Abstract
Background
Maternal air pollution exposure has been related to orofacial clefts but the literature is equivocal. Potential chronic preconception effects have not been studied.
Objectives
Criteria air pollutant exposure during three months preconception and gestational weeks 3–8 was studied in relation to orofacial defects.
Methods
Among 188,102 live births and fetal deaths from the Consortium on Safe Labor (2002–2008), 63 had isolated cleft palate (CP) and 159 had isolated cleft lip with or without cleft palate (CL ± CP). Exposures were estimated using a modified Community Multiscale Air Quality model. Logistic regression with generalized estimating equations adjusted for site/region and maternal demographic, lifestyle and clinical factors calculated the odds ratio (OR) and 95% CI per interquartile increase in each pollutant.
Results
Preconception, carbon monoxide (CO; OR = 2.24; CI: 1.21, 4.16) and particulate matter (PM) ≤10 μm (OR = 1.72; CI: 1.12, 2.66) were significantly associated with CP, while sulfur dioxide (SO2) was associated with CL ± CP (OR = 1.93; CI: 1.16, 3.21). During gestational weeks 3–8, CO remained a significant risk for CP (OR = 2.74; CI: 1.62, 4.62) and nitrogen oxides (NOx; OR = 3.64; CI: 1.73, 7.66) and PM ≤2.5 μm (PM2.5; OR = 1.74; CI: 1.15, 2.64) were also related to the risk. Analyses by individual week revealed that positive associations of NOx and PM2.5 with CP were most prominent from weeks 3–6 and 3–5, respectively.
Conclusions
Exposure to several criteria air pollutants preconception and during early gestation was associated with elevated odds for CP, while CL ± CP was only associated with preconception SO2 exposure.
Keywords: Air pollution, Preconception exposure, Organogenesis, Cleft palate, Cleft lip
1. Introduction
A steadily growing body of literature has implicated maternal exposure to air pollution as a potential causal factor in offspring adverse birth outcomes, including infant mortality, low birth-weight, and preterm birth (Proietti et al., 2013; Shah et al., 2011; Stieb et al., 2012). Emerging data also suggests a link between air pollution and congenital anomalies (E.K. Chen et al., 2014; Vrijheid et al., 2011). A leading cause of infant mortality (Matthews and MacDorman, 2013), congenital anomalies also contribute significantly to childhood and adult morbidity. Moreover, given that ambient air pollution affects large populations and is difficult to modify at the individual level, it is of great public health significance to improve our understanding of the associations between air pollutants and congenital anomalies.
Orofacial defects (i.e., cleft palate and cleft lip with or without cleft palate) are common but have received less attention than congenital heart defects in studies of air pollution. Positive associations between air pollutants and orofacial defects have been observed (Gilboa et al., 2005; Hansen et al., 2009; Hwang and Jaakkola, 2008; Marshall et al., 2010), but no significant pooled associations were found in meta-analyses (E.K. Chen et al., 2014; Vrijheid et al., 2011). The overall null associations may be attributable to the heterogeneity in outcome ascertainment and exposure assessment, varied confounders, and the small number of studies.
Among the nine previous studies on air pollution and orofacial clefts, eight were single-region studies with relatively small geographic coverages (Gilboa et al., 2005; Hansen et al., 2009; Hwang and Jaakkola, 2008; Marshall et al., 2010; Padula et al., 2013; Rankin et al., 2009; Ritz et al., 2002; Schembari et al., 2014) except for one covering four regions in England (Dolk et al., 2010). In addition, most previous studies averaged air pollutant levels over the organogenesis period from gestational weeks 3–8 (Gilboa et al., 2005; Hansen et al., 2009; Marshall et al., 2010; Schembari et al., 2014); others used monthly (Hwang and Jaakkola, 2008; Ritz et al., 2002), trimester (Padula et al., 2013; Rankin et al., 2009) or annual averages (Dolk et al., 2010), which might mask the temporal associations between air pollutants and orofacial defects. Moreover, given that teratogen exposure before pregnancy can be associated with increased risk of congenital anomalies (Shaw et al., 1999; Sun et al., 2014), the investigation of potential preconception effects of air pollution on orofacial defects is warranted but has not been addressed in previous studies.
Therefore, the objective of this study was to examine the associations of maternal exposure to criteria air pollutants [carbon monoxide (CO), nitrogen oxides (NOx), ozone (O3), particulate matter with aerodynamic diameter ≤2.5 and 10 μm (PM2.5 and PM10), and sulfur dioxide (SO2)] with risks of orofacial defects in a large, contemporary, multi-site/region US cohort. The exposure windows of interest were three months preconception and early gestation, including both an average over weeks 3–8 of gestation to be comparable to previous studies and an exploration of individual weekly averages from weeks 1 through 10 given that the lip and palate form between weeks 5–9 of gestation (Nanci, 2012).
2. Methods
2.1. Study population and outcome
The Consortium on Safe Labor (CSL) is a retrospective cohort study of labor and delivery conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. As previously described in detail (Zhang et al., 2010), data on maternal demographic characteristics, medical history, labor, delivery, and obstetric and neonatal outcomes of 228,562 deliveries (233,736 live births and fetal deaths) at ≥23 weeks of gestation (January 1, 2002 to January 31, 2008) were extracted from electronic medical records. Newborn discharge diagnoses, in International Classification of Diseases-9 (ICD-9) codes, were linked to each infant. The study was approved by the institutional review boards of all participating institutions, whose names and locations can be found in the acknowledgments.
Infants with missing discharge summaries and no ICD-9 code data (n = 28,753), chromosomal anomalies (n = 424), and congenital anomalies other than orofacial defects (n = 16,457) were excluded, rendering a pool of 188,102 live births and fetal deaths. Determination of orofacial defect status for each infant was obtained via ICD-9 discharge codes (see Supplemental material, Table S1). Each case without any additional major defects was classified as isolated, although there could have been minor defects as de-fined by the National Birth Defects Prevention Study guidelines (Rasmussen et al., 2003). Infants with orofacial defects who also had at least one other major defect either in the same or a different organ system constitute the multiple groupings.
2.2. Exposure assessment
Due to the anonymity of the CSL data, maternal ambient air pollution exposures were based on the average air pollutant concentrations in her delivery hospital referral region (415–312,644 km2) (The Dartmouth Atlas of Health Care, 2013) during each of the specified exposure windows. A modified version of the Community Multi-scale Air Quality (CMAQ) model 4.7.1 (Foley et al., 2010) was used to estimate criteria air pollutant levels in the 15 non-overlapping hospital referral regions involved in the CSL with a 36-km horizontal resolution domain (G. Chen et al., 2014). The CMAQ simulations were based on meteorology data derived by the Weather Research and Forecasting model and emission data by National Emissions Inventories provided by the US Environmental Protection Agency (US EPA), respectively, and model results were weighted to reflect population density within the hospital referral region, discounting areas where women were unlikely to live and work.
Despite the wide use of the CMAQ model in estimating regional air quality, potential biases in meteorology and emission inputs, uncertainties of other model components, and issues with spatial resolution can compromise the precision in estimation (G. Chen et al., 2014). Thus, we used an inverse distance weighting based method to adjust raw CMAQ estimations using observational air quality data retrieved from the US EPA Air Quality System. This observation-fused technique led to significant improvement of the model performance and was demonstrated to best account for spatial variation in air pollutants and population density as compared to four other exposure estimation methods (G. Chen et al., 2014). This analysis included a three-month preconception period window, a 6-week average during organogenesis at weeks 3–8 of gestation, and weekly averages for weeks 1–10 of gestation. Gestational age in weeks was calculated from gestational age at delivery using the best obstetrical estimate as recorded in the medical record.
2.3. Statistical methods
Descriptive statistics for subject characteristics were presented as percentages for categorical variables. Differences in subject characteristics between each case group and controls were assessed by Fisher’s exact test. Distributions of air pollutant concentrations were presented by quartile and interquartile range (IQR) averaged over three months preconception and weeks 3–8 of gestation. Logistic regression models were fitted to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for orofacial defects per IQR increase for each air pollutant. Outcomes of interest (isolated/multiple cleft palate and cleft lip with or without cleft palate) were analyzed separately with each of the exposure windows of interest. Generalized estimating equations were used to calculate robust standard errors accounting for clustering due to multiple pregnancies of the same woman (3.9% women contributed more than one pregnancy). We selected a priori covariates including site/region, maternal age (<20, 20–24, 25–29, 30–34, ≥35 years), race/ethnicity (White, Black, Hispanic, other/unknown), marital status (married or not), insurance (private, public or other/none), prepregnancy body mass index (BMI; <18.5, 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2 or missing), nulliparity (yes or no), season of conception (spring, summer, fall, winter), smoking and/or alcohol consumption during pregnancy (yes or no), multiple birth (yes or no), preexisting or gestational diabetes mellitus (yes or no). Covariates were missing for <5% of the study population except prepregnancy BMI which was missing for 36.2%. We included an indicator level for missing data for categorical covariates, if necessary. Given that the CMAQ model accounts for biochemical reactions among air pollutants, effects of weather, and long-term sources of pollutants (Foley et al., 2010), air pollutants were fitted in the model separately during each exposure window of interest.
To evaluate the robustness of the findings, we performed sensitivity analyses excluding multiple gestation pregnancies and infants/fetuses born to women with preexisting or gestational diabetes, respectively. We further performed simulation extrapolation procedures (Cook and Stefanski, 1994) to correct for the potential exposure misclassification assuming a measurement error rate of 10% or 20% within each hospital referral region, respectively. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
Among 188,102 infants/fetuses, we identified 63 with isolated cleft palate, 159 isolated cleft lip with or without cleft palate, and 187,819 without any congenital malformations (Table 1). In addition, 26/35 had multiple cleft palate/cleft lip with or without cleft palate (see Supplemental material, Table S2). Infants with isolated cleft palate were more likely to be born to women who smoked during pregnancy (12.7 vs. 6.4%) as compared to non-malformed controls (n = 187,819). The cleft lip with or without cleft palate group were more likely to be White and less likely to be Black than controls. In addition, multiple gestations and male births were more prevalent in the isolated cleft lip group. The distribution of criteria air pollutants concentrations averaged over three months preconception and weeks 3–8 of gestation by orofacial defect grouping are presented in Table 2. Pairwise spearman correlation coefficients showed that all air pollutants were significantly correlated with each other both in the preconception and early gestation exposure windows (see Supplemental material, Table S3). The high correlation between CO and NOx (rho = 0.74 for both time windows) may reflect the common source of emissions from transport, whereas the negative correlations of O3 with other pollutants (except PM10) is likely a function of the chemical reactions among air pollutants.
Table 1.
Subject characteristics by isolated orofacial defect status for live births and fetal deaths from the Consortium of Safe Labor (n = 188,102), 2002–2008a
| Maternal age, years | Controls (n = 187,819) |
Isolated cleft palate (n = 63) |
Isolated cleft lip with or without cleft palate (n = 159) |
|---|---|---|---|
| <20 | 16160 (8.6) | 4 (6.3) | 17 (10.7) |
| 20–24 | 46503 (24.8) | 18 (28.6) | 44 (27.7) |
| 25–29 | 52916 (28.2) | 14 (22.2) | 47 (29.6) |
| 30–35 | 43400 (23.1) | 16 (25.4) | 31 (19.5) |
| ≥35 | 28546 (15.2) | 11 (17.5) | 20 (12.6) |
| Missing | 391 (0.2) | 0 (0.0) | 0 (0.0) |
| Race/ethnicity | |||
| White | 106178 (56.5) | 42 (66.7) | 106 (66.7) |
| Black | 36512 (19.4) | 7 (11.1) | 13 (8.2) |
| Hispanic | 25637 (13.6) | 11 (17.5) | 26 (16.4) |
| Other | 10784 (5.7) | 2 (3.2) | 11 (6.9) |
| Missing/unknown | 8708 (4.6) | 1 (1.6) | 3 (1.9) |
| Married/living with a partner | 117237 (62.4) | 41 (65.1) | 104 (65.4) |
| Private insurance | 116504 (62.0) | 36 (57.1) | 90 (56.6) |
| Smoking during pregnancy | 12007 (6.4) | 8 (12.7) | 15 (9.4) |
| Alcohol consumption during pregnancy | 3444 (1.8) | 2 (3.2) | 1 (0.6) |
| Prepregnancy body mass index, kg/m2 | |||
| <18.5 | 6672 (3.6) | 2 (3.2) | 4 (2.5) |
| 18.5–24.9 | 65304 (34.8) | 25 (39.7) | 60 (37.7) |
| 25–29.9 | 26445 (14.1) | 12 (19.0) | 24 (15.1) |
| ≥30.0 | 21479 (11.4) | 6 (9.5) | 26 (16.4) |
| Missing | 67919 (36.2) | 18 (28.6) | 45 (28.3) |
| Nulliparity | 74899 (39.9) | 25 (39.7) | 55 (34.6) |
| Season of conception | |||
| Spring (Mar–May) | 43818 (23.3) | 10 (15.9) | 16 (17.8) |
| Summer (Jun–Aug) | 48526 (25.8) | 22 (34.9) | 29 (32.2) |
| Fall (Sep–Nov) | 52343 (27.9) | 17 (27.0) | 22 (24.4) |
| Winter (Dec–Feb) | 43132 (23.0) | 14 (22.2) | 23 (25.6) |
| Multiple birth | 7255 (3.9) | 2 (3.2) | 15 (9.4) |
| Infant sex, male | 95106 (50.6) | 26 (41.3) | 94 (59.1) |
| Preexisting diabetes | 3573 (1.9) | 3 (4.8) | 2 (1.3) |
| Gestational diabetes | 5967 (3.2) | 1 (1.6) | 7 (4.4) |
Values are n (%).
Table 2.
Concentrations of criteria air pollutants by orofacial defect status during the three months preconception and weeks 3–8 of gestation for live births and fetal deaths from the Consortium of Safe Labor (n = 188, 102), 2002–2008.
| 3 months preconception
|
Weeks 3–8 of gestation
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | IQR | 25th | 50th | 75th | IQR | |
| CO (ppm) | ||||||||
| Controls | 0.40 | 0.51 | 0.67 | 0.27 | 0.39 | 0.52 | 0.69 | 0.30 |
| Isolated cleft palate | 0.41 | 0.47 | 0.72 | 0.30 | 0.42 | 0.54 | 0.82 | 0.40 |
| Isolated cleft lip with or without cleft lip | 0.39 | 0.47 | 0.61 | 0.23 | 0.37 | 0.52 | 0.68 | 0.31 |
| NOx (ppb) | ||||||||
| Controls | 15.88 | 24.42 | 42.12 | 26.23 | 15.54 | 24.80 | 45.46 | 29.92 |
| Isolated cleft palate | 15.97 | 24.79 | 38.94 | 22.97 | 18.92 | 25.56 | 55.89 | 36.97 |
| Isolated cleft lip with or without cleft lip | 15.45 | 23.50 | 32.29 | 16.84 | 15.58 | 22.99 | 44.84 | 29.25 |
| O3 (ppb) | ||||||||
| Controls | 24.00 | 30.38 | 36.38 | 12.38 | 22.24 | 30.08 | 36.53 | 14.29 |
| Isolated cleft palate | 27.51 | 33.39 | 40.70 | 13.19 | 25.35 | 32.49 | 39.53 | 14.18 |
| Isolated cleft lip with or without cleft lip | 26.84 | 34.32 | 41.32 | 14.48 | 25.33 | 32.57 | 39.82 | 14.49 |
| PM10 (ug/m3) | ||||||||
| Controls | 18.36 | 21.45 | 24.94 | 6.58 | 17.99 | 21.46 | 25.44 | 7.45 |
| Isolated cleft palate | 19.35 | 23.63 | 26.75 | 7.40 | 17.64 | 23.27 | 27.54 | 9.90 |
| Isolated cleft lip with or without cleft lip | 18.36 | 21.38 | 24.70 | 6.34 | 19.12 | 22.37 | 25.48 | 6.35 |
| PM2.5 (ug/m3) | ||||||||
| Controls | 9.20 | 11.74 | 14.38 | 5.18 | 8.89 | 11.73 | 14.47 | 5.58 |
| Isolated cleft palate | 7.48 | 10.37 | 13.21 | 5.74 | 7.61 | 11.70 | 14.95 | 7.34 |
| Isolated cleft lip with or without cleft lip | 7.19 | 10.93 | 12.79 | 5.60 | 7.65 | 10.61 | 13.93 | 6.29 |
| SO2 (ppb) | ||||||||
| Controls | 2.13 | 3.45 | 5.59 | 3.46 | 2.08 | 3.41 | 5.56 | 3.48 |
| Isolated cleft palate | 2.00 | 2.62 | 3.97 | 1.97 | 1.95 | 2.59 | 3.86 | 1.90 |
| Isolated cleft lip with or without cleft lip | 1.96 | 2.61 | 5.14 | 3.18 | 1.95 | 2.65 | 5.28 | 3.33 |
Abbreviation: IQR, interquartile range.
After adjustment for site/region and maternal demographic, lifestyle and clinical factors, maternal exposure to CO was consistently associated with an increased risk of isolated cleft palate during both preconception (OR = 2.24; 95% CI: 1.21, 4.16 per IQR increase) and early gestation (OR = 2.74; 95% CI: 1.62, 4.62) exposure windows (Table 3). Exposure to PM10 during the three months preconception, but not in early gestation, was positively associated with the risk of cleft palate (OR = 1.72; 95% CI: 1.12, 2.66). During weeks 3–8 of gestation, increased odds of cleft palate were also observed for NOx (OR = 3.64; 95% CI: 1.73, 7.66) and PM2.5 (OR = 1.74; 95% CI: 1.15, 2.64). In addition, a significant and positive association between cleft lip with or without cleft palate and SO2 was observed during three months preconception (OR = 1.93; 95% CI: 1.16, 3.21) but not weeks 3–8 of gestation (OR = 1.03; 95% CI: 0.60, 1.74). We observed similar associations in sensitivity analyses excluding women with multiple gestations and preexisting/gestational diabetes, respectively (data not shown). As for multiple orofacial defects, we observed overall null associations with air pollutants except for NOx which was positively associated with multiple cleft palate (OR = 3.77; 95% CI: 1.36, 10.5; data not shown). When we examined the potential impact of 10–20% exposure misclassification using simulation extrapolation procedures, we found similar results for preconception CO exposure and risk of isolated cleft palate (see Supplemental material, Table S4 and Fig. S3).
Table 3.
Adjusted ORs (95% CIs) of orofacial defects per IQR increment of criteria air pollutants in three months preconception and weeks 3–8 of gestation, the Consortium on Safe Labor study (n = 188,102), 2002–2008a
| Air pollutants | Isolated cleft palate (n = 63)
|
Isolated cleft lip with or without cleft palate (n = 159)
|
||
|---|---|---|---|---|
| 3 months preconception | Weeks 3–8 of gestation | 3 months preconception | Weeks 3–8 of gestation | |
| CO | 2.24 (1.21, 4.16) | 2.74 (1.62, 4.62) | 1.14 (0.76, 1.70) | 1.43 (0.98, 2.10) |
| NOx | 1.58 (0.70, 3.55) | 3.64 (1.73, 7.66) | 0.89 (0.53, 1.50) | 1.37 (0.88, 2.13) |
| O3 | 0.53 (0.23, 1.23) | 0.73 (0.38, 1.44) | 1.35 (0.81, 2.27) | 1.01 (0.65, 1.57) |
| PM10 | 1.72 (1.12, 2.66) | 1.34 (0.82, 2.19) | 1.17 (0.85, 1.61) | 1.07 (0.84, 1.38) |
| PM2.5 | 1.14 (0.61, 2.13) | 1.74 (1.15, 2.64) | 1.03 (0.65, 1.65) | 0.97 (0.74, 1.27) |
| SO2 | 1.64 (0.52, 5.15) | 1.78 (0.53, 5.93) | 1.93 (1.16, 3.21) | 1.03 (0.60, 1.74) |
Abbreviation: IQR, interquartile range.
Adjusted for site/region, maternal age (<20, 20–24, 25–29, 30–34, ≥35 years), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other/unknown), marital status (married/living with a partner or not), insurance (private or other/none), prepregnancy BMI (<18.5, 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2 or missing), nulliparity (yes or no), season of conception (spring, summer, fall, winter), smoking and/or alcohol consumption during pregnancy (yes or no), multiple birth (yes or no), preexisting or gestational diabetes mellitus (yes or no).
Further exploration by individual gestational week revealed that CO exposure was consistently and positively associated with risk of isolated cleft palate from gestational weeks 3 through 10 with associations being most prominent at week 4 (Fig. 1). In contrast, the risk for cleft lip with or without cleft palate was associated with a less consistent pattern of weekly risk for CO exposure with significant elevated risks for exposure at weeks 3, 5 and 10. Exposure to NOx and PM2.5 were associated with increased odds of isolated cleft palate primarily during the first few weeks of gestation (i.e. weeks 3–6 and 3–5 for NOx and PM2.5, respectively). No weekly variability in the odds of isolated orofacial defects was observed for other air pollutants (see Supplemental material, Fig. S1).
Fig. 1.
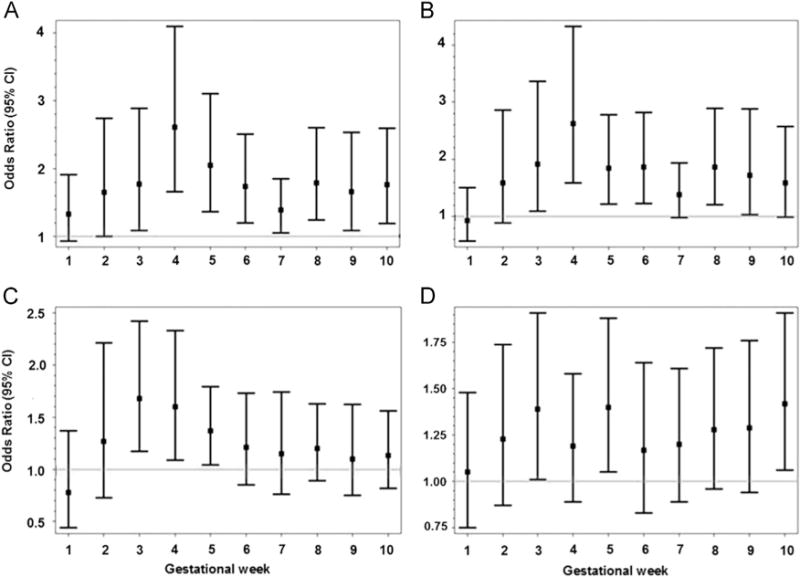
Adjusted ORs and 95% CIs for the associations between isolated orofacial defects and criteria air pollutants per interquartile range increase from week 1 through 10 of gestation, Consortium on Safe Labor study, 2002–2008. (A) CO and isolated cleft palate. (B) NOx and isolated cleft palate. (C) PM2.5 and isolated cleft palate. (D) CO and isolated cleft lip with or without palate. Each model was adjusted for site/region, maternal age, race/ethnicity, marital status, insurance, prepregnancy body mass index, nulliparity, season of conception, smoking and/or alcohol consumption during pregnancy, multiple birth, and preexisting or gestational diabetes.
4. Discussion
In this large retrospective cohort, we observed significant and positive associations between ambient air pollution and risk of orofacial defects in the offspring when mothers were exposed during three months preconception and the weeks during formation of the lip and palate. Specifically, exposures to CO, NOx, and PM2.5 during weeks 3–8 of gestation were positively associated with isolated cleft palate. As transport emissions are the major sources of these pollutants, our findings add to the growing body of epidemiological evidence regarding traffic-related air pollutants and adverse birth outcomes. Given that traffic pollution is relatively ubiquitous, it is of great public health significance to better understand the potential teratogenicity of this common exposure. On the other hand, exposures to air pollutants were not associated with multiple oral clefts except for a positive association between NOx and risk of multiple cleft palate. The null associations between air pollutants and multiple case groupings could be partially attributable to lack of sufficient statistical power with small case numbers.
We also observed for the first time the positive associations of exposures to CO and NOx with isolated cleft palate and SO2 with isolated cleft lip with or without cleft palate during the three months preconception exposure window, as a proxy of chronic exposure to air pollution. Previous data from animal studies demonstrated that chronic maternal exposure to CO during pregnancy can cause oxygen deficiency in utero and adversely affect organ development of the fetus (Garvey and Longo, 1978). However, such data on chronic preconception exposure are limited. One study on 370 women in France reported that maternal exposure to air pollution during the three months preconception period altered immunophenotypic profile in the cord blood (Baiz et al., 2011). Immune dysregulation, in turn, may be involved in disruptions of fetal organ development and result in an increased risk of congenital malformations (Cappon et al., 2003).
Examination of weekly air pollution exposure and risk of orofacial defects gives some additional insight into the variation in potential gestational week-specific windows of susceptibility, especially in the early stage of embryonic formation (i.e., weeks 3–5 of gestation). In spite of the borderline significant association between CO exposure over weeks 3–8 of gestation and isolated cleft lip with or without cleft palate, CO exposure was positively associated with cleft lip at weeks 3 and 5 of gestation, respectively. Findings suggest that future studies might benefit from examining the temporal variability in the associations between air pollution exposure and time-dependent outcomes such as birth defects, while using the averaged exposure over the whole window of interest could result in an attenuated overall association. In addition, our data suggest that weeks 3–6 and 3–5 of gestation appear to be important for NOx and PM2.5 exposure in association with risk of cleft palate, respectively. Of particular note, these susceptibility windows are prior to the fusion of palatine shelves and formation of the secondary palate at weeks 6–7 of gestation (Sadler, 2010), which indicates the temporal-specific susceptibility to air pollutants at particular stages of organogenesis. Additional studies are needed to replicate our findings.
In spite of accumulating data, previous findings on air pollution exposure and orofacial defects are equivocal and recent meta-analyses suggest null effects (E.K. Chen et al., 2014; Vrijheid et al., 2011). Among the four studies conducted in the US, two in California observed null associations between criteria air pollutants and orofacial defects (Padula et al., 2013; Ritz et al., 2002), whereas the other two reported positive associations of cleft lip with PM10 in Texas (29–45% increased risk) (Gilboa et al., 2005) and with SO2 (60% increase) and CO (40% increase) in New Jersey (Marshall et al., 2010) during weeks 3–8 of gestation. Although these significant quartile-specific associations are not directly comparable to ours, the range of exposures were generally similar in Texas and somewhat higher in New Jersey compared to our exposure levels. Studies outside the US also exhibited inconsistent findings with positive associations observed between cleft lip and O3 during the first two months of gestation in Taiwan (Hwang and Jaakkola, 2008) and between cleft lip and SO2 during weeks 3–8 of gestation in Australia (Hansen et al., 2009), in contrast to the null associations reported in two UK studies (Dolk et al., 2010; Rankin et al., 2009) and one study in Spain (Schembari et al., 2014). The effect sizes of the associations between air pollutant exposures during weeks 3–8 of gestation and oral clefts were greater in our study compared to previous data, although the confidence intervals of our estimates were wide and overlap with some prior positive findings. The differential effect sizes could be partially attributable to the variation in exposure distributions across studies. For instance, the mean concentrations of NOx during weeks 3–8 of gestation were much lower in Brisbane, Australia (Hansen et al., 2009) than in our study (8.2 vs. 30.4 ppb), the former of which observed a null association between maternal NO2 exposure at gestational weeks 3–8 and cleft palate (OR = 0.73; 95% CI: 0.46–1.15 per 4 ppb increase). It is possible that there is a threshold effect of air pollutants on health outcomes. In addition, the observation on CO exposure at gestational weeks 3–8 and cleft lip (OR = 1.4; 95% CI: 1.0–1.9 comparing the highest versus lowest quartile) in New Jersey was in line with our data (OR = 1.47; 95% CI: 1.01, 2.12 per IQR increase), although with a higher level of CO exposure during weeks 3–8 of gestation (mean concentrations: 0.85 ppm in New Jersey vs 0.56 ppm across sites/regions in our study). In sum, the inconsistency in previous findings could be attributed to (1) distinct geographic and meteorologic features of study regions (as shown in our study; see Supplemental material, Fig. S2); (2) different sources of air pollutants and composition of particulate matter; (3) differences in case ascertainment and exposure assignment; and (4) varied sociodemographic context of the study population. In spite of this variability, these studies are helpful to inform standard setting and encourage data harmonization (Ritz and Wilhelm, 2008).
Although there is a lack of well-established animal models to formulate a priori hypotheses, experimental data from animal studies suggest the potential teratogenicity of air pollutants is biologically plausible (Kannan et al., 2006). For instance, inhaled SO2 and CO may cause oxidative damage and induce dysmorphogenesis in multiple organs of mice, not limited to the respiratory system (Meng, 2003; Singh et al., 1993), while reactive oxygen species induced by oxidative stress may adversely disrupt cell signaling and gene transcription and result in embryopathic consequences (Wells et al., 2010). Other studies revealing associations of air pollutants with inflammation (Hajat et al., 2015), coagulation (Chen et al., 2015; Pekkanen et al., 2000), and endothelial function (Brook et al., 2003; Hajat et al., 2015) also provide biological rationale for the assessment of air pollution’s impact on congenital anomalies.
Our study has a number of notable strengths. The air pollution data was estimated using a modified CMAQ model fused with observed data which accounts for chemical reactions between air pollutants and the impact of weather and pollutant sources. The model exposure approach also allowed us to include women from areas without fixed-site monitoring stations. In contrast, most previous studies used observed air quality data from the nearest air monitors to maternal residence with a maximum radius ranging between 36–54 km and thus excluded births in areas where air monitors were not available. To our knowledge, our study is the first one in the US assessing the associations between air pollution and orofacial defects in a multi-site/region scale with a great geographic coverage, whereas the previous four were limited to a single state. Additionally, we reported for the first time potential effects of air pollution exposure during the preconception exposure window in addition to the vulnerable period of organogenesis at weeks 3–8 of gestation. Furthermore, the major advantage of our study was the ability to conduct weekly exposure analysis during weeks 1–10 of gestation in an effort to unmask temporal variability in the associations between air pollutants and orofacial defects.
Several limitations of the study should be noted. Data on stillbirths and terminated pregnancies before 23 weeks of gestation were not available. Also, we were unable to rule out the possibility of case misclassification and had no follow-up diagnoses beyond the delivery admission. However, orofacial defects are relatively easy to diagnose at birth and only a small percentage (7%) of isolated orofacial defects initially ascertained at birth were later reclassified to have other major associated anomalies at one-year follow-up (Rittler et al., 2011). In addition, the prevalence of total (i.e., isolated and multiple) cleft palate in our study was 4.78 per 10,000 live/stillborn births, slightly lower than the national prevalence of 5.77–6.45 per 10,000 live births at gestational weeks 20 or later (2004–2006) (Parker et al., 2010); whereas the study prevalence of total cleft lip with or without cleft palate (10.31 per 10,000 live/stillborn births) was comparable to the national prevalence of 8.74–10.89 per 10,000 live births. Misclassification of exposure is possible if maternal movement and migration occurred during the exposure window of interest in our study, which however, is unlikely to be associated with case status. Further, the mobility during pregnancy tends to be within short distances (median often <10 km) (Bell and Belanger, 2012) which would likely leave women in the same hospital referral region. Reassuringly, the simulation results illustrated that the positive and significant association between CO levels during three months preconception and risk of isolated cleft palate were robust under the assumption of 10–20% error in the exposure measurement. In this study, prepregnancy BMI was not available for all women but missing data were due to differences in medical record abstraction which are not likely to be related to air pollution or case status.
In conclusion, maternal exposure to ambient air pollution during both preconception and early gestation were significantly associated with an elevated risk of orofacial defects in the offspring, particularly for isolated cleft palate which exhibited an increase in risk associated with every pollutant studied except ozone. Weekly analyses illustrated that the magnitude of associations appeared to be most pronounced during early weeks of organogenesis for some pollutants (i.e., gestational weeks 3–5 and 3–6 for PM2.5 and CO, respectively). Our findings revealed positive associations between exposures to air pollution during the three months preconception period and orofacial defects, suggesting the need for future research to understand the potential adverse effects of chronic preconception exposure to air pollution on later embryo-fetal development.
Supplementary Material
Acknowledgments
We thank all the participating study centers involved in the Consortium on Safe Labor, including Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, NY; Me-troHealth Medical Center, Cleveland, OH; Summa Health System, Akron City Hospital, Akron, OH; University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, Texas. We also thank the EMMES Corporation, Rockville, MD that provided data coordination and the Texas A&M Supercomputing Facility and the Texas Advanced Computing Center that provided computing resources essential to completing the air quality simulations for exposure estimations in this study.
Funding sources: This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health; including Contract No. HHSN267200603425C (Consortium on Safe Labor), Contract No. HHSN275200800002I, and Task Order No. HHSN27500008 (Air Quality and Reproductive Health).
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2015.06.002.
Footnotes
Competing interests
The authors declare they have no actual or potential competing financial interests.
References
- Baiz N, et al. Maternal exposure to air pollution before and during pregnancy related to changes in newborn’s cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregnancy Childbirth. 2011;11:87. doi: 10.1186/1471-2393-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Belanger K. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol. 2012;22:429–438. doi: 10.1038/jes.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, et al. Air pollution: The “heart” of the problem. Curr Hypertens Rep. 2003;5:32–39. doi: 10.1007/s11906-003-0008-y. [DOI] [PubMed] [Google Scholar]
- Cappon GD, et al. Relationship between cyclooxygenase 1 and 2 selective inhibitors and fetal development when administered to rats and rabbits during the sensitive periods for heart development and midline closure. Birth Defects Res Part B-Dev Reprod Toxicol. 2003;68:47–56. doi: 10.1002/bdrb.10008. [DOI] [PubMed] [Google Scholar]
- Chen EK, et al. Effects of air pollution on the risk of congenital anomalies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014a;11:7642–7668. doi: 10.3390/ijerph110807642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, et al. Evaluation of observation-fused regional air quality model results for population air pollution exposure estimation. Sci Total Environment. 2014b;485:563–574. doi: 10.1016/j.scitotenv.2014.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, et al. Size-fractionated particulate air pollution and circulating biomarkers of inflammation, coagulation, and vasoconstriction in a panel of young adults. Epidemiology. 2015;26:328–336. doi: 10.1097/EDE.0000000000000273. [DOI] [PubMed] [Google Scholar]
- Cook JR, Stefanski LA. Simulation-Extrapolation Estimation in Parametric Measurement Error Models. Journal of the American Statistical Association. 1994;89:1314–1328. [Google Scholar]
- Dolk H, et al. Ambient air pollution and risk of congenital anomalies in England, 1991–1999. Occup Environ Med. 2010;67:223–227. doi: 10.1136/oem.2009.045997. [DOI] [PubMed] [Google Scholar]
- Foley KM, et al. Incremental testing of the Community Multiscale Air Quality (CMAQ) modeling system version 4.7. Geosci Model Dev. 2010;3:205–226. doi: 10.5194/gmd-10-1703-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey DJ, Longo LD. Chronic low level maternal carbon monoxide exposure and fetal growth and development. Biol Reprod. 1978;19:8–14. doi: 10.1095/biolreprod19.1.8. [DOI] [PubMed] [Google Scholar]
- Gilboa SM, et al. Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997–2000. Am J Epidemiol. 2005;162:238–252. doi: 10.1093/aje/kwi189. [DOI] [PubMed] [Google Scholar]
- Hajat A, et al. Long-term exposure to air pollution and markers of in-flammation, coagulation, and endothelial activation: a repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) Epidemiology. 2015 doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CA, et al. Ambient air pollution and birth defects in Brisbane, Australia. PLoS One. 2009;4:e5408. doi: 10.1371/journal.pone.0005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BF, Jaakkola JJ. Ozone and other air pollutants and the risk of oral clefts. Environ Health Perspect. 2008;116:1411–1415. doi: 10.1289/ehp.11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, et al. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006;114:1636–1642. doi: 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EG, et al. Oral cleft defects and maternal exposure to ambient air pollutants in New Jersey. Birth Defects Res A Clin Mol Teratol. 2010;88:205–215. doi: 10.1002/bdra.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews TJ, MacDorman MF. Infant mortality statistics from the 2010 period linked birth/infant death data set. Natl Vital Stat Rep. 2013;62:1–26. [PubMed] [Google Scholar]
- Meng Z. Oxidative damage of sulfur dioxide on various organs of mice: sulfur dioxide is a systemic oxidative damage agent. Inhal Toxicol. 2003;15:181–195. doi: 10.1080/08958370304476. [DOI] [PubMed] [Google Scholar]
- Nanci A. Ten Cate’s Oral Histology: Development, Structure, and Function. Mosby; St. Louis: 2012. [Google Scholar]
- Padula AM, et al. The association of ambient air pollution and traffic exposures with selected congenital anomalies in the San Joaquin valley of California. Am J Epidemiol. 2013;177:1074–1085. doi: 10.1093/aje/kws367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- Pekkanen J, et al. Daily concentrations of air pollution and plasma fibrinogen in London. Occup Environ Med. 2000;57:818–822. doi: 10.1136/oem.57.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti E, et al. Air pollution during pregnancy and neonatal outcome: a review. J Aerosol Med Pulm Drug Deliv. 2013;26:9–23. doi: 10.1089/jamp.2011.0932. [DOI] [PubMed] [Google Scholar]
- Rankin J, et al. Maternal exposure to ambient air pollutants and risk of congenital anomalies. Environ Res. 2009;109:181–187. doi: 10.1016/j.envres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, et al. Guidelines for case classification for the national birth defects prevention study. Birth Defects Research Part a-Clin Mol Teratol. 2003;67:193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- Rittler M, et al. Associated anomalies among infants with oral clefts at birth and during a 1-year follow-up. Am J Med Genet Part A. 2011;155A:1588–1596. doi: 10.1002/ajmg.a.34046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Wilhelm M. Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic Clin Pharmacol Toxicol. 2008;102:182–190. doi: 10.1111/j.1742-7843.2007.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, et al. Ambient air pollution and risk of birth defects in Southern California. Am J Epidemiol. 2002;155:17–25. doi: 10.1093/aje/155.1.17. [DOI] [PubMed] [Google Scholar]
- Sadler TW. Langman’s Medical Embryology. Lippincott Williams & Wilkins; Philadelphia, PA: 2010. [Google Scholar]
- Schembari A, et al. Traffic-related air pollution and congenital anomalies in Barcelona. Environ Health Perspect. 2014;122:317–323. doi: 10.1289/ehp.1306802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS, et al. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37:498–516. doi: 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Shaw GM, et al. Maternal pesticide exposure from multiple sources and selected congenital anomalies. Epidemiology. 1999;10:60–66. [PubMed] [Google Scholar]
- Singh J, et al. Teratogenicity and developmental toxicity of carbon monoxide in protein-deficient mice. Teratology. 1993;48:149–159. doi: 10.1002/tera.1420480209. [DOI] [PubMed] [Google Scholar]
- Stieb DM, et al. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Sun Y, et al. Trimethoprim use before pregnancy and risk of congenital malformation: reanalyzed using a case-crossover design and a case-time-control design. Pharmacoepidemiol Drug Saf. 2014;23:1076–1083. doi: 10.1002/pds.3691. [DOI] [PubMed] [Google Scholar]
- The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice. 2013 [Google Scholar]
- Vrijheid M, et al. Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environ Health Perspect. 2011;119:598–606. doi: 10.1289/ehp.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells PG, et al. Oxidative DNA damage and repair in teratogenesis and neurodevelopmental deficits. Birth Defects Res C Embryo Today. 2010;90:103–109. doi: 10.1002/bdrc.20177. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326e1–326e10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


