Abstract
Harbors and marinas are well known gateways for species introductions in marine environments but little work has been done to ascertain relationships between species diversity, harbor type, and geographic distance to uncover patterns of secondary spread. Here, we sampled ascidians from 32 harbors along ca. 300 km of the NW Mediterranean coast and investigated patterns of distribution and spread related to harbor type (marina, fishing, commercial) and geographic location using multivariate techniques. In total, 28 ascidians were identified at the species level and another 9 at the genus level based on morphology and genetic barcoding. Eight species were assigned to introduced forms, 15 were given native status and 5 were classified as cryptogenic. Aplidium accarense was reported for the first time in the Mediterranean Sea and was especially abundant in 23 of the harbors. Introduced and cryptogenic species were abundant in most of the surveyed harbors, while native forms were rare and restricted to a few harbors. Significant differences in the distribution of ascidians according to harbor type and latitudinal position were observed. These differences were due to the distribution of introduced species. We obtained a significant correlation between geographic distance and ascidian composition, indicating that closely located harbors shared more ascidian species among them. This study showed that harbors act as dispersal strongholds for introduced species, with native species only appearing sporadically, and that harbor type and geographic location should also be considered when developing management plans to constrain the spread of non-indigenous species in highly urbanized coastlines.
Electronic supplementary material
The online version of this article (doi:10.1007/s10530-014-0821-z) contains supplementary material, which is available to authorized users.
Keywords: Tunicates, Introduced species, Barcoding, Artificial substrates, Distribution, Diversity
Introduction
Maritime activity has been spreading non-native species around the globe since early attempts to voyage by sea (Hewitt et al. 2009). However, recent increases in the number of artificial substrates available to non-indigenous species have greatly accelerated the introduction process (Glasby and Connell 1999). Once a species is well established in a new area, local fishing and recreational boating potentially facilitate further range expansion (Wasson et al. 2001; Darbyson et al. 2009; Davidscon et al. 2010). Thus, harbors and marinas play crucial roles in the introduction of marine species, including the initial inoculation of a species from another area and the subsequent spread at a local level (also called pre-border and post-border processes; Forrest et al. 2009). To date, most studies have focused on cataloguing the exotic species observed in a given location or harbor (e.g. Arenas et al. 2006; Callahan et al. 2010; Carman et al. 2010; Sephton et al. 2011; Pyo et al. 2012); while a surprisingly low number of studies have explored the links between these harbors, including patterns of species turnover (beta-diversity), harbor type (recreational, fishing, commercial or mixtures thereof), or temporal or geographic trends (e.g. Lambert and Lambert 2003; Cohen et al. 2005; Grey 2009a).
The Mediterranean is the largest enclosed sea on Earth and is connected to most parts of the world by substantial maritime traffic (Kaluza et al. 2010; Keller et al. 2011), although vessels from the North Atlantic represent over 55 % of all entries (CIESM 2002). The shipping industry is largely responsible for the introduction of alien species from distant areas into the Mediterranean Sea and is one of the major vectors of spread, second only to corridors such as the Suez Canal (Zenetos et al. 2012). In addition, the highly urbanized Mediterranean Sea supports a dense network of harbors and marinas, especially along the northwestern coast (Airoldi and Beck 2007). Thus, the Mediterranean Sea is a well-suited location to test the importance of harbors as entrance gates to exotic species, while the densely packed northwestern coast and its high number of harbors and marinas allow testing relationships between species diversity, harbor type, and geographic distance to uncover patterns of secondary spread. Moreover, the enclosed nature of Mediterranean harbors allows for immediate quarantine and confined attempts of eradication should a known invader arrive. In this sense, knowledge of the processes of secondary spread can be used to define internal borders (Forrest et al. 2009), and direct contingency responses to maximize efficiency.
Ascidians or sea-squirts (Chordata, Tunicata) are sessile invertebrates ideally suited for the study of introduction processes as related to harbor dynamics. Firstly, ascidians are especially abundant on artificial substrates and are among the taxa with the highest recorded number of introduced species (Lambert and Lambert 1998, 2003; Paulay et al. 2002; Callahan et al. 2010; Aldred and Clare 2014). Secondly, ascidian larvae are short-lived and usually settle within a few hours or days (Svane and Young 1989; Ayre et al. 1997; Rius et al. 2010a, b) so these animals mostly rely on human transport for their long-distance dispersal. Furthermore, recurrent introductions are common in ascidians, increasing propagule pressure and, therefore, the probability of success of an introduction (Dupont et al. 2010; Goldstein et al. 2011; Pineda et al. 2011; Rius et al. 2012).
Successfully introduced ascidians have a series of biological characteristics that enable them to quickly become established in a new habitat, including the ability to outcompete resident species (Rius et al. 2009b) and high growth and reproductive outputs (Rius et al. 2009a; Morris and Carman 2012; Pineda et al. 2013). The long-term establishment of a non-indigenous ascidian also depends on both the physical (e.g., temperature, salinity) and biological (resident biota) conditions characterizing the new habitat (Brunetti et al. 1980; Vázquez and Young 2000; Whitlatch and Osman 2009; Bullard and Whitlatch 2009; Pineda et al. 2012a, b). To date there are few instances of introduced ascidians becoming invasive and spreading to natural habitats (Castilla et al. 2004; Turon et al. 2007; Rius et al. 2009b; Lambert 2009; Morris et al. 2009; Morris and Carman 2012; Stefaniak et al. 2009, 2012), but plenty of ascidians have established themselves on artificial substrates as fouling organisms, increasing management costs and impairing the normal development of commercial species in aquaculture facilities (reviewed in Aldred and Clare 2014).
The main aim of this study was to uncover patterns of secondary (post-border) spread of introduced benthic species in highly urbanized areas since some harbor types are known to be reservoirs for further spread while others act as sinks for migrants (Dupont et al. 2009). To achieve this goal, we performed a thorough inventory of the ascidian fauna in 32 Mediterranean harbors spanning the highly urbanized Catalan shores (NE Iberian Peninsula). These data were used to (1) characterize the presence and abundance of introduced species (2) analyze the role of harbors in the spread of introduced species by assessing patterns of diversity as a function of harbor type and geographic distance, and (3) establish a baseline for future studies.
Materials and methods
Sample collection
Thirty-two harbors along ca. 300 km of the Catalan coast (NE Iberian Peninsula) were surveyed between November 2012 and April 2013 (Fig. 1) and classified in three categories according to the type of activities observed (Table 1): (1) recreational marina, (2) marina and fishing, and (3) marina, fishing and commercial (vessels from local businesses; e.g. diving boats, tourist boats). Both fishing and commercial vessels in the investigated harbors operate daily and do not normally navigate overnight or internationally. The surveyed harbors provide a broad representation of small- to medium-sized harbors along the Western Mediterranean coast, ranging from 118 m (linear length) of concrete docks to 3,271 m (data obtained either from the harbor’s website or measured from aerial photographs using the software ImageJ; Table 1). The two largest commercial ports in Catalonia are located in the cities of Barcelona and Tarragona, and to date they are the only ones housing big cruise vessels, cargo ships, oil tankers and other vessels traveling internationally for trade. Unfortunately, these two ports could not be surveyed due to logistic reasons but their absence should not prevent us from observing patterns of secondary spread, since these are more likely to be dictated by the intense local traffic between medium and small harbors.
Fig. 1.
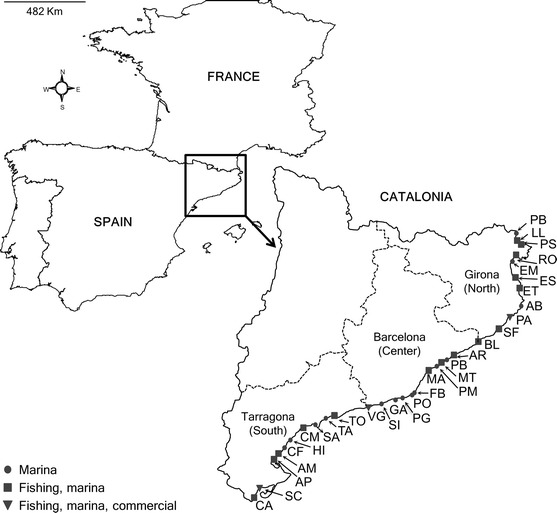
Map of the study area indicating the harbors surveyed (codes as in Table 1)
Table 1.
List of the 32 harbors surveyed in this study, with name, code, type (1: marina, 2: marina and fishing, 3: marina, fishing and commercial), sampling date, geographic region (or province, north: Girona; central: Barcelona; south: Tarragona), GPS position, total linear length (meters) of all docks per harbor, and number of species observed at each harbor
| Harbor name | Code | Type | Sampling date | Location | GPS position | Dock length | No. of species |
|---|---|---|---|---|---|---|---|
| St. Carles de la Ràpita | SC | 3 | November 9, 2012 | South | 40°34.50′N; 0°33.20′E | 2,286 | 10 |
| Port Balís | PL | 1 | December 31, 2012 | Center | 41°33.50′N; 2°30.50′E | 2,327 | 8 |
| Arenys de Mar | AR | 2 | January 19, 2013 | Center | 41°34.30′N; 2°33.30′E | 2,314 | 9 |
| Aiguablava | AB | 1 | January 29, 2013 | North | 41°56.00′N; 3°13.00′E | 118 | 4 |
| Estartit | ET | 2 | January 29, 2013 | North | 42°03.10′N; 3°12.40′E | 1,100 | 7 |
| L’Escala | ES | 2 | January 30, 2013 | North | 42°07.00′N; 3°08.60′E | 2,923 | 16 |
| Roses | RO | 2 | January 31, 2013 | North | 42°15.20′N; 3°10.60′E | 2,780 | 11 |
| Empuriabrava | EM | 1 | January 31, 2013 | North | 42°14.60′N; 3°08.10′E | 557 | 5 |
| Port de la Selva | PS | 2 | February 1, 2013 | North | 42°20.20′N; 3°11.90′E | 1,041 | 11 |
| Portbou | PB | 1 | February 2, 2013 | North | 42°25.70′N; 3°10.00′E | 573 | 6 |
| Blanes | BL | 2 | March 21, 2013 | North | 41°40.30′N; 2°47.80′E | 1,486 | 8 |
| Fòrum Barcelona | FB | 1 | March 22, 2013 | Center | 41°24.91′N; 2°13.72′E | 1,485 | 9 |
| Garraf | GA | 1 | March 1, 2013 | Center | 41°14.97′N; 1°54.04′E | 956 | 6 |
| Llançà | LL | 2 | February 1, 2013 | North | 42°22.00′N; 3°09.00′E | 1,648 | 7 |
| Masnou | MA | 2 | February 26, 2013 | Center | 41°28.50′N; 2°18.60′E | 2,679 | 9 |
| Mataró | MT | 2 | February 16, 2013 | Center | 41°32.00′N; 2°26.00′E | 1,796 | 9 |
| Port Ginesta | PG | 1 | March 12, 2013 | Center | 41°15.50′N; 1°55.50′E | 3,271 | 7 |
| Port Olímpic | PO | 1 | March 14, 2013 | Center | 41°23.12′N; 2°12.60′E | 1,864 | 9 |
| Premià de Mar | PM | 1 | March 25, 2013 | Center | 41°29.00′N; 2°21.00′E | 1,132 | 10 |
| Salou | SA | 1 | March 27, 2013 | South | 41°04.40′N; 1°07.80′E | 502 | 8 |
| Sant Feliu de Guíxols | SF | 2 | March 17, 2013 | North | 41°46.30′N; 3°01.54′E | 1,202 | 7 |
| Sitges (Aiguadolç) | SI | 1 | March 2, 2013 | Center | 41°13.90′N; 1°49.40′E | 1,879 | 6 |
| Port Nàutic Tarragona | TA | 1 | March 27, 2013 | South | 41°06.20′N; 1°15.80′E | 955 | 9 |
| Torredembarra | TO | 2 | January 28, 2013 | South | 41°08.03′N; 1°24.15′E | 1,363 | 6 |
| Vilanova i la Geltrú | VG | 3 | January 28, 2013 | South | 41°12.30′N; 1°43.70′E | 3,012 | 6 |
| Cambrils | CM | 2 | March 28, 2013 | South | 41°03.70′N; 1°03.80′E | 1,482 | 9 |
| Hospitalet de l’Infant | HI | 1 | March 28, 2013 | South | 40°59.23′N; 0°55.40′E | 1,081 | 11 |
| Calafat | CF | 1 | March 29, 2013 | South | 40°55.90′N; 0°51.20′E | 754 | 5 |
| Ametlla de Mar | AM | 2 | March 29, 2013 | South | 40°52.00′N; 0°47.00′E | 1,477 | 8 |
| Ampolla | AP | 2 | March 29, 2013 | South | 40°48.00′N; 0°43.00′E | 1,809 | 10 |
| Cases d’Alcanar | CA | 2 | March 30, 2013 | South | 40°33.20′N; 0°32.00′E | 992 | 9 |
| Palamós | PA | 3 | April 4, 2013 | North | 41°50.50′N; 3°07.10′E | 1,549 | 7 |
Sampling was achieved using a variant of the Rapid Assessment Method described by Campbell et al. (2007) and consisted of monitoring at least 6 docks for each harbor (always including a central dock, an inner dock, and the dock located closest to the harbor entrance). When a marina (recreational activity) had more than 6 docks, then we monitored 6 docks plus one of every two (e.g. if a harbor had 12 docks, we monitored 9). All docks dedicated to fishing and commercial activities were sampled since there were not as numerous. Surveys were always conducted by the same 2–4 people (all well-trained in recognizing ascidian species) and lasted between 30 min (Aiguablava) to over 5 h (L’Escala), depending on the harbor size and type.
At each harbor, we recorded all ascidian species observed by pulling ropes, examining submerged structures, and turning around partially submerged buoys. Other substrata such as tires and plastic structures were occasionally observed hanging from docks and these were also monitored. Surveying organism diversity from the surface has been proven to be highly efficient (Grey 2009b) but, when possible, we also surveyed ship hulls and the harbor’s walls and bottom by submerging an underwater camera and observing the resulting digital pictures. Underwater photos were used with the sole purpose of verifying that all species have been sampled and to identify other substrata favored by ascidians since our goal was to maximize coverage and obtain exhaustive species lists from each sampled harbor. In addition, salinity was measured at −0.20 m with a refractometer.
At the end of each survey, relative abundance was estimated according to the number of individuals (or colonies) observed: (1) rare: one or a few specimens observed; (2) common: species frequently observed but not overly abundant; and (3) abundant: species occurring frequently and in great numbers. When the species was not readily recognized or we had any doubt about its taxonomic identification, we collected samples and preserved them in 4 % formaldehyde. Identification of preserved samples was based on standard taxonomic keys for ascidians and, particularly, on comprehensive faunistic studies of ascidians in the area (e.g., Turon 1987; Ramos-Esplá 1988).
Ascidian barcoding
Most ascidian species retrieved in this study were barcoded using the standard 5′ “barcode region” of the mitochondrial gene cytochrome oxidase I (COI) to facilitate future identifications. Some rare species for which we only had material fixed in formaldehyde could not be sequenced (Table 2) and we choose not to sequence Styela plicata because hundreds of sequences have been recently obtained for this species in the same study area (Pineda et al. 2011). For each of the other species, at least one individual or colony was immediately preserved in absolute ethanol and stored at −20 °C until processed. To maximize DNA extractions, specimens were dissected under a stereomicroscope to separate zooids from the tunic for colonial species and a piece of the branchial sac for solitary ones. DNA was extracted from the zooid fraction or the branchial sac tissue using the DNeasy Blood and Tissue kit (Qiagen). A ca. 600 bp fragment of the COI gene was amplified using either the primer set Tun_forward and Tun_reverse2 described by Stefaniak et al. (2009) or the primer set LCO1490 and HCO2198 described by Folmer et al. (1994). Total PCR volume was 25 µl, including 5 pmol of each primer, 5 nmol of each dNTP, 1× reaction buffer (Ecogen), and 2.5 units of BIOTAQ polymerase (Ecogen). Amplification conditions included initial DNA denaturing at 94 °C for 5 min, followed by 40 amplification cycles of 94 °C for 30 s, annealing at 40 °C for 30 s, and extension at 72 °C for 1 min, and a final extension step at 72 °C for 10 min. PCR cleaning and sequencing reactions were performed at Macrogen, Inc. (Seoul, Korea). The resulting 136 sequences were deposited in the GenBank database (accession numbers KF309529–KF309664).
Table 2.
Ascidian species (classified at least to the genus level) found in the 32 surveyed harbors
| Order | Species | Origin | Acc. num. | # Harbors |
|---|---|---|---|---|
| Aplousobranchia | Clavelina oblonga | Introduced | KF309648 | 1 |
| Clavelina lepadiformis | Introduced | KF309563, -638 | 32 | |
| Clavelina sabbadini | Native | KF309535, -645 | 2 | |
| Diplosoma listerianum | Introduced | KF309531, -561, -581, -605, -616, -638, -639, -660, -664 | 30 | |
| Diplosoma spongiforme | Native | KF309624 | 2 | |
| Trididemnum cereum | Native | KF309632 | 3 | |
| Didemnum sp. 1 | – | KF309573 | 1 | |
| Didemnum sp. 2 | – | KF309622 | 1 | |
| Didemnum fulgens | Native | KF309576 | 1 | |
| Morchellium argus | Native | KF309620-21 | 1 | |
| Aplidium accarense | Introduced | KF309553, -558, -571, -574, -584–586, -597–599, -601, -646, -618, -625–27, -629–630, -640, -654–657, -663 | 23 | |
| Aplidium sp. 1 | – | – | 1 | |
| Aplidium sp. 2 | – | KF309633 | 1 | |
| Phlebobranchia | Ascidia virginea | Native | KF309647 | 1 |
| Ascidia sp. | – | – | 1 | |
| Ascidiella aspersa | Introduced | KF309533-34, -555, -559, -562, -568, -594, -606, -617, -631, -637, -653, -661 | 27 | |
| Ascidiella scabra | Cryptogenic | KF309529, -556, -560, -572, -650 | 5 | |
| Phallusia ingeria | Native | – | 1 | |
| Phallusia sp. | – | – | 1 | |
| Phallusia mammillata | Native | KF309607 | 1 | |
| Phallusia fumigata | Native | KF309548 | 1 | |
| Ciona intestinalis | Cryptogenic | KF309614, -532, -554, -570, -574, -578, -580, -587, -591, -593, -602–604, -613, -628, -651, -658, -662 | 28 | |
| Ciona sp. | – | KF309636 | 1 | |
| Stolidobranchia | Botrylloides leachii | Cryptogenic | KF309549, -551, -608–611, -641–642, -644 | 3 |
| Botryllus schlosseri | Cryptogenic | KF309536-47, -530, -564–567, -579, -592, -615, -659 | 30 | |
| Polyandrocarpa zorritensis | Introduced | KF309643 | 1 | |
| Distomus variolosus | Native | KF309623 | 2 | |
| Styela plicata | Introduced | – | 21 | |
| Styela canopus | Cryptogenic | KF309590 | 2 | |
| Polycarpa pomaria | Native | – | 2 | |
| Polycarpa sp. | – | KF309588 | 1 | |
| Molgula bleizi | Native | – | 2 | |
| Molgula occidentalis | Native | – | 1 | |
| Molgula sp. | – | – | 1 | |
| Microcosmus squamiger | Introduced | KF309550, -552, -595, -600, -612, -619, -634–635, -649, -652 | 24 | |
| Pyura dura | Native | KF309596 | 4 | |
| Pyura squamulosa | Native | – | 2 |
Species were further classified according to their origin: native, introduced, and cryptogenic (see Appendix 1). GenBank accession numbers of the COI sequences generated in this study and the number of harbors in which the species have been found are also indicated
Data analysis
Once identified, each species was classified as native, introduced, or cryptogenic (Carlton 1996), following relevant literature (see Appendix 1). In short, introduced species were those for which distributional or genetic data existed supporting an alien origin. Native species were those endemic to the Mediterranean, or with Atlanto-Mediterranean distribution, known to inhabit natural substrata not adjacent to artificial structures. Finally, cryptogenic species were those for which there was insufficient information to unambiguously decide whether they were introduced or native. The species classified as cryptogenic were widely distributed and generally abundant within harbors, so an introduced origin was suspected in most cases. However, since no study to date has determined their native area and in order to avoid errors, cryptogenic species were not included in further analyses comparing introduced and native species.
A linear regression analysis was performed to determine whether there was a relationship between harbor size (as linear length of docks) and number of species recorded. Analyses of variance (ANOVA) were used to test for differences in linear length among harbor types or geographic zones. Likewise, tests were done to compare species abundance across harbor types and geographic zones. When the assumptions of normality or homogeneity of variances were not met, the non-parametric Kruskal–Wallis test was used instead of ANOVA. All tests were performed using the software SigmaStat v 3.5. To compare ascidian diversity and structure across harbors two similarity matrices were constructed. The first considered the relative abundance of each species within each harbor using the Bray-Curtis index (no transformation of data was applied, as our original data was semi-quantitative). The second dataset consisted of presence–absence data analyzed with the Jaccard index. Analyses were carried out using the Primer v6.1.10 statistical package (Clarke and Gorley 2006) with the PERMANOVA + β20 module (Anderson et al. 2008) incorporated. Permutational analyses of variance (PERMANOVA) were applied to assess the significance of the factors: geographic location (North, Center, South, which corresponded to the three provinces in the area: Girona, Barcelona, and Tarragona, respectively) and harbor type (three levels, see above). In the case of significant factors, we ran permutational pairwise tests on levels of these factors. Likewise, we analyzed differences in relative dispersion among the groups determined by levels of significant factors using PERMDISP. This was done to verify that the significant outcome in PERMANOVA was due to differences in location in multivariate space, not to differences in dispersion among the groups. These analyses were performed for three datasets, one comprising all species (32 harbors), one with only introduced species (32 harbors), and one with just the species identified as native (a restricted set of the 15 harbors were native species were found).
Results were visualized with non-metric multidimensional scaling (nMDS) plots. These analyses were done with R v 2.14.2 (R Development Core Team 2012). The similarity matrices were transformed to distances for input into the vegan 2.0-7 package (Oksanen et al. 2013). We used the metaMDS function of vegan with default parameters. Unlike MDS programs that find a single configuration by iteration and thus can get trapped in local optima, metaMDS performs different (20) random starts and compares them to find a stable solution. MDS configurations were obtained separately for the entire dataset comprising all species, for the introduced species, and for the native species. The analyses were also run separately for relative abundance and presence–absence data and compared using Procrustes analysis (Peres-Neto and Jackson 2001) as implemented in vegan (function Protest), and the significance of the correlations found was tested by permutation. For the native species dataset, the final solutions reached were not stable among runs due to the low number of data, even after increasing the number of random starts (parameter trymax) to one hundred. For this reason MDS plots for the native species are not shown.
Additionally, Mantel tests were conducted to test for correlations between geographic distances among harbors (in kilometers) and species dissimilarity for both the abundance and the presence–absence data involving all species, introduced species, and native species. Shortest surface distances between pairs of harbors were calculated using free software developed by Byers (1997). The Mantel tests were performed using the ade4 package for R (function mantel.rtest) and its significance tested by permutation (Dray and Dufour 2007).
Results
Ascidian diversity
In the 32 harbors investigated, we identified 28 ascidians at the species level and another 9 at the genus level (Table 2; Fig. S1). Samples that could not be assigned a species name were normally single specimens at immature stages, so key morphological characters could not be observed (and, for those barcoded, no perfect match was found in the databases either). Consequently, these taxa could not be further classified according to their origin and were only used in analyses using the global dataset. On average, Catalan harbors contained 8.18 ± 2.33 (SD) ascidian species. The harbor that presented the highest species richness was l’Escala (n = 16), followed by Roses, Port de la Selva and Hospitalet de l’Infant, each with 11 species (Table 1). In contrast, we only found 5 species in Empuriabrava and Calafat, and 4 in Aiguablava (Table 1). A significant relationship between harbor size and species richness was observed (linear regression, p < 0.05), with harbor size explaining 17.2 % of observed variance in species richness among harbors (Fig. S2). However, no significant differences in size were observed between harbor types (ANOVA, F = 3.060, DF = 2,29, p = 0.062) or between geographic zones (F = 1.928, DF = 2,29, p = 0.164). The data on species and abundances found at each harbor are listed in Table S1. Most species were either known introduced ascidians (n = 8) or were considered native from the area (n = 15), while 5 were assigned cryptogenic status (Table 2; Appendix 1). All species found had been previously reported in the Mediterranean Sea, with the exception of Aplidium accarense (see further taxonomic remarks in Appendix 2).
No significant differences in species number were found according to harbor type (Fig. 2, ANOVA F = 2.179, DF = 2,29, p = 0.131) or geographical area (F = 0.016, DF = 2,29, p = 0.984). Introduced and cryptogenic species were the most common in all harbor types (Fig. 2). The geographic span of the three groups of species (introduced, cryptogenic and native) was clearly different (Fig. 3). Introduced species were present in many more harbors (an average ca. 20 harbors) than native species (average of 1.7 harbors), while cryptogenic species were found in 13.6 harbors on average. The differences in spread between native and the other two groups of species were significant, but not between introduced and cryptogenic forms (Kruskal–Wallis test, H = 10.371, DF = 2, p = 0.006, followed by Dunn’s pairwise tests at p = 0.05).
Fig. 2.
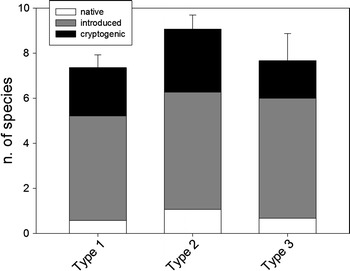
Mean number of ascidian species found at each harbor type (type 1: marina; type 2: marina and fishing; type 3: marina, fishing and commercial) and for each category of species. Bars are standard errors
Fig. 3.
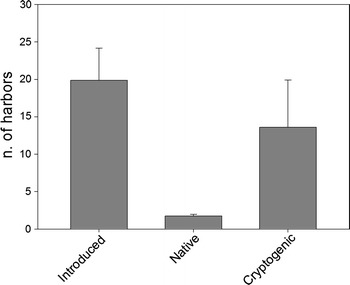
Mean number of harbors in which each species was found as per type of species (introduced, native, cryptogenic). Bars are standard errors
Both colonial and solitary ascidians were widely distributed among harbors (Table 2). The ascidian Clavelina lepadiformis was found in all 32 sampled harbors, while Botryllus schlosseri and Diplosoma listerianum were observed in 30. The most common solitary ascidian was Ciona intestinalis (present in 28 harbors) followed by Ascidiella aspersa (27 harbors) and Microcosmus squamiger (24 harbors). All these species were classified as introduced or cryptogenic and were observed colonizing a wide range of substrata, including ropes, buoys, tires, boat hulls and metal ladders. On the other hand, the least common ascidians (identified at the species level) were the colonial forms Clavelina oblonga, Morchellium argus, Didemnum fulgens, and Polyandrocarpa zorritensis; and the solitary species Molgula occidentalis, Ascidia virginea, Phallusia ingeria, P. mammillata and P. fumigata. These species were observed in a single harbor and were exclusively found on ropes; except for P. ingeria and D. fulgens that grew in mussels and P. zorritensis that was also observed under buoys and attached to dock walls.
Twenty-seven species were identified genetically (Table 2). The sequences generated for Clavelina lepadiformis corresponded to the introduced Atlantic clade defined in Turon et al. (2003). All sequences obtained for Diplosoma listerianum corresponded to clade A (Pérez-Portela et al. 2013), sequences for C. intestinalis matched species A described in Caputi et al. (2007) and Nydam and Harrison (2007), and sequences for B. schlosseri corresponded to clade 5 in López-Legentil et al. (2006), except for KF309545 that matched clade 1, and two sequences (KF309592, KF309530) that presented 98 % identity (BLASTn) with a USA specimen (GU065352, Callahan et al. 2010). Identification of Ascidiella scabra and A. aspersa was made based on morphological characters following a recent review (Nishikawa et al. 2014). However, while the COI sequences generated for A. aspersa clustered within the A. aspersa clade of Nishikawa et al. (2014), those of A. scabra formed a well-supported clade separated from the corresponding clade in that work, which only included European Atlantic specimens (Fig. S3).
Permutational analyses considering both geographic location and harbor type as variables showed that there was a significant effect of both factors on ascidian community structure, which together explained about 28–30 % of the variation found among harbors (Table 3). No significant interaction between these two variables was found, indicating that the effect of each variable on our data was independent of the other. The results were similar when considering relative abundance and presence–absence data. The PERMDISP analyses were not significant (Table 3), indicating homogeneity in data dispersion across levels of the considered factors, except for the geographic region factor with the presence–absence data matrix (p = 0.036). When the analyses were run separately for introduced and native species, the same results were obtained for the introduced species dataset (Table 3), and in this case no evidence for heterogeneity of dispersion between groups was found (non-significant PERMDISP outcome). The native species distribution did not show any significant pattern according to harbor type or geographic location.
Table 3.
Permutational statistical analyses (PERMANOVA) of ascidian similarity among harbors according to their type (marina; marina and fishing, and marina, fishing and commercial) and geographic region (north: Girona; central: Barcelona; south: Tarragona)
| df | Abundance | Presence–absence | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SS | pseudo-F | p value | Permdisp | SS | pseudo-F | p value | Permdisp | ||
| All species | |||||||||
| Region | 2 | 3,685.5 | 2.578 | 0.006 | 0.128 | 4,408 | 2.462 | 0.009 | 0.036 |
| Harbor type | 2 | 3,252.4 | 2.275 | 0.020 | 0.366 | 3,484.6 | 1.946 | 0.038 | 0.714 |
| Interaction | 4 | 3,384.8 | 1.184 | 0.287 | – | 4,879.6 | 1.363 | 0.138 | – |
| Residual | 23 | 16,440 | 20,588 | ||||||
| Introduced species | |||||||||
| Region | 2 | 3,759.8 | 3.306 | 0.003 | 0.308 | 3,871.8 | 4.513 | 0.004 | 0.249 |
| Harbor type | 2 | 2,513.2 | 2.210 | 0.042 | 0.190 | 3,871.8 | 4.513 | 0.004 | 0.249 |
| Interaction | 4 | 2,597.4 | 1.142 | 0.375 | – | 3,171.5 | 1.848 | 0.095 | – |
| Residual | 23 | 13,079 | 9,866.4 | ||||||
| Native species | |||||||||
| Region | 2 | 10,382 | 1.238 | 0.227 | – | 10,057 | 1.153 | 0.318 | – |
| Harbor type | 2 | 9,128.7 | 1.089 | 0.356 | – | 9,376.1 | 1.076 | 0.361 | – |
| Interaction | 2 | 9,485.8 | 1.132 | 0.347 | – | 9,390.6 | 1.077 | 0.378 | – |
| Residual | 8 | 33,533 | 34,867 | ||||||
Analyses were performed for abundance (Bray-Crutis index) and presence–absence (Jaccard index) data, and for the global dataset (32 harbors), the introduced species dataset (32 harbors), and the native species dataset (15 harbors). PERMISP probabilities of homogeneity of dispersion were also given for significant factors
Pairwise comparisons of levels of the significant main factors revealed that, for the geographic regions, there were significant differences between the North and the other two zones (Center and South), which were not different among themselves (Table 4). For the presence–absence dataset, the PERMDISP analyses also showed a significantly higher dispersion of the data in the North and South than in the Center. Pairwise comparisons between harbor types revealed significant differences in the comparison between marinas (type 1) and marina and fishing harbors (type 2) when considering relative abundance values for both the total species and the introduced species datasets (Table 5). Results were less clear-cut for the presence–absence data, as no comparison was significant when considering all species, and only the comparison between harbor types 2 and 3 (marina, fishing and commercial) was significant for the introduced species (Table 5).
Table 4.
Permutational pairwise comparisons of ascidian similarity among harbors according to geographic zone (north: Girona; central: Barcelona; south: Tarragona) for all species and for the introduced species dataset
| Abundance | Presence–absence | |||
|---|---|---|---|---|
| t | p value | t | p value | |
| All species | ||||
| South–Center | 1.283 | 0.180 | 1.471 | 0.091 |
| South–North | 1.599 | 0.024 | 1.531 | 0.025 |
| Center–North | 1.810 | 0.017 | 1.681 | 0.018 |
| Introduced species | ||||
| South–Center | 1.078 | 0.360 | 1.265 | 0.229 |
| South–North | 1.925 | 0.011 | 2.264 | 0.006 |
| Center–North | 2.118 | 0.010 | 2.401 | 0.010 |
Table 5.
Permutational pairwise comparisons of ascidian similarity among harbors according to their type (type 1: marina; type 2: marina and fishing; type 3: marina, fishing and commercial) for all species and for the introduced species dataset
| Abundance | Presence–absence | |||
|---|---|---|---|---|
| t | p value | t | p value | |
| All species | ||||
| Type 1–type 2 | 1.585 | 0.027 | 1.365 | 0.066 |
| Type 1–type 3 | 1.443 | 0.118 | 1.412 | 0.102 |
| Type 2–type 3 | 1.452 | 0.074 | 1.424 | 0.090 |
| Introduced species | ||||
| Type 1–type 2 | 1.711 | 0.042 | 1.664 | 0.091 |
| Type 1–type 3 | 1.345 | 0.168 | 1.584 | 0.121 |
| Type 2–type 3 | 1.149 | 0.293 | 1.992 | 0.038 |
Non-metric MDS plots constructed from relative abundance data (using Bray–Curtis index) showed better differentiation among harbors than those based on presence–absence data (Jaccard index, Fig. 4). The differences observed in PERMANOVA analyses according to geographic location are graphically represented by a separation of the group centroids; the northern harbors in particular tended to be separated from the center and southern harbors, which clustered more closely (Fig. 4). Differences according to harbor type were less evident, with all group centroids relatively close together. Some type 1 harbors appeared consistently separated from the other harbors, while a type 3 harbor (SC: Sant Carles) was usually set apart at one extreme of the spatial ordination (Fig. 4), due to the presence of some particular species at this harbor that were not found in other harbors (see Discussion). Overall, the spatial harbor arrangement was coherent between the MDS plots of the whole datasets and those of introduced species and, to a lesser degree, between abundance and presence–absence data (Fig. 4). This was further corroborated by the results of the Procrustes analyses (Table 6), which showed high correlations (r > 0.84) between the whole species and the introduced species configurations (p < 0.001), and lower (r > 0.56), although significant (p < 0.001), correlations between the abundance and the presence–absence configurations (Table 6).
Fig. 4.
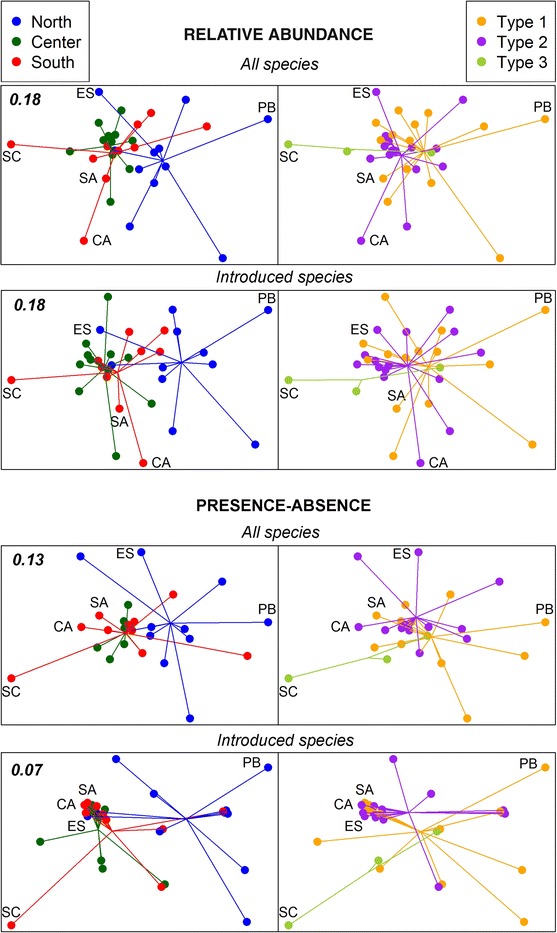
Non-metric MDS plots of the harbors studied obtained from the relative abundance and the presence–absence data, for the whole dataset and for the introduced species. Every plot is color-coded for the geographic region (left; north Girona; central Barcelona; south Tarragona) and for the type of harbor (right; type 1 marina; type 2 marina and fishing; type 3 marina, fishing and commercial). Lines join harbors with their weighed group centroid as for the corresponding factor. Coincident positions of the presence–absence plots were slightly displaced as overlapping groups for clarity. Some harbors are identified (codes as in Table 1) to ease comparison of the configurations. MDS plots with full code names are given in Fig. S4. Stress values are given for each plot (upper left)
Table 6.
Statistical comparison of the MDS configurations obtained. Procrustes sum of squares, correlation, and p value obtained by permutation are given
| Sum of squares | Correlation | p value | |
|---|---|---|---|
| Abundance versus presence–absence | |||
| All species | 0.634 | 0.603 | <0.001 |
| Introduced species | 0.656 | 0.586 | <0.001 |
| All species versus introduced species | |||
| Abundance | 0.124 | 0.936 | <0.001 |
| Presence–absence | 0.293 | 0.841 | <0.001 |
Ascidian distribution along the Catalan coast
The shortest surface distance between the northernmost (Portbou) and southernmost (Cases d’Alcanar) harbors was 299.49 km, and between the closest harbors (Garraf and Port Ginesta) 2.26 km. A Mantel test showed a significant correlation between geographic distance and species dissimilarity (r = 0.321 for the relative abundance matrix, r = 0.325 for the presence–absence matrix, p < 0.001 in both cases). Similar results were obtained when correlating geographic distances with dissimilarity based only on introduced species (Mantel test, r = 0.271 for the relative abundance matrix, r = 0.270 for the presence–absence matrix, p < 0.001 in both cases). For the harbors with native species, the correlations between geographic distances and distances based on diversity of native ascidians were weaker and non significant (relative abundance data, r = 0.166, p = 0.080; presence–absence data, r = 0.165, p = 0.062).
Discussion
This study uncovered an unexpected diversity of ascidians in northwestern Mediterranean harbors. Our survey of 32 small- to medium-sized harbors identified 28 ascidians at the species level and another 9 at the genus level. A recent review placed the total number of ascidian species in the Mediterranean at 229 (Coll et al. 2010), thus in just a single type of habitat along ca. 300 km of shoreline, we have found ca. 16 % of the total recorded biodiversity in the whole Mediterranean Sea. We also found a clear pattern of ascidian distribution, in which introduced (and cryptogenic) species tended to be present in many more harbors than native species, while native species abundance was low overall. This pattern reinforces the general understanding that harbors are not good habitats for native species and are instead populated by highly tolerant introduced forms. Thus, harbor connectivity through shipping does not contribute to the spread of indigenous species, but rather harbors and marinas are strongholds for dispersion of introduced forms. In addition, we found a significant and positive relationship between harbor size and species richness, indicating that larger harbors tended to contain more ascidian species. We did not observe, however, significant differences in the number of ascidian species according to harbor type or geographic zone.
A significant correlation between geographic distance and ascidian diversity in the harbors studied was detected. This correlation was mostly due to the distribution of introduced species, and was weaker and not significant when native species were considered. This pattern is likely a result of the short-range movement of vessels among the small- to medium-sized harbors that enable species dispersal, while species establishment is facilitated by environmental similarity between close-by harbors. It is noteworthy that, even in habitats subject to anthropogenic transport (which usually “breaks” isolation by distance patterns), differences can still be retrieved at the scale considered here (i.e. 300 km). This observation has important implications for secondary spread of introduced species and points to stepping-stone processes that are highly relevant for future preventive actions (see below).
Permutational analyses of variance revealed that harbor type and latitudinal position had significant effects on ascidian community structure, with patterns driven by the introduced species (both factors were not significant for native species). For the factor ‘harbor type’, pairwise tests showed significant (or marginally so) differences for many comparisons in one or another analysis (considering all species and introduced species, as well as relative abundance and presence–absence data), a fact likely reflecting the different size, boating dynamics, and maintenance levels of the different harbor types. Alternatively, the different number of harbors scored in each category (e.g., only three in category 3) may have also influenced some of the p values obtained.
For the factor ‘geographic location’, pairwise comparisons consistently showed a different composition between harbors located in the North (Girona), and the central and southern zones (Barcelona and Tarragona, respectively). Seawater temperatures in southern Catalonia are 0.1–2 °C warmer than in the North, depending on the season and year (López-Legentil et al. 2005; Sabatés et al. 2006). Some of the ascidian species found here are known to be very sensitive to changes in seawater temperature and feature resistance forms during summer (e.g. Didemnum fulgens, López-Legentil et al. 2013), while others such as Styela plicata are able to thrive in habitats featuring seasonal temperature variations of 23 °C (Pineda et al. 2012b). Thus, the absence of some species in northern or southern Catalonia could be due to differences in seawater temperatures, as found for other ascidian species (Lambert 2005). Alternatively, species that are present in just southern or northern harbors could be recent introductions that have not yet spread to harbors located further away.
In spite of a significant effect of the factors analyzed, together they explain ca. 30 % of the variability found, so other abiotic (e.g. salinity, pollution) and biotic factors are influencing ascidian populations. Salinity appears to be an important factor in determining the distribution of some introduced species (Dybern 1969; Lowe 2002; Epelbaum et al. 2009; Pineda et al. 2012a). Our salinity measurements were taken at one point in time and under different weather conditions and thus can only be considered ‘snapshots.’ Not surprisingly, we did not find any correlation between number of species and salinity values (r 2 = 0.001; results not shown). A potential exception was observed for the northern section of the harbor of Sant Carles de la Rapita that received a freshwater rivulet and for which we recorded a salinity of 15 ‰, although it is likely that salinity drops are episodic there. Notably, this section of the harbor was very different from the rest (34 ‰) and was colonized by only two species (out of a total of 10 recorded in that harbor): Clavelina oblonga and Styela plicata, both known to tolerate salinities <34 ‰ (Rocha et al. 2009; Pineda et al. 2012b).
Pollution is also known to shape benthic communities in harbors, especially heavy metals (Piola and Johnston 2009). Information about pollution levels at the investigated Catalan harbors is scarce, but the few studies conducted so far had reported moderate to low levels of heavy metals (De Caralt et al. 2002; Cebrian et al. 2007). It is known that some ascidian species such as Ciona intestinalis, Microcosmus squamiger, Styela plicata, Diplosoma listerianum, Botrylloides leachii and Botryllus schlosseri are able to tolerate high levels of pollution and that this tolerance has been key for their successful introduction in new habitats (Naranjo et al. 1996; Lambert and Lambert 2003; Piola and Johnston 2008; Pineda et al. 2012a). Finally, biotic factors such as competition and predation (Whitlatch and Osman 2009; Ordóñez et al. 2013) are also likely to have an impact on overall species abundance and distribution between and within harbors and their importance remains to be tested.
In general, relative abundance data (here given as semi-quantitative ranks) and presence–absence data tended to give similar information in the analyses performed. However, the ordination configurations with presence–absence data were less resolved and tended to clutter harbors, accentuating the importance of acquiring abundance data whenever possible. MDS plots showed that the overall ordination of harbors was largely driven by the distribution pattern of introduced species. Some northern harbors tended to appear separated from the rest, with the harbor from Sant Carles de la Ràpita (SC) offset from the others in ordination space. This separation was explained by the presence of some exclusive (Polyandrocarpa zorritensis, Clavelina oblonga) or almost exclusive (Clavelina sabbadini, Botrylloides leachii) species in this harbor. SC harbor is the home base of fishermen working in the nearby aquaculture facilities of the Ebro Delta, a large artificial setup hosting several introduced species (Ordóñez 2013). Thus, at least some of the species retrieved in SC may have come from the nearby aquaculture settings, and SC (which is large and sustains recreational activities, a considerable fishing fleet and some commercial ships) may now be acting as a focal point for further expansions. This observation revealed a complex interplay among harbor types, aquaculture activities in the vicinity, and secondary spread of introduced species.
In conclusion, we have uncovered an unexpected diversity of ascidian species in a relatively restricted but vastly developed stretch of coast in the Western Mediterranean. We also found an effect of harbor type, size and geographic area on ascidian diversity and distribution, as well as a pattern of higher similarity in geographically closer harbors. Thus, highly urbanized coastlines and their associated network of harbors and marinas act as dispersal strongholds for introduced species with little impact for the rarely observed native ascidians. Cataloguing species and establishing periodic surveys of artificial structures are easily achieved first-steps to prevent spreading of detrimental species and are critical for the development of cost-effective management and contingency plans. Species inventories should not only incorporate taxonomic surveys (for which complementary genetic data is mandatory in the face of taxonomic conundrums), but also a thorough assessment of inter-harbor distribution patterns in order to define efficient internal borders for further action.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study would not have been possible without the collaboration of the authorities of the 32 Catalan harbors that kindly allowed sampling and entrance to their facilities. Ruth Oliveras helped with some of the sampling. Dr. Marie Nydam provided useful information to identify C. intestinalis genetic type. Dr. Rosana Rocha provided images of Aplidium accarense for comparison. Two anonymous reviewers greatly contributed to improve this manuscript. This study was funded by the Marie Curie International Reintegration Grant FP7-PEOPLE-2010-RG 277038 and by the COCONET project #287844, both within the 7th European Community Framework Program, by the Spanish Government project CTM2013-48163, and by the Catalan Government Grant 2014SGR-336 for Consolidated Research Groups.
References
- Airoldi L, Beck MW. Loss, status and trends for coastal marine habitats of Europe. Oceanogr Mar Biol Annu Rev. 2007;45:345–405. [Google Scholar]
- Aldred N, Clare AS. Impact and dynamics of surface fouling by solitary and compound ascidians. Biofouling. 2014 doi: 10.1080/08927014.2013.866653. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Gorley RN, Clarke KR. PERMANOVA for PRIMER: guide to software and statistical methods. Plymouth: PRIMER-E; 2008. [Google Scholar]
- Arenas F, Bishop JDD, Carlton JT, Dyrynda PJ, Farnham WF, et al. Alien species and other notable records from a rapid assessment survey of marinas on the south coast of England. J Mar Biol Assoc UK. 2006;86:1329–1337. doi: 10.1017/S0025315406014354. [DOI] [Google Scholar]
- Ayre DJ, Davis AR, Billingham M, Llorens T, Styan C. Genetic evidence for contrasting patterns of dispersal in solitary and colonial ascidians. Mar Biol. 1997;130:51–61. doi: 10.1007/s002270050224. [DOI] [Google Scholar]
- Brunetti R, Beghi L, Bressan M, Marin MG. Combined effects of temperature and salinity on colonies of Botryllus schlosseri and Botrylloides leachi (Ascidiacea) from the venetian lagoon. Mar Ecol Prog Ser. 1980;2:303–314. doi: 10.3354/meps002303. [DOI] [Google Scholar]
- Bullard SG, Whitlatch RB. In situ growth of the colonial ascidian Didemnum vexillum under different environmental conditions. Aquat Invasions. 2009;4(1):275–278. doi: 10.3391/ai.2009.4.1.27. [DOI] [Google Scholar]
- Byers JA (1997) Surface distance between two points of latitude and longitude. http://www.chemical-ecology.net/java/lat-long.htm. Accessed April 2013
- Callahan AG, Deibel D, McKenzie CH, Hall JR, Rise ML. Survey of harbours in Newfoundland for indigenous and non-indigenous ascidians and an analysis of their cytochrome c oxidase I gene sequences. Aquat Invasions. 2010;5(1):31–39. doi: 10.3391/ai.2010.5.1.5. [DOI] [Google Scholar]
- Campbell ML, Gould B, Hewitt CL. Survey evaluations to assess marine bioinvasions. Mar Poll Bull. 2007;55:360–378. doi: 10.1016/j.marpolbul.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Caputi L, Andreakis N, Mastrototaro F, Cirino P, Vassillo M, Sordino P. Cryptic speciation in a model invertebrate chordate. Proc Natl Acad Sci USA. 2007;104(22):9364–9369. doi: 10.1073/pnas.0610158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JT. Biological invasions and cryptogenic species. Ecology. 1996;77:1653–1655. doi: 10.2307/2265767. [DOI] [Google Scholar]
- Carman MR, Morris JA, Karney RC, Grunden DW. An initial assessment of native and invasive tunicates in shellfish aquaculture of the North American east coast. J Appl Ichthyol. 2010;26(2):8–11. doi: 10.1111/j.1439-0426.2010.01495.x. [DOI] [Google Scholar]
- Castilla JC, Lagos NA, Cerda M. Marine ecosystem engineering by the alien ascidian Pyrua praeputialis on a mid-intertidal rocky shore. Mar Ecol Prog Ser. 2004;268:119–130. doi: 10.3354/meps268119. [DOI] [Google Scholar]
- Cebrian E, Uriz MJ, Turon X. Sponges as biomonitors of heavy metals in spatial and temporal surveys in northwestern Mediterranean: multispecies comparison. Environ Toxicol Chem. 2007;26:2430–2439. doi: 10.1897/07-292.1. [DOI] [PubMed] [Google Scholar]
- CIESM (2002) The Mediterranean Science Commission. http://www.ciesm.org/. Accessed May 2013
- Clarke KR, Gorley RN. Primer v6: user manual/tutorial. Plymouth: Primer-E; 2006. [Google Scholar]
- Cohen AN, Harris LH, Bingham BL, Carlton JT, Chapman JW, et al. Rapid assessment survey for exotic organisms in southern California bays and harbors, and abundance in port and non-port areas. Biol Invasions. 2005;7:995–1002. doi: 10.1007/s10530-004-3121-1. [DOI] [Google Scholar]
- Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F, et al. The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS One. 2010;5(8):e11842. doi: 10.1371/journal.pone.0011842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbyson E, Locke A, Hanson JM, Willison JHM. Marine boating habits and the potential for spread of invasive species in the Gulf of St. Lawrence. Aquat Invasions. 2009;4:87–94. doi: 10.3391/ai.2009.4.1.9. [DOI] [Google Scholar]
- Davidscon IC, Zabin CJ, Chang AL, Brown CW, Sytsma MD, Ruiz GM. Recreational boats as potential vectors of marine organisms at an invasion hotspot. Aquat Biol. 2010;11:179–191. doi: 10.3354/ab00302. [DOI] [Google Scholar]
- De Caralt S, López-Legentil S, Tarjuelo I, Uriz MJ, Turon X. Contrasting biological traits of Clavelina lepadiformis (Ascidiacea) populations from inside and outside harbors in western Mediterranean. Mar Ecol Prog Ser. 2002;244:125–137. doi: 10.3354/meps244125. [DOI] [Google Scholar]
- Dray S, Dufour A. The ade4 package: implementing the duality diagram for ecologists. J Stat Softw. 2007;22:1–20. [Google Scholar]
- Dupont L, Viard F, Dowell MJ, Wood C, Bishop JDD. Fine- and regional-scale genetic structure of the exotic ascidian Styela clava (Tunicata) in southwest England, 50 years after its introduction. Mol Ecol. 2009;18:442–453. doi: 10.1111/j.1365-294X.2008.04045.x. [DOI] [PubMed] [Google Scholar]
- Dupont L, Viard F, Davis MH, Nishikawa T, Bishop JD. Pathways of spread of the introduced ascidian Styela clava (Tunicata) in Northern Europe, as revealed by microsatellite markers. Biol Invasions. 2010;12:2707–2721. doi: 10.1007/s10530-009-9676-0. [DOI] [Google Scholar]
- Dybern BI. Distribution and ecology of ascidians in Kviturdvikpollen and Vagsbopollen on the west coast of Norway. Sarsia. 1969;37:21–40. [Google Scholar]
- Epelbaum A, Herborg LM, Therriault TW, Pearce CM. Temperature and salinity effects on growth, survival, reproduction, and potential distribution of two non-indigenous botryllid ascidians in British Columbia. J Exp Mar Biol Ecol. 2009;369:43–52. doi: 10.1016/j.jembe.2008.10.028. [DOI] [Google Scholar]
- Folmer O, Hoeh W, Black M, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- Forrest BM, Gardner JPA, Taylor MD. Internal borders for managing invasive marine species. J Appl Ecol. 2009;46:46–54. doi: 10.1111/j.1365-2664.2008.01544.x. [DOI] [Google Scholar]
- Glasby TM, Connell SD. Urban structures as marine habitats. Ambio. 1999;28:595–598. [Google Scholar]
- Goldstein SJ, Dupont L, Viard F, Hallas PJ, Nishikawa T, et al. Global phylogeography of the widely introduced North West Pacific ascidian Styela clava. PLoS One. 2011;6(2):e16755. doi: 10.1371/journal.pone.0016755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey EK. Scale-dependent relationships between native richness, resource stability and exotic cover in dock fouling communities of Washington, USA. Divers Distrib. 2009;15:1073–1080. doi: 10.1111/j.1472-4642.2009.00608.x. [DOI] [Google Scholar]
- Grey EK. Do we need to jump in? A comparison of two surveys of exotic ascidians on docks. Aquat Invasions. 2009;4:81–86. doi: 10.3391/ai.2009.4.1.8. [DOI] [Google Scholar]
- Hewitt CL, Gollasch S, Minchin D. The vessel as a vector—biofouling, ballast water and sediments. In: Rilov GR, Crooks JA, editors. Biological invasions in marine ecosystems. Berlin: Springer; 2009. pp. 117–131. [Google Scholar]
- Kaluza P, Kölzsch A, Gastner MT, Blasius B. The complex network of global cargo ship movements. J R Soc Interface. 2010;6(7):1093–1103. doi: 10.1098/rsif.2009.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller RP, Geist J, Jeschke JM, Kühn I. Invasive species in Europe: ecology, status and policy. Environ Sci Eur. 2011;23:23–39. doi: 10.1186/2190-4715-23-23. [DOI] [Google Scholar]
- Lambert G. Ecology and natural history of the protochordates. Can J Zool. 2005;83:34–50. doi: 10.1139/z04-156. [DOI] [Google Scholar]
- Lambert G. Adventures of a sea squirt sleuth: unraveling the identity of Didemnum vexillum, a global ascidian invader. Aquat Invasions. 2009;4(1):5–28. doi: 10.3391/ai.2009.4.1.2. [DOI] [Google Scholar]
- Lambert CC, Lambert G. Non-indigenous ascidians in southern California harbors and marinas. Mar Biol. 1998;130:675–688. doi: 10.1007/s002270050289. [DOI] [Google Scholar]
- Lambert CC, Lambert G. Persistence and differential distribution of non-indigenous ascidians in harbors of the Southern California Bight. Mar Ecol Prog Ser. 2003;259:146–161. doi: 10.3354/meps259145. [DOI] [Google Scholar]
- López-Legentil S, Ruchy M, Doménech A, Turon X. Life cycles and growth rates of two morphotypes of Cystodytes (Ascidiacea) in the western Mediterranean. Mar Ecol Prog Ser. 2005;296:219–228. doi: 10.3354/meps296219. [DOI] [Google Scholar]
- López-Legentil S, Turon X, Planes S. Genetic structure of the sea star squirt, Botryllus schlosseri, introduced in southern European harbors. Mol Ecol. 2006;15:3957–3967. doi: 10.1111/j.1365-294X.2006.03087.x. [DOI] [PubMed] [Google Scholar]
- López-Legentil S, Erwin PM, Velasco M, Turon X. Growing or reproducing in a temperate sea: optimization of resource allocation in a colonial ascidian. Invertebr Biol. 2013;132(1):69–80. doi: 10.1111/ivb.12013. [DOI] [Google Scholar]
- Lowe AJ (2002) Microcosmus squamiger: a solitary ascidian introduced to southern California harbors and marinas: salinity tolerance and phylogenetic analysis. PhD dissertation, California State University, Fullerton
- Morris JA, Carman MR. Fragment reattachment, reproductive status, and health indicators of the invasive colonial tunicate Didemnum vexillum with implications for dispersal. Biol Invasions. 2012;14:2133–2140. doi: 10.1007/s10530-012-0219-8. [DOI] [Google Scholar]
- Morris JA, Carman MR, Hoagland E, Green-Beach ERM, Karney RC. Impact of the invasive colonial tunicate Didemnum vexillum on the recruitment of the bay scallop (Argopecten irradians irradians) and implications for recruitment of the sea scallop (Placopecten magellanicus) on Georges Bank. Aquat Invasions. 2009;4(1):207–211. doi: 10.3391/ai.2009.4.1.21. [DOI] [Google Scholar]
- Naranjo SA, Carballo JL, García-Gómez JC. Effects of environmental stress on ascidian populations in Algeciras Bay (southern Spain) Possible marine bioindicators? Mar Ecol Prog Ser. 1996;144:119–131. doi: 10.3354/meps144119. [DOI] [Google Scholar]
- Nishikawa T, Oohara I, Saitoh K, Shigenobu Y, Hasegawa N, et al. Molecular and morphological discrimination between and invasive ascidian, Ascidiella aspersa and its congener A. scabra (Urochordata: Ascidiacea) Zool Sci. 2014;31(3):180–185. doi: 10.2108/zsj.31.180. [DOI] [PubMed] [Google Scholar]
- Nydam ML, Harrison RG. Genealogical relationships within and among shallow-water Ciona species (Ascidiacea) Mar Biol. 2007;151(5):1839–1847. doi: 10.1007/s00227-007-0617-0. [DOI] [Google Scholar]
- Oksanen JF, Blanchet G, Kindt R, Legendre P, Minchin PR et al (2013) Vegan: community ecology package. R package version 2.0-7. http://CRAN.R-project.org/package=vegan
- Ordóñez V (2013) Ecology and genetics of invasive ascidians in Western Mediterranean. PhD dissertation, University of Barcelona, Barcelona
- Ordóñez V, Rius M, McQuaid CD, Pineda MC, Pascual M, Turon X. Early biotic interactions among introduced and native benthic species reveal cryptic predation and shifts in larval behavior. Mar Ecol Prog Ser. 2013;488:65–79. doi: 10.3354/meps10416. [DOI] [Google Scholar]
- Paulay G, Kirkendale L, Lambert G, Meyer C. Anthropogenic biotic interchange in a coral reef ecosystem: a case study from Guam. Pac Sci. 2002;56(4):403–422. doi: 10.1353/psc.2002.0036. [DOI] [Google Scholar]
- Peres-Neto PR, Jackson DA. How well do multivariate data sets match? The advantages of a Procrustean superimposition approach over the Mantel test. Oecologia. 2001;129:169–178. doi: 10.1007/s004420100720. [DOI] [PubMed] [Google Scholar]
- Pérez-Portela R, Arranz V, Rius M, Turon X. Cryptic speciation or global spread? The case of a cosmopolitan ascidian with limited dispersal capabilities. Sci Rep. 2013;3:3197. doi: 10.1038/srep03197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda MC, López-Legentil S, Turon X. The whereabouts of an ancient wanderer: global phylogeography of the solitary ascidian Styela plicata. PLoS One. 2011;6(9):e25495. doi: 10.1371/journal.pone.0025495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda MC, Turon X, López-Legentil S. Stress levels over time in the introduced ascidian Styela plicata: the effects of temperature and salinity variations on hsp70 gene expression. Cell Stress Chaperon. 2012;17:435–444. doi: 10.1007/s12192-012-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda MC, McQuaid CD, Turon X, López-Legentil S, Ordóñez V, Rius M. Tough adults, frail babies: an analysis of stress sensitivity across early life-history stages of widely introduced marine invertebrates. PLoS One. 2012;7(10):e46672. doi: 10.1371/journal.pone.0046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda MC, López-Legentil S, Turon X. Year-round reproduction in a seasonal sea: biological cycle of the introduced ascidian Styela plicata in the Western Mediterranean. Mar Biol. 2013;160:221–230. doi: 10.1007/s00227-012-2082-7. [DOI] [Google Scholar]
- Piola RF, Johnston EL. Pollution reduces native diversity and increases invader dominance in marine hard-substrate communities. Divers Distrib. 2008;14:329–342. doi: 10.1111/j.1472-4642.2007.00430.x. [DOI] [Google Scholar]
- Piola RF, Johnston EL. Comparing differential tolerance of native and non-indigenous marine species to metal pollution using novel assay techniques. Environ Pollut. 2009;157:2853–2864. doi: 10.1016/j.envpol.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Pyo J, Lee T, Shin S. Two newly recorded invasive alien ascidians (Chordata, Tunicata, Ascidiacea) based on morphological and molecular phylogenetic analysis in Korea. Zootaxa. 2012;3368:211–228. [Google Scholar]
- R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/
- Ramos-Esplá AA (1988) Littoral ascidians of the Iberian Mediterranean. Faunistics, Ecology, and Biogeography. PhD dissertation, University of Barcelona, Barcelona
- Rius M, Pineda MC, Turon X. Population dynamics and life cycle of the introduced ascidian Microcosmus squamiger in the Mediterranean Sea. Biol Invasions. 2009;11:2181–2194. doi: 10.1007/s10530-008-9375-2. [DOI] [Google Scholar]
- Rius M, Turon X, Marshall DJ. Non-lethal effects of an invasive species in the marine environment: the importance of early life-history stages. Oecologia. 2009;159:873–882. doi: 10.1007/s00442-008-1256-y. [DOI] [PubMed] [Google Scholar]
- Rius M, Branch GM, Griffiths CL, Turon X. Larval settlement behaviour in six gregarious ascidians in relation to adult distribution. Mar Ecol Prog Ser. 2010;418:151–163. doi: 10.3354/meps08810. [DOI] [Google Scholar]
- Rius M, Turon X, Dias GM, Marshall DJ. Propagule size effects across multiple life-history stages in a marine invertebrate. Funct Ecol. 2010;24:685–693. doi: 10.1111/j.1365-2435.2009.01668.x. [DOI] [Google Scholar]
- Rius M, Turon X, Ordóñez V, Pascual M. Tracking invasion histories in the sea: facing complex scenarios using multilocus data. PLoS One. 2012;7:e35815. doi: 10.1371/journal.pone.0035815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha RM, Kremer LP, Baptista MS, Metri R. Bivalve cultures provide habitat for exotic tunicates in southern Brazil. Aquat Invasions. 2009;4(1):195–205. doi: 10.3391/ai.2009.4.1.20. [DOI] [Google Scholar]
- Sabatés A, Martín P, Lloret J, Raya V. Sea warming and fish distribution: the case of the small pelagic fish, Sardinella aurita, in the western Mediterranean. Glob Change Biol. 2006;12:2209–2219. doi: 10.1111/j.1365-2486.2006.01246.x. [DOI] [Google Scholar]
- Sephton D, Vercaemer B, Nicolas JM, Keays J. Monitoring for invasive tunicates in Nova Scotia, Canada (2006–2009) Aquat Invasions. 2011;6:391–403. doi: 10.3391/ai.2011.6.4.04. [DOI] [Google Scholar]
- Stefaniak L, Lambert G, Gittenberger A, Zhang H, Lin S, et al. Genetic conspecificity of the worldwide populations of Didemnum vexillum Kott, 2002. Aquat Invasions. 2009;4:29–44. doi: 10.3391/ai.2009.4.1.3. [DOI] [Google Scholar]
- Stefaniak L, Zhang H, Gittenberger A, Smith K, Holsinger K, et al. Determining the native region of the putatively invasive ascidian Didemnum vexillum Kott, 2002. J Exp Mar Biol Ecol. 2012;422–423:64–71. doi: 10.1016/j.jembe.2012.04.012. [DOI] [Google Scholar]
- Svane IB, Young CM. The ecology and behaviour of ascidian larvae. Oceanogr Mar Biol Annu Rev. 1989;27:45–90. [Google Scholar]
- Turon X (1987) Ascidians from the shores of Catalonia and Balearic Islands. PhD dissertation, University of Barcelona, Barcelona
- Turon X, Tarjuelo I, Duran S, Pascual M. Characterising invasion processes with genetic data: an Atlantic clade of Clavelina lepadiformis (Ascidiacea) introduced into Mediterranean harbours. Hydrobiologia. 2003;503:29–35. doi: 10.1023/B:HYDR.0000008481.10705.c2. [DOI] [Google Scholar]
- Turon X, Nishikawa T, Rius M. Spread of Microcosmus squamiger (Ascidiacea: Pyuridae) in the Mediterranean Sea and adjacent waters. J Exp Mar Biol Ecol. 2007;342:185–188. doi: 10.1016/j.jembe.2006.10.040. [DOI] [Google Scholar]
- Vázquez E, Young CM. Effects of low salinity on metamorphosis in estuarine colonial ascidians. Invertebr Biol. 2000;119(4):433–444. doi: 10.1111/j.1744-7410.2000.tb00113.x. [DOI] [Google Scholar]
- Wasson K, Zabin CJ, Bedinger L, Diaz CM, Pearse JS. Biological invasions of estuaries without international shipping: the importance of intraregional transport. Biol Conserv. 2001;102:143–153. doi: 10.1016/S0006-3207(01)00098-2. [DOI] [Google Scholar]
- Whitlatch RB, Osman RW. Post-settlement predation on ascidian recruits: predator responses to changing prey density. Aquat Invasions. 2009;4(1):121–131. doi: 10.3391/ai.2009.4.1.13. [DOI] [Google Scholar]
- Zenetos A, Gofas S, Morri C, Rosso A, Violanti D et al (2012) Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr Mar Sci 13(2):328–352
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


