Abstract
Background
Acute severe ulcerative colitis (ASUC), the most aggressive presentation ulcerative colitis (UC), occurs in 15 percent of adults and children with UC. First line therapy with intravenous corticosteroids is ineffective in half of adults and one third of children. Therapeutic monoclonal antibodies against TNF (anti-TNF therapy) are emerging as a common treatment for ASUC due to their similar efficacy to calcineurin inhibitors and more favorable adverse effect profile.
Aim
To comprehensively review the evidence for anti-TNF therapy for ASUC in children and adults with regard to outcomes and pharmacokinetics.
Methods
PubMed and recent conference proceedings were searched using the terms “ulcerative colitis”, “acute severe ulcerative colitis”, “anti-TNF”, “pharmacokinetics”, and the generic names of specific anti-TNF agents.
Results
Outcomes after anti-TNF therapy for ASUC remain suboptimal with aboutone half of children and adults undergoing colectomy. While several randomized controlled trials have demonstrated the efficacy of anti-TNF therapy for ambulatory patients with moderate to severely active UC, patients in these studies were less ill than those with ASUC. Patients with ASUC may exhibit more rapid clearance of anti-TNF biologics due pharmacokinetic mechanisms influenced by disease severity.
Conclusions
Conventional weight-based dosing effective in patients with moderately to severely active UC, may not be equally effective in those with ASUC. Personalized anti-TNF dosing strategies that integratepatient factors and early measures of pharmacokinetics and response hold promise for ensuring sustained drug exposure and maximizing early mucosal healing in patients with ASUC.
Keywords: ulcerative colitis, anti-TNF, tumor necrosis factor, pharmacokinetics
1. Introduction
Ulcerative colitis (UC) affects approximately 600,000 individuals in the United States, 20,000 of whom are children.1,2UC is clearly a global disease, as its incidence is rising in nations around the world.3Furthermore, as previously low-incident countries become more developed, the rate of inflammatory bowel disease (IBD) increases beginning with the emergence of increased UC cases.4 Across various cohorts, between 14 and 47% of adults with UC will develop pan-colitis and 12-15% will develop aggressive or severe disease requiring hospitalization.5,6In contrast, pan-colitis occurs in 80% of children with UC, a much higher frequency than in adults, with 15% exhibiting severe disease.7Intravenous (IV) corticosteroids are first line treatment for acute severe UC (ASUC) requiring hospitalization in children and adults. Approximately one third of children and one half of adults hospitalized for acute severe UC (ASUC) will prove refractory to IV corticosteroids.8-11Therapeutic monoclonal antibodies against tumor necrosis factor (anti-TNF therapy) are emerging as the predominant treatment for ASUC refractory to IV corticosteroids; however, colectomy rates still remain high.9Approximately 30% of adults with ASUC undergo colectomy within 60 days of admission.12In children with ASUC, 10% undergo colectomy prior to discharge, with a cumulative colectomy rate at 1 year of 20%.9This review will focus on the evidence supporting the use of anti-TNF therapy for ASUC, limitations of previous large randomized clinical trials with regard to ASUC, and how understanding the pharmacokinetics (PK) and pharmacodynamics (PD) of anti-TNF biologics can lead to improvements in how we use this class of drugs to treat ASUC.
2. Treatment of Steroid-Refractory ASUC Before Anti-TNF Therapy
In 1992, a landmark randomized controlled trial of the calcineurin inhibitor cyclosporine for ASUC refractory to IV corticosteroids was ended early after enrollment of only 20 patients due to an 82 percent response rate in the treatment arm compared to 0 percent in the placebo arm.13Due to frequent serious adverse effects association with chronic cyclosporine use (hypertension, hyperkalemia, neuropathies, and infection) it is generally used as a bridge therapy to thiopurines,with the colectomy rates in the subsequent 18 months remaining high at 34 percent.14The macrolide calcineurin inhibitor tacrolimus has also been an appealing option for the treatment of ASUC given the growing comfort with the drug for the prevention of transplant rejection, and its more favorable adverse effect profile and oral bioavailability compared to cyclosporine. In an open-label single arm trial in children with ASUC, 69 percent responded to tacrolimus, but 44 of responders underwent colectomy by 1 year.15 In a randomized controlled trial in adults with ASUC, tacrolimus induced a clinical response in 50 percent of patients and mucosal healing in 44 percent.16 While calcineurin inhibitors are used in many centers for the treatment of ASUC, treatment with anti-TNF biologics has become more common due to their more favorable adverse effect profile, indication as a maintenance therapy, and familiarity with the drug for the treatment of less severe UC and Crohn’s disease (CD). In fact, a long-awaited head-to-head randomized controlled trial comparing cyclosporine to infliximab for the treatment of ASUC in adults demonstrated a similar rates of treatment failure by Day 7 of 60 and 54 percent in the cyclosporine and infliximab arms, respectively.17Interestingly, a key feature of calcineurin inhibitor treatment in ASUC, which has not been generally been employed with anti-TNF biologics,is the measurement of trough drug levels to optimize and individualize dosing.18,19The evaluation of individual pharmacokinetic parameters may explain reported response rates as high as 80-90% with calcineurin inhibitors, and may be an important lesson that can be applied to maximize responses to anti-TNF therapy.13,19
3. Randomized Controlled Trials of Anti-TNF biologics for Moderate to Severely Active UC: Relevance to ASUC
Infliximab treatment regimens used for ASUC are based on those in the Active Ulcerative Colitis Trials (ACT) 1 and 2, which demonstrated efficacy in moderate to severely active ulcerative colitis in adults. Across both trials, clinical remission was achieved in 34-40 percent and mucosal healing in 60-62 percent of patients at week 8.20However, the patient population treated in ACT 1 and 2 were very different from patients ASUC. Fifty-six percent of patients in ACT 1 and 2 had only left-sided disease, and on average patients had a Mayo score consistent with moderate disease severity. Similarly, outcomes after infliximab in children hospitalized ASUC are not reflected by the major pediatric randomized controlled trial of infliximab for moderate to severely active UC, which demonstrated 40 and 68 percent remission and mucosal healing rates, respectively.21While most patientsin this trialdid have extensive involvement (extensive disease is more common in children), children with ASUC were specifically excluded, and 75% of the children with a clinical response at 8 weeks exhibitedonly moderate disease severity.Two subcutaneous anti-TNF biologics, adalimumab and golimumab, are also efficacious for moderately to severely active UC in ambulatory patients. In the adalimumab trial, remission at week 8 was achieved in 18.5% of patients treated with adalimumab at the 160/80 mg induction regimen.22While this remission rate was lower than that in ACT 1 and 2, it is important to note that patients in the adalimumab trial may have had more severe disease as reflected by a higher baseline median c-reactive protein (CRP) levels and a lower percentage of left-sided disease (38%), and, in these regards, were more similar to patients with ASUC. The PURSUIT-SC golimumab trial population was more similar at baseline to those of ACT 1 and 2, and the rate of clinical remission and endoscopic healing was 18 percent and 45 percent, respectively, at 6 weeks.23 Interestingly, insecondary analyses in both these trials, higher CRP and higher baseline Mayo scores were associated with decreased rates of remission. Given the more moderate disease severity of the study populations in these trials, and the results of secondary analyses indicating disease severity may impact response, it follows that anti-TNF dosing regimens for ambulatory children and adults with moderate to severely active UC may not be similarly effective in those hospitalized with ASUC.
4. Evidence Supporting Anti-TNF Therapy for ASUC
There have been two randomized controlled trials of infliximab for ASUC in adults. Sands and colleagues randomized patients to a single infusion of placebo or infliximab 5, 10, or 20 mg/kg, but stopped enrollment after only 11 patients because of slow accrual.24 Amongst patients treated with infliximab, 4 of 8 achieved a clinical response by 2 weeks, compared to 0 of 3 of those treated with placebo, all of whom underwent colectomy. A later, larger clinical trial by Järnerotand colleaguesrandomized 45 patients with an acute exacerbation of moderate-severely active UC refractory to intravenous corticosteroids to a single dose of infliximab 5 mg/kg or placebo.25Twenty-nine percent of patients in the infliximab arm underwent colectomy by 30 days compared to 66 percent in the placebo arm. However, when only the 28 patients who met all the criteria for fulminant colitis were examined, 47 percent of patients in the infliximab arm underwent colectomy compared to 69 percent in the placebo arm. There have also been a number of observational studies reporting a wide range of short-term colectomy rates after rescue therapy with infliximab for ASUC (Table 1). In pediatrics, Turner and colleagues performed a prospective multicenter cohort study of children with ASUC in which 33 patients were treated with infliximab after failing to respond to intravenous corticosteroids. In those treated with rescue infliximab, 24 percent underwent colectomy prior to discharge and an additional 33 percentunderwent colectomy or continued to be steroid-dependent at 1 year.9Collectively, these studies support that, while infliximab is effective as rescue therapy for ASUC, near- and long-term outcomes remain poor in a large fraction of patients.
Table 1.
Studies of Infliximab for ASUC refractory to intravenous corticosteroids
| Study | Study Design | IFX Dosing* | n# | Short-term Response§ (%) |
Long-term Colectomy- free† (%) |
|---|---|---|---|---|---|
|
| |||||
| Adult Studies | |||||
| Sands, et al.
(2001)24 |
RCT | Single dose(5, 10, or 20 mg/kg) |
11 | 50 | – |
| Kohn, et al.
(2002)53 |
Prospective cohort |
Single dose | 13 | 77 | – |
| Järnerot,et al.
(2005) 200525 |
RCT | Single dose | 15 (of 24) |
53 | – |
| Regueiro, et al.
(2006) |
Retrospective cohort |
3 dose induction and maintenance |
12 | 25 | – |
| Lees, et al.
(2007)45 |
Retrospective cohort |
1-3 dose induction, repeated as needed |
39 | 66 | 62 |
| Kohn, et al.
(2007)54 |
Prospective cohort |
1-3 doses | 83 | 85 | 70 |
| Aratari, et al.
(2008)55 |
Retrospective cohort |
3 dose induction |
11 | 100 | 81 |
| Bressler, et al.
(2008)56 |
Retrospective cohort |
Single dose ± maintenance |
21 | 76 | 62 |
| Ho, et al.
(2009)38 |
Prospective cohort |
Single dose | 21 | 52 | – |
| Mortensen, et al.
(2011)57 |
Retrospective cohort |
1-3 dose induction ± maintenance |
56 | 82 | 61 |
| Monterubbianesi et al.(2014)58 |
Prospective cohort |
3 dose induction ± maintenance |
113 | 82 | 75 |
| Gibson et al.
(2014) |
Retrospective cohort |
3 dose induction ± maintenance / accelerated dosing‡ |
35/15 | 40/7 | 76/72 |
|
| |||||
| Pediatric Studies | |||||
|
| |||||
| Mamulaet al.
(2002 & 2004)59,60 |
Retrospective cohort |
2 dose induction ± maintenance |
5 (of 17) |
100 | – |
| Russell et al.
(2004)61 |
Retrospective cohort |
3 dose induction ± maintenance |
9 (of 14) |
88 | – |
| Fanjianget al. (2007)62 |
Retrospective cohort |
Induction and maintenance |
16 (of 27) |
– | 75 |
| Cucchiara, et al.
(2008)63 |
Retrospective cohort |
Induction and maintenance |
4 (of 22) |
0 | |
| McGinnis et al.
(2008)64 |
Retrospective cohort |
3 dose induction (5- 10 mg/kg) ± maintenance |
27 (of 39) |
70 | 61 |
| Hyamset al.
(2010)7,8 |
Prospective cohort |
1-3 dose induction ± maintenance |
25 (of 52) |
68 | 50 |
| Turner et al.
(2010)9 |
Prospective cohort |
3 dose induction ± maintenance |
33 | 76 | 55 |
| Falaiyeet al.
(2014)39 |
Retrospective cohort |
3 dose induction ± maintenance |
17 (of 29) |
– | 41 |
Dose = 5 mg/kg unless otherwise noted
Number of patients with ASUC (of total) treated with infliximab
Short-termresponse outcome ranged from prior to discharge to 3 months
Long-term colectomy-free outcome represents median follow-up of ranging 1-2 years
Accelerated induction = 3 induction doses given based on clinical statuswithin 24 day period
IFX, infliximab; RCT, randomized controlled trial
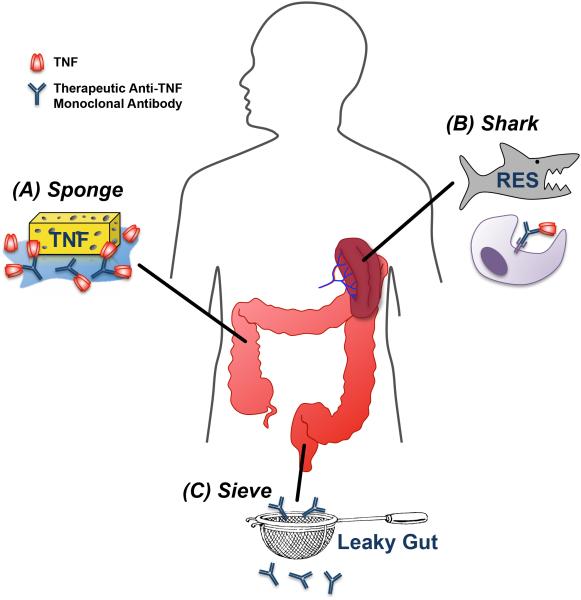
5. Considering Anti-TNF Biologic Pharmacokinetics in ASUC: The Sponge, The Shark, and the Sieve
Multiple studies have demonstrated the association between higher serum concentrations of anti-TNF biologics and better outcomes.26-28The importance of serum anti-TNF concentrations was first reported by Seow and colleagues who showed that in a cohort of adult UC patients treated with infliximab, rates of remission and endoscopic improvement were substantially higher, and rates of colectomy were substantially lower, in those with detectable trough serum infliximab concentrations.28Subsequently, in an analysis of patients in the ACT 1 and 2 trials, serum infliximab concentrationswere significantly higher in patients with clinical response, mucosal healing, and/or clinical remission at all time points studied.26 The authors reported that an approximate trough serum infliximab concentration of 41 µg/ml at 8 weeks(two weeks after the third induction dose), and 3.7 µg/ml at maintenance steady state was associated with optimal outcomes. Similarly, analysis of the pediatric UC infliximab trial revealed higher rates of clinical response, remission, and mucosal healing in those in the highest week 8 serum infliximab concentration quartile (>41 µg/ml) compared to those in the lowest quartile (<18.1 µg/ml).27
Consideration of factors influencing anti-TNF PK/PD and their relation to disease severity may shed light on how we may more optimally administer these drugs to treat ASUC. If patients with ASUC exhibit accelerated clearance of therapeutic monoclonal antibodies, they may benefit from alternative dosing strategies to optimize their exposure to the drug. As outlined below and in Figure 1, a helpful metaphor may be to consider the patient with ASUC as a Sponge, a Shark, and a Sieve with regard to how she handles anti-TNF biologic drugs.
Figure 1.
Conceptual framework for hypothesized mechanisms of rapid clearance of therapeutic anti-TNF biologics in ASUC. (A) High concentrations of circulating and tissue TNF may act as a “sponge” that rapidly “absorbs” or neutralizes standard dose of anti-TNF biologics. (B) Mononuclear cell “sharks” within the upregulated RES may rapidly “chew through” drug-TNF complexes by phagocytosis and proteolyticdegradation. (C) Leaky guts associated with severe colonic inflammation may act as a “sieve”, permitting the excessive fecal loss of therapeutic monoclonal antibody.
5.1. The Sponge: High TNF Burden
Patients with ASUC likely have a higher serum and mucosal TNF burden that acts as a “sponge” to quickly absorb and bind anti-TNF monoclonal antibodies, which may lead to more rapid drug clearance. Serum TNF is elevated in UC and correlates with disease severity. In one study, peak serum TNF levels were 2.5 fold higher in severe compared to moderate UC.29 Similarly, mucosal TNF from lymphocytes and macrophages isalso highly correlated with UC disease severity.30In the recent ATLAS study, the investigators measuredserum anti-TNF levels along with mucosal TNF and anti-TNF levels in adult IBD patients on anti-TNF therapy undergoing endoscopy.31Patients with severe inflammation exhibited higher mucosal TNF levels and lower mucosal anti-TNF levels than those with moderate inflammation, resulting in the lowest ratio of mucosal anti-TNF to TNF. Furthermore, patients with a mismatch between serum and mucosal anti-TNF levels were most likely to exhibit active mucosal disease. Collectively, these findings support that high mucosal TNF levels negatively influence mucosal anti-TNF drug levels and therapeutic effect locally at the site of inflammation.It may simply follow that patients with ASUC require higher and/or more frequent dosing of anti-TNF therapy than ambulatory patients with less severe disease in order to sufficiently neutralizehigher levels of tissue and circulating TNF. In a prospective study of patients with moderate to severely active UC treated with infliximab, mucosal healing was achieved in 82%, 64% and 42% of patients with low, middle and high pre-treatment mucosal TNF gene expression, respectively.32Similarly, higher serum TNF levels have been associated with poorer response to infliximab in rheumatoid arthritis and fistulizing CD.33,34
5.2. The Shark: Proteolytic Degradation by the Reticuloendothelial System
As IgG1 monoclonal antibodies, anti-TNF biologics used in the treatment of UC form immune complexes with TNF that are likely cleared through Fc receptor-mediated endocytosis and proteolytic degradation by mononuclear phagocytes of the reticuloendothelial system (RES).35,36Alternatively, therapeutic monoclonal antibodies may bind cell-surface expressed TNF on immune cells, opsonizing these cells for phagocytosis. It is suspected that RES activity is influenced by degree of inflammation; therefore, the severe inflammatory burden of ASUC may lead to increased activity of RES phagocyte “sharks” that “chew” through anti-TNF therapeutic monoclonal antibodies leading to more rapid clearance.35 Supporting this notion, elevated CRP is associated with faster infliximab clearance, and higher fecal calprotectin levels are associated with poorer response to infliximab in adults with UC.37,38 In a study by our group of children hospitalized with acute UC or Crohn’s colitis, erythrocyte sedimentation rate was highly predictive of need for infliximab dose escalation, also suggesting more rapid clearance in those with higher degrees of systemic inflammation.39
The RES does contain an important recycling mechanism for IgG. Both IgG (including therapeutic IgG antibodies) and albumin bound to the neonatal Fc receptor (FcRn) expressed on RES vascular endothelial and myeloid cells are protected from lysosomal catabolism and returned to the circulation.35This salvage mechanism, which may prolong the half-life of IgG therapeutic monoclonal antibodies, can be saturated in the setting of high circulating IgG concentrations. Therefore, others have proposed that in the setting of severe inflammation, such as that in ASUC, high circulating endogenous IgG may saturate FcRn binding sites and reduce retention of therapeutic monoclonal antibodies.35
5.3. The Sieve: Gut Leakage
Patients with ASUC are known to have protein losses through the diseased colon, which partially explainsthehypoalbuminemia commonly seen in these patients.40There is now increasing recognition that therapeutic monoclonal antibodies may also pass through the diseased colon mucosa into the stool. Accordingly, patients with ASUC may act as “sieves” with a proportion of the biologic drug being lost to the stool as soon as it is being administered. Early studies using nuclearscintigraphy studies demonstratedthat technetium-labeled human immunoglobulin accumulated in the colons of UC patients, supporting the notion of immunoglobulin gut loss in UC.41Brandseand colleagues were the first to report detectable infliximab in the feces of patients with IBD treated with their first dose of infliximab, with the highest levels in the first few days after the infusion.42They reported in a follow-up study of adult patients with moderate-severely active UC that patients without endoscopic response at week 6-8 exhibited higher Day 1 fecal infliximab concentrations, lower serum infliximab levels at week 6, and in some cases, early development of antibodies to infliximab.43 While these are preliminary reports, fecal loss of therapeutic monoclonal antibodies, especially in patients with the most severe disease, warrants further investigation.Low serum albumin, which may serve as a biomarker for stool protein loss, has been associated with low serum infliximab levels, early infliximab dose escalation, and infliximab non-response in children and adults with UC.23,27,39,44-46
6. Baseline Factors Associated with Anti-TNF Pharmocokinetics
In order to individualize dosing of anti-TNF biologics to achieve early optimal drug exposure, we must ultimately incorporate informative baseline patient and disease factors into our algorithms. Many factors have already been associated with low levels of anti-TNF biologics, poor response, or both in patients with UC (Table 2). Low serum albumin is the most consistently identified disease factor associated with rapid anti-TNF clearance.26,27,44,47With regard to patient factors, weight exhibitsa non-linear relationship with anti-TNF clearance such that small patients under 40 kg are more likely to exhibit low trough levels with conventional dosing.47 Male sex has also been associated with more rapid clearance.37 Interestingly, although concomitant treatment with an immunomodulator(thiopurine or methotrexate) is associated with slower anti-TNF clearance in patients with CD, the same relationship has not been observed in UC.37,48
Table 2.
Baseline factors reported to be associated with drug PK and/or clinical outcomes after anti-TNF therapy for UC
| Baseline Factor | Association with PK | Association with outcomes |
|---|---|---|
| Age | – | Inversely associated with clinical response to IFX 65 |
| Sex | IFX clearance faster in men37 | Increased rates of clinical response and remission in females treated with GLM 23 |
| Race | – | White race associated with higher rates of GLM clinical response 23 |
| Weight | Directly associated with IFX volume of distribution 37 |
Inversely associated with frequency of IFX dose escalation in children 39 |
| High body weight associated with higherIFX clearance; low body weight associated with low trough IFX levels since relationship between weight and clearance is nonlinear47 |
||
| Directly associated with serum IFX levels in children 27 |
||
| Albumin | Low albuminassociated withlow serum IFX concentrations rapid clearance26,44,47 |
Low serum albumin associated with lower IFX response rates44, increased colectomy rates46, and increased frequency of IFX dose escalation in children39 |
| CRP | CRP inversely associated with IFX levels 26 |
CRP inversely associated with GLM response23 |
| ESR | – | High ESR associated with increased frequency of IFX dose escalation in children39 |
| Fecal inflammatory markers |
– | Fecal lactoferrin inversely associated with GLM response23 |
| Fecal calprotectin inversely related to IFX response in ASUC38 |
||
| Mayo Score | Inversely associated with IFX levels26 |
Inversely associated with incidence of clinical remission after treatment with IFX or GLM23,28 |
| pANCA | – | Positive pANCA associated with decreased rates of clinical response to IFX65 |
| TNF | – | Mucosal TNF gene expression inversely associated with response to IFX32 |
| Mucosal gene expression |
– | Panel of 5 genes (TNFRSF11B, STC1, PTGS2, IL13RA2 and IL11) predicted response to IFX66 |
| Gene expression principle component representing UC molecular disturbance associated with non- response.67 |
IFX, infliximab; GLM, golimumab; CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; pANCA, pronuclear anti-neutrophil cytoplasmic antibodies
7. The Future of Anti-TNF Therapy for ASUC
Accelerated clearance of anti-TNF biologics in children and adults with ASUC may explain high treatment failure rates with conventional weight-based dosing developed for treatment of ambulatory patients with moderate to severely active disease. Alternative dosing regimens that ensure sustained optimal biologic exposure, especially early in the treatment of ASUC when inflammatory burden and colon injury is highest, may lead to improved outcomes. As a quality improvement intervention, Gibson and colleagues introduced an “accelerated” infliximab induction regimen for the treatment of ASUC.46 In this regimen, subsequent induction doses of infliximab (5 mg/kg) were administered based on worsening clinical symptoms or inflammatory markers, instead of the standard regimen of doses at 0, 2, and 6 weeks. The rate of early colectomy was 6.7% in patients treated with the accelerated induction regimen, compared to 40% in a group of similar historical controls treated with the standard induction regimen; although, long-term colectomy rates were similar between the two groups. While this study serves as an important proof of principal that alternative dosing regimens may be needed in patients with ASUC, doses were still administered in a reactive fashion in response to deteriorating clinical signs.
Given the clear association between serum levels of anti-TNF biologics and patient outcomes, it would seem rational to monitor levels and specified time points and adjust doses to achieve optimal cut-off levels. This therapeutic monitoringapproach is being actively investigated in patients with moderate to severely active UC and CD based mainly on post-induction levels obtained at 6-14 weeks associated with improved outcomes.49-51However, time is of the essence in patients with ASUC, and measurement of serum drug levels after induction will not be helpful for the 25-30% of ASUC patients who will undergo colectomy in the first 2-4 weeks after treatment.9,25Therefore, there is a need to develop approaches to optimize anti-TNF dosing at the outset of treatment for ASUC.
We hypothesize such optimized anti-TNF regimens for ASUC will be achieved by individualized dosing and pro-active adjustment based on early measurement of levels and biomarkers of response. At this time, however, optimal time-points and targets for early anti-TNF levels (i.e. within the first week of treatment) are unknown. The largest anti-TNF PK-PD studies from UC clinical trials have analyzed primarily trough and peak blood samples with each infusion, with no additional early measurements between the first two infusions.27,37Therefore, we propose that the first step toward developing optimized dosing strategies in ASUC will be to assemble a cohort of anti-TNF-naïve patients with steroid-refractory ASUC being initiated on an anti-TNF biologic to assess the following: 1) individual baseline patient and disease parameters hypothesized to predict PK, 2) serial measurementsof anti-TNF levels within the first week of treatment, and 3) measures of early clinical response and longer term clinical remission. With such data, one could determine whether variability in early anti-TNF exposure and clearance influences initial clinical response, and generate a predictive model that relates anti-TNF PKback to clinically relevant baseline parameters (e.g. dose, weight, albumin, TNF, inflammatory markers, etc.).
Looking toward the future, software decisions support tools or “dashboards” that incorporate a predictive PK model may be tested to tailor anti-TNF dosing regimens to individual patients with ASUC, and reduce the variability in effective drug exposure.52Dashboard systems can incorporate baseline covariates into a PK model to reduce unexplained variability, and propose a dosing regimen estimated to result in optimal drug exposure for a given patient.52As proof of this principle, in adult patients with UC and CD of varying severity, the incorporation of individual weight and albumin parameters increased the accuracy of predicted serum infliximab concentrations.47Once the individualized dosing regimen is applied, early proactive monitoring of serum drug concentrations and biomarkers of treatmentresponse (e.g. CRP) can be used to update the PK model and guide subsequent dosing in real time (adaptive dosing). Patients with ASUC are an ideal population for the clinical application of therapeutic dashboards since they likely have profound inter-individual variability in anti-TNF PK, and the severity of their condition requires earlyproactive effective dosing. The future development and clinical application of PK modeling in the form of dashboards that account for TNF burden (the Sponge), inflammation-induced RES activation (the Shark), and intestinal losses (the Sieve), will likely result in sustained exposure to the drug, mucosal healing, and fewer colectomies in children and adults with ASUC.
Acknowledgments
Declaration of funding interests:
(i) Michael J. Rosen’s effort was supported in part by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number K23DK09483.
(ii) Phillip Minar’s effort was supported in part by and the National institutes of Health Child Health Research Career Development Award under award Number K12HD028827.
Footnotes
Statement of Interests
- (i) Michael J. Rosen has served on a scientific advisory board for Abbvie, Inc.
References
- 1.Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–9. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2012;58:519–25. doi: 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 5.Magro F, Rodrigues A, Vieira AI, Portela F, Cremers I, Cotter J, et al. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012;18:573–83. doi: 10.1002/ibd.21815. [DOI] [PubMed] [Google Scholar]
- 6.Daperno M, Sostegni R, Rocca R, Rigazio C, Scaglione N, Castellino F, et al. Review article: medical treatment of severe ulcerative colitis. Aliment Pharmacol Ther. 2002;16(Suppl 4):7–12. doi: 10.1046/j.1365-2036.16.s4.2.x. [DOI] [PubMed] [Google Scholar]
- 7.Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Stephens M, Evans J, et al. Outcome following infliximab therapy in children with ulcerative colitis. Am J Gastroenterol. 2010;105:1430–6. doi: 10.1038/ajg.2009.759. [DOI] [PubMed] [Google Scholar]
- 8.Turner D, Griffiths AM. Acute severe ulcerative colitis in children: a systematic review. Inflamm Bowel Dis. 2011;17:440–9. doi: 10.1002/ibd.21383. [DOI] [PubMed] [Google Scholar]
- 9.Turner D, Mack D, Leleiko N, Walters TD, Uusoue K, Leach ST, et al. Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010;138:2282–91. doi: 10.1053/j.gastro.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 10.Daperno M, Sostegni R, Scaglione N, Ercole E, Rigazio C, Rocca R, et al. Outcome of a conservative approach in severe ulcerative colitis. Dig Liver Dis. 2004;36:21–8. doi: 10.1016/j.dld.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Ho G-T, Mowat C, Goddard CJR, Fennell JM, Shah NB, Prescott RJ, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19:1079–87. doi: 10.1111/j.1365-2036.2004.01945.x. [DOI] [PubMed] [Google Scholar]
- 12.Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to Corticosteroids in Severe Ulcerative Colitis: A Systematic Review of the Literature and a Meta-Regression. Clinical Gastroenterology and Hepatology. 2007;5:103–10. doi: 10.1016/j.cgh.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–5. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 14.Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol. 1999;94:1587–92. doi: 10.1111/j.1572-0241.1999.01149.x. [DOI] [PubMed] [Google Scholar]
- 15.Bousvaros A. Oral tacrolimus treatment of severe colitis in children. The Journal of Pediatrics. 2000;137:794–9. doi: 10.1067/mpd.2000.109193. [DOI] [PubMed] [Google Scholar]
- 16.Ogata H, Kato J, Hirai F, Hida N, Matsui T, Matsumoto T, et al. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2012;18:803–8. doi: 10.1002/ibd.21853. [DOI] [PubMed] [Google Scholar]
- 17.Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012 doi: 10.1016/S0140-6736(12)61084-8. [DOI] [PubMed] [Google Scholar]
- 18.Lichtiger S, Present DH. Preliminary report: cyclosporin in treatment of severe active ulcerative colitis. The Lancet. 1990;336:16–9. doi: 10.1016/0140-6736(90)91521-b. [DOI] [PubMed] [Google Scholar]
- 19.Watson S, Pensabene L, Mitchell P, Bousvaros A. Outcomes and adverse events in children and young adults undergoing tacrolimus therapy for steroid-refractory colitis. Inflamm Bowel Dis. 2011;17:22–9. doi: 10.1002/ibd.21418. [DOI] [PubMed] [Google Scholar]
- 20.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 21.Hyams J, Damaraju L, Blank M, Johanns J, Guzzo C, Winter HS, et al. Induction and Maintenance Therapy With Infliximab for Children With Moderate to Severe Ulcerative Colitis. Clinical Gastroenterology and Hepatology. 2012;10:391–1. doi: 10.1016/j.cgh.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Reinisch W, Sandborn WJ, Hommes DW, D'Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780–7. doi: 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 23.Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95. doi: 10.1053/j.gastro.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 24.Sands BE, Tremaine WJ, Sandborn WJ, Rutgeerts PJ, Hanauer SB, Mayer L, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2001;7:83–8. doi: 10.1097/00054725-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, Grännö C, et al. Infliximab as Rescue Therapy in Severe to Moderately Severe Ulcerative Colitis: A Randomized, Placebo-Controlled Study. Gastroenterology. 2005;128:1805–11. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Adedokun OJ, Sandborn WJ, Feagan BG, Rutgeerts P, Xu Z, Marano CW, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296–1307.e5. doi: 10.1053/j.gastro.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Adedokun OJ, Xu Z, Padgett L, Blank M, Johanns J, Griffiths A, et al. Pharmacokinetics of Infliximab in Children with Moderate-to-Severe Ulcerative Colitis. Inflamm Bowel Dis. 2013;19:2753–62. doi: 10.1097/01.MIB.0000435438.84365.f7. [DOI] [PubMed] [Google Scholar]
- 28.Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 29.Gardiner KR, Halliday MI, Barclay GR, Milne L, Brown D, Stephens S, et al. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsen T, Goll R, Cui G, Husebekk A, Vonen B, Birketvedt GS, et al. Tissue levels of tumor necrosis factor-alpha correlates with grade of inflammation in untreated ulcerative colitis. Scand J Gastroenterol. 2007;42:1312–20. doi: 10.1080/00365520701409035. [DOI] [PubMed] [Google Scholar]
- 31.Yarur AJ, Jain A, Sussman DA, Barkin JS, Quintero MA, Princen F, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut. 2015 doi: 10.1136/gutjnl-2014-308099. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Olsen T, Goll R, Cui G, Christiansen I, Florholmen J. TNF-alpha gene expression in colorectal mucosa as a predictor of remission after induction therapy with infliximab in ulcerative colitis. Cytokine. 2009;46:222–7. doi: 10.1016/j.cyto.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Edrees AF, Misra SN, Abdou NI. Anti-tumor necrosis factor (TNF) therapy in rheumatoid arthritis: correlation of TNF-alpha serum level with clinical response and benefit from changing dose or frequency of infliximab infusions. Clin Exp Rheumatol. 2005;23:469–74. [PubMed] [Google Scholar]
- 34.Martínez-Borra J, López-Larrea C, González S, Fuentes D, Dieguez A, Deschamps EM, et al. High serum tumor necrosis factor-alpha levels are associated with lack of response to infliximab in fistulizing Crohn's disease. Am J Gastroenterol. 2002;97:2350–6. doi: 10.1111/j.1572-0241.2002.05990.x. [DOI] [PubMed] [Google Scholar]
- 35.Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF Monoclonal Antibodies in Inflammatory Bowel Disease: Pharmacokinetics-Based Dosing Paradigms. Clin Pharmacol Ther. 2012;91:635–46. doi: 10.1038/clpt.2011.328. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Wang EQ, Balthasar JP. Monoclonal Antibody Pharmacokinetics and Pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–58. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 37.Fasanmade AA, Adedokun OJ, Ford J, Hernandez D, Johanns J, Hu C, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009;65:1211–28. doi: 10.1007/s00228-009-0718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho G-T, Lee HM, Brydon G, Ting T, Hare N, Drummond H, et al. Fecal Calprotectin Predicts the Clinical Course of Acute Severe Ulcerative Colitis. Am J Gastroenterol. 2009;104:673–8. doi: 10.1038/ajg.2008.119. [DOI] [PubMed] [Google Scholar]
- 39.Falaiye TO, Mitchell KR, Lu Z, Saville BR, Horst SN, Moulton DE, et al. Outcomes following infliximab therapy for pediatric patients hospitalized with refractory colitis-predominant IBD. J Pediatr Gastroenterol Nutr. 2014;58:213–9. doi: 10.1097/MPG.0b013e3182a98df2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grill BB, Hillemeier AC, Gryboski JD. Fecal alpha 1-antitrypsin clearance in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1984;3:56–61. doi: 10.1097/00005176-198401000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Sarikaya I, Bektas A, Ibis E, Yasa MH, Bastemir M, Ormeci N, et al. Tc-99m dextran and Tc-99m HIG findings in patients with ulcerative colitis. Clinical nuclear medicine. 1999;24:243–7. doi: 10.1097/00003072-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Brandse JF, Wildenberg M, de Bruyn JR, Wolbink G-J, Lowenberg M, Ponsioen C, et al. 2013;144:S–36–36. [Google Scholar]
- 43.Brandse JF, van der Kleij D, Wolbink G-J, Rigter IM, Baars PA, Lowenberg M, et al. 786 The Pharmacokinetics of Infliximab Induction Therapy in Patients With Moderate to Severe Ulcerative Colitis. Gastroenterology. 2014;146:S–134–134. [Google Scholar]
- 44.Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48:297–308. doi: 10.5414/cpp48297. [DOI] [PubMed] [Google Scholar]
- 45.Lees CW, Heys D, Ho G-T, Noble CL, SHAND AG, Mowat C, et al. A retrospective analysis of the efficacy and safety of infliximab as rescue therapy in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2007;26:411–9. doi: 10.1111/j.1365-2036.2007.03383.x. [DOI] [PubMed] [Google Scholar]
- 46.Gibson DJ, Heetun ZS, Redmond CE, Nanda KS, Keegan D, Byrne K, et al. An Accelerated Infliximab Induction Regimen Reduces the Need for Early Colectomy in Patients With Acute Severe Ulcerative Colitis. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.07.041. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Dotan I, Ron Y, Yanai H, Becker S, Fishman S, Yahav L, et al. Patient Factors That Increase Infliximab Clearance and Shorten Half-life in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2014;20:2247–59. doi: 10.1097/MIB.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 48.Fasanmade AA, Adedokun OJ, Blank M, Zhou H, Davis HM. Pharmacokinetic properties of infliximab in children and adults with Crohn's disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011;33:946–64. doi: 10.1016/j.clinthera.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Singh N, Rosenthal CJ, Melmed GY, Mirocha J, Farrior S, Callejas S, et al. Early Infliximab Trough Levels Are Associated with Persistent Remission in Pediatric Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2014;20:1708–13. doi: 10.1097/MIB.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 50.Roblin X, Rinaudo M, Del Tedesco E, Phelip JM, Genin C, Peyrin-Biroulet L, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol. 2014;109:1250–6. doi: 10.1038/ajg.2014.146. [DOI] [PubMed] [Google Scholar]
- 51.Paul S, Del Tedesco E, Marotte H, Rinaudo-Gaujous M, Moreau A, Phelip J-M, et al. Therapeutic Drug Monitoring of Infliximab and Mucosal Healing in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2013;19:2568–76. doi: 10.1097/MIB.0b013e3182a77b41. [DOI] [PubMed] [Google Scholar]
- 52.Mould DR, Upton RN, Wojciechowski J. Dashboard Systems: Implementing Pharmacometrics from Bench to Bedside. AAPS J. 2014;16:925–37. doi: 10.1208/s12248-014-9632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohn A, Prantera C, Pera A, Cosintino R, Sostegni R, Daperno M. Anti-tumour necrosis factor alpha (Infliximab) in the treatment of severe ulcerative colitis: result of an open study on 13 patients. Digestive and Liver Disease. 2002;34:626–30. doi: 10.1016/s1590-8658(02)80204-3. [DOI] [PubMed] [Google Scholar]
- 54.Kohn A, Daperno M, Armuzzi A, CAPPELLO M, BIANCONE L, ORLANDO A, et al. Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment Pharmacol Ther. 2007;26:747–56. doi: 10.1111/j.1365-2036.2007.03415.x. [DOI] [PubMed] [Google Scholar]
- 55.Aratari A, Papi C, Clemente V, Moretti A, Luchetti R, Koch M, et al. Colectomy rate in acute severe ulcerative colitis in the infliximab era. Digestive and Liver Disease. 2008;40:821–6. doi: 10.1016/j.dld.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Bressler B, Law JK, Nahdi Sheraisher Al N, Atkinson K, Byrne MF, Chung HV, et al. The use of infliximab for treatment of hospitalized patients with acute severe ulcerative colitis. Canadian Journal of Gastroenterology. 2008;22:937. doi: 10.1155/2008/749547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mortensen C, Caspersen S, Christensen NL, Svenningsen L, Thorsgaard N, Christensen LA, et al. Treatment of acute ulcerative colitis with infliximab, a retrospective study from three Danish hospitals. J Crohns Colitis. 2011;5:28–33. doi: 10.1016/j.crohns.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Monterubbianesi R, Aratari A, Armuzzi A, Daperno M, Biancone L, Cappello M, et al. Infliximab three-dose induction regimen in severe corticosteroid-refractory ulcerative colitis: Early and late outcome and predictors of colectomy. J Crohns Colitis. 2014;8:852–8. doi: 10.1016/j.crohns.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Mamula P, Markowitz JE, Brown KA, Hurd LB, Piccoli DA, Baldassano RN. Infliximab as a novel therapy for pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2002;34:307–11. doi: 10.1097/00005176-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 60.Mamula P, Markowitz JE, Cohen LJ, Allmen von D, Baldassano RN. Infliximab in pediatric ulcerative colitis: two-year follow-up. J Pediatr Gastroenterol Nutr. 2004;38:298–301. doi: 10.1097/00005176-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 61.Russell GH, Katz AJ. Infliximab is effective in acute but not chronic childhood ulcerative colitis. J Pediatr Gastroenterol Nutr. 2004;39:166–70. doi: 10.1097/00005176-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Fanjiang G, Russell GH, Katz AJ. Short- and long-term response to and weaning from infliximab therapy in pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2007;44:312–7. doi: 10.1097/MPG.0b013e31802e98d4. [DOI] [PubMed] [Google Scholar]
- 63.Cucchiara S, Romeo E, Viola F, Cottone M, Fontana M, Lombardi G, et al. Infliximab for pediatric ulcerative colitis: a retrospective Italian multicenter study. Digestive and Liver Disease. 2008;40:S260–4. doi: 10.1016/S1590-8658(08)60535-6. [DOI] [PubMed] [Google Scholar]
- 64.McGinnis JK, Murray KF. Infliximab for Ulcerative Colitis in Children and Adolescents. J Clin Gastroenterol. 2008;42:875–9. doi: 10.1097/MCG.0b013e3181354417. [DOI] [PubMed] [Google Scholar]
- 65.Ferrante M, Vermeire S, Katsanos KH, Noman M, van Assche G, Schnitzler F, et al. Predictors of early response to infliximab in patients with ulcerative colitis. Inflamm Bowel Dis. 2007;13:123–8. doi: 10.1002/ibd.20054. [DOI] [PubMed] [Google Scholar]
- 66.Arijs I, Li K, Toedter G, Quintens R, Van Lommel L, Van Steen K, et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut. 2009;58:1612–9. doi: 10.1136/gut.2009.178665. [DOI] [PubMed] [Google Scholar]
- 67.Halloran B, Chang J, Shih DQ, Mcgovern D, Famulski K, Evaschesen C, et al. Molecular Patterns in Human Ulcerative Colitis and Correlation with Response to Infliximab. Inflamm Bowel Dis. 2014;20:2353–63. doi: 10.1097/MIB.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]