Abstract
Hypoglossal (XII) motoneurons innervate muscles of the tongue whose tonic and inspiratory modulated activity protects the upper airway from collapse in patients affected by the obstructive sleep apnea (OSA) syndrome. Both norepinephrine and serotonin provide wakefulness-related excitatory drives that maintain activity in XII motoneurons, with the noradrenergic system playing a particularly prominent role in rats. When noradrenergic and serotonergic drives are antagonized, no further decline of XII nerve activity occurs during pharmacologically induced rapid eye movement (REM) sleep-like state. This is the best evidence to date that, at least in this model, the entire REM sleep-related decline of upper airway muscle tone results from withdrawal of these two excitatory inputs. A major component of noradrenergic input to XII motoneurons originates from pontine noradrenergic neurons that have state-dependent patterns of activity, maximal during wakefulness, and minimal, or absent during REM sleep. Our data suggest that not all ventrolateral medullary catecholaminergic neurons follow this pattern, with adrenergic C1 neurons probably increasing their activity during REM sleep. When rats are subjected to chronic-intermittent hypoxia, noradrenergic drive to XII motoneurons is increased by mechanisms that include sprouting of noradrenergic terminals in the XII nucleus, and increased expression of α1-adrenoceptors; an outcome that may underlie the elevated baseline activity of upper airway muscles during wakefulness in OSA patients.
Keywords: adrenergic receptors, atonia, norepinephrine, genioglossus, obstructive sleep apnea, REM sleep
1 INTRODUCTION
The finding that sleep-disordered breathing occurs when upper airway muscle activity declines, whereas obstructive episodes are resolved when upper airway muscle activity is restored (Remmers et al., 1978; Sauerland and Harper 1976) had a profound influence on subsequent basic and clinical research on the mechanisms underlying the obstructive sleep apnea (OSA) syndrome. OSA patients generate adequate ventilation when they are wake but, during sleep, decrements of upper airway muscle activity, combined with the anatomical predisposition of the upper airway to collapse, result in recurrent periods of respiratory flow limitation or a complete loss of upper airway patency. Thus, the depressant effect of sleep on upper airway muscle tone plays a key role in the disorder. Some OSA patients experience obstructive events predominantly during slow-wave sleep, whereas in others, flow limitations and complete upper airway obstructions occur mainly during rapid eye movement (REM) sleep. These differences may depend on the severity of the disorder, strength of the reflexes that act to restore upper airway muscle tone, and anatomical factors. Obstructive episodes during REM sleep predominate in children and certain adult OSA patients, and they often result in the most severe oxyhemoglobin desaturations (Conwell et al., 2012; Muraki et al., 2008; Spruyt and Gozal, 2012).
The brainstem contains both the neuronal network responsible for the generation of REM sleep (Brown et al., 2012; Jouvet, 1962; Siegel, 2009), and also most of the neuronal systems responsible for the central regulation of breathing (Feldman et al., 2003; Ramirez and Viemari, 2005; von Euler, 1986). Hence, studies of the interaction between these two networks have been based on the conceptual frameworks derived from the extensive studies of each of these two systems conducted separately. In our studies, we focus on the effects of REM sleep on hypoglossal (XII) motoneurons because they innervate the muscles of the tongue, including the genioglossus, and the position and stiffness of the tongue is a major determinant of upper airway patency in persons whose upper airway anatomy predisposes them to sleep-related respiratory disorders (Brouillette and Thach, 1979; Eisele et al., 2003; Remmers et al., 1978; Saboisky et al., 2007; Sauerland and Harper, 1976).
The depression of upper airway muscle tone during REM sleep is often seen as a special case of postural muscle atonia that is one of the hallmarks of this state of sleep. Accordingly, the concepts regarding the depression of upper airway muscle tone during REM sleep have been derived from studies of the mechanisms causing the depression of activity in postural motoneurons. These studies suggested that the atonia of postural muscles is caused by an active, postsynaptic inhibition of motoneurons mediated by glycine, because the frequency and amplitude of glycine-mediated inhibitory postsynaptic potentials increase in spinal motoneurons during REM sleep when compared to non-REM sleep (Chase et al., 1989; Morales et al., 1987). However, none of the studies that tested the application of this concept to the motor output to upper airway muscles yielded supportive results. In one study in cats, infusion of antagonists of either glycinergic or GABAA inhibitory receptors did not abolish the depressant effect of REM sleep on reflexly evoked activation of trigeminal motoneurons (Soja et al., 1987). In another study, a pharmacologically induced REM sleep-like depression of spontaneous activity of XII motoneurons was not diminished by microinjections into the XII motor nucleus of antagonists of either of these two receptors, and the authors concluded the REM sleep-related depression of XII motoneuronal activity was not caused by active inhibition mediated by either glycine or GABAA receptors (Kubin et al., 1993). Subsequent studies in rats have led to the same conclusion for both XII and trigeminal motoneurons (Brooks and Peever, 2008; Fenik et al., 2005a; Morrison et al., 2003).
The absence of evidence for active postsynaptic inhibition being the cause of the depression of upper airway muscle tone during REM sleep prompted a search for alternative mechanisms. Based on data showing that serotonin (5-HT)- and norepi-nephrine (NE)-containing neurons are maximally active during wakefulness and stop firing during REM sleep, and that both 5-HT and NE activate XII and trigeminal motoneurons, we conducted studies in cats (Kubin et al., 1992, 1993, 1994) which have led us to propose that the REM sleep-related depression of activity in motoneurons innervating upper airway muscles is a result of disfacilitation, rather than active inhibition, caused by state-dependent withdrawal of endogenous activation mediated by 5-HT and NE (Kubin et al., 1998). We then developed and validated a novel pharmacological model of REM sleep-like depression of activity in XII motoneurons and used it to determine the role of NE in the depression of XII motoneuronal activity during REM sleep. We also measured endogenous NE-mediated activation of XII motoneurons under baseline conditions and following chronic treatment with intermittent hypoxia as a model of the conditions experienced by OSA patients. In this chapter, I describe our animal model and then summarize our recent findings.
2 REM SLEEP-LIKE STATE IN URETHANE-ANESTHETIZED RATS
The model of REM sleep that we developed is based on urethane-anesthetized rats in which microinjections of a cholinergic agonist, carbachol placed into a discrete site within the dorsomedial pons trigger multiple electrophysiological changes that are typical of REM sleep, including the depression of upper airway muscle tone (Kubin, 2001; Lu et al., 2007). Importantly, our studies are conducted under neuromuscular paralysis and with artificial ventilation at a constant rate and volume. Consequently, any neural changes observed during the carbachol-induced REM sleep-like state can be interpreted as resulting from activation of the central network responsible for the generation of REM sleep, rather than as secondary to muscle relaxation or changes in ventilation that accompany changes in behavioral state. We see this as an advantage of our model when used to study the basic effects of REM sleep on the cardiorespiratory and motor systems. Figure 1 shows an example of an REM sleep-like episode elicited by pontine carbachol injection in a urethane-anesthetized, paralyzed, and artificially ventilated rat.
FIGURE 1.
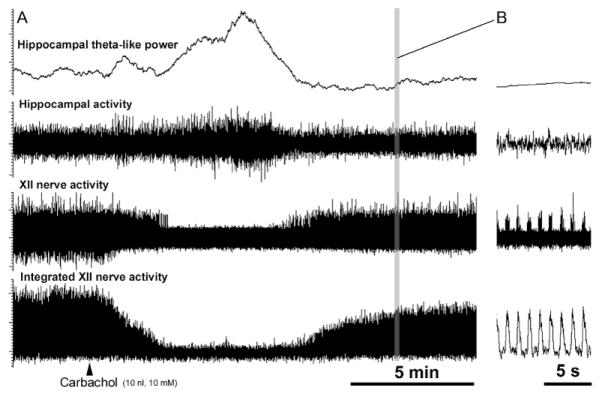
Example of an REM sleep-like episode elicited by carbachol microinjection into the dorsomedial pontine tegmentum in a urethane-anesthetized, paralyzed, and artificially ventilated rat. (A) Continuous recording before and after carbachol injection that elicited a typical REM sleep-like episode. The top trace shows the running average of the power of hippocampal activity in the 2.5–4.0 Hz frequency range, which corresponds to theta frequency in anesthetized rats (Vertes et al., 1993). In this case, inspiratory activity of the XII nerve was transiently abolished at the peak of the carbachol effect. (B) Expanded portion of the record in (A) showing individual inspiratory bursts of XII nerve activity and its integrated version recorded after the episode, as marked by the grey vertical line in (A). All signals are scaled in arbitrary units.
**Unpublished record from Stettner et al. (2013).
As during natural REM sleep, carbachol-induced REM sleep-like state in anesthetized rats is characterized by activation of cortical EEG and the appearance of theta-like rhythm in the hippocampus (Kubin, 2001; Lu et al., 2007). The effective region for carbachol microinjections is well defined (Fenik and Kubin, 2009; Fenik et al., 2005b; Kubin, 2001) and corresponds to the dorsal pontine tegmental region where carbachol enhances REM sleep in behaving rats (Bourgin et al., 1995; Gnadt and Pegram, 1986). Noradrenergic (NA) cells of the locus coeruleus (LC) are silenced during the carbachol-induced REM sleep-like state in anesthetized rats (Kubin, 2001) and during natural REM sleep (Aston-Jones and Bloom, 1981). Ventral medullary inspiratory cells often have increased activity during carbachol-induced REM sleep-like state in anesthetized rats (Woch et al., 2000), and they are also activated during natural REM sleep (Orem, 1994; Orem et al., 2005). Activation of cells in the wake-promoting region of the posterior, lateral hypothalamus blocks the ability of pontine carbachol to elicit the REM sleep-like state in urethane-anesthetized rats (Lu et al., 2007) and, similarly, optogenetic or pharmacological activation of cells in the same hypothalamic region suppresses generation of natural REM sleep in behaving rats (Adamantidis et al., 2007; Alam et al., 2005). Thus, the anesthetized rat carbachol model of REM sleep mimics at many levels the processes underlying the initiation and maintenance of REM sleep (except phasic events, such as muscle twitches or rapid variation of respiratory rate and blood pressure; see discussion in Kimura et al., 1990; Kubin, 2001; Orem and Kubin, 2005). Individual REM sleep-like episodes last 4–8 min, and can be triggered repeatedly, thus allowing one to explore single cell activity and conduct within-subject pharmacological experiments.
3 NE AND SEROTONIN PROVIDE A MAJOR ENDOGENOUS DRIVE TO XII MOTONEURONS
When we proposed that REM sleep-related depression of activity of XII motoneurons is caused by disfacilitation, we considered both 5-HT and NE as the two major neuromodulators whose withdrawal could cause decrements of upper airway muscle tone during REM sleep (Kubin et al., 1992, 1998). This hypothesis proved to be correct, but additional intricacies emerged. It now appears that, in cats and dogs, the endogenous, wakefulness-related drive to upper airway motoneurons mediated by 5-HT is very strong (Kubin et al., 1992; Neuzeret et al., 2009; Veasey et al., 1996), whereas the drive dependent on endogenous NE is of a lesser magnitude (Neuzeret et al., 2009). In contrast, in rats, endogenous excitation of XII motoneurons mediated by 5-HT is relatively less powerful than that mediated by NE. This is the case in both chronically instrumented, behaving rats (Chan et al., 2006; Sood et al., 2005), and in urethane-anesthetized rats (Fenik et al., 2005b). Figure 2 compares the effects of local microinjections of an α1-adrenergic receptor antagonist, prazosin, and a broad-spectrum 5-HT receptor antagonist, methysergide, into the XII nucleus on spontaneous activity of the medial branch of the XII nerve in urethane-anesthetized rats.
FIGURE 2.
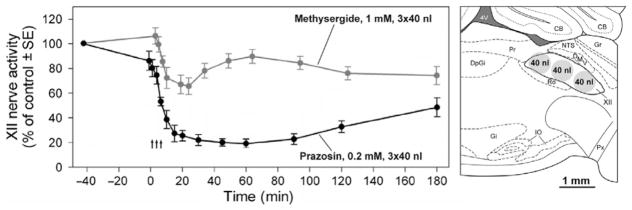
Comparison of the effects of the α1-adrenergic receptor antagonist, prazosin, and a broad-spectrum 5-HT receptor antagonist, methysergide, microinjected into the XII nucleus on spontaneous activity of the medial branch of the XII nerve in urethane-anesthetized rats. Given the elongated shape of the XII nucleus, the compounds were injected into the center of the nucleus at three rostro-caudal levels to ensure antagonism of relevant receptors on all XII motoneurons and their dendrites. Spontaneous activity of the XII nerve was measured relative to its level prior to the injections. The injections were made during the first ~10 min after time zero (arrows). Both antagonists reduced XII nerve activity, with the minimum attained ~30 min after the injections. Prazosin had a stronger depressant effect than methysergide, suggesting that NE provided more endogenous excitation to XII motoneurons than 5-HT. In addition, methysergide caused a delayed activation. XII nerve activity partially recovered during180 min after the injections. Each curve is based on data from six animals (Fenik et al., 2005b). The panel on the right shows a lateral view of the XII nucleus and surrounding structures adapted from a rat brain atlas (Paxinos and Watson, 2007). The diameters of the circles representing the injection sites are scaled to show the initial distribution of the drugs calculated with the assumption that 25% of tissue volume is available to accommodate 40 nl volume of the injectate (Nicholson, 1985). Abbreviations: CB, cerebellum; DMV, dorsal motor nucleus of the vagus nerve; DpGi, dorsal paragigantocellular nucleus; Gi, gigantocellular reticular nucleus; Gr, gracile nucleus; IO, inferior olive; NTS, nucleus of the solitary tract; Pr, prepositus nucleus; Px, pyramidal tract; Ro, nucleus of roller; 4V, 4th ventricle.
Thus, in rat XII motoneurons, the relative contribution of endogenous activation mediated by NE is larger than that mediated by 5-HT. In humans, neither the absolute nor the relative magnitude of 5-HT and NE drives to upper airway motoneurons has been investigated. Furthermore, animals with fully patent upper airway were used in most of the studies of the effects of NE and 5-HT on motoneurons innervating upper airway muscles. In OSA patients, the chronic sleep and respiratory disruption may alter the roles 5-HT and NE play in the control of upper airway motoneurons. In addition, rats are distinctly nocturnal animals. Therefore, it is possible that the magnitudes of these two aminergic drives vary with circadian time. We base this suggestion on our data showing that mRNA and protein levels for the excitatory 5-HT2A receptor are higher in the XII nucleus during the active period (night in rats) than during the rest period, whereas NE receptor mRNA does not exhibit circadian variation (Volgin et al., 2013). These issues need further investigation.
4 FUNCTIONAL EQUIVALENCE BETWEEN A COMBINED WITHDRAWAL OF ENDOGENOUS NA AND SEROTONERGIC DRIVES AND REM SLEEP-RELATED DEPRESSION OF XII MOTONEURONAL ACTIVITY
Using urethane-anesthetized rats with REM sleep-like episodes repeatedly elicited by pontine carbachol injections, we obtained evidence that REM sleep-related depression of XII motoneuronal activity can be fully and sufficiently explained by a combined withdrawal from motoneurons of NE- and 5-HT-mediated excitations (Fenik et al., 2005b). The premise of these experiments was that, if the REM sleep-like depression of activity in XII motoneurons is the result of withdrawal of motoneuronal excitation mediated by NE and 5-HT, then no additional REM sleep-like depression should occur following pharmacological antagonism of their receptors at the level of the XII nucleus. To test this hypothesis, we designed experiments in which REM sleep-like episodes were elicited by pontine carbachol injections under the baseline conditions, then after combined microinjections of NE and 5-HT receptor antagonists directly into the XII nucleus, and then again when the effects of the antagonists started to dissipate (cf. Fig. 2). As antagonists, we used the α1-adrenergic receptor blocker, prazosin, and a broad-spectrum 5-HT receptor antagonist, methysergide because α1-adrenergic and 5-HT2 receptors have been identified as the main aminergic receptors in the XII nucleus (Fenik and Veasey, 2003; Okabe et al., 1997; Volgin et al., 2001, 2003; Zhan et al., 2002).
The key finding from these studies was that, when both NE and 5-HT receptors located within and adjacent to the XII nucleus were antagonized, XII nerve activity was reduced to approximately the same level as that attained by XII nerve activity during the REM sleep-like episodes elicited by carbachol, and no further reduction of XII nerve activity occurred during the episodes. Importantly, while no additional depression of XII nerve activity occurred, other characteristic effects, such as hippocampal activation and respiratory rate changes, occurred as usual, and the depressant effect of REM sleep-like episodes on XII nerve activity gradually recovered over the subsequent 3 h as the antagonists diffused away (Fig. 3). This outcome indicated that an occlusion occurred at the level of the XII nucleus between the depressant effect of REM sleep-like state and the NE and 5-HT receptor antagonism.
FIGURE 3.
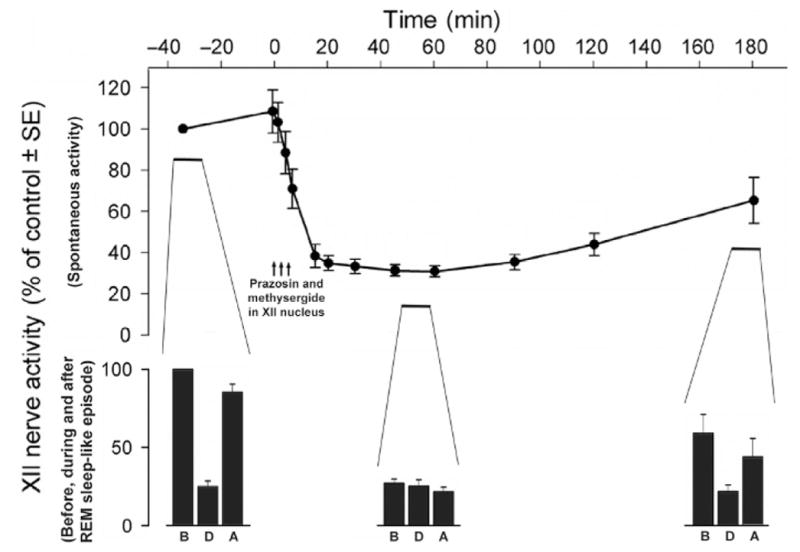
When endogenous activation of XII motoneurons mediated by NE and 5-HT is blocked, there is no additional depression of XII nerve activity during the REM sleep-like episodes elicited in urethane-anesthetized rats by pontine carbachol injections. The top graph shows the effect of combined microinjections of the α1-adrenergic receptor antagonist, prazosin, and a broad-spectrum 5-HT receptor antagonist, methysergide, into the XII nucleus on spontaneous XII nerve activity. REM sleep-like episodes were elicited repeatedly at three times during each experiment, first prior to the antagonists injections, then at the time of peak antagonist effect (~50 min after injections), and then again when XII nerve activity partially recovered from the effect of the antagonists (~170 min after injections). The three bar graphs at the bottom show XII nerve activity before (B), during (D), and after (A) each of the three REM sleep-like episodes elicited at these three times. The middle set of bars shows that no depression of XII nerve activity occurs at the time when the antagonists exert their maximal effect. Average data from six rats.
Adapted from Fenik et al. (2005b).
The experiments with NE and 5-HT receptor antagonists indicated that both receptor groups need to be antagonized because, when, in separate experiments, only prazosin or only methysergide were used, a residual but statistically significant depression of XII nerve activity still occurred during the carbachol-induced REM sleep-like episodes (Fenik et al., 2005b). It is also of note that, when the XII nucleus was simultaneously infiltrated with prazosin, methysergide, GABAA, and glycine receptor antagonists (bicuculline and strychnine, respectively), the carbachol-induced, REM sleep-like depression did not occur (Fenik et al., 2004). This finding, albeit not surprising in the face of the result with prazosin and methysergide only, was an important control experiment, because the same result was obtained while the baseline level of XII nerve activity was elevated by the antagonists of inhibitory transmission (bicuculline and strychnine). Thus, the absence of any depression of XII nerve activity during the REM sleep-like episodes elicited following microinjections of prazosin and methysergide only into the XII nucleus was not secondary to the low level of XII nerve activity resulting from the antagonism of the α1-adrenergic and 5-HT receptors.
Thus, in rats, the pontine carbachol-triggered, REM sleep-like depression of XII motoneuronal activity is caused by withdrawal of motoneuronal excitation mediated by only two modulators, NE and 5-HT. Whether this is also the case in intact, unanesthetized rats, other animal species, and humans remains to be determined. Indeed, additional mechanisms are likely to operate in these more complex conditions. For example, in chronically instrumented, behaving rats, scopolamine (a muscarinic cholinergic type 1 receptor antagonist) administered by reverse microdialysis into the XII nucleus region resulted in a significant increase of lingual muscle activity that was most prominent during REM sleep when compared to either non-REM sleep or wakefulness, and a similar result was obtained with the peptide blocker of inwardly rectifying and Ca2+-activated large conductance K+ channels, tertiapin-Q (Grace et al., 2013). These findings suggest that a muscarinic cholinergic inhibitory mechanism that acts through K+ channels suppresses excitatory inputs to XII motoneurons predominantly during REM sleep. Consistent with this interpretation, many XII premotor neurons located in the reticular formation ventrolateral to the XII nucleus use acetylcholine as their transmitter and express mRNA for multiple muscarinic cholinergic receptors (Volgin et al., 2008). These premotor neurons may interact with adjacently located glutamatergic neurons that are a known source of inspiratory drive to XII motoneurons (Woch et al., 2000), and some may also express mRNA for muscarinic receptors (Volgin et al., 2008). These interactions may be pre-synaptic and occur at the level of the XII nucleus, although it is also possible that effects exerted by scopolamine and tertiapin-Q on the reflex transmission at the level of the nucleus of the solitary tract located just dorsal to the XII nucleus contributed to the results reported by Grace et al. (2013).
5 THE SOURCES OF NA EXCITATORY INPUT TO XII MOTONEURONS AND THEIR STATE DEPENDENCE
The findings summarized in the preceding sections revealed that endogenous NA excitatory drive has a major role in setting the baseline level of activity in XII motoneurons. This conclusion prompted us to investigate the sources of NA projections to the XII nucleus, and whether these sources are all state dependent in a manner previously established for only two groups of pontine NA neurons—LC cells (A6 group) and cells of the sub-coeruleus (SubC) region (Aston-Jones and Bloom, 1981; Reiner, 1986).
One earlier study found that NA axonal projections to the XII nucleus originate mainly in the pontine A5, SubC, and A7 groups, that the projections are bilateral, and that those from the LC are negligible (Aldes et al., 1992). We reinvestigated the issue with the goal to include ventrolateral medullary NA neurons, and to gain quantitative insights into the relative contributions of different NA groups to the total NA input to XII motoneurons. We used either Fluoro-Gold or B subunit of cholera toxin as retrograde tracers, and tyrosine hydroxylase immunohistochemistry to determine the distribution of NA neurons with efferent projections to the XII nucleus (Rukhadze and Kubin, 2007).
Our results generally confirmed the findings of Aldes et al. (1992), who reported that NA projections to the XII nucleus originate primarily in the SubC region (69% of all retrogradely labeled NA neurons), followed by the A7 (21%) and A5 (10%) groups, but we also found quantitative differences. In our study, A5 group contained 43.5% of all NA cells retrogradely labeled from the XII nucleus, SubC 21.0%, A7 group 15%, and LC only 1.7% (Fig. 4A). Additionally, we found substantial projections from the ventrolateral medullary A1/C1 group (18.5%) that was not previously reported. Since there are no adrenergic fibers in the XII nucleus (Kalia et al., 1985), the projections from the ventrolateral medulla must have originated from the NA neurons of the A1 group. The relatively high proportion of projections from the A5 group that we found might have been caused by retrograde labeling from the periphery of the XII nucleus, rather than from its center, because our retrograde tracers could diffuse more readily than the iontophoretically deposited tracer used by Aldes et al. (1992). Consistent with this interpretation, in a separate antidromic mapping study of A5 cell projections to the XII nucleus, no A5 cell axonal branches were localized in the center of the XII nucleus (Fenik et al., 2002). Therefore, any projections from the A5 group to the XII nucleus are more likely to target distal dendrites of XII motoneurons than their cell bodies.
FIGURE 4.
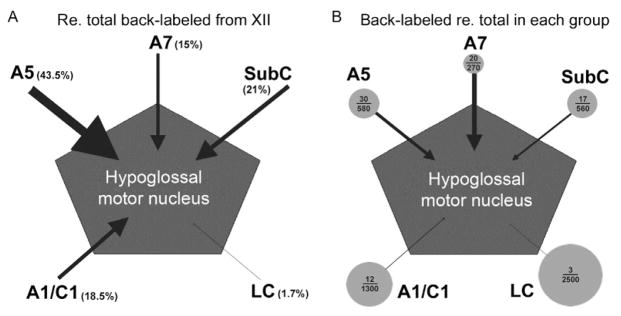
Quantitative representation of afferent projections to the XII nucleus from distinct groups of pontomedullary noradrenergic (NA) neuronal groups. (A) When expressed relative to the average total numbers of retrogradely labeled NA neurons following tracer injections into the XII nucleus, cells of the A5 group have strongest projections, followed by sub-coeruleus (SubC), A1/C1 and A7 groups, whereas the projections from the locus coeruleus (LC) are negligible. (B) When the numbers of NA cells retrogradely labeled from the XII nucleus are expressed relative to the average total numbers of neurons present in each cell group, the A7 group contains the highest percentage of cells that send axons to the XII nucleus. In this panel, circles are scaled proportionally to the square root of the numbers of cells counted bilaterally in each NA group and arrow thickness is proportional to the percentage of cells in each group that were retrogradely labeled from the XII nucleus. The numbers inside each circle represent the average numbers of retrogradely labeled cells found in each group per animal and the average total numbers of NA cells in each group, as determined by Rukhadze and Kubin (2007). Consistent with the representation of axonal projections to the XII nucleus shown in (B), pharmacological inhibition of A7 neurons resulted in a significant reduction of spontaneous XII nerve activity (Fenik et al., 2008), whereas local microinjections of clonidine to inhibit A5 or SubC neurons did not result in significant changes (Fenik et al., 2002, 2008).
An alternative way of quantifying the contribution of axonal projections from different groups of NA neurons to the XII nucleus to the one based on the numbers of retrogradely labeled cells in each group can be obtained by relating the numbers of retrogradely labeled cells to the total numbers of NA cells in each group. Using this approach, we determined that 7.5% of all A7 cells, 5.1% of A5 cells, 3.1% of SubC cells, 0.9% of A1/C1 cells, and 0.1% of LC cells were retrogradely labeled from the XII nucleus. This quantification suggests that, compared to other NA groups, the pontine A7 group has a particularly high preference to target the XII nucleus relative to its other efferent projections. Figure 4 schematically depicts the contributions of different NA groups to axonal projections to the XI nucleus, as quantified by these two different approaches.
The percentages of cells located in different NA cell groups that have axonal projections to the XII nucleus provide useful estimates of their potential contributions to the total NA input to XII motoneurons. However, for a more complete picture, one will need to determine the density and pattern of branching within the XII nucleus of NA axons that originate in different cell groups, the preferential locations of axon terminals relative to XII motoneuronal cell bodies and dendrites (cf. Funk et al., 2011) and, ultimately, the magnitude of postsynaptic effects produced by afferents from different NA groups.
In separate pharmacological experiments, we made an attempt to estimate the relative strength of the endogenous NA excitatory drive to XII motoneurons from different NA cell groups. Based on the evidence that most NA cells have the inhibitory α2-adrenergic autoreceptors (Huangfu and Guyenet, 1997; Reiner, 1985), we conducted local microinjections of the α2-adrenergic receptor agonist, clonidine, into selected sites of origin of NA projections to the XII nucleus as the means of selectively silencing NA cells in different groups. To date, we tested the effects of clonlidine microinjections on the level of spontaneous XII nerve activity in urethane-anesthetized, paralyzed, and artificially ventilated rats by placing the injections into the A5, SubC, or A7 groups (Fenik et al., 2002, 2008, 2012). We found that only the injections placed in the A7 group significantly reduced spontaneous XII nerve activity, by 31% following unilateral injections, and that the effect was blocked when clonidine injections were preceded by microinjections into the same site of a selective α2-adrenergic receptor agonist (Fenik et al., 2008). In contrast, neither clonidine microinjections placed at sites surrounding the A7 group nor those placed into the SubC or the A5 regions had any tonic effect of XII nerve activity. Thus, unilateral pharmacological inhibition of A7 cells resulted in significant decrements of XII nerve activity, whereas silencing of A5 or SubC cells did not despite the anatomical evidence for NA projections from all these groups to the XII nucleus and direct evidence that at least A5 cells are spontaneously active in our rat model and are inhibited by clonidine (Fenik et al., 2002). This demonstrates that the presence of axonal projections does not necessarily translate into significant functional effects.
The concept that activity of NA cells predictably changes with behavioral states, so that it is highest during active wakefulness, partially declines during slow-wave sleep and ceases during REM sleep has been well supported by recordings from LC and SubC neurons (Aston-Jones and Bloom, 1981; Reiner, 1986; Takahashi et al., 2010). However, it has not been determined whether the same is the case for other brainstem NA and adrenergic neurons. Since our anatomical and pharmacological data indicated that the A7 and possibly also A5 and A1 groups may provide endogenous excitation to XII motoneurons, it became important to determine whether all brainstem catecholaminergic cells are depressed during REM sleep. In the case of A5 neurons, we addressed this by recording from these cells during carbachol-induced REM sleep-like episodes and determined that A5 cells are indeed silenced (Fenik et al., 2002). To test whether this is also the case for A7 and other catecholaminergic neuronal groups, we used the carbachol model of REM sleep and c-Fos expression as an indirect marker of the level of cell activity. In different rats, we produced varying cumulative durations of REM sleep-like state by repeatedly injecting carbachole (or saline) into the dorsomedial pons. At the end of the experiment, the animals were perfused and the percentages of tyrosine hydroxylase-positive cells expressing c-Fos were determined for each catecholaminergic group and correlated with the total time spent in REM sleep-like state during 140-min-long period prior to perfusion (Rukhadze et al., 2008). For the SubC and pontine part of the A5 group, we found a significant negative correlation between the level of c-Fos expression and the amount of time spent in REM sleep-like state. This was consistent with electrophysiological data indicating that these cells are silenced during REM sleep (Fenik et al., 2002; Reiner, 1986). We also found significant negative correlations for A7 neurons. Furthermore, for A7, pontine A5 and dorsomedial medullary A2/C2 neurons, there was a positive correlation between the time that elapsed between the last REM sleep-like episode and the time of perfusion, which indicated that nuclear c-Fos accumulation increased in these cells with time after each REM sleep-like episode. This was consistent with the cells increasing their activity after each episode. In contrast, the correlations were not significant for catecholaminergic cells located in the caudal part of the A5 group and ventrolateral medullary A1 and C1 neurons. Although interpretation of these results is based on the assumption that the level of c-Fos expression reflects the level of cell activity, the consistency of c-Fos and electrophysiological data in support of this assumption for SubC and A5 neurons strongly suggests that pontine A7 and medullary A2/C2 neurons are also suppressed or silenced during REM sleep, whereas the ventrolateral medullary A1/C1 neurons and neurons of the caudal part of the A5 group are not. This would indicate heterogeneity of the behaviors of pontomedullary catecholaminergic neurons during REM sleep. Alternatively, our negative results with A1/C1 and caudal A5 neurons could be caused by the absence of spontaneous activity in these neurons in urethane-anesthetized rats. However, data show that adrenergic C1 neurons have spontaneous activity in urethane-anesthetized rats (Huangfu et al., 1992; Verberne et al., 1999). Our recent electrophysiological data confirm that C1 neurons are spontaneously active and show that they are activated during the REM sleep-like episodes (Stettner et al., 2013). It is possible that the lack of significant correlation between c-Fos expression and the amount of prior time spent in REM sleep-like episodes for the combined group of A1 and C1 neurons was caused by opposite behaviors of A1 and C1 neurons and our limited ability to distinguish between A1 and C1 neurons based solely on anatomical landmarks and tyrosine hydroxylase immunohistochemistry (Rukhadze et al., 2008). Thus, whether A1 neurons are spontaneously active and how their activity changes during REM sleep remains to be determined.
6 NA CONTROL OF THE UPPER AIRWAY FOLLOWING EXPOSURE TO CHRONIC-INTERMITTENT HYPOXIA
The findings reported in the preceding sections revealed the mechanisms that may importantly determine the level of activity in XII motoneurons across sleep–wake states in normal rats, thus in an animal model that differs in many ways from subjects with OSA, which is an almost uniquely human disorder. Although rats and humans have similar fundamental mechanisms that regulate sleep–wake behavior and the control of breathing, rats’ upper airway anatomy is different (their hyoid bone is not mobile like it is in humans), and rats do not exhibit any propensity for sleep-related upper airway obstructions. With NA control identified as a potentially important determinant of upper airway muscle tone in normal rats, we conducted studies with rats subjected to chronic-intermittent hypoxia (CIH) in an attempt to determine how this major pathophysiological condition of OSA affects the otherwise normal control of the upper airway.
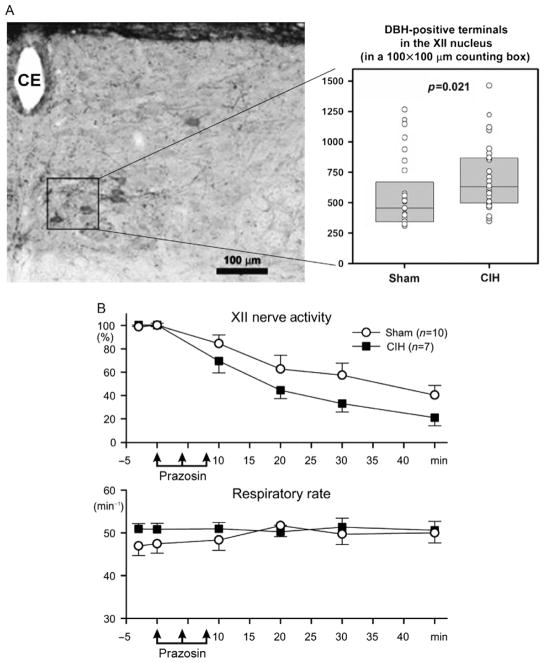
Rats were subjected to CIH for 35 days, with O2 oscillating with 180-s period between a mild hyperoxia (24%) and nadirs of 6.9% daily from 7 am to 5 pm, while control rats were subjected to a matching sham treatment (Rukhadze et al., 2010). About 17 h after the last exposure, the animals were perfused and brain sections containing the XII nucleus were subjected to tyrosine hydroxylase or α1-adrenoceptor immunohistochemistry. We quantified the density of NA terminals in the ventrome-dial quadrant of the XII nucleus, where these terminals are particularly numerous in normal rats (Aldes et al., 1988) and where most XII motoneurons that innervate tongue protruder muscles are located (Altschuler et al., 1994; Dobbins and Feldman, 1995). We also counted XII motoneurons expressing α1-adrenoceptor-like protein throughout the entire cross sections through the XII nucleus in CIH- and sham-exposed rats. We found that CIH rats had significantly higher density of NA terminals in the ventromedial XII nucleus than sham-treated animals (Fig. 5A). CIH rats also had a significantly higher percentage of α1-adrenoceptor-expressing XII motoneurons in the dorsal half of the XII nucleus and a similar trend was present in the ventral half. In complementary studies, we determined that CIH-exposed rats had elevated NA terminal density in the spinal trigeminal sensory nucleus (Sp5) and a trend in the same direction in the trigeminal motor nucleus (V), but the magnitude of these effects was not as large as in the XII nucleus (39% increase in the ventro-medial quadrant of the XII nucleus, 18% in the Sp5, and 9% in the V) (Mody et al., 2011; Rukhadze et al., 2010).
FIGURE 5.
Rats exposed to chronic-intermittent hypoxia (CIH) for 35 days have increased density of NA terminals in the ventromedial quadrant of the XII nucleus and increased dependence of spontaneous XII nerve activity on the endogenous excitatory drive mediated by α1-adrenergic receptors. (A) Position of the axon terminal counting box (100×100 μm) in the XII nucleus and the average numbers of dopamine-β-hydroxylase (DBH) terminals counted in 24 matched for the anteroposterior level pairs of brain sections from eight pairs of CIH/ sham-treated rats. Several XII motoneurons retrogradely labeled from the base of the tongue are present within and adjacent to the counting box. CE, central canal. (B) Microinjections of the α1-adrenergic receptor antagonist, prazosin into the XII nucleus resulted in larger decrements of spontaneous XII nerve activity in anesthetized, paralyzed, and artificially ventilated rats tested ~20 h after the last exposure to CIH than in sham-treated animals. The injections did not cause any changes of the central respiratory rate.
(A) Data from Rukhadze et al. (2010) and (B) data from Stettner et al. (2012).
These results suggested that NE terminal sprouting, combined with increased expression of α1-adrenergic receptors may act together to enhance endogenous NA activation of XII motoneurons following exposure to CIH. To test whether this is the case, in urethane-anesthetized, paralyzed, and artificially ventilated rats previously subjected to CIH or sham treatment for 35 days, we tested the effects of α1-adrenoreceptor agonists and antagonists injected into the XII nucleus on spontaneous XII nerve activity (Stettner et al., 2012). Microinjections of the α1-adrenoceptor agonist, phenylephrine into the XII nucleus predictably increased XII nerve activity. However, the magnitude of the effect did not differ between the CIH- and sham-treated rats, possibly due to the large intersubject variability of the effect. In contrast, microinjections of the α1-adrenoceptor antagonist, prazosin caused larger declines of spontaneous XII nerve activity in the CIH- than sham-treated rats (Fig. 5B). This finding was consistent with CIH-enhancing endogenous NA excitatory drive to XII motoneurons that could last at least for 20 h after the last CIH exposure. Interestingly, our related c-Fos study did not support the hypothesis that CIH exposure leads to a lasting increase of activity in central catecholamineric neurons. We found no c-Fos increase in any of the pontomedullary catecholaminergic neuronal groups in rats that were gently kept awake for 2.5 h and then perfused 20 h after the last exposure to CIH when compared to sham-treated animals. Indeed, the percentage of catecholaminergic cells with nuclear c-Fos expression tended to be lower in the CIH than sham-treated animals in all medullary groups (significant for the A1 group) (Benincasa Herr et al., 2013). Thus, CIH may lead to enhanced central catecholaminergic transmission through sprouting of central terminals and increased expression of α1-adrenoceptors, whereas the level of spontaneous activity of catecholaminergic cells does not appear to be persistently increased following CIH.
These studies show that CIH may enhance central catecholaminergic tone, thereby increasing NA drive to upper airway motoneurons during wakefulness and probably slow-wave sleep, that is, when NA neurons are active. This possibility offers a mechanistic explanation for the observation that OSA patients have elevated upper airway muscle tone when compared to persons with fully patent upper airway (Katz and White, 2004; Mezzanotte et al., 1992; Suratt et al., 1988). CIH may also affect catecholaminergic modulation of transmission in other pathways. For example, transmission of reflexes affecting XII motoneurons and NA modulation of other cardiorespiratory functions and sleep–wake behavior may be altered following CIH exposure, as well as in OSA patients. Demonstration of these possible outcomes will require additional studies.
7 CONCLUSIONS AND FUTURE DIRECTIONS
Both NE and 5-HT provide excitatory drives that maintain spontaneous activity of XII motoneurons, with the NA system playing a relatively more prominent role than 5-HT in rats. When these two drives are antagonized at the level of the XII nucleus, no further decline of XII nerve activity occurs during pharmacologically induced REM sleep-like episodes. This finding shows that, at least in our experimental model, the entire REM sleep-related decline of upper airway muscle tone is caused by the withdrawal of these two neurochemically distinct excitatory inputs. A major part of the NA input to XII motoneurons originates in pontine NA groups that have state-dependent activity, maximal during active wakefulness and minimal or absent during REM sleep. Our data also suggest that ventrolateral medullary catecholaminergic neurons do not have this pattern; indeed, adrenergic C1 neurons may increase their activity during REM sleep. After a period of exposure to CIH, NE drive to XII motoneurons is increased by mechanisms that include sprouting of NA terminals in the XII nucleus and increased expression of α1-adrenoceptors.
One challenge for future studies will be to determine to what extent these findings apply to upper airway control in healthy humans and OSA patients. Comparison of the role of NE and 5-HT in the control of XII motoneurons in rats and cats suggests that there are at least quantitative species differences. Furthermore, in subjects with an anatomically compromised upper airway, reflex regulation of upper airway muscle tone initiated by flow limitation is superimposed on the central drives that set the baseline upper airway muscle activity. Not only CIH but also recurrent sleep disruptions may alter the role NE plays in the control of upper airway muscles and other aspects of cardiorespiratory and sleep–wake controls. Thus, our studies in rats provide reference points for future investigations of the role of NE in humans and animal models with spontaneous- or experimentally induced sleep-related respiratory disorders.
Acknowledgments
Our studies summarized in this review were supported by the following grants from the National Institutes of Health: HL-47600, HL-60287, and HL-74385.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol (Lond) 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldes LD, Chronister RC, Marco LA, Haycock JW, Thibault J. Differential distribution of biogenic amines in the hypoglossal nucleus of the rat. Exp Brain Res. 1988;73:305–314. doi: 10.1007/BF00248222. [DOI] [PubMed] [Google Scholar]
- Aldes LD, Chapman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull. 1992;29:931–942. doi: 10.1016/0361-9230(92)90168-w. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Miselis RR. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J Comp Neurol. 1994;342:538–550. doi: 10.1002/cne.903420404. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep–waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benincasa Herr K, Stettner GM, Kubin L. Reduced c-Fos expression in medullary catecholaminergic neurons in rats 20 hours after exposure to chronic intermittent hypoxia. Am J Physiol. 2013;304:R514–R522. doi: 10.1152/ajpregu.00542.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin P, Escourrou P, Gaultier C, Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. Neuroreport. 1995;6:532–536. doi: 10.1097/00001756-199502000-00031. [DOI] [PubMed] [Google Scholar]
- Brooks PL, Peever JH. Glycinergic and GABAA-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28:3535–3545. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol. 1979;46:772–779. doi: 10.1152/jappl.1979.46.4.772. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep–wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9:743–751. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conwell W, Patel B, Doeing D, Pamidi S, Knutson KL, Ghods F, Mokhlesi B. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16:519–526. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- Eisele DW, Schwartz AR, Smith PL. Tongue neuromuscular and direct hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Clin North Am. 2003;36:501–510. doi: 10.1016/s0030-6665(02)00178-0. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik VB, Kubin L. Differential localization of carbachol- and bicuculline-sensitive pontine sites for eliciting REM sleep-like effects in anesthetized rats. J Sleep Res. 2009;18:99–112. doi: 10.1111/j.1365-2869.2008.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- Fenik V, Marchenko V, Janssen P, Davies RO, Kubin L. A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol. J Appl Physiol. 2002;93:1448–1456. doi: 10.1152/japplphysiol.00225.2002. [DOI] [PubMed] [Google Scholar]
- Fenik V, Davies RO, Kubin L. Combined antagonism of aminergic excitatory and amino acid inhibitory receptors in the XII nucleus abolishes REM sleep-like depression of hypoglossal motoneuronal activity. Arch Ital Biol. 2004;142:237–249. [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res. 2005a;14:419–429. doi: 10.1111/j.1365-2869.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005b;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik VB, Rukhadze I, Kubin L. Inhibition of pontine noradrenergic A7 cells reduces hypoglossal nerve activity in rats. Neuroscience. 2008;157:473–482. doi: 10.1016/j.neuroscience.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik VB, Marchenko V, Davies RO, Kubin L. Inhibition of A5 neurons facilitates the occurrence of REM sleep-like episodes in urethane-anesthetized rats: a new role for noradrenergic A5 neurons? Front Neurol. 2012;3:1–12. Article 119. doi: 10.3389/fneur.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Zwicker JD, Selvaratnam R, Robinson DM. Noradrenergic modulation of hypoglossal motoneuron excitability: developmental and putative state-dependent mechanisms. Arch Ital Biol. 2011;149:426–453. doi: 10.4449/aib.v149i4.1271. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Pegram GV. Cholinergic brainstem mechanisms of REM sleep in the rat. Brain Res. 1986;384:29–41. doi: 10.1016/0006-8993(86)91216-3. [DOI] [PubMed] [Google Scholar]
- Grace KP, Hughes SW, Shahabi S, Horner RL. K+ channel modulation causes genioglossus inhibition in REM sleep and is as strategy for reactivation. Respir Physiol Neurobiol. 2013;188:277–288. doi: 10.1016/j.resp.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Guyenet PG. Alpha 2-adrenergic autoreceptors in A5 and A6 neurons of neonate rats. Am J Physiol. 1997;273:H2290–H2295. doi: 10.1152/ajpheart.1997.273.5.H2290. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Verberne AJM, Guyenet PG. Rostral ventrolateral medullary neurons projecting to locus coeruleus have cardiorespiratory inputs. Brain Res. 1992;598:67–75. doi: 10.1016/0006-8993(92)90169-a. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Recherches sur les structures nerveuses et les mécanismes responsables des différentes phases du sommeil physiologique. Arch Ital Biol. 1962;100:125–206. [PubMed] [Google Scholar]
- Kalia M, Fuxe K, Goldstein M. Rat medulla oblongata. III Adrenergic (C1 and C2) neurons, nerve fibers and presumptive terminal processes. J Comp Neurol. 1985;233:333–349. doi: 10.1002/cne.902330304. [DOI] [PubMed] [Google Scholar]
- Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:553–560. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- Kimura H, Kubin L, Davies RO, Pack AI. Cholinergic stimulation of the pons depresses respiration in decerebrate cats. J Appl Physiol. 1990;69:2280–2289. doi: 10.1152/jappl.1990.69.6.2280. [DOI] [PubMed] [Google Scholar]
- Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- Kubin L, Reignier C, Tojima H, Taguchi O, Pack AI, Davies RO. Changes in serotonin level in the hypoglossal nucleus region during the carbachol-induced atonia. Brain Res. 1994;645:291–302. doi: 10.1016/0006-8993(94)91663-2. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- Lu JW, Fenik VB, Branconi JL, Mann GL, Rukhadze I, Kubin L. Disinhibition of perifornical hypothalamic neurones activates noradrenergic neurones and blocks pontine carbachol-induced REM sleep-like episodes in rats. J Physiol (Lond) 2007;582:52–67. doi: 10.1113/jphysiol.2007.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody P, Rukhadze I, Kubin L. Rats subjected to chronic-intermittent hypoxia have increased density of noradrenergic terminals in the trigeminal sensory and motor nuclei. Neurosci Lett. 2011;505:176–179. doi: 10.1016/j.neulet.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales FR, Boxer P, Chase MH. Behavioral state-specific inhibitory postsynaptic potentials impinge on cat lumbar motoneurons during active sleep. Exp Neurol. 1987;98:418–435. doi: 10.1016/0014-4886(87)90252-4. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol (Lond) 2003;552:965–980. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki M, Kitaguchi S, Ichihashi H, Haraguchi R, Iwanaga T, Kubo H, Higashiyama A, Tohda Y. Apnoea-hypopnoea index during rapid eye movement and non-rapid eye movement sleep in obstructive sleep apnoea. J Int Med Res. 2008;36:906–913. doi: 10.1177/147323000803600506. [DOI] [PubMed] [Google Scholar]
- Neuzeret PC, Sakai K, Gormand F, Petitjean T, Buda C, Sastre JP, Parrot S, Guidon G, Lin JS. Application of histamine and serotonin to the hypoglossal nucleus increases genioglossus activity across the wake-sleep cycle. J Sleep Res. 2009;18:113–121. doi: 10.1111/j.1365-2869.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in the brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Okabe S, Mackiewicz M, Kubin L. Serotonin receptor mRNA expression in the hypoglossal motor nucleus. Respir Physiol. 1997;110:151–160. doi: 10.1016/s0034-5687(97)00080-7. [DOI] [PubMed] [Google Scholar]
- Orem J. Central respiratory activity in rapid eye movement sleep: augmenting and late inspiratory cells. Sleep. 1994;17:665–673. doi: 10.1093/sleep/17.8.665. [DOI] [PubMed] [Google Scholar]
- Orem J, Kubin L. Respiratory physiology: central neural control. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Elsevier-Saunders; Philadelphia: 2005. pp. 213–223. [Google Scholar]
- Orem JM, Lovering AT, Vidruk EH. Excitation of medullary respiratory neurons in REM sleep. Sleep. 2005;28:801–807. doi: 10.1093/sleep/28.7.801. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Elsevier; Amsterdam: 2007. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Viemari JC. Determinants of inspiratory activity. Respir Physiol Neurobiol. 2005;147:145–157. doi: 10.1016/j.resp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Reiner PB. Clonidine inhibits central noradrenergic neurons in unanesthetized cats. Eur J Pharmacol. 1985;115:249–257. doi: 10.1016/0014-2999(85)90697-1. [DOI] [PubMed] [Google Scholar]
- Reiner PB. Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats. Brain Res. 1986;378:86–96. doi: 10.1016/0006-8993(86)90288-x. [DOI] [PubMed] [Google Scholar]
- Remmers JE, DeGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Kubin L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J Chem Neuroanat. 2007;33:23–33. doi: 10.1016/j.jchemneu.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Fenik VB, Branconi JL, Kubin L. Fos expression in pontomedullary catecholaminergic cells following REM sleep-like episodes elicited by pontine carbachol in urethane-anesthetized rats. Neuroscience. 2008;152:208–222. doi: 10.1016/j.neuroscience.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukhadze I, Fenik VB, Benincasa KE, Price A, Kubin L. Chronic intermittent hypoxia alters density of aminergic terminals and receptors in the hypoglossal motor nucleus. Am J Respir Crit Care Med. 2010;182:1321–1329. doi: 10.1164/rccm.200912-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, McKenzie DK, Gorman RB, Trinder JA, White DP, Gandevia SC. Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol (Lond) 2007;585:135–146. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Siegel JM. The neurobiology of sleep. Semin Neurol. 2009;29:277–296. doi: 10.1055/s-0029-1237118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soja PJ, Finch DM, Chase MH. Effect of inhibitory amino acid antagonists on masseteric reflex suppression during active sleep. Exp Neurol. 1987;96:178–193. doi: 10.1016/0014-4886(87)90179-8. [DOI] [PubMed] [Google Scholar]
- Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–1347. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- Spruyt K, Gozal D. REM and NREM sleep-state distribution of respiratory events in habitually snoring school-aged community children. Sleep Med. 2012;13:178–184. doi: 10.1016/j.sleep.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Fenik VB, Kubin L. Effect of chronic intermittent hypoxia on noradrenergic activation of hypoglossal motoneurons. J Appl Physiol. 2012;112:305–312. doi: 10.1152/japplphysiol.00697.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Lei Y, Benincasa Herr K, Kubin L. Evidence that adrenergic ventrolateral medullary cells are activated whereas precerebellar lateral reticular nucleus neurons are suppressed during REM sleep. PLoS One. 2013;8 (4):e62410. doi: 10.1371/journal.pone.0062410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am Rev Respir Dis. 1988;137:889–894. doi: 10.1164/ajrccm/137.4.889. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience. 2010;169:1115–1126. doi: 10.1016/j.neuroscience.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med. 1996;153:776–786. doi: 10.1164/ajrccm.153.2.8564132. [DOI] [PubMed] [Google Scholar]
- Verberne AJ, Stornetta RL, Guyenet PG. Properties of C1 and other ventrolateral medullary neurones with hypothalamic projections in the rat. J Physiol (Lond) 1999;517:477–494. doi: 10.1111/j.1469-7793.1999.0477t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Colom LV, Fortin WJ, Bland BH. Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Exp Brain Res. 1993;96:419–429. doi: 10.1007/BF00234110. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Mackiewicz M, Kubin L. α1B receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. J Chem Neuroanat. 2001;22:157–166. doi: 10.1016/s0891-0618(01)00124-7. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2A and 2C receptors in brainstem motoneurons. Eur J Neurosci. 2003;17:1179–1188. doi: 10.1046/j.1460-9568.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Rukhadze I, Kubin L. Hypoglossal premotor neurons of the intermediate medullary reticular region express cholinergic markers. J Appl Physiol. 2008;105:1576–1584. doi: 10.1152/japplphysiol.90670.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgin DV, Stettner GM, Kubin L. Circadian dependence of receptors that mediate wake-related excitatory drive to hypoglossal motoneurons. Respir Physiol Neurobiol. 2013;188:301–307. doi: 10.1016/j.resp.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Euler C. Brain stem mechanisms for generation and control of breathing pattern. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology. Section 3: The Respiratory System, vol. II: Control of Breathing, Part 1. American Physiological Society; Bethesda: 1986. pp. 1–67. [Google Scholar]
- Woch G, Ogawa H, Davies RO, Kubin L. Behavior of hypoglossal inspiratory pre-motor neurons during the carbachol-induced, REM sleep-like suppression of upper airway motoneurons. Exp Brain Res. 2000;130:508–520. doi: 10.1007/s002219900244. [DOI] [PubMed] [Google Scholar]
- Zhan G, Shaheen F, Mackiewicz M, Fenik P, Veasey SC. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neuroscience. 2002;113:145–154. doi: 10.1016/s0306-4522(02)00137-9. [DOI] [PubMed] [Google Scholar]