Abstract
Background
Previous clinical trials indicate that probiotic consumption may improve blood glucose control, however, results from randomized trials on glycemic control have been inconsistent.
Objective
To investigate the effects of probiotics on glycemic control in a systematic review and meta-analysis of randomized controlled trials.
Data Sources
PubMed, Embase, Cochrane Library, and Clinicaltrial.gov through October 2014.
Data Extraction and Synthesis
Two independent reviewers extracted relevant data and assessed study quality and risk of bias. Data were pooled using a random-effects model and expressed as mean differences (MD) with 95% CI. Heterogeneity was assessed (Cochran Q-statistic) and quantified (I 2).
Results
Seventeen randomized controlled trials were included, in which 17 fasting blood glucose (n = 1105), 11 fasting plasma insulin (n = 788), 8 homeostasis model assessment of insulin resistance (n = 635) comparisons were reported. Probiotic consumption, compared with placebo, significantly reduced fasting glucose (MD = -0.31 mmol/L; 95% CI 0.56, 0.06; p = 0.02), fasting plasma insulin (MD = -1.29 μU/mL; 95% CI -2.17, -0.41; p = 0.004), and HOMA-IR (MD = 0.48; 95% CI -0.83, -0.13; p = 0.007).
Conclusions
Probiotic consumption may improve glycemic control modestly. Modification of gut microbiota by probiotic supplementation may be a method for preventing and control hyperglycemia in clinical practice.
Introduction
Abnormal glucose metabolism is causally related to a greater risk of several chronic disorders, including diabetes, obesity, dyslipidemia, and cardiovascular diseases. Blood glucose can be controlled through diet and lifestyle modification to prevent diabetes or related complications and evidence suggests that dietary constituents and supplements such as omega-3 fatty acids [1], dairy products [2], pistachio [3] and coffee [4] can improve glycemic control or reduce an individual’s risk of diabetes.
Probiotics are defined as live microorganisms with potential health benefits for the host if consumed in adequate amounts [5]. Probiotic benefits have been investigated for improving immune function [6], lowering blood pressure [7], and improving lipids [8]. Data from animal models suggest that probiotics can reduce blood glucose and insulin resistance [9]. Interestingly, research shows that gut microbiota are involved in diabetes and metabolic disorders, revealing that diabetic patients have altered gut microbiota compared to non-diabetic counterparts [10]. Probiotics can be used to alter gut microbiota, and their ability to lower glucose is of interest to researchers [11–13]. However, human clinical trials of probiotics and glucose have yielded mixed results. For instance, some studies indicate that probiotic yogurt ingestion for 6 weeks can significantly improve glucose [12], whereas other studies concluded that this approach had no meaningful effects [14, 15]. Such inconsistent findings complicate approaches to and conclusions about probiotic use. In order to provide better evidence-based guidance on the role of probiotics on glycemic control, a systematic review and meta-analysis of randomized controlled trials (RCTs) was performed to assess the effect of probiotics on the endpoints of fasting glucose, fasting insulin, and homeostasis model assessment of insulin resistance (HOMA-IR).
Materials and Methods
1. Literature search
The online databases PubMed, The Cochrane Library, EMBASE, and Clinicaltrial.gov were searched until October 2014 for relevant studies. The following terms were used to search for relevant publications: ‘probiotic’, ‘lactobacilli’, ‘bifidobacter’, ‘bacillus’, ‘saccharomyces’, ‘enterococcus’, ‘streptococcus’, ‘yogurt’, ‘yoghurt’, ‘sour milk’, ‘fermented milk’, ‘gut microbiota’ in combination with ‘glucose’, ‘blood sugar’, ‘glycemic’, and ‘hyperglycemia’. We supplemented the literature search by scanning reference lists of relevant articles. The methodology of this systematic review was specified in advance and documented in a protocol that was published in a prospective register of systematic reviews, PROSPERO (www.crd.york.ac.uk/PROSPERO; ref CRD42014014498).
2. Study selection
Studies were included if they meet the following criteria: (1) human RCTs, (2) included adults ≥ 18 years-of-age with or without hyperglycemia, (3) use of probiotic products as an intervention group, (4) mean fasting blood glucose (+ SD) were reported for the intervention and control groups, (5) subjects had not received intestinal surgery. Studies were excluded if the total number of probiotic bacteria was not reported, if the probiotic contained prebiotics as the intervention product, or if they were not in English.
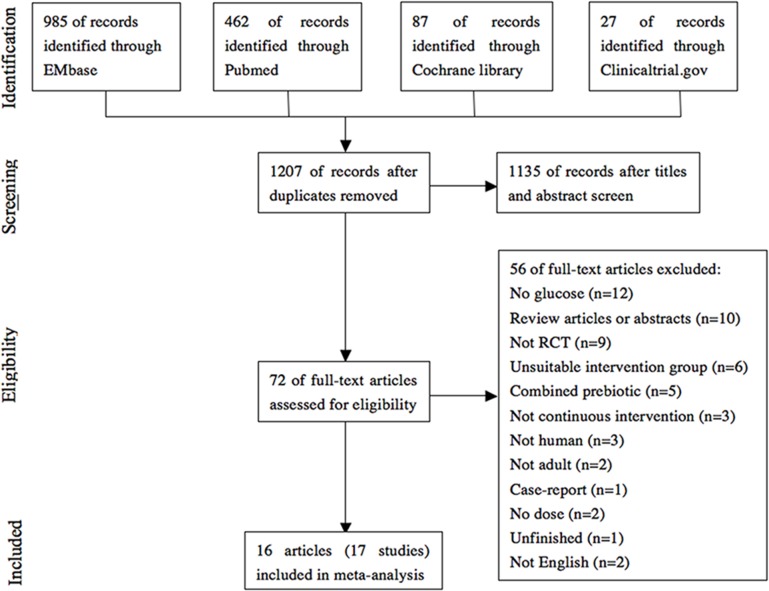
YTR and JH conducted an initial screening of studies based on titles and then reviewed abstracts and the full text to assess eligibility criteria independently. Final eligibility was determined through agreement between the 2 reviewers, with any disagreement resolved in consultation with HC. A PRISMA flow chart summarizes these decisions (Fig 1).
Fig 1. Flowchart for the selection of eligible studies.
3. Data extraction
YTR and JH independently extracted these data from eligible publications: probiotics, duration of intervention, sample size, subjects’ characteristics including age, sex, body mass index (BMI), baseline blood glucose, and antidiabetic medication use; probiotics or their fermented dairy products dosage; intervention and treatment results on glucose. We also noted data on baseline and follow-up insulin concentrations and HOMA-IR to measure any correlation between probiotics and glycemic control.
For trials containing multiple intervention arms, a weighted average was applied to combine them in order to create pair-wise comparisons. Ivey and coworkers [14] compared the effect of probiotic in four arms (1 probiotic yoghurt plus probiotic capsules group; 1 probiotic yoghurt plus placebo capsules group; 1 control milk plus probiotic capsules group and 1 control milk plus placebo capsules group). We extracted the data and analyzed subsets separately (probiotic yoghurt plus probiotic capsules group vs. probiotic yoghurt plus placebo capsules group; control milk plus probiotic capsules group vs. control milk plus placebo capsules group). Therefore, 16 articles and 17 RCTs were included in this meta-analysis.
4. Data analyses
Statistical analysis was performed according to the Cochrane Handbook for Statistical Review of Interventions (Version 5.0.2). The difference between the intervention and control arm’s change from baseline value was derived from each trial for endpoints fasting glucose, fasting insulin, and HOMA-IR. Mean differences (MD) of glucose, insulin and HOMA-IR were pooled respectively using a random-effects model due to study heterogeneity. The meta-analysis was performed using RevMan software (Cochrane Review Manager, version 5.2). Statistical tests were two-sided (p < 0.05).
Heterogeneity was tested and measured with a Q-test and with I 2 statistics. In general, we regarded heterogeneity as substantial if the I 2 > 50% or I 2 > 25% with a low p value (< 0.10). We explored sources of heterogeneity by comparing mean differences in each endpoint (fasting glucose, fasting insulin, and HOMA-IR) between subgroups stratified by hyperglycemia, pregnancy, probiotic dose, species, and sources and duration of treatment. To test data robustness, sensitivity analyses were performed in which each individual trial was removed from the meta-analysis and the effect size was recalculated with the remaining trials. Sensitivity analyses also undertook in which small studies (sample size < 20 for each group) were removed from the meta-analysis.
Risk of bias was assessed using the Cochrane Risk of Bias tool (S1 Fig). Potential publication bias was assessed using visual inspection of funnel plots and quantitatively assessed using egger’s tests performed by STATA 10.0 software, where a p-value < 0.10 was considered evidence of small study effects.
Results
1. Characteristics of Included Studies
Seventeen clinical trials involving 1,105 participants (551 probiotics, 554 control) were included and these trials were parallel RCTs that were similar with regard to baseline characteristics, indicating successful randomization. Sixteen studies were double-blind design [12, 14–27]; and one was a single-blind design [28]. Seventeen trials reported data for fasting glucose (n = 1105), 11 for fasting insulin (n = 788), and 8 for HOMA-IR (n = 635). In twelve trials, dropout reasons and numbers were noted [12, 14, 16, 18–21, 25–28].
Table 1 displays the characteristics of the included trials. The average baseline fasting blood glucose (FBG) across the studies was 5.89 mmol/L in probiotic group and 5.83 mmol/L in the control group. In five trials [12, 16, 22, 25, 27], patients used antidiabetic medications but they did not change their medications during the study. One of the five studies was used antidiabetic medication (metformin) to treat non-alcoholic steatohepatitis patients, while others were included T2DM patients. The duration of the studies ranged from 3 to 24 weeks and nutrition intake was measured in 7 studies [12, 16, 20–22, 25, 28]; no differences in energy or nutrient intake between intervention and control groups were found. The remainder of the studies only reported that participants were advised to maintain their diet, except for 2 studies in which subjects were instructed to modify dietary intake in both groups [20, 26]. Probiotic species and dose used varied between studies. Eight studies used a single species of probiotics, whereas the others used a combination of equal or more than 2 species. All studies reported good compliance with no side effects from consuming probiotics, except 2 studies that reported subject flatulence, loose stools or constipation [18, 20].
Table 1. Characteristics of included studies.
| Study | Design, Location | Probiotic Source | Duration (weeks) | Participant, Age (No. of Intervention/No. of Control) | Baseline Characteristics | Probiotic | Dose, CFU | Antidiabetic Medication Use | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose (mmol/l) | BMI (kg/m 2 ) | Insulin (μU/ml) | HOMA-IR | ||||||||
| Asemi et al. (16) | DB,PC,P, Iran | C | 8 | T2DM, 35–70, (27/27) | 7.73 | 30.89 | 5.76 | 2.01 | L. acidophilus, L. rhamnosus, L. casei, L. bulgaricus, B. longum, S. thermophilus | 3.92×1010 | YES |
| Asemi et al. (28) | SB,PC,P, Iran | Y | 9 | Pregnant, 18–30, (37/33) | 5.21 | ND | 7.90 | 1.82 | S. thermophilus, L. bulgaricus, L. acidophilus, B. animalis | 1×107 | NO |
| Bukowska et al. (17) | DB,PC,P, Poland | Fermented oatmeal soups | 6 | HC, men, 40–45, (15/15) | 5.94 | 26.25 | ND | ND | L. plantarum | 1×1010 | NO |
| Ejtahed et al. (12) | DB,PC,P, Iran | Y | 6 | T2DM, 30–60, (30/30) | 7.71 | 29.05 | 6.89 | ND | L. acidophilus, B. lactis | 3.98×109 | YES |
| Ivey et al. (14)a | DB,PC,P, Australia | C, Y | 6 | OB, 56–77, (40/37) | 5.58 | 30.41 | 9.79 | 2.47 | L. acidophilus, B. animalis subsp lactis | 6×109 | NO |
| Ivey et al. (14)b | DB,PC,P, Australia | C | 6 | OB, 56–77, (40/39) | 5.47 | 30.80 | 9.88 | 2.44 | L. acidophilus, B. animalis subsp lactis | 3×109 | NO |
| Jones et al. (18) | DB,PC,P, Canada | C | 9 | HC, 20–75, (62/62) | 5.35 | 27.30 | ND | ND | L. reuteri | 5.8×109 | NO |
| Jung et al. (19) | DB,PC,P, Korea | C | 6 | OB, 19–60, (22/28) | 5.75 | 29.16 | 10.21 | ND | L. Gasseri | 6×1010 | NO |
| Laitinen et al. (20) | DB,PC,P, Finland | C | 20 | Pregnant, 25–35, (66/70) | 4.53 | ND | 5.67 | 1.17 | L. rhamnosus, B. lactis | 1×1010 | NO |
| Lindsay et al. (21) | DB,PC,P, Ireland | C | 4 | OB, pregnant, 31–36, (63/75) | 4.73 | 33.55 | 15.36 | 3.27 | L. salivarius | 1×109 | NO |
| Mohamadshahi al (22) | DB,PC,P, Iran | Y | 8 | OB, T2DM, 42–59, (20/20) | 10.07 | 28.79 | ND | ND | L. Bb12, L. acidophilus | 1.11×109 | YES |
| Naruszewicz et al. (15) | DB,PC,P, Sweden | D | 6 | Healthy smoker, 35–45, (18/18) | 5.89 | 25.3 | 9.7 | ND | L. plantarum | 2×1010 | NO |
| Rajkumar et al. (24) | DB,PC,P, India | C | 6 | OB, 40–60, (15/15) | 4.93 | 28.79 | 18.15 | 3.95 | L. acidophilus, L. paracasei, L. delbrueckii, L. plantarum, B. longum, B. infantis, B. breve | 1.13×1011 | NO |
| Rajkumar et al. (23) | DB,PC,P, Japan | C | 6 | Health, 20–25, (15/15) | 4.70 | 22.53 | 18.77 | 3.80 | L. salivarius | 4×109 | NO |
| Shavakhi et al. (27) | DB,PC,P, Iran | C | 24 | NASH, 18–75, (31/32) | 5.52 | 28.40 | ND | ND | L. acidophilus, L. rhamnosus L. casei, L. bulgaricus, L. rhamnosus, L. bulgaricus | 9.5×108 | YES |
| Shakeri et al. (25) | DB,PC,P, Iran | Bread | 8 | T2DM, 35–70, (26/26) | 8.27 | 30.05 | ND | ND | L. sporogenes | 1.30×1010 | YES |
| Sharafedtinov et al. (26) | DB,PC,P, Estonia | Cheese | 3 | Met.S, 30–69, (25/11) | 7.06 | 37.27 | ND | ND | L. plantarum | 7.5×1012 | NO |
C: capsule; Y: yogurt; D: drink; CFU, colony-forming unit; DB, double blind; HC, hypercholesterolemia; Met.S, metabolic syndrome; NASH, non-alcoholic steatohepatitis; OB, obesity; P, parallel; PC, placebo control; SB, single blind; T2DM, type 2 diabetes mellitus.
2. Fasting Blood Glucose
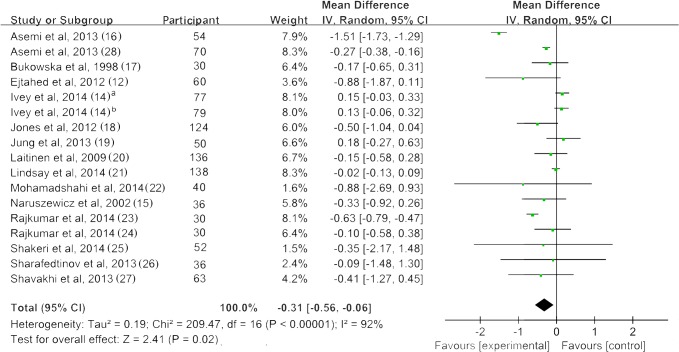
Fig 2 shows a forest plot of the pooled effect of probiotics on fasting blood glucose. All studies reported changes in fasting blood glucose (FBG). Of the seventeen trials, four studies reported a significant reduction of FBG after probiotic intervention, with mean differences ranging from -0.15 to -1.51 mmol/L [12, 16, 20, 24]. Our meta-analysis of 17 trials indicated a significant reduction of FBG of 0.31 mmol/L (95% CI: 0.56, 0.06; p = 0.02) compared with control groups. However, significant evidence of inter-study heterogeneity was observed across studies (I 2 = 92%, p < 0.01).
Fig 2. Forest plot of randomized controlled trials comparing the effect of probiotics on fasting blood glucose with placebo/comparator.
Weighted mean differences (95% CIs) for fasting blood glucose are shown. Pooled estimates (diamonds) calculated by the random effects method. IV, inverse variance.
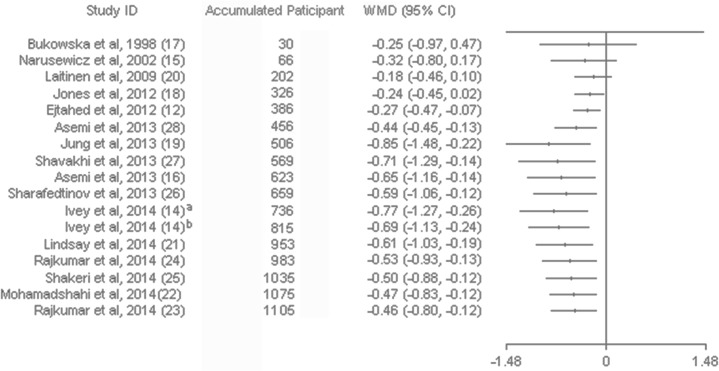
Sensitivity analysis of systematically removing individual trials showed that the removal of four trials with high heterogeneity [14, 16, 24] revealed significance in the overall effect as well (MD = -0.16 mmol/L, p < 0.01). Sensitivity analysis also showed that removing studies with small sample sizes (n < 20 for each group) [15, 17, 23, 24, 26] did not change the significance of the pooled effect (p = 0.04) (S1 Table). Subgroup analysis of studies with hyperglycemic patients revealed a significant reduction of FBG, and these results were not reported in normoglycemic patients. The effect of probiotic on glucose was significant only in the using antidiabetic medications subgroup; however, the non-taking antidiabetic medications subgroup had substantially higher heterogeneity. For trials that included multispecies probiotics revealed significant reduction of glucose while no effect was observed in the single species of probiotic. Trials of > 8 weeks showed a significant effect on glucose reduction. Although significant advantages for intervention ≤ 8 weeks were not observed, there was trend of glucose lowing effect for trials of ≤ 8 weeks (p = 0.06). There was no solid evidence for an association between treatment effect of probiotics and the daily dose, source of probiotics or pregnant status (Table 2). A cumulative meta-analysis for FBG indicated that the combined mean difference has tended to be stable since 2013, lingering around a small effect between -0.4 to -0.5. The trend indicated a positive effect of probiotics along time (Fig 3).
Table 2. Result of subgroup analysis of included randomized, controlled trials in meta-analysis.
| Groups | Fasting blood glucose (mmol/L) | Insulin (μU/ml) | HOMA-IR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | WMD (95%CI) | P | I 2 (%) | P heterogeneity | n | WMD (95%CI) | P | I 2 (%) | P heterogeneity | n | WMD (95%CI) | P | I 2 (%) | P heterogeneity | |
| Hyperglycemic | |||||||||||||||
| YES | 4 | -1.46 (-1.67, -1.25) | <0.01 | 11 | 0.34 | 2 | -2.01 (-2.46, -1.56) | <0.01 | 36 | 0.21 | 1 | -1.60 (-1.87, -1.33) | <0.01 | ||
| NO | 13 | -0.15 (-0.33, 0.02) | 0.09 | 82 | <0.01 | 9 | -1.20 (-2.36, -0.03) | 0.05 | 91 | <0.01 | 7 | -0.32 (-0.65, 0.00) | 0.05 | 91 | <0.01 |
| Use of antidiabetic medications | |||||||||||||||
| YES | 5 | -0.98 (-1.58, -0.37) | <0.01 | 54 | 0.07 | 2 | -2.01 (-2.46, -1.56) | <0.01 | 36 | 0.21 | 1 | -1.60 (-1.87, -1.33) | <0.01 | ||
| NO | 12 | -0.14 (-0.32, 0.04) | 0.12 | 82 | <0.01 | 9 | -1.20 (-2.36, -0.03) | 0.05 | 91 | <0.01 | 7 | -0.32 (-0.65, 0.00) | 0.05 | 91 | <0.01 |
| Pregnant participant | |||||||||||||||
| YES | 3 | -0.15 (-0.35, 0.05) | 0.16 | 81 | <0.01 | 3 | -1.47 (-4.31, 1.38) | 0.31 | 93 | <0.01 | 3 | -0.37 (-0.98, 0.24) | 0.23 | 93 | <0.01 |
| NO | 14 | -0.36 (-0.74, 0.01) | 0.06 | 93 | <0.01 | 8 | -1.15 (-1.78, -0.52) | <0.01 | 67 | <0.01 | 5 | -0.53 (-1.12, 0.06) | 0.08 | 95 | <0.01 |
| Species | |||||||||||||||
| Single species | 8 | -0.05 (-0.14, 0.05) | 0.36 | 0 | 0.64 | 4 | -0.83 (-2.94, 1.28) | 0.44 | 66 | 0.03 | 2 | -0.08 (-0.84, 0.69) | 0.85 | 82 | 0.02 |
| Multispecies | 9 | -0.44 (-0.83, -0.05) | 0.03 | 96 | <0.01 | 7 | -1.46 (-2.51, -0.41) | <0.01 | 93 | <0.01 | 6 | -0.60 (-0.98, -0.22) | <0.01 | 94 | <0.01 |
| Duration | |||||||||||||||
| > 8 weeks | 4 | -0.27 (-0.37, -0.17) | <0.01 | 0 | 0.78 | 2 | -2.90 (-4.88, -0.93) | <0.01 | 85 | 0.01 | 2 | -0.67 (-1.15, -0.20) | <0.01 | 89 | <0.01 |
| ≤ 8 weeks | 13 | -0.32 (-0.67, 0.02) | 0.06 | 94 | <0.01 | 9 | -0.92 (-1.61, -0.23) | <0.01 | 73 | <0.01 | 6 | -0.40 (-0.96, 0.17) | 0.17 | 95 | <0.01 |
| Daily dose | |||||||||||||||
| ≥ 1011 CFU | 2 | -0.62 (-0.78, -0.47) | <0.01 | 0 | 0.45 | 1 | -1.17 (-1.52, -0.82) | <0.01 | 1 | -0.77 (-0.94, -0.60) | <0.01 | ||||
| < 1011 CFU | 15 | -0.28 (-0.56, -0.01) | 0.04 | 92 | <0.01 | 10 | -1.28(-2.35, -0.21) | 0.02 | 88 | <0.01 | 7 | -0.42 (-0.89, 0.04) | 0.07 | 94 | <0.01 |
| Source of probiotic | |||||||||||||||
| Capsule | 9 | -0.34 (-0.74, 0.06) | 0.10 | 96 | <0.01 | 7 | -1.18 (-1.81, -0.54) | <0.01 | 70 | <0.01 | 6 | -0.51 (-0.99, -0.03) | 0.04 | 93 | <0.01 |
| Others | 8 | -0.18 (-0.43, 0.07) | 0.16 | 61 | 0.01 | 4 | -1.84 (-4.54, 0.86) | 0.18 | 92 | <0.01 | 2 | -0.35 (-1.46, 0.77) | 0.54 | 96 | <0.01 |
| Total | 17 | -0.31 (-0.56, -0.05) | 0.02 | 92 | <0.01 | 11 | -1.29(-2.17, -0.41) | <0.01 | 90 | <0.01 | 8 | -0.48 (-0.83, -0.13) | <0.01 | 93 | <0.01 |
Data were meta-analyzed by using a random-effects model or fixed-effects model as appropriate and are presented as WMD. Statistical heterogeneity was assessed by using the chi-square test and quantified by using the I2 statistic. WMD: Weight mean difference.
Fig 3. Cumulative Meta-analysis of the probiotics for fasting blood glucose.
Error bars indicate the 95% CI of the cumulative meta-analysis estimates as randomized patients accumulate through time. WMD, weight mean difference.
3. Fasting Plasma Insulin
Fig 4 shows a forest plot of the pooled effect of probiotics on fasting plasma insulin. Eleven studies reported the changes in insulin, with 3 studies reporting a significant reduction of insulin after probiotic use [23, 24, 28]. The mean difference ranged from -0.36 to -3.8 μU/mL. The pooled mean difference was -1.29 μU/mL (95% CI -2.17, -0.41; p = 0.004) for insulin. Nevertheless, significant inter-study heterogeneity was observed in the overall analysis (I 2 = 90%, p < 0.01).
Fig 4. Forest plot of randomized controlled trials comparing the effect of probiotics on fasting plasma insulin with placebo/comparator.
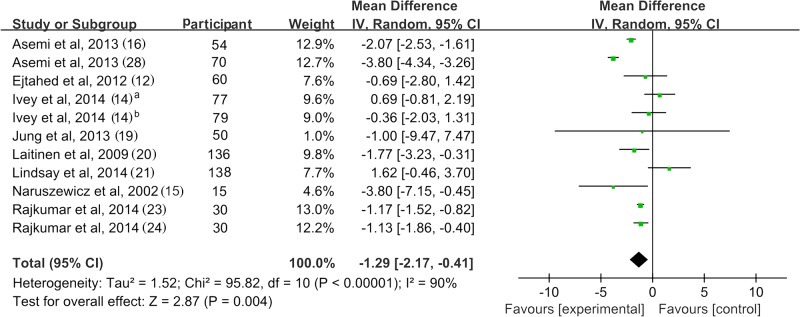
Weighted mean differences (95% CIs) for fasting plasma insulin are shown. Pooled estimates (diamonds) calculated by the random effects method. IV, inverse variance.
Sensitivity analysis drew the same conclusion after removing the trials with high heterogeneity [14,16,21,28]. However, removing the trials of small sample size [15,23,24] led to a loss of significance in the overall effect (MD = -1.09 μU/mL; p = 0.09) (S1 Table). Subgroup analysis showed that hyperglycemic patients received better effect of insulin reduction than the normoglycemic patients. However, there were only 2 studies included in the hyperglycemic subgroup may result in unreliable conclusions. Subgroup analysis of trials with multispecies probiotics found a significant reduction of insulin. Those trials using a single species of probiotic did not show significant effect compared with control groups. Using capsule as the source of probiotic resulted in significant reduction in insulin; similar results were not found for other sources of probiotics. There was no solid evidence for an association between insulin lowing effect of probiotics and the daily dose or duration due to small number of study and high inter-study heterogeneity (Table 2).
4. Homeostasis model assessment of insulin resistance
Fig 5 shows a forest plot of the pooled effect of probiotics on HOAM-IR. Eight of 17 studies reported changes in HOMA-IR, with 4 studies reporting a significant reduction of HOMA-IR after consuming probiotics[16, 23, 24, 28]. The mean difference ranged from -0.41 to -1.60. The pooled mean difference was -0.48 (95% CI -0.83, -0.13; p = 0.007) for HOMA-IR. Significant evidence of inter-study heterogeneity was observed across studies (I 2 = 93%, p < 0.01).
Fig 5. Forest plot of randomized controlled trials comparing the effect of probiotics on HOMA-IR with placebo/comparator.
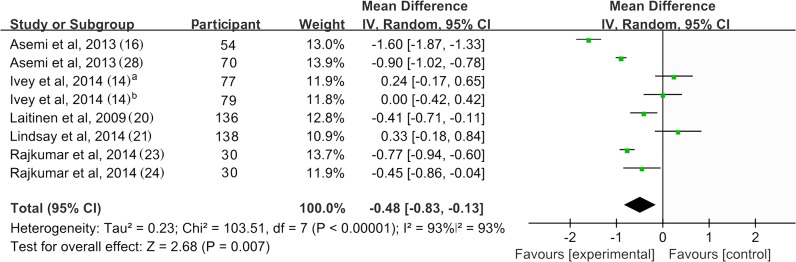
Weighted mean differences (95% CIs) for HOMA-IR are shown. Pooled estimates (diamonds) calculated by the random effects method. IV, inverse variance.
Sensitivity analysis was performed to evaluate the reliability of the pooled mean difference. Results remained consistent after removing the trials with high heterogeneity [14,16,21,23,28]. However, removing the trials of small sample size [23,24] led to a loss of significance in the overall effect (MD = -0.42; p = 0.12) (S1 Table). Subgroup analysis of trials with multispecies probiotics found a significant reduction of HOMA-IR. Those trials using a single species of probiotic did not show significant effect compared with control groups. Using capsule as the source of probiotic resulted in significant reduction in HOAM-IR; similar results were not found for other sources of probiotics. There was no solid evidence for an association between HOMA-IR lowing effect of probiotics and the hyperglycemic status, pregnant status, daily dose or duration due to small number of study and high inter-study heterogeneity (Table 2).
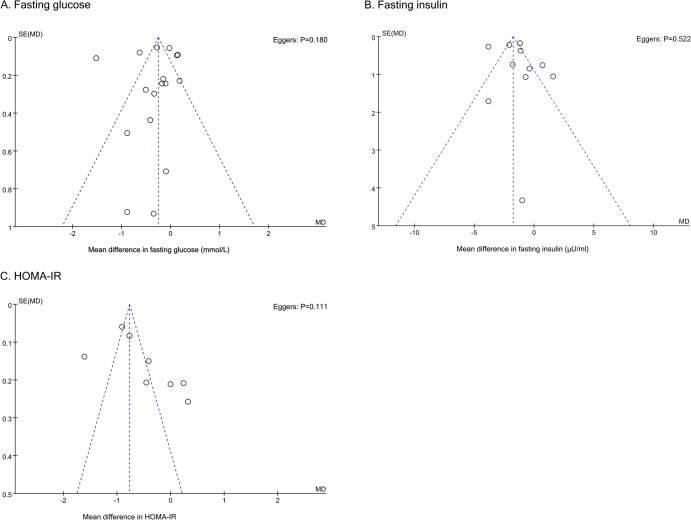
5. Publication bias
Funnel plot and the Egger’s regression were performed to detect potential publication bias. As shown in the Fig 6, funnel plots for fasting glucose and insulin were approximately symmetric while an asymmetry was detected for HOMA-IR. Egger’s regression indicated no significant publication bias for all three indices with p-value equal to 0.180, 0.522 and 0.111.
Fig 6. Publication bias funnel plots.
Publication bias funnel plots for fasting glucose (A), fasting insulin (B) and HOMA-IR (C). The solid line represents the pooled effect estimate expressed as the weighted mean difference for each analysis. The dashed lines represent pseudo-95% confidence limits. P-values displayed in the top right corner of each funnel plot are derived from quantitative assessment of publication bias by Egger’s test.
Discussion
This is the first study to systematically analyze the effect of probiotics on glycemic control. Overall, probiotics significantly reduced FBG by 0.31 mmol/L, insulin by 1.17 μU/mL and improved HOMA-IR by 0.48, indicating a modest effect of probiotics on glycemic control; however, even small glucose reductions may provide health benefits. Abnormal glucose metabolism carries crucial risks for many metabolic diseases, such as obesity, diabetes, obstructive sleep apnea-hypopnea syndrome (OSAHS), and cardiovascular disease.
The hypothesis that probiotics may be involved in maintenance of healthy gut microbiota and glucose management has received much attention. The ratio of bacteroidetes species in T2DM correlates positively with plasma glucose [29] and alterations in gut microbiota have recently been reported in patients with T2DM, and this may be reversible with probiotic supplement [10]. Dietary supplementation of probiotics for high fructose- and streptozotocin-induced diabetes in rats improved glucose and lipid metabolism, suppressed glucose intolerance and delayed the onset of hyperglycemia, hyperinsulinemia, dyslipidemia, and oxidative stress [30, 31]. Yun’s group [32] found that FBG and 2-hour blood glucose were significantly lower after probiotic ingestion for 3 weeks in db/db mice. In our study, we observed that probiotics had a greater effect on FBG in people with diabetes and there were only trends of glucose-lowing effect in those without diabetes, supporting the notion that probiotics supplementation may generate a greater benefit in individuals with higher FBG levels. We further assessed potential associations of the treatment effect with antidiabetic agents in the subgroup analysis. Four of five trials are included hyperglycemic participants and one included NASH patients. Interestingly, the significant effect on glucose was only observed in those with antidiabetic medications subgroup, which means that it may exist confounding effects on glucose lowing between probiotics and antidiabetic medications. Another explanation may relate to a well understanding phenomenon that the higher baseline glucose levels, the greater reduction with anti-hyperglycemic agents.
There was evidence of substantial inter-study heterogeneity in the overall effect for FBG, insulin and HOMA-IR. Subgroup analysis indicated that diabetes status partially explained the heterogeneity in the overall analysis. Moreover, not all studies reported beneficial effects of probiotics, and thus caution is needed regarding the species, sources and dose to be used, which may have important ramifications on the effects observed and help to explain the heterogeneity across the studies. Subgroup analysis of studies using multispecies of probiotic indicated a more pronounced reduction in FBG, fasting plasma insulin and HOMA-IR; however, it seems to be no effect of probiotics on these endpoints for those trials using a single species of probiotic. The findings of present meta-analysis are in line with the previous studies, both suggesting a combination of probiotic species are more effective than single species products [33]. Although these findings may provide important information for future interventions using probiotics, caution is required because high heterogeneity was observed in the multispecies subgroup. Unfortunately, the lack of trials on species and strains of probiotics made it not practical to analyze the effect of different probiotic species on glycemic control. Secondary, administration of probiotic sources varied among trials with most trials using encapsulated probiotic supplements. Subgroup analysis of studies using the probiotic capsule didn’t reveal significant reductions in FBG compared with other sources. However, an inadequate number of studies that used other sources of probiotics (yogurt, rose-hip drinks, probiotic cheese, etc.) limit these conclusions for the best source of probiotics. In addition, there seems to be no trend between the daily dose of probiotics consumed and change in FBG or insulin or HOMA-IR. However, finding from the subgroup analysis indicate that the reduction in FBG may be greater when the daily dose of probiotics consumption ≥ 1011 CFU. This finding may be because of the bias of the low number of trials in the high daily dose subgroup.
Another important observation we made was that the improvements in FBG and HOMA-IR were restricted to trials > 8 weeks, while better insulin reduction was obtained in trials > 8 weeks compared to trials ≤ 8 weeks. However, further studies with longer treatment durations are required to confirm this result because the group with duration > 8 weeks was small with only 2 to 4 studies. Thus, there was no solid evidence for an association between glycemic control of probiotics and the treatment durations. Finally, pregnant women are susceptible to increased insulin resistance and glucose, so a subgroup analysis was conducted to pregnant women. In human clinical trials, supplementation of probiotics combined with dietary counseling has been shown to positively affect glucose metabolism in normoglycemic pregnant women [20]. However, the subgroup analysis of probiotics on FBG, fasting insulin and HOMA-IR was not significant among pregnant women, which might be explained by the inter-individual differences of pregnancy. Furthermore, probiotic strain differences, dose, and treatment duration across different studies might explain differences in outcomes.
How probiotics lower glucose is unclear. They may be related to decreased oxidative stress [12], which is shown to be present in hyperglycemia [34]. Specific strains of lactic acid bacteria have antioxidant properties [35, 36]. For example, Yadav and colleagues [30] reported that probiotic dahi, a fermented milk containing Lactobacillus acidophilus and L. casei delayed the progression of glucose intolerance, hyperglycemia, hyperinsulinemia via decreased oxidative stress in animal models. Also, low-grade chronic inflammation is observed in diabetic and obese individuals and the immune system is crucial for regulation of glucose metabolism. Thus, probiotics may modulate immune responses and systemic low-grade inflammation, in particular by reducing cytokines [37] and suppressing the NF-κB pathway, which mediates immune system microbial activation via toll-like receptors [38]. Laitinen’s group [20] observed pronounced effects of probiotics on reduced glucose and attributed this to immunoregulatory properties. Five of the included studies suggest that the consumption of probiotics decreased inflammatory markers, including hsCRP, IL-6, and TNF-α [15, 16, 22–24]. Also, other studies indicate that systemic inflammation was reduced and intestinal endotoxin (a potential inflammatory stimulant) was decreased with probiotics, lowering insulin resistance and hyperglycemic incidences [39]. Probiotics may attenuate circulating endotoxin, subsequently affecting glucose metabolism [40, 41]
Our work has several limitations. First, we could not obtain data from unpublished literature or non-English published material, which may lead to potential publication bias. However, Egger’s regression showed no significant publication bias, indicating that the unpublished evidence didn’t affect the results of the meta-analysis. Second, some studies had fewer than 20 participants for each experimental group. Forest plots show possible bias, favoring small trials with extreme effects. However, these trials had small weights in our meta-analysis and excluding them only slightly modified probiotic-induced effects on glucose. Third, there was evidence of substantial inter-study heterogeneity in this meta-analysis, which was not explained by most of the priori subgroup analyses. In addition, majority of subgroup analyses were unpowered and it was not possible to assess the effect of other factors that may influence glycemic control due to small number of study.
Thus, our meta-analysis revealed a moderate beneficial effect of probiotics on glycemic control along with lower insulin and HOMA-IR, data that are consistent with a recent meta-analysis suggesting that yogurt intake was associated with an 18% lower risk of T2DM [42]. Modification of gut microbiota by probiotic supplementation may be a method for preventing and control hyperglycemia in clinical practice.
Supporting Information
(PDF)
(DOCX)
(PDF)
(DOCX)
(PDF)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Natural Science Foundation of China, (81300689, 81403215). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Moosheer SM, Waldschutz W, Itariu BK, Brath H, Stulnig TM. A protein-enriched low glycemic index diet with omega-3 polyunsaturated fatty acid supplementation exerts beneficial effects on metabolic control in type 2 diabetes. Primary care diabetes. 2014. Epub 2014/03/25. 10.1016/j.pcd.2014.02.004 . [DOI] [PubMed]
- 2. Gao D, Ning N, Wang C, Wang Y, Li Q, Meng Z, et al. Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response meta-analysis. PloS one. 2013;8(9):e73965 Epub 2013/10/03. 10.1371/journal.pone.0073965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernandez-Alonso P, Salas-Salvado J, Baldrich-Mora M, Juanola-Falgarona M, Bullo M. Beneficial Effect of Pistachio Consumption on Glucose Metabolism, Insulin Resistance, Inflammation, and Related Metabolic Risk Markers: a Randomized Clinical Trial. Diabetes care. 2014. Epub 2014/08/16. 10.2337/dc14-1431 . [DOI] [PubMed] [Google Scholar]
- 4. Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes care. 2014;37(2):569–86. Epub 2014/01/25. 10.2337/dc13-1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, et al. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS immunology and medical microbiology. 2003;35(2):131–4. Epub 2003/03/12. . [DOI] [PubMed] [Google Scholar]
- 6. Moro-Garcia MA, Alonso-Arias R, Baltadjieva M, Fernandez Benitez C, Fernandez Barrial MA, Diaz Ruisanchez E, et al. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age (Dordrecht, Netherlands). 2013;35(4):1311–26. Epub 2012/05/31. 10.1007/s11357-012-9434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64(4):897–903. Epub 2014/07/23. 10.1161/hypertensionaha.114.03469 . [DOI] [PubMed] [Google Scholar]
- 8. Guo Z, Liu XM, Zhang QX, Shen Z, Tian FW, Zhang H, et al. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trials. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2011;21(11):844–50. Epub 2011/09/21. 10.1016/j.numecd.2011.04.008 . [DOI] [PubMed] [Google Scholar]
- 9. Tabuchi M, Ozaki M, Tamura A, Yamada N, Ishida T, Hosoda M, et al. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Bioscience, biotechnology, and biochemistry. 2003;67(6):1421–4. Epub 2003/07/05. 10.1271/bbb.67.1421 . [DOI] [PubMed] [Google Scholar]
- 10. Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS one. 2010;5(2):e9085 Epub 2010/02/09. 10.1371/journal.pone.0009085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moroti C, Souza Magri LF, de Rezende Costa M, Cavallini DC, Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids in health and disease. 2012;11:29 Epub 2012/02/24. 10.1186/1476-511x-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition (Burbank, Los Angeles County, Calif). 2012;28(5):539–43. Epub 2011/12/02. 10.1016/j.nut.2011.08.013 . [DOI] [PubMed] [Google Scholar]
- 13. Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Moller K, Svendsen KD, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. The British journal of nutrition. 2010;104(12):1831–8. Epub 2010/09/08. 10.1017/s0007114510002874 . [DOI] [PubMed] [Google Scholar]
- 14. Ivey KL, Hodgson JM, Kerr DA, Lewis JR, Thompson PL, Prince RL. The effects of probiotic bacteria on glycaemic control in overweight men and women: a randomised controlled trial. European journal of clinical nutrition. 2014;68(4):447–52. Epub 2014/02/27. 10.1038/ejcn.2013.294 . [DOI] [PubMed] [Google Scholar]
- 15. Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. The American journal of clinical nutrition. 2002;76(6):1249–55. Epub 2002/11/27. . [DOI] [PubMed] [Google Scholar]
- 16. Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Annals of nutrition & metabolism. 2013;63(1–2):1–9. Epub 2013/08/01. 10.1159/000349922 . [DOI] [PubMed] [Google Scholar]
- 17. Bukowska H, Pieczul-Mroz J, Jastrzebska M, Chelstowski K, Naruszewicz M. Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis. 1998;137(2):437–8. Epub 1998/06/11. . [DOI] [PubMed] [Google Scholar]
- 18. Jones ML, Martoni CJ, Di Pietro E, Simon RR, Prakash S. Evaluation of clinical safety and tolerance of a Lactobacillus reuteri NCIMB 30242 supplement capsule: a randomized control trial. Regulatory toxicology and pharmacology: RTP. 2012;63(2):313–20. Epub 2012/05/09. 10.1016/j.yrtph.2012.04.003 . [DOI] [PubMed] [Google Scholar]
- 19. Jung SP, Lee KM, Kang JH, Yun SI, Park HO, Moon Y, et al. Effect of Lactobacillus gasseri BNR17 on Overweight and Obese Adults: A Randomized, Double-Blind Clinical Trial. Korean journal of family medicine. 2013;34(2):80–9. Epub 2013/04/06. 10.4082/kjfm.2013.34.2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laitinen K, Poussa T, Isolauri E. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. The British journal of nutrition. 2009;101(11):1679–87. Epub 2008/11/20. 10.1017/s0007114508111461 . [DOI] [PubMed] [Google Scholar]
- 21. Lindsay KL, Kennelly M, Culliton M, Smith T, Maguire OC, Shanahan F, et al. Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). The American journal of clinical nutrition. 2014;99(6):1432–9. Epub 2014/03/22. 10.3945/ajcn.113.079723 . [DOI] [PubMed] [Google Scholar]
- 22. Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. BioImpacts: BI. 2014;4(2):83–8. Epub 2014/07/19. 10.5681/bi.2014.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajkumar H, Kumar M, Das N, Kumar SN, Challa HR, Nagpal R. Effect of Probiotic Lactobacillus salivarius UBL S22 and Prebiotic Fructo-oligosaccharide on Serum Lipids, Inflammatory Markers, Insulin Sensitivity, and Gut Bacteria in Healthy Young Volunteers: A Randomized Controlled Single-Blind Pilot Study. Journal of cardiovascular pharmacology and therapeutics. 2014. Epub 2014/10/22. 10.1177/1074248414555004 . [DOI] [PubMed]
- 24. Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, Myakala SP. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators of inflammation. 2014;2014:348959 Epub 2014/05/06. 10.1155/2014/348959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shakeri H, Hadaegh H, Abedi F, Tajabadi-Ebrahimi M, Mazroii N, Ghandi Y, et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49(7):695–701. Epub 2014/04/08. 10.1007/s11745-014-3901-z . [DOI] [PubMed] [Google Scholar]
- 26. Sharafedtinov KK, Plotnikova OA, Alexeeva RI, Sentsova TB, Songisepp E, Stsepetova J, et al. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients—a randomized double-blind placebo-controlled pilot study. Nutrition journal. 2013;12:138 Epub 2013/10/15. 10.1186/1475-2891-12-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shavakhi A, Minakari M, Firouzian H, Assali R, Hekmatdoost A, Ferns G. Effect of a Probiotic and Metformin on Liver Aminotransferases in Non-alcoholic Steatohepatitis: A Double Blind Randomized Clinical Trial. International journal of preventive medicine. 2013;4(5):531–7. Epub 2013/08/10. [PMC free article] [PubMed] [Google Scholar]
- 28. Asemi Z, Samimi M, Tabassi Z, Naghibi Rad M, Rahimi Foroushani A, Khorammian H, et al. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. European journal of clinical nutrition. 2013;67(1):71–4. Epub 2012/11/29. 10.1038/ejcn.2012.189 . [DOI] [PubMed] [Google Scholar]
- 29.Gomes AC, Bueno AA, de Souza RG, Mota JF. Gut microbiota, probiotics and diabetes. Nutrition journal. 2014;13:60. Epub 2014/06/19. 10.1186/1475-2891-13-60 [DOI] [PMC free article] [PubMed]
- 30. Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition (Burbank, Los Angeles County, Calif). 2007;23(1):62–8. Epub 2006/11/07. 10.1016/j.nut.2006.09.002 . [DOI] [PubMed] [Google Scholar]
- 31. Yadav H, Jain S, Sinha PR. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. The Journal of dairy research. 2008;75(2):189–95. Epub 2008/05/14. 10.1017/s0022029908003129 . [DOI] [PubMed] [Google Scholar]
- 32. Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. Journal of applied microbiology. 2009;107(5):1681–6. Epub 2009/05/22. 10.1111/j.1365-2672.2009.04350.x . [DOI] [PubMed] [Google Scholar]
- 33. Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? European journal of nutrition. 2011;50(1):1–17. Epub 2011/01/14. 10.1007/s00394-010-0166-z . [DOI] [PubMed] [Google Scholar]
- 34. Ferreira L, Teixeira-de-Lemos E, Pinto F, Parada B, Mega C, Vala H, et al. Effects of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in an animal model of type 2 diabetes (ZDF rat). Mediators of inflammation. 2010;2010:592760 Epub 2010/07/24. 10.1155/2010/592760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amaretti A, di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Applied microbiology and biotechnology. 2013;97(2):809–17. Epub 2012/07/14. 10.1007/s00253-012-4241-7 . [DOI] [PubMed] [Google Scholar]
- 36. Uskova MA, Kravchenko LV. Antioxidant properties of lactic acid bacteria—probiotic and yogurt strains. Voprosy pitaniia. 2009;78(2):18–23. Epub 2009/06/12. . [PubMed] [Google Scholar]
- 37. de Moreno de Leblanc A, Perdigon G. The application of probiotic fermented milks in cancer and intestinal inflammation. The Proceedings of the Nutrition Society. 2010;69(3):421–8. Epub 2010/06/17. 10.1017/s002966511000159x . [DOI] [PubMed] [Google Scholar]
- 38. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of clinical investigation. 2006;116(11):3015–25. Epub 2006/10/21. 10.1172/jci28898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathologie-biologie. 2008;56(5):305–9. Epub 2008/01/08. 10.1016/j.patbio.2007.09.008 . [DOI] [PubMed] [Google Scholar]
- 40. Burcelin R, Luche E, Serino M, Amar J. The gut microbiota ecology: a new opportunity for the treatment of metabolic diseases? Frontiers in bioscience (Landmark edition). 2009;14:5107–17. Epub 2009/06/02. . [DOI] [PubMed] [Google Scholar]
- 41. Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes care. 2010;33(10):2277–84. Epub 2010/09/30. 10.2337/dc10-0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, et al. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC medicine. 2014;12(1):215 Epub 2014/11/26. 10.1186/s12916-014-0215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(PDF)
(DOCX)
(PDF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.