Abstract
Spasticity is a common cause of long-term disability in poststroke hemiplegic patients. We investigated whether intermittent theta burst stimulation (iTBS) could reduce upper-limb spasticity after a stroke. Fifteen hemiplegic stroke patients were recruited for a double-blind sham-controlled cross-over design study. A single session of iTBS or sham stimulation was delivered on the motor hotspot of the affected flexor carpi radialis muscle in a random and counterbalanced order with a 1-week interval. Modified Ashworth scale (MAS), modified Tardieu scale (MTS), H-wave/M-wave amplitude ratio, peak torque (PT), peak torque angle (PTA), work of affected wrist flexor, and rectified integrated electromyographic activity of the flexor carpi radialis muscle were measured before, immediately after, 30 min after, and 1 week after iTBS or sham stimulation. Repeated-measures analysis of variance showed a significant interaction between time and intervention for the MAS, MTS, PT, PTA, and rectified integrated electromyographic activity (P<0.05), indicating that these parameters were significantly improved by iTBS compared with sham stimulation. However, the H-wave/M-wave amplitude ratio and work were not affected. MAS and MTS significantly improved for at least 30 min after iTBS, but the other parameters only improved immediately after iTBS (P<0.05). In conclusion, iTBS on the affected hemisphere may help to reduce poststroke spasticity transiently.
Keywords: hemiplegia, intermittent theta burst stimulation, muscle spasticity, stroke, transcranial magnetic stimulation
Introduction
Spasticity is a common disorder, with a prevalence of about 35% in stroke patients 1, and it increases functional impairment and significantly impacts activities and quality of daily life 2. Although many therapies are available, none is universally effective.
Spasticity can be defined as a velocity-dependent increase in muscle tone, and its pathophysiology is commonly believed to involve lesions of upper motor neurons, which modulate the activity of the spinal circuit 2. Thus, reduced inhibitory upper motor neuron activity may lead to overactivation of α and γ motor neurons and a large group of interneurons at the spinal level 1. Therefore, techniques that modulate the excitability of upper motor neurons (e.g. transcranial magnetic stimulation) may be a treatment option for spasticity.
Repetitive transcranial magnetic stimulation (rTMS) can noninvasively alter the cortical excitability at the site of stimulation 3. Some previous studies showed that conventional rTMS can reduce the spasticity, as measured by a modified Ashworth scale (MAS) 4–6 and the F-wave/M-wave amplitude ratio 7,8. However, these studies were limited to clinical and/or electrophysiological measurements. Moreover, theta burst stimulation (TBS), a novel rTMS stimulation protocol that requires less stimulation time than conventional rTMS, has not been attempted.
Thus, our aim was to determine whether intermittent TBS (iTBS) may significantly reduce upper-limb spasticity after a stroke on the basis of clinical, electrophysiological, and biomechanical assessments.
Methods
Participants
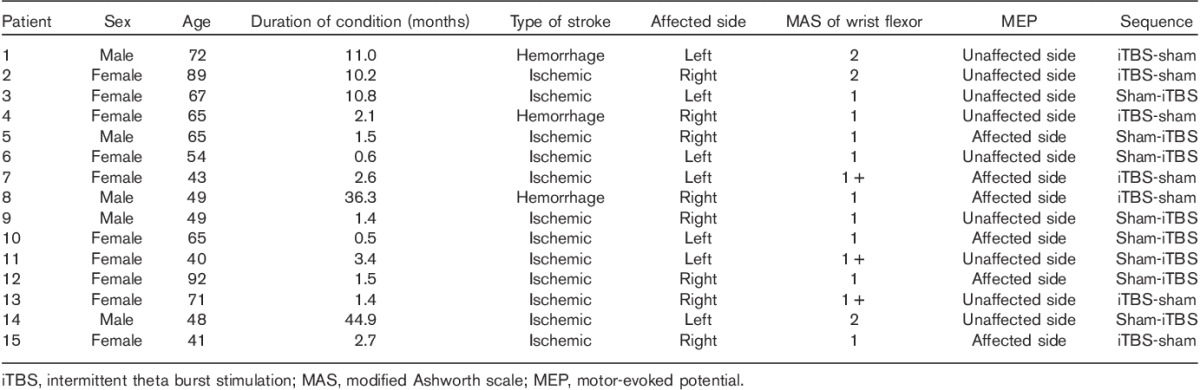
Fifteen stroke patients (10 women, five men) who fulfilled the following criteria were recruited: (a) a first ever unilateral stroke, as confirmed by MRI or computed tomography; (b) supratentorial stroke; (c) at least 18 years of age; (d) wrist flexor spasticity with a MAS score of 2 or less; (e) suspended antispastic medications and/or physiotherapy at least 1 month ago; and (f) cognitive function sufficient to allow cooperation. Exclusion criteria were as follows: (a) seizure history or ictal wave form on the electroencephalogram; (b) contraindications for rTMS 9 (e.g. intracranial implants, cardiac pacemakers, or pregnancy, implanted medication pumps); or (c) coexisting neurological and orthopedic disease. The age of the patients was 60.7±8.7 years (range=40–92 years) and the duration since the stroke was 8.7±13.6 months. The patients’ characteristics are summarized in Table 1. The group size was comparable with that of other studies of rTMS and spasticity 2,8. The study was approved by our Institutional Research Ethics Committee for Human Subjects. Informed consent was obtained from all patients before the study.
Table 1.
General characteristics of the participants
Experimental design
This study used a double-blind, sham-controlled, cross-over design. Each patient underwent two different stimulations of the affected hemisphere: iTBS and sham. The order of these stimulations was randomized and counterbalanced for all patients. The iTBS and sham stimulation sessions were separated by a 1-week interval to minimize carryover effects.
To familiarize patients with the outcome measurements, the same procedures were performed more than five times to obtain stable data on the day before the stimulation session; data of last trials were obtained (T−1). The outcome measurements were evaluated by an experienced physiatrist before (T0), immediately after (T1), 30 min after (T2), and 1 week after stimulation (T3). We could not find a significant difference in any of the outcome measurements between on the day before the stimulation (T−1) and before stimulation on the experimental day (T0). The outcome measurements were completed within 10 min after the stimulation session. The physician who performed the stimulation was instructed not to disclose the stimulation order to the patients and another physician who assessed the parameters.
Intermittent theta burst stimulation
iTBS was administered using a Magstim Rapid magnetic stimulator (Magstim Company, Whitland, UK) connected to a figure-of-eight coil (diameter=70 mm). It was delivered over the motor hotspot of the affected flexor carpi radialis muscle (FCR). The stimulation intensity was 80% of the active motor threshold 10. The iTBS protocol consisted of 10 bursts, each of which was composed of three stimuli at 50 Hz, repeated at a theta frequency of 5 Hz every 10 s for a total of 600 stimuli (200 s) 10. If no motor-evoked potential (MEP) was detectable from the FCR of the affected limb, the stimulation location was established as the equivalent point of the scalp in the affected hemisphere relative to the unaffected motor hot spot of the FCR. In these cases, the stimulation intensity was set to 50% of the maximum stimulator output 2. For the sham stimulation, iTBS was delivered (80% of the active motor threshold) with the coil held close over the motor area corresponding to the affected limb as determined for iTBS, but tilted ∼90° so that no current was induced in the brain 2. The stimulation intensity was 48.1±2.7 of the maximum stimulator output. The MEP from the FCR in the affected limb were detected in six patients (Table 1).
Outcome measurements
The wrist flexor spasticity was assessed systematically by clinical, electrophysiological, and biomechanical measurements. Clinical assessments were performed using MAS and the modified Tardieu scale (MTS). MAS was scored using a six-point (0, 1, 1.5, 2, 3, 4) scale 4 and MTS involving scoring of R1 and R2 was also assessed 11. Electrophysiological measurements were obtained using a Synergy system EMG unit (VIASYS HealthCare Inc., Old Woking, UK) with a bandpass filter at 20 Hz to 2 kHz, sweep speed at 5 ms/division, and sensitivity at 200–500 μV. Compound muscle action potentials, Hoffmann (H) reflex, and electromyographic (EMG) activity were recorded using Ag–AgCl surface electrodes on affected FCR. Compound muscle action potentials and H reflex were evoked 10 times by electrical stimulation of the median nerve through a bipolar electrode placed in the antecubital fossa (1 ms duration, 20–40 mA intensity when set at the minimal M wave) 12. The H-wave/M-wave amplitude ratio (H/M ratio) was calculated and averaged across 10 occurrences. Biomechanical measurements including the rectified integrated EMG activity were performed using an isokinetic dynamometer (Lumex Inc., Ronkonkoma, New York, USA) and an MP100 system (BIOPAC Inc., Goleta, California, USA). To assess these measurements, 10 continuous passive wrist movements from 45° flexion to 35° extension were completed at a velocity of 30°/s. Peak torque (PT) was measured on the basis of the resistance force applied to the lever during the passive motion of the wrist. PT (Nm), peak torque angle (PTA) (°), work (J), and EMG activity (mV) of FCR were measured simultaneously. The raw data for the EMG activity were integrated after being rectified using the Acknowledgement 4.2 program (BIOPAC Inc.).
Statistical analysis
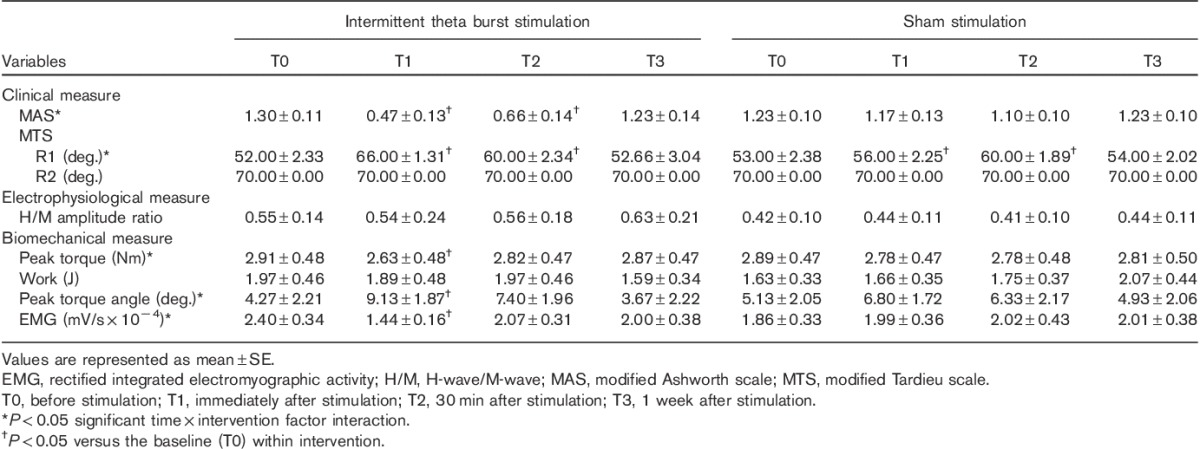
Data analysis was completed using SPSS, version 19.0 (SPSS Inc., Chicago, Illinois, USA). Repeated-measures analysis of variance (RM-ANOVA) was used to evaluate the effects of time (T0–T3) and intervention (iTBS and sham) on spasticity because each patient underwent two stimulation conditions (iTBS and sham stimulation) and evaluations were performed at four time points (from T0 to T3). The Wilks’ λ correction was used to correct for violations of sphericity. When significant differences were found, post-hoc testing was performed and corrected for multiple comparisons (Bonferroni). For comparisons with baseline, an independent t-test was used for the baseline data of iTBS and sham stimulation. A paired t-test was used for all parameters between T−1 and T0 to confirm familiarization. The level for statistical significance was set at P value less than 0.05. All data are expressed as the mean±SE (Table 2).
Table 2.
Changes in clinical, electrophysiological, and biomechanical measures of spasticity after stimulation
Results
Clinical measurements
MAS and MTS at baseline did not differ significantly between iTBS and sham stimulation. RM-ANOVA showed a significant interaction between time (T0–T3) and intervention (iTBS/sham) with respect to MAS (F=11.752, P<0.001) and R1 of MTS (F=13.365, P<0.001), indicating the significant improvements on MAS and R1 of MTS after iTBS in comparison with the sham stimulation. However, R2 of MTS did not show any improvement. On comparison with T0, post-hoc comparisons showed that MAS was improved significantly immediately after (T1) and 30 min after iTBS (T2), but was not improved at 1 week after stimulation (T3), and R1 of MTS was also improved significantly immediately after (T1) and 30 min after both iTBS and sham stimulation (T2) (P<0.001 with Bonferroni correction), but was not improved at 1 week after stimulation (T3) (Table 2 and Fig. 1).
Fig. 1.
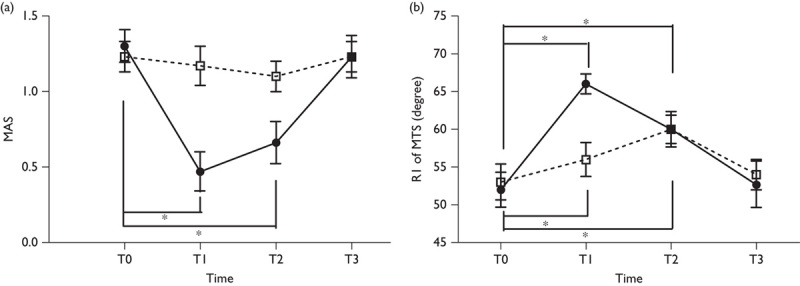
Effects of a single intermittent theta burst stimulation on the modified Ashworth scale (a) and the modified Tardieu scale (b). *P<0.05 versus the baseline (T0) within intervention. MAS, modified Ashworth scale; MTS, modified Tardieu scale.
Electrophysiological measurements
The H/M ratio at baseline did not differ significantly between iTBS and sham stimulation. RM-ANOVA did not indicate a significant interaction between time (T0–T3) and intervention (iTBS/sham) on the H/M ratio (F=0.405, P=0.751) (Table 2 and Fig. 2).
Fig. 2.
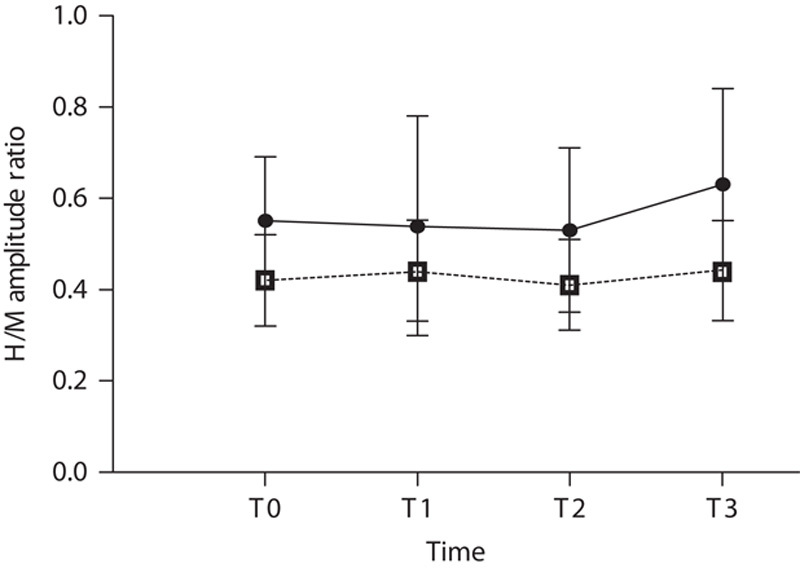
Effects of a single intermittent theta burst stimulation on the H/M amplitude ratio. H/M, H-wave/M-wave.
Biomechanical measurements
No significant difference in PT, PTA, work, and rectified integrated EMG activity at baseline could be found between iTBS and sham stimulation. RM-ANOVA showed a significant interaction between time (T0–T3) and intervention (iTBS/sham) with respect to PT (F=3.798, P=0.022), PTA (F=3.137, P=0.042), and rectified integrated EMG activity (F=8.078, P=0.001), indicating that these parameters were significantly improved after iTBS compared with sham stimulation. However, work was not improved. On comparison with T1, post-hoc comparisons showed that PT, PTA, and rectified integrated EMG activity were improved immediately after iTBS (T1) (P<0.001 with Bonferroni correction), but were not improved at 30 min after (T2) or 1 week after (T3) stimulation (Table 2 and Fig. 3).
Fig. 3.
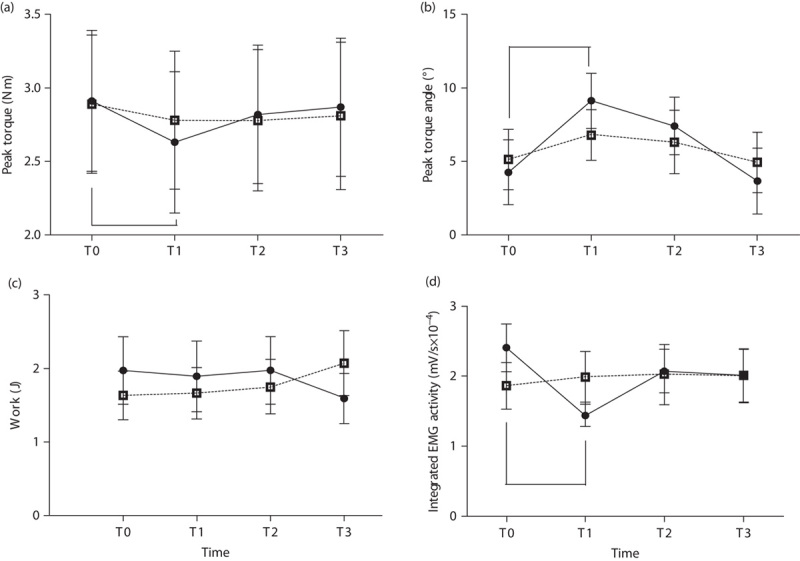
Effects of a single intermittent theta burst stimulation on peak torque (a), peak torque angle (b), work (c), and EMG activity (d). EMG, rectified integrated electromyographic activity. *P<0.05 versus the baseline (T0) within intervention.
Discussion
This study showed that a single session of iTBS over the affected motor cortex caused a transient reduction in spasticity after a stroke. These results are consistent with previous studies reporting a significant reduction of upper-limb spasticity after conventional rTMS in patients after a stroke 4,7,8. However, all previous studies were open-labeled studies such that the researchers and participants all knew which stimulation was being administered, or the outcome measurements in these studies were limited to clinical or electrophysiological measures. Many measurements of spasticity were introduced by researchers. These measurements could be classified into clinical, electrophysiological, and biomechanical measures. Among these, biomechanical measurements may offer better quantification of spasticity compared with clinical and electrophysiological measurements 12,13 because clinical measures are highly dependent on the examiner’s judgment 14 and because electrophysiological measures have high variability and low reliability 15. This is the first randomized sham-controlled study to quantitatively assess the effect of rTMS on spasticity using biomechanical measurements.
A significant improvement was found in clinical and biomechanical assessments after iTBS. However, we failed to observe a reduction of spasticity as assessed by electrophysiological measurement. The lack of electrophysiological changes may be attributed to several factors. First, the H reflex has high variability and low reliability for measuring spasticity 12,15. Second, the stimulation intensity during active iTBS (80% active motor threshold of FCR or 50% of maximal stimulation output) was lower than the intensity to induce electrophysiological changes in the studies with conventional rTMS, which showed positive effects on spasticity. Huang et al. 10 also reported similar findings with TBS that continuous TBS had no effect on H reflexes evoked in FCR, whereas MEPs were suppressed in normal individuals. Third, the interneurons at the spinal level, which are not directly involved in the H reflex, may play a much larger role in spasticity reduction after iTBS 3. Fourth, the symptoms related to the spasticity were similar irrespective of underlying diseases, but different mechanisms in spasticity may occur depending on the specific underlying disease of the central nervous system 3. A reduction in the H/M ratio has been reported following iTBS stimulation of the motor cortex in multiple sclerosis patients 2, but no changes in the H/M ratio were reported in patients with spinal-cord injury 3.
We also found that the reduction in spasticity lasted less than 30 min after a single session of iTBS. These results were in line with the studies measuring the upper-limb motor performance after iTBS in patients after a stroke 16,17. Talelli et al. 16 reported that a single session of iTBS reduced the reaction time by up to 30 min, but changes in MEP size had a duration of ∼0 min. Huang et al. 18 also observed the transient changes in MEP size for 20 min after iTBS 18. These results support the use of iTBS in future longer-term trials for the modulation of spasticity after a stroke.
The mechanism underlying the anti-spastic effect of iTBS remains to be determined. iTBS is well-known to modulate cortical excitability 10, and the increased neural activity of the corticospinal tract after iTBS may project to local inhibitory interneurons of the spinal cord. The corticospinal tract has widespread terminations in the spinal gray matter, thereby controlling motor neurons through monosynaptic but also polysynaptic connections involving local interneurons and sensory afferents 19. iTBS may also result in changes in the levels of endogenous neurotransmitters such as γ-aminobutyric acid, glutamate, and dopamine, which are all involved in synaptic plasticity 19. Although the mechanisms underlying the effect of iTBS are not yet completely understood, it is increasingly accepted that they could be analogous to long-term potentiation plastic changes recorded in neurons of animal models. Further studies are needed to explore this issue.
Conclusion
iTBS may be useful to reduce spasticity transiently in post-stroke patients. The potential clinical relevance is unclear, but TBS with a shorter stimulation time relative to conventional rTMS may offer a clinical advantage in the treatment of spasticity. Finally, further studies are necessary to explore the underlying mechanisms of iTBS, to induce longer carry-over effects, and to establish the benefits in clinical settings.
Acknowledgements
The authors would like to thank Kyunghwa Han, PhD (Biostatistician), for her help with the statistical analysis.
Author contributions: Deog Young Kim designed the experimental plan and prepared the manuscript; Dae Hyun Kim collected and analyzed the data; Tae-Min Jung helped to conduct the experiment and Ji Cheol Shin and Seungsoo Jung supervised this work.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mori F, Koch G, Foti C, Bernardi G, Centonze D. The use of repetitive transcranial magnetic stimulation (rTMS) for the treatment of spasticity. Prog Brain Res 2009; 175:429–439. [DOI] [PubMed] [Google Scholar]
- 2.Mori F, Codeca C, Kusayanagi H, Monteleone F, Boffa L, Rimano A, et al. Effects of intermittent theta burst stimulation on spasticity in patients with multiple sclerosis. Eur J Neurol 2010; 17:295–300. [DOI] [PubMed] [Google Scholar]
- 3.Kumru H, Murillo N, Samso JV, Valls-Sole J, Edwards D, Pelayo R, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair 2010; 24:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakuda W, Abo M, Kobayashi K, Momosaki R, Yokoi A, Fukuda A, et al. Anti-spastic effect of low-frequency rTMS applied with occupational therapy in post-stroke patients with upper limb hemiparesis. Brain Inj 2011; 25:496–502. [DOI] [PubMed] [Google Scholar]
- 5.Barros Galvao SC, Borba Costa dos Santos R, Borba dos Santos P, Cabral ME, Monte-Silva K. Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper-limb spasticity in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil 2014; 95:222–229. [DOI] [PubMed] [Google Scholar]
- 6.Kakuda W, Abo M, Kobayashi K, Momosaki R, Yokoi A, Fukuda A, et al. Low-frequency repetitive transcranial magnetic stimulation and intensive occupational therapy for poststroke patients with upper limb hemiparesis: preliminary study of a 15-day protocol. Int J Rehabil Res 2010; 33:339–345. [DOI] [PubMed] [Google Scholar]
- 7.Kondo T, Kakuda W, Yamada N, Shimizu M, Abo M. Effects of repetitive transcranial magnetic stimulation and intensive occupational therapy on motor neuron excitability in poststroke hemiparetic patients: a neurophysiological investigation using F-wave parameters. Int J Neurosci 2015; 125:25–31. [DOI] [PubMed] [Google Scholar]
- 8.Wupuer S, Yamamoto T, Katayama Y, Motohiko H, Sekiguchi S, Matsumura Y, et al. F-wave suppression induced by suprathreshold high-frequency repetitive transcranial magnetic stimulation in poststroke patients with increased spasticity. Neuromodulation 2013; 16:206–211. Discussion 211. [DOI] [PubMed] [Google Scholar]
- 9.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 1998; 108:1–16. [DOI] [PubMed] [Google Scholar]
- 10.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005; 45:201–206. [DOI] [PubMed] [Google Scholar]
- 11.Haugh AB, Pandyan AD, Johnson GR. A systematic review of the Tardieu scale for the measurement of spasticity. Disabil Rehabil 2006; 28:899–907. [DOI] [PubMed] [Google Scholar]
- 12.Pisano F, Miscio G, del Conte C, Pianca D, Candeloro E, Colombo R. Quantitative measures of spasticity in post-stroke patients. Clin Neurophysiol 2000; 111:1015–1022. [DOI] [PubMed] [Google Scholar]
- 13.Pierce SR, Lauer RT, Shewokis PA, Rubertone JA, Orlin MN. Test-retest reliability of isokinetic dynamometry for the assessment of spasticity of the knee flexors and knee extensors in children with cerebral palsy. Arch Phys Med Rehabil 2006; 87:697–702. [DOI] [PubMed] [Google Scholar]
- 14.Kim DY, Park CI, Chon JS, Ohn SH, Park TH, Bang IK. Biomechanical assessment with electromyography of post-stroke ankle plantar flexor spasticity. Yonsei Med J 2005; 46:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voerman GE, Gregoric M, Hermens HJ. Neurophysiological methods for the assessment of spasticity: the Hoffmann reflex, the tendon reflex, and the stretch reflex. Disabil Rehabil 2005; 27:33–68. [DOI] [PubMed] [Google Scholar]
- 16.Talelli P, Greenwood RJ, Rothwell JC. Exploring theta burst stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol 2007; 118:333–342. [DOI] [PubMed] [Google Scholar]
- 17.Ackerley SJ, Stinear CM, Barber PA, Byblow WD. Priming sensorimotor cortex to enhance task-specific training after subcortical stroke. Clin Neurophysiol 2014; 125:1451–1458. [DOI] [PubMed] [Google Scholar]
- 18.Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex 2008; 18:563–570. [DOI] [PubMed] [Google Scholar]
- 19.Mori F, Ljoka C, Magni E, Codeca C, Kusayanagi H, Monteleone F, et al. Transcranial magnetic stimulation primes the effects of exercise therapy in multiple sclerosis. J Neurol 2011; 258:1281–1287. [DOI] [PubMed] [Google Scholar]


