
CDME dihydrochloride and CysME hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). CDME solution was prepared fresh daily (1 mg/ml in water) and 200 μl was administered to knockout male mice (body weight 20 g) by gavage daily for four weeks using a 20G needle (Instech Laboratories, Inc., Plymouth Meeting, PA). This dose is equivalent to 10 mg/kg or 29 μmol/kg. To assess high dose toxicity, we administered CDME (10 mg/ml) to wild-type male and female mice daily for one week and four weeks as above. CDME (5 mg/ml) was also administered in the water supply to control and knockout mice for four weeks. Mice were then sacrificed and selected tissues placed in 10% formalin for histology. Limited studies were carried out with CysME, under the same conditions as above.
Urine collection and analysis
Urine samples were collected by placing the mice in metabolic cages for four hours or longer. Samples were frozen at −20°C and then analyzed for amino acids by ion exchange chromatography. Amino acid levels were normalized to nmol/mg creatinine. Cystine data only are presented here.
Micro computed tomography
Mice were sacrificed after urine collection and the bladder and kidneys removed and placed in 10% formalin. The bladder was scanned ex vivo using a SkyScan 1172 micro CT scanner (Bruker Corp., Billerica, MA). The SkyScan reconstruction program NRecon was used for image reconstruction. The output images were imported into the Bruker CT-Analyzer (CTAn) program (version 1.13), which was then used to assess quantitative parameters such as bladder volume, stone volume, and stone number.
Estimation of stone size and number
After micro CT scanning, bladders were weighed and the stones removed, weighed, counted, and measured in the longest dimension.
Analysis of stones by scanning electron microscopy
A Zeiss Merlin field-emission scanning electron microscope was used to compare stones retrieved from a CDME- and from a water-treated mouse with respect to the habit and size of crystals within the stones.
Analysis of urine and stone extracts by UPLC-MS
We evaluated ultra-performance liquid chromatography (UPLC) coupled with atmospheric pressure chemical ionization ion trap mass spectrometer (APCI/ITMS) for separating CDME and its reduced derivative CysME spiked into control urine. Liquid-liquid extraction of these analytes from urine was carried out using acetonitrile and methanol followed by UPLC in a Hypersil gold column and detection by MS-MS. We also developed an approach for measuring total thiols by reduction and chemical derivatization of disulfides with tris(2-carboxyethyl)phosphine (TCEP) and N-ethylmaleimide (NEM), respectively. Stone material was extracted with water (1 mg/30 μl) via homogenization and sonication and the extract analyzed on a Waters Aquity UPLC system operating in reverse phase (BEH C18 column and alkaline mobile phase) coupled to a Waters Q-Tof Premier mass spectrometer operating in electrospray ionization mode. Reduced and derivatized stone extracts were compared with derivatized thiol standards to assess stone composition.
Data analysis
Fisher’s exact test was used to assess the number of mice with or without stones in the two groups. A two-tailed t-test with unequal variance was used to assess differences in bladder weight, stone weight, and stone number between the two groups. Stone size distribution was assessed using the likelihood ratio test allowing for error (LRTae)18,19. Significance level was set at 5%.
RESULTS
CDME treatment decreases stone size but increases stone number
In the CDME-treated group (n = 14), seven mice had small stones and seven did not have any stones. In the water-treated group (n = 11), six mice had small-to-large stones and five had no stones (Table 1). Student’s t-tests demonstrated no significant difference in the number of mice with or without stones in the two groups (p = 1), and in mice that did not form stones, there was no difference in bladder weight between the two groups (p = 0.94), indicating that CDME treatment had no effect on the bladder in the absence of urolithiasis. In mice with stones, the bladder weight was slightly lower in the CDME group, but the difference was not significant (p = 0.07). Also, the difference in total stone weight between the two groups did not reach significance (p = 0.06).
Table 1.
Comparison of CDME- and water-treated groups.
| Parameter | CDME group (n=14) | Water group (n=11) | P value | Significant |
|---|---|---|---|---|
| Urinary cystine (nmol/mg creatnine) | 4343 +/− 1577 (n=11) | 5410 +/− 2124 (n=8) | 0.23 | No |
| Mice with stones | 7/14 (50%) | 6/11 (54%) | 1.0 | No |
| Bladder weight, stones absent (mg) | 18.57 +/−5.18 (n=7) | 18.78 +/− 2.35 (n=5) | 0.93 | No |
| Bladder weight, stones present (mg) | 65.39 +/− 18.59 (n=7) | 82.93 +/− 10.80 (n=6) | 0.067 | No |
| Stone weight (mg) | 29.23 +/− 14.80 (n=7) | 55.17 +/− 27.95 (n=6) | 0.056 | No |
| Stone number | 25.86 +/− 10.96 (n=181) | 7.83 +/− 6.46 (n=47) | 0.005 | Yes |
| Stone size range (mm) | 0.5–2.0: 180 stones | 0.5–2.0: 41 stones | 0.0002 | Yes |
| 2.0–3.0: 1 stone | 3.1–4.0: 3 stones | |||
| 4.1–5.0: 1 stone | ||||
| 6.1–7.0: 1 stone | ||||
| 8.1–9.0: 1 stone |
Data are presented as mean and standard deviation. Stone size ranges (e.g., 5.1–6.0 mm) that did not include any stones have been omitted from the table.
The number of stones per bladder in the CDME group ranged from 16–45 and the total number of stones in the seven mice was 181. The number of stones per bladder in the water group ranged from 1–19 and the total number of stones in the six mice was 47. This difference was highly significant (p = 0.005). All stones in the CDME group were in the size range 0.5 to 2.0 mm except one, which was 3.0 mm long. There was a wide variation in stone size range in the water group, ranging from 0.5 to 2.0 mm (41 stones) to 9.0 mm (1 stone). Using the LRTae test18,19 the difference in stone size distribution between the two groups was highly significant (p = 0.0002), even after correcting for multiple tests. The category that showed the largest difference was in the size range 0.5–2.0 mm. There were a higher proportion of stones in this category in the CDME group (0.994) than in the water group (0.872). However, the category that contributed the most to the observed difference was the 3.1–4.0 mm category, since there were no stones in this category in the CDME group whereas the proportion of stones in the water group was 0.064. These observations are consistent with our prior in vitro study15 demonstrating that CDME inhibits cystine crystal growth, as well as our hypothesis that CDME can serve as an inhibitor of cystine stone formation in vivo.
Stone weight per bladder in the CDME group ranged from 14.2 to 52.3 mg (median 29.0 mg) and the average weight per stone was 1.1 mg. Stone weight per bladder in the water group ranged from 26.8 to 101.8 mg (median 53.6 mg) and the average weight per stone was 7.0 mg. Thus, in the treated mice, CDME reduced the stone burden to 0.54 (29.0/53.6) multiples of the median (MOM) relative to the water-treated group. Smaller stones resulting from CDME treatment may be more clinically manageable.
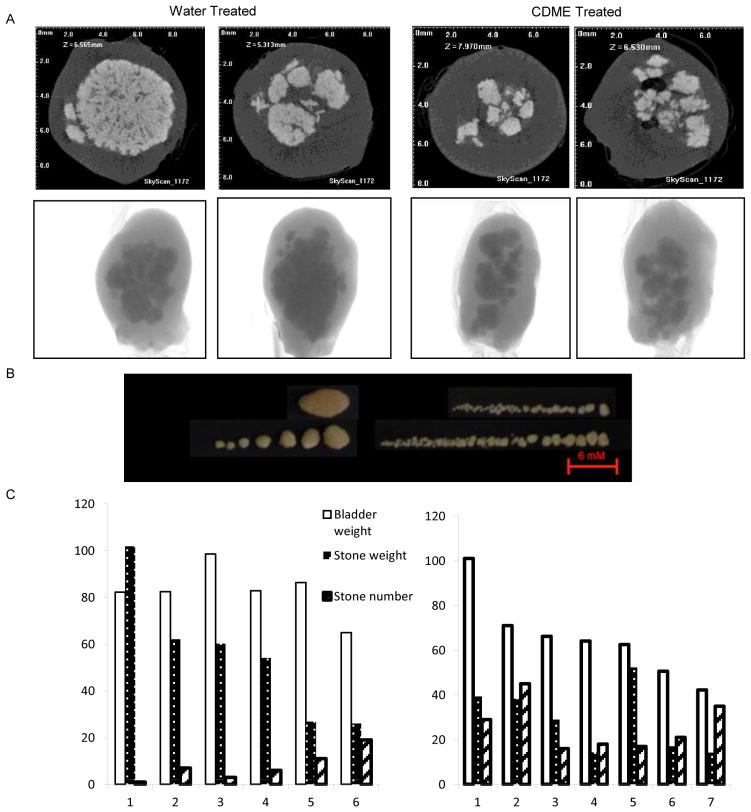
The difference in stone burden in the bladder between the CDME- and water-treated groups was clearly evident by micro CT (Figure 2A), but quantitative parameters of stone burden could not be reliably established with the available CT analysis software. Upon dissection, gross examination of the bladder confirmed the micro CT findings that stones from CDME-treated animals were smaller but more numerous than those from the water group (Figure 2B). Figure 2C quantifies the difference in bladder weight, stone weight, and stone number between the two groups. These studies strongly suggest that CDME inhibits the growth of cystine stones in vivo. The presence of smaller stones suggests that CDME might also inhibit crystal aggregation and agglomeration. Thus, CDME or its derivatives have potential for the treatment of human cystinuria.
Figure 2. Analysis of cystine stones.
(A). Micro CT images of bladders from two Slc3a1 knockout mice treated with water (left panel) or CDME (right panel). For each mouse, the top panel is a cross-sectional image and the bottom panel is the intact organ. (B). Stones retrieved from the bladder of a water- or CDME-treated mouse. (C). Quantification of bladder weight, stone weight, and stone number in the water- and CDME-treated groups.
CDME-treated mice demonstrate no gross pathological changes
No pathological changes were observed in the kidneys, bladder, or liver from wild-type mice exposed to 2 mg CDME daily for one week or four weeks (Supplementary Figures 1 and 2). We also added CDME to the water supply (5 mg/ml) for four weeks. Male mice drink 4–5 ml water per day. Assuming average water consumption, this is equivalent to a dose that is up to 100 times greater than that used in the treatment protocol. We observed a reduction in stone formation in the CDME-treated male mice, and our veterinary staff did not observe any gross pathological changes at necropsy.
Mice without bladder stones have higher levels of cystine in the urine
When analyzing data without respect to the presence or absence of stones in the bladder, there was no difference in cystine excretion in the urine from the CDME- or water-treated groups (p = 0.23, Table 1) and this was verified using one-way ANOVA (F = 2.82, p = 0.07). These data suggest that CDME does not affect the metabolism or synthesis of cystine. Our sample sizes are small (4–6 mice per group), but when the cystine urine data were partitioned into individual groups (water no stones, water with stones, CDME no stones, and CDME with stones), cystine excretion in the water group without stones was higher than that in the other three groups. This is the expected result due to the sequestration of cystine in the stone material.
CDME treatment alters the shape and size of cystine crystals in cystine stones
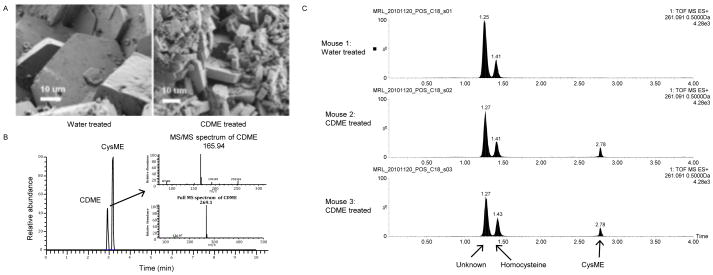
Scanning electron microscopy (SEM) was used to compare the stones retrieved from water- or CDME-treated mice with respect to the habit and size of crystals within the stones (Figure 3). Figure 3A (right) confirms that CDME treatment affects stone formation at the molecular level, as seen by both the decreased size of crystals and the increased number of crystal fragments in the CDME-treated sample compared with the water-treated sample (Figure 3A, left). These results are consistent with the data from the gross pathological analyses above demonstrating that CDME treatment leads to more numerous but smaller cystine stones and further support our hypothesis that CDME inhibits crystal growth both in vitro and in vivo.
Figure 3. Assessment of the effects of CDME on cystine stone formation.
(A). Scanning electron microscopy of bladder stones retrieved from a water-treated (left) and a CDME-treated (right) mouse. Note the decrease in crystal size, increase in crystal number, and the change in crystal morphology in the scan on the right. (B). Preliminary validation of UPLC-MS without chemical derivatization for differentiating and quantifying CDME and CysME added to control urine. (C). Application of UPLC-MS for the analysis of stone extracts following reduction and chemical derivatization with TCEP and NEM, respectively. CysME (retention time 2.78 min) was found in the stone extract from CDME-treated mice only, whereas homocysteine (1.43 min) and an unidentified thiol (1.27 min) were detected in extracts from both the CDME- and water-treated mice.
CDME is extensively metabolized following absorption
Preliminary studies using control urine spiked with CDME and CysME validated our separation technique by demonstrating an 80–90% recovery for both compounds and retention times of 2.93 and 3.18 min for CDME and CysME, respectively (Figure 3B). Precursor/product ion pairing gave m/z values of 269.1/165.9 for CDME and 136.0/118.9 for CysME in MS/MS mode, providing unambiguous quantitation with a method detection limit of 0.0015 and 0.017 μg/ml urine, respectively.
Preliminary analysis of chemically reduced stone extracts from CDME-treated mice by UPLC-MS identified cysteine, homocysteine (a structural isomer of CysME), and CysME in the extract (Figure 3C). Similarly, in urine samples from two CDME-treated mice, CysME was detected as the major metabolite, but CDME was not detected. Further analysis of the extracts in the native oxidized state of the urine indicated that CysME was present only as a cysteine-bound heterodimer, not as CDME. These findings demonstrate that CDME is absorbed from the intestinal tract and they suggest that a metabolite of CDME, as yet unidentified, may be the active inhibitor.
CysME is a poor crystal growth inhibitor compared with CDME
We treated mice with CysME under the same conditions as for CDME and demonstrated that CysME inhibited stone formation, but it was less effective than CDME (0.72 MOM for CysME versus 0.54 for CDME). This observation is in agreement with our in vitro studies that revealed poorer crystal growth inhibition for CysME compared with CDME15.
COMMENT
Two European groups have developed mouse models of cystinuria20,21, and penicillamine has been evaluated as an anti-lithogenic agent in one of these models22. In the present study, we tested CDME in vivo as a representative of a class of novel cystine crystal growth inhibitors for the treatment of cystinuria-induced urolithiasis.
Application of AFM to study crystal formation at the molecular level has given unprecedented insight into how certain molecules can perturb crystal formation15. Further, comparative experiments modifying specific moieties of parent compounds have demonstrated which moieties may enhance or reduce the ability to interfere with crystal formation (Ward et al., unpublished data). Together, these advances have allowed us to rationally select compounds for testing in vitro and choose an optimal candidate for testing in our mouse model. Here, we show that the administration of CDME in vivo leads to the formation of smaller but more numerous stones, consistent with the disruption of crystallization seen in vitro. Identification of molecular entities that inhibit crystal aggregation in addition to crystal growth would represent a major advance in this field.
UPLC-MS analysis demonstrated that the CDME metabolite CysME eluted uniquely from stones in the CDME-treated group. Incorporation of CysME or an unidentified CDME metabolite into the crystalline structure appears to alter the solubility, shape and size of subsequent stones. Homocysteine and an unidentified compound were present in stone extracts from both the CDME- and water-treated mice. The presence or absence of homocysteine and other endogenous thiols may influence stone burden in cystinuria patients, and hence the characterization of these metabolites may provide clues for the development of more potent stone inhibitors.
Clinical management of cystine stones is challenging as they are often resistant to extracorporeal shockwave lithotripsy and may require multiple rounds of treatment, with concomitant risk of kidney injury3. Despite the inability of CDME to prevent cystine stone formation, treatment with this compound does appear to inhibit the formation of large and complex stones. A retrospective study reviewed shockwave lithotripsy treatments in 149 pediatric patients aged 3 to 17 years. Results of multivariate analysis indicated that only total stone diameter independently predicted treatment success23. Extrapolating this evidence to cystine stones, CDME treatment may prove critical in the successful management of cystinuria-induced uroliths by complimenting other treatment strategies.
Further studies in this field will have a two-pronged approach to the progression of cystine mimics toward clinical use: continued testing of CDME in vivo and more comprehensive assessment of other candidate compounds. Furthermore, research strategies utilizing CT and magnetic resonance imaging may allow monitoring of stone development and progression in vivo. Finally, studies similar to those described above for CDME are underway for other candidate compounds that exhibit a pronounced disruption of cystine crystal growth. An exploratory investigational new drug application to perform a pilot phase I human study of CDME is under consideration. Together these efforts will represent significant progress towards the clinical implementation of this class of compounds for the treatment of cystinuria.
CONCLUSIONS
Although more extensive studies on efficacy, tolerability, and long-term toxicity are required, CDME and other cystine ester mimics may herald a promising new medical treatment for cystinuria-induced urolithiasis.
Supplementary Material
Light micrographs of H and E-stained kidney, bladder, and liver sections from representative wild-type male mice treated with water alone or with 200 μl of 10 mg/ml CDME for four weeks. No differences in histology were noted between the control and treated mice. The mouse tag numbers are indicated in the figure.
Light micrographs of H and E-stained kidney, bladder, and liver sections from representative wild-type female mice treated with water alone or with 200 μl of 10 mg/ml CDME for four weeks. Tissue histology in the treated mice was indistinguishable from that in the control mice. The mouse tag numbers are indicated in the figure.
Acknowledgments
This work was supported in part by a pilot project grant (No. 434056) from the Rare Kidney Stone Consortium (U54DK083908), which is a part of the NIH Rare Diseases Clinical Research Network, supported through collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science and NIDDK. This work was also supported in part by NIEHS Center Grant P30ES005022. We thank Michael Jones, Children’s National Medical Center, Washington, DC, for urine amino acid analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eggermann T, Venghaus A, Zerres K. Cystinuria: an inborn cause of urolithiasis. Orphanet J Rare Dis. 2012;7:19. doi: 10.1186/1750-1172-7-19. Published online Apr 5, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisceglia L, Purroy J, Jiménez-Vidal M, et al. Cystinuria type I: identification of eight new mutations in SLC3A1. Kidney Int. 2001;59:1250–1256. doi: 10.1046/j.1523-1755.2001.0590041250.x. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed K, Dasgupta P, Khan MS. Cystine calculi: challenging group of stones. Postgrad Med J. 2006;82:799–801. doi: 10.1136/pgmj.2005.044156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knoll T, Zollner A, Wendt-Nordahl G, et al. Cystinuria in childhood and adolescence: recommendations for diagnosis, treatment, and follow-up. Pediatr Nephrol. 2005;20:19–24. doi: 10.1007/s00467-004-1663-1. [DOI] [PubMed] [Google Scholar]
- 5.Joly D, Rieu P, Méjean A, et al. Treatment of cystinuria. Pediatr Nephrol. 1999;13:945–950. doi: 10.1007/s004670050736. [DOI] [PubMed] [Google Scholar]
- 6.Biyani C, Cartledge J. Cystinuria diagnosis and management. EAU-EBU Update Series. 2006;4:175–183. [Google Scholar]
- 7.Kit LC, Filler G, Pike J, et al. Pediatric urolithiasis: experience at a tertiary care pediatric hospital. Can Urol Assoc J. 2008;2:381–386. doi: 10.5489/cuaj.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattoo A, Goldfarb DS. Cystinuria. Semin Nephrol. 2008;28:181–191. doi: 10.1016/j.semnephrol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb DS. Potential pharmacologic treatments for cystinuria and for calcium stones associated with hyperuricosuria. Clin J Am Soc Nephrol. 2011;6:2093–2097. doi: 10.2215/CJN.00320111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chillarón J, Font-Llitjós M, Fort J, et al. Pathophysiology and treatment of cystinuria. Nat Rev Nephrol. 2010;6:424–434. doi: 10.1038/nrneph.2010.69. [DOI] [PubMed] [Google Scholar]
- 11.Bröer A, Friedrich B, Wagner CA, et al. Association of 4F2hc with light chains LAT1, LAT2 or y+LAT2 requires different domains. Biochem J. 2001;355:725–731. doi: 10.1042/bj3550725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz BF, Stoller ML. The vesical calculus. Urol Clin North Am. 2000;27:333–346. doi: 10.1016/s0094-0143(05)70262-7. [DOI] [PubMed] [Google Scholar]
- 13.Schwentner C, Oswald J, Lunacek A, et al. Giant cystine stone in an infant bladder with no evidence of cystinuria - valence of possible pathomechanisms. Urol Int. 2005;75:285–287. doi: 10.1159/000087810. [DOI] [PubMed] [Google Scholar]
- 14.DeBerardinis RJ, Coughlin CR, Kaplan P. Penicillamine therapy for pediatric cystinuria: experience from a cohort of American children. J Urol. 2008;180:2620–2623. doi: 10.1016/j.juro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rimer JD, An Z, Zhu Z, et al. Crystal growth inhibitors for the prevention of L-cystine kidney stones through molecular design. Science. 2010;330:337–341. doi: 10.1126/science.1191968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coe FL, Asplin JR. Stopping the stones. Science. 2010;330:325–326. doi: 10.1126/science.1197207. [DOI] [PubMed] [Google Scholar]
- 17.Ercolani M, Sahota A, Schuler C, et al. Bladder outlet obstruction in male cystinuria mice. Int Urol Nephrol. 2010;42:57–63. doi: 10.1007/s11255-009-9597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barral S, Haynes C, Stone M, et al. LRTae: improving statistical power for genetic association with case/control data when phenotype and/or genotype misclassification errors are present. BMC Genet. 2006;7:24. doi: 10.1186/1471-2156-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon D, Yang Y, Haynes C, et al. Increasing power for tests of genetic association in the presence of phenotype and/or genotype error by use of double-sampling. Stat Appl Genet Mol Biol. 2004;3:Article 26. doi: 10.2202/1544-6115.1085. [DOI] [PubMed] [Google Scholar]
- 20.Peters T, Thaete C, Wolf S, et al. A mouse model for cystinuria type I. Hum Mol Genet. 2003;12:2109–2120. doi: 10.1093/hmg/ddg189. [DOI] [PubMed] [Google Scholar]
- 21.Feliubadaló L, Arbonés ML, Mañas S, et al. Slc7a9-deficient mice develop cystinuria non-I and cystine urolithiasis. Hum Mol Genet. 2003;12:2097–2108. doi: 10.1093/hmg/ddg228. [DOI] [PubMed] [Google Scholar]
- 22.Font-Llitjós M, Feliubadaló L, Espino M, et al. Slc7a9 knockout mouse is a good cystinuria model for antilithiasic pharmacological studies. Am J Physiol Renal Physiol. 2007;293:F732–F740. doi: 10.1152/ajprenal.00121.2007. [DOI] [PubMed] [Google Scholar]
- 23.McAdams S, Kim N, Ravish IR, et al. Stone size is only independent predictor of shock wave lithotripsy success in children: a community experience. J Urol. 2010;184:659–664. doi: 10.1016/j.juro.2010.03.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Light micrographs of H and E-stained kidney, bladder, and liver sections from representative wild-type male mice treated with water alone or with 200 μl of 10 mg/ml CDME for four weeks. No differences in histology were noted between the control and treated mice. The mouse tag numbers are indicated in the figure.
Light micrographs of H and E-stained kidney, bladder, and liver sections from representative wild-type female mice treated with water alone or with 200 μl of 10 mg/ml CDME for four weeks. Tissue histology in the treated mice was indistinguishable from that in the control mice. The mouse tag numbers are indicated in the figure.