Abstract
Brucellosis is the most common bacterial zoonosis, and causes a considerable burden of disease in endemic countries. Cardiovascular involvement is the main cause of mortality due to infection with Brucella spp., and most commonly manifests as endocarditis, peripheral and cerebrovascular aneurysms, or arterial and venous thrombosis. We report a case of brucellosis presenting as bacteremia and aortic endarteritis 18 years after the last known exposure to risk factors for brucella infection. The patient was treated with doxycycline, rifampin, and gentamicin, and underwent surgical repair of a penetrating aortic ulcer, with a good clinical recovery. We review the signs and symptoms, diagnostic approach, prognosis, and treatment of brucella arteritis. We draw attention to the absence of consensus about the optimum therapy for vascular brucellosis, and the urgent need for additional studies and renewed scientific interest in this major pathogen.
INTRODUCTION
Brucellosis is the most common bacterial zoonosis, with more than 500,000 cases annually worldwide.1 The infection is endemic in the Mediterranean area, eastern Europe, the Middle East, Africa, South and Central America, and Asia.2,3 Scientific interest in human brucellosis has been renewed in the past 5 years, because of recognition of the substantial burden of disease in endemic countries,1,4 and classification of Brucella species as potential agents of bioterrorism. Brucellosis has a broad range of clinical presentations, from acute undifferentiated febrile illnesses to chronic infections that most commonly affect the CNS, cardiovascular system, or skeletal system. Although cardiovascular involvement with brucellosis is rare, complications due to cardiac involvement are the most common cause of morbidity and mortality in patients with brucellosis.
In this Grand Round we present the case of a patient who had longstanding, unrecognised, chronic brucellosis, whose endovascular infection was diagnosed after examination of a penetrating ascending aortic ulcer. Although the presentation and treatment of brucella endocarditis, disseminated brucellosis, and spinal brucellosis have been well described,3,5–13 data for less common sites of infection are scarce, despite the high morbidity associated with such infections. We review published work to define the clinical presentation, diagnostic approach, prognosis, and treatment of brucella arteritis.
CASE PRESENTATION
A 73-year-old Ecuadorian man who had been treated for coronary artery disease for 3 years at the US National Institutes of Health (NIH) returned to the NIH in July, 2010, with 2 months of accelerating angina pectoris.
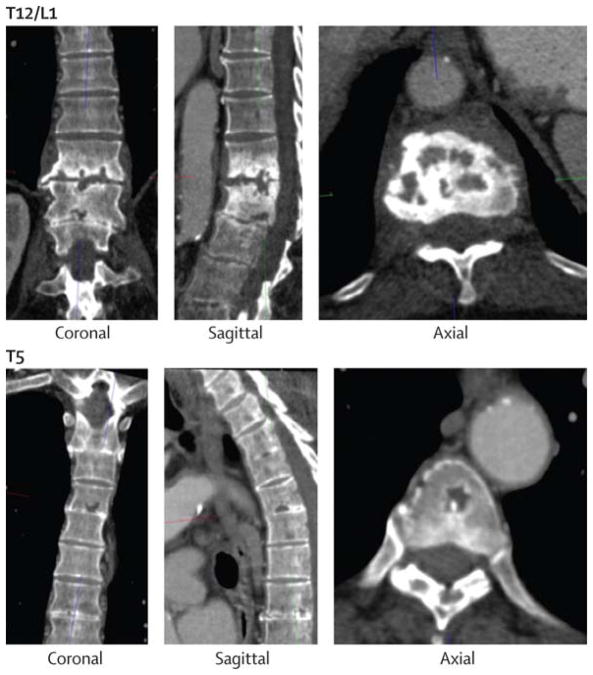
The man’s medical history included chronic normocytic anaemia, a transient ischaemic attack, a 3 cm infrarenal abdominal aortic aneurysm, and hypertension. He had also had lower-back pain for 18 years. In July, 2009, a research cardiac CT scan incidentally identified several lytic lesions in the fifth and 12th thoracic and first lumbar vertebrae (Figure 1). Assessment of these lytic spine lesions included a prostate serum antigen concentration and serum protein electrophoresis, which were normal. The patient’s alkaline phosphatase was raised at 327 U/L (normal 37–116 U/L). The examining rheumatologist attributed the findings to degenerative disease and recommended expectant management.
Figure 1. Vertebral lesions.
CT scan showing lytic lesions in the 12th thoracic (T12) and first lumbar (L1) vertebrae (top images), and in the fifth thoracic (T5) vertebra (bottom images).
The patient had lived in Ecuador until the age of 63 years when he moved to Washington, DC, USA. From the age of 14 to 55 years he had worked in a slaughterhouse in Ecuador, where he handled carcasses of cattle, goats, and other animals, and occasionally drank bull’s blood. He retired from the abattoir aged 55 years because of intractable lower-back pain that had developed a year before. He denied having travelled outside the Washington area since moving to the USA. His only pets were two canaries, and he had had no contact with other animals since retiring from the abattoir. He had not knowingly consumed unpasteurised milk.
The patient had normal vital signs. The examination was notable for a 3/6 low-pitched, late-peaking aortic systolic ejection murmur with normal carotid upstrokes. Examination also showed tenderness to palpation of the central sacral area, but was otherwise normal.
Results of laboratory studies showed normocytic anaemia with haemoglobin of 119 g/L, a platelet count of 149 000 per μL, and leucopoenia with a white-blood-cell count of 3570 per μL. Routine chemistry and liver enzyme test results were normal, with the exception of increased alkaline phosphatase at 209 U/L. HIV-1 and HIV-2 ELISA was negative. C-reactive protein was 13·9 mg/L (normal <10 mg/L), and erythrocyte sedimentation rate was 23 mm/h.
Coronary arteriography identified an intramural haematoma with an ulcer in the ascending aorta immediately distal to the left coronary artery ostium (figure two). The operator avoided contact with the ulcer and recommended surgical consultation for early operative repair. 6 h afterwards, the patient had a temperature of 39·3°C, but was not tachycardic despite his high temperature. Two sets of blood cultures were obtained, and 60 h later small Gram-negative bacilli had grown in both aerobic bottles. Within 3 days the organism was identified as a Brucella spp, on the basis of morphology and a rapid and strongly positive urease test. Amplification with universal bacterial 16S rRNA PCR primers followed by sequencing confirmed the diagnosis of Brucella spp. The isolate was sent to the US Centers for Disease Control and Prevention (Atlanta, GA, USA) for speciation, and identified by PCR as Brucella abortus. B abortus IgG and IgM were positive by ELISA and agglutination antibody tests, with an agglutination titre of 1/640. Repeat blood cultures 5 days later and before initiation of antibiotics were negative.
Figure 2. Invasive coronary arteriography (A) and cardiac CT scan (B).
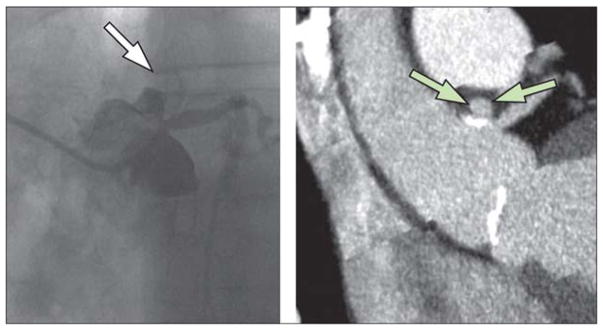
Arrows indicate the penetrating intramural aortic ulcer immediately distal to the ostium of the left coronary artery.
Transoesophageal echocardiography showed the penetrating aortic ulcer, but no valvular vegetation or paravalvular abnormality was present. The consensus decision was to delay surgical repair to allow a period of antimicrobial therapy. Treatment was started with doxycycline 100 mg twice daily, rifampicin 600 mg daily, and gentamicin 5 mg/kg intravenously daily. After a 2 week course of triple therapy, the patient was discharged on doxycycline and rifampicin.
4 months after diagnosis, the patient had surgical repair of the penetrating aortic ulcer. The ascending aorta showed generalised atherosclerosis and a posterior penetrating ulcer associated with dense surrounding inflammatory tissue adherent to the left main coronary and pulmonary arteries. The inflammatory tissue was resected, the ascending aorta replaced by a prosthetic graft, and a two-vessel coronary artery bypass graft done. Pathology of the aorta showed partially calcified atheromatous plaque and mild perivascular inflammation involving the vasa vasorum. PCR of the surgical aortic specimen was negative for Brucella spp. The abdominal aortic aneurysm was not thought to be involved because it had been stable in size for at least 3 years.
Within 3 months of the start of therapy, and before surgical treatment, the patient’s anti-brucella IgM had reached the lower limit of detection. 6 months into treatment, his white blood cell and C-reactive protein concentrations had normalised, his anaemia had substantially improved, and his agglutination antibody test was negative. 15 months into treatment, and after 19 years of back pain, the patient became pain-free and was no longer taking analgesics; repeat spine imaging was not done. He continued on doxycycline and rifampicin with a plan for lifelong therapy in view of the indwelling graft material in a presumed infected area.
Brucellosis
Brucellosis is an anthropozoonotic infection caused by small, intracellular, Gram-negative bacteria of the genus Brucella. Because of a high morbidity rate in both human beings and animals, brucellosis represents a serious public health issue in endemic countries. As a result of increased international tourism and migration, and the potentially slow clinical progression of disease, brucellosis is also a pathogen of potential importance in non-endemic countries.1,4 The primary method of infection with Brucella spp is by occupational exposure to animals. The different pathogenic species are host-specific, with Brucella melitensis (the species that most commonly infects human beings) spread by sheep and goats, B abortus spread by cows, and Brucella suis transmitted by pigs. Human beings are also infected through ingestion of unpasteurised dairy products and (especially in non-endemic regions) through laboratory exposure. 1,4 B abortus might have a more indolent presentation in human beings than does B melitensis,14 as was the case with our patient.
Brucellosis is a disease that has protean clinical manifestations. The clinical course can be divided into acute, undulant, and chronic forms.2 Acute brucellosis typically presents as an undifferentiated fever, often accompanied by malaise, diaphoresis, and arthralgias.15 The so-called classic or undulant form occurs after about 2 months of infection, and is characterised by an undulating fever.16
The chronic form of brucellosis consists of either localised disease or a distinct syndrome characterised by cyclical bouts of back pain, arthralgias, and sweating.16 Chronic disease can present many years after initial exposure. This long latency period is probably a result of the slow, intracellular growth of the organism.17 Localised disease due to chronic brucellosis most commonly manifests (in decreasing order of frequency) as spondylitis, hepatitis, epididymitis, or endocarditis.4 Although cardiovascular involvement occurs in only 1–2% of all cases, cardiac complications are the main cause of mortality from brucellosis.2,4,15,18–22
Brucella endarteritis
Demographics of brucella endarteritis cases
The most common cardiovascular manifestation of brucella infection is endocarditis.2 However, brucella can also cause arterial and venous thrombosis, cutaneous vasculitis, and endarteritis, leading to peripheral and cerebrovascular aneurysms.2,23 The bacteria can directly infect endothelial cells and cause a sustained pro-inflammatory response, although the precise role of this event in the pathogenesis of endovascular infection is unclear.24 34 cases of brucella endarteritis have been published;25–59 the table lists the demographics of these cases of vascular brucellosis. Patients who present with brucella arteritis tend to be young (mean age of published cases is 42·9 years) and predominantly male. The reason for the male predominance of infection is unknown, but has been recorded in other studies of localised chronic brucellosis,15,60 and could indicate differing occupational exposures between the sexes, or differing access to care.
The geographical origin of cases of brucella arteritis does not differ significantly from the known distribution of brucella endemicity; most reported cases come from the Middle East and the Mediterranean region. The most common causative species of endarteritis among reported cases include B melitensis (62·5%), B abortus (16·7%), B suis (16·7%), and Brucella canis (4·0%). In most cases, patients report exposure to a known risk factor for brucellosis, most commonly occupational exposure to animals, followed by ingestion of unpasteurised dairy products, travel to endemic regions, and non-occupational contact with animals.
Cases of endarteritis due to brucella infection occur frequently in individuals who have underlying disease that might have predisposed them to cardiac involvement (table). The most common underlying conditions are atherosclerosis, hypertension, and rheumatic heart disease. Endocarditis also commonly accompanies endarteritis in patients with brucellosis.
Table.
| Data for cases | |
|---|---|
| Demographic | |
| Mean age (years; SD, range) | 42.9 (17.2;11.0–78.0) |
| Sex | |
| Men | 30 (80%) |
| Women | 4 (12%) |
| Geographical origin | |
| Turkey | 6 |
| USA | 5 |
| Saudi Arabia | 4 |
| Spain | 3 |
| Italy | 3 |
| Mexico | 2 |
| Iran | 2 |
| France | 2 |
| Portugal | 1 |
| Corsica | 1 |
| Morocco | 1 |
| Poland | 1 |
| Korea | 1 |
| Greece | 1 |
| Macedonia | 1 |
| Exposure history | |
| Any | 25 (74%) |
| Occupational | 14 (41%) |
| Ingestion of unpasteurized dairy products | 10 (29%) |
| Travel to endemic regions | 4 (12%) |
| Non-occupational contact with animals | 2 (6%) |
| Comorbid conditions | |
| Any | 14 (41%) |
| Atherosclerotic heart disease | 6 (18%) |
| Hypertension | 4 (12%) |
| Rheumatic fever | 3 (9%) |
| Smoker | 2 (6%) |
| Diabetes | 1 (3%) |
| Subaortic membrane | 1 (3%) |
| Presentation | |
| Vessel involvement | |
| Aorta | 23 (68%) |
| Aortic aneurysm | 18 (53%) |
| Aortic root abscess | 4 (12%) |
| Aortic thrombus | 1 (3%) |
| Lower extremity vessel | 6 (18%) |
| Femoral artery thrombus | 1 (3%) |
| Femoral artery aneurysm | 2 (6%) |
| Popliteal artery aneurysm | 2 (6%) |
| Posterior tibial/peroneal artery aneurysms | 1 (3%) |
| Upper extremity vessel | 3 (9%) |
| Radial artery aneurysm | 1 (3%) |
| Brachial artery aneurysm | 1 (3%) |
| Subclavian artery thrombus | 1 (3%) |
| Carotid aneurysm | 1 (3%) |
| Superior mesenteric artery aneurysm | 2 (6%) |
| Presenting symptoms | |
| Warmth/subjective fever | 16 (47%) |
| Abdominal pain | 10 (29%) |
| Back pain | 9 (26%) |
| Weight loss | 10 (29%) |
| Diaphoresis | 8 (24%) |
| Arthralgias | 6 (18%) |
| Malaise | 6 (18%) |
| Chills | 4 (12%) |
| Dyspnoea on exertion | 4 (12%) |
| Chest pain | 2 (6%) |
| Abnormal findings at physical examination | |
| Heart murmur | 15 (44%) |
| Hepatosplenomegaly | 10 (29%) |
| Pulsatile mass | 7 (21%) |
| Absent distal pulse | 6 (18%) |
| Neurological findings (weakness, decreased reflexes) | 4 (12%) |
| Skin rash | 2 (6%) |
| Temperature, geometric mean (°C; SD, range) | 38·2 (0·78, 37·0–39·5) |
| Mean white blood cell count 103 per μL (SD, range) | 7.1 (3.7, 3·1–16.7) |
| Mean haemoglobin g/L (SD, range) | 100 (22, 60–138) |
| Mean ESR mm/h (SD, range) | 53.7 (31.6, 6.0–120.0) |
Data are number (%), unless otherwise indicated. ESR=erythrocyte sedimentation rate. The “any” category under exposure history and comorbid condition refers to the number of cases in which this history was provided in the case presentation. Cases might have had more than one exposure history or comorbid condition. Therefore, the numbers do not necessarily add up to 34.
Manifestations of infection
The presenting signs and symptoms of localised brucellosis are non-specific and unlikely to be helpful for diagnosis. The most prominent symptom is subjective fever, which is present in about 47% of published cases. Other common presenting symptoms of patients who have brucella endarteritis are abdominal pain (29%), weight loss (29%), back pain (26%), and diaphoresis (24%). No symptom had greater than 50% sensitivity for diagnosis of localised brucellosis in these cases. Physical examination findings are similarly non-specific, and when present seem to signify concomitant endocarditis and not the endarteritis itself. The most common physical examination findings include heart murmur; hepatosplenomegaly, a palpable, pulsatile mass, and absent distal pulses.
One challenge in diagnosis of endarteritis due to brucella is that traditional markers of infection such as fever and white blood cell count are frequently absent. Contrary to the common notion of brucellosis as a febrile illness, only 63% of known cases had a fever on admission. Additionally, most patients had normal white blood cell counts on presentation. The most common laboratory abnormalities are non-specific, and include anaemia and increased sedimentation rate. The most common site of arterial involvement is the aorta, followed by lower extremity and upper extremity arteries (table). Most cases are identified in the setting of degenerative aneurysms in those vessels.
Multiorgan involvement of brucella is probably under-recognised. In a series of 1028 cases of brucellosis, 36·1% of cases had focal involvement, most commonly in the bone, CNS, or epididymis.2 In most published cases of brucella endarteritis, there was no explicit indication of a search for concurrent extravascular foci of brucellosis. However, when additional sites of infection were investigated, they were present in most cases.25,28,30,38–40,44,50,54 The most common extravascular sites of infection among patients who had endarteritis were bone (87·5%), kidney (12·5%), and testes (12·5%). Despite this high proportion of focal involvement, and the increased risk of relapse with such involvement,5 few of the published cases (37%) describe an assessment for the possibility of extravascular infection.
The clinical presentation of brucella endarteritis challenges the common perception of brucellosis as an acute febrile illness with short incubation. Our patient’s primary risk factor for brucellosis occurred 18 years before his presentation with aortic endarteritis. His back pain was not investigated for many years, and when imaging studies showed vertebral abnormalities they were not recognised as being infectious. His positive blood culture drew attention to the infection; in a review, positive blood cultures were associated with survival in patients with brucella endocarditis because they led to earlier diagnosis.61 Our review identified two additional cases with remarkably long intervals between exposure and diagnosis.25,27 These cases of prolonged, low-grade infection are unsurprising in view of the pathophysiology of brucella. Brucella exist as intracellular organisms in walled-off compartments within macrophages, which probably helps the organism to evade the immune system for long periods.17,62 The time from exposure seems to be unhelpful in excluding brucellosis as a possible diagnosis.
Diagnosis
Serology is the primary method of diagnosis, and was used in most published cases of endarteritis. Although some studies have shown that serology seems to be less sensitive in localised disease than in disseminated infection, this does not seem to be the case with endarteritis, possibly because many cases present late in the course of infection.27,31,37 Brucella serology, whether by agglutination test or ELISA, is positive in 92% of patients with endarteritis (among those who had serological testing). Agglutination titres can be measured by several different assays, including standard tube or Wright’s agglutination assay, Coombs’ test, Rose Bengal test, and the 2-mercaptoethanol test.1,4 Agglutination assays had a sensitivity of 91% in reported cases of endarteritis; brucella ELISA had a reported sensitivity of 100%. Findings from only one study showed poor concordance between serological tests in brucella endartertitis.25 Three cases, however, had initial negative serologies that turned positive in repeated tests.31,37.50 Thus, if brucellosis is a strong diagnostic consideration, repetition of an initial negative serological test seems reasonable.
PCR for brucella is a newer diagnostic test that can help to confirm the diagnosis. Although this test was rarely done in previous published studies, it seems to have adequate sensitivity (80%).27,44,45,51,55 The potential diagnostic usefulness of PCR is exemplified by one case with negative blood cultures in which the diagnosis of brucellosis was made from a positive PCR on resected tissue.27 Despite the fact that brucella is traditionally thought to be a fastidious organism on culture, blood and tissue cultures are sensitive and useful tests for diagnosis of brucella in these cases. In the published cases we reviewed, blood cultures had an average sensitivity of 68% (ie, 25 of 34 cases did this analysis, 17 of which were positive); tissue cultures had a mean sensitivity of 71%. In the case of our patient, brucella PCR was attempted from a tissue specimen that had been fixed in formalin after the patient had already received several months of multidrug treatment, possibly explaining the negative result from molecular testing.
Treatment
Treatment of brucella arteritis varies substantially (figure 3). Generally, antibiotic regimens included a combination of two or three of five antibiotics: fluoroquinolones, sulfanilamides, tetracyclines, rifampicin, and aminoglycosides. Most patients had at least one regimen change during their course of therapy; in most cases the change in therapy was due to intolerance to the initial regimen, although treatment failure or relapse were the attributed causes in several cases.26,27,41 Although length of therapy varied substantially, on average patients were treated for about 3 months.
Figure 3. Antibiotics used to treat brucellosis.
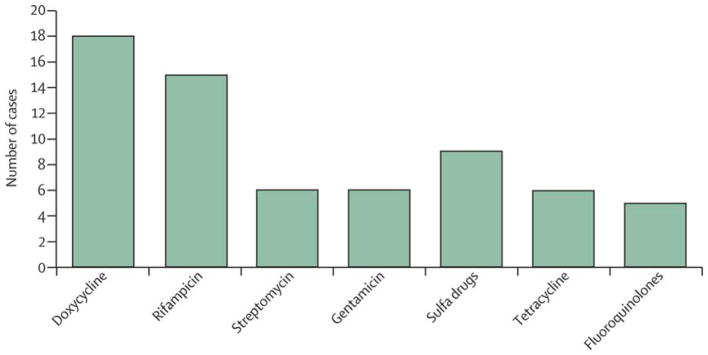
The number of patients treated with each class of antibiotic among 30 published cases in which antibiotic regimens were described.25–32, 35–45,47,49,50,52,53,55,56,57,58 Sulfa drugs include co-trimoxazole, terfonyl, and sulfanilamide.
In the absence of specific data for the treatment of brucella localised to the cardiovascular system, treatment of brucella arteritis according to the recommendations for generalised infection seems reasonable.5,6 WHO recommends doxycycline and rifampicin for 6 weeks as standard therapy for brucellosis; however, doxycycline for 6 weeks with streptomycin for the initial 3 weeks is also an acceptable alternative, and is the regimen used for the Ioannina recommendations.50
Results of two systematic reviews showed that doxycycline with streptomycin might have improved outcomes compared with doxycycline and rifampicin.63,64 Although the doxycycline with streptomycin regimen was associated with more light-to-moderate side-effects than was doxycycline and rifampicin, the rate of serious side-effects between these two treatment regimens did not differ.63 Additionally, the rate of relapses and treatment failures was higher in the doxycycline and rifampin group than in those given doxycycline with streptomycin.63 Fluoroquinolones seem to be less effective for the treatment of brucellosis and are not recommended.3 Despite these clear treatment recommendations, many of the published cases of brucella endarteritis we have described used alternative antimicrobial regimens. Investigators noticed a trend towards improved survival in cases in which treatment followed one of these recommended regimens.
Most reported cases were treated surgically and medically. The most common surgeries included in-situ aortic graft, extra-anatomical vascular bypass, aneurysmectomy of peripheral artery, valve replacement, and fistula repair. The indications for surgery included haemodynamic instability, limb ischaemia, and impending aneurysmal rupture, which were similar to those used for surgical management of mycotic aneurysms and other intravascular infections. Most patients were taken to surgery as soon as their infections were identified. Results of a review of brucella endocarditis showed a dramatic survival advantage when patients underwent surgery and medical therapy.61 Thus, whether any circumstances exist in which patients who have brucella arteritis could be treated with antibiotics alone is unclear.
Prognosis
Findings from our review of published work suggests that patients with brucella endarteritis have high morbidity and mortality rates. The mortality rate in published cases is 21%, and many patients’ infection progressed despite adequate therapy.32,41,50,52,55 A review of published cases suggested that several factors seem to be associated with increased likelihood of mortality with brucella arteritis. Survival seems to be lower in those infected with B suis than in those infected with other strains. Death rates are also higher in those who had concomitant endocarditis.
Of the published cases we identified, patients who died had, on average, been treated with fewer antibiotics than had those who survived; however, this finding could have been affected by the fact that, in some cases, patients presented with acute cardiovascular collapse and died before appropriate antimicrobial therapy could be provided.26,56 Additionally, the short follow-up for some cases could prevent a full picture of the longer-term mortality from recrudescence or relapse of infection.
Although few cases were treated with medical therapy alone, the mortality rate for those treated solely with medical therapy was much higher than for those treated surgically (75% vs 16%). However, this finding is also likely to be skewed by cases in which patients died before surgery could be done and by other possible differences in characteristics of the patients who underwent surgical procedures compared with those who did not.26
Brucella endocarditis
The concomitant presence of endocarditis represents one of the key risk factors for poor outcome in cases of endarteritis. Indeed, brucella endocarditis is the main cause of mortality related to brucellosis.21,22 Endocarditis is found most often on the aortic valve, followed by the mitral valve. Only one case of prosthetic valve endocarditis in conjunction with endarteritis has been reported.31 Compared with other causes of endocarditis, brucella is associated with a high proportion of intracardiac abscess and congestive heart failure.60 Brucella endocarditis is typically a slowly progressive, destructive process associated with large vegetations. As with cases of endarteritis, cases of endocarditis seem to have an increased mortality when treated with medical therapy alone.61 Indications for surgery include bulky vegetations, abscess, aneurysm formation, valvular malfunction, and prosthetic valve endocarditis.21 Some cases of concurrent endocarditis and endarteritis have been treated with cardiac surgery alone, and without surgical management of the infected vessel.55
Conclusion
Brucellosis is a disease with protean manifestations that can be undetected for long periods before becoming clinically apparent. A diagnosis of intravascular infection with brucella warrants a search for extravascular sites of infection with careful physical examination and imaging of suspected sites of involvement. Although most would agree that a course of multidrug therapy is usually warranted in brucella arteritis, duration of therapy has not been established. Surgery for endarteritis might confer a mortality benefit, as is the case for brucella endocarditis, but possible selection bias weakens this conclusion. For our patient’s unique circumstances, we chose long-term multidrug suppression because of the delicate location of prosthetic intravascular graft material in the site of infection, as well as the patient’s age and ability to tolerate the medications in the long-term. Clinical studies are needed to elucidate the optimum therapeutic approach for this uncommon but life-threatening manifestation of brucellosis.
Search strategy and selection criteria.
We identified data by searching the National Library of Medicine through PubMed for the terms “brucella” AND “aortitis” OR “aneurysm” OR “arteritis” OR “endarteritis”. We searched references of identified articles for further articles. Additionally, we searched Web of Science, Scopus, Embase, Biosis, Agricola, Cochrane Library, Cinahl Plus, ClinicalTrials.gov, Conference Papers Index, Gideon, PsycINFO, Google Scholar, and Oaister with the terms “brucella” AND “abortus” OR “canis” OR “ovis” OR “suis” OR “melitensis” AND “artery” OR “arteries”. We included studies published in any language and at any date up to July 31, 2013, and we did not exclude studies on the basis of quality screens. We included all study designs that provided information about cases of brucella arteritis, including randomised controlled trials, cohort studies, case-control studies, and case series.
Acknowledgments
We thank the Fellows Editorial Board of the NIH for their valuable assistance with editing of this Grand Round. This research was supported [in part] by the intramural research program of the National Institute of Allergy and Infectious Disease. JP is supported by the Collaborative Clinical Research Branch, Clinical Research Directorate/CMRP, SAIC-Frederick. This project was supported by federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and from the National Cancer Institute, National Institutes of Health, under contract number HHSN261200800001E. The content of this publication does not necessarily represent the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organisations imply endorsement by the U.S. Government.
Footnotes
Contributors
BS and JHP did the primary literature search. JAH, JHP, and TNP created the protocol, and reviewed and abstracted data from each selected article. JAH created the figures, analysed the data, and wrote the first draft of the paper. TNP, RJL, and JHP all assisted in editing the manuscript.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 2.Buzgan T, Karahocagil MK, Irmak H, et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis. 2010;14:e469–e478. doi: 10.1016/j.ijid.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Skalsky K, Yahav D, Bishara J, Pitlik S, Leibovici L, Paul M. Treatment of human brucellosis: systematic review and meta-analysis of randomised controlled trials. BMJ. 2008;336:701–704. doi: 10.1136/bmj.39497.500903.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq JA. Therapeutic options for human brucellosis. Expert Rev Anti Infect Ther. 2008;6:109–120. doi: 10.1586/14787210.6.1.109. [DOI] [PubMed] [Google Scholar]
- 6.Ariza J, Bosilkovski M, Cascio A, et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 2007;4:e317. doi: 10.1371/journal.pmed.0040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas G, Christou L, Akritidis N, Tsianos EV. Quinolones for brucellosis: treating old diseases with new drugs. Clin Microbiol Infect. 2006;12:823–825. doi: 10.1111/j.1469-0691.2006.01442.x. [DOI] [PubMed] [Google Scholar]
- 8.Solera J. Treatment of human brucellosis. J Med Liban. 2000;48:255–263. [PubMed] [Google Scholar]
- 9.Solera J, Espinosa A, Geijo P, et al. Treatment of human brucellosis with netilmicin and doxycycline. Clin Infect Dis. 1996;22:441–445. doi: 10.1093/clinids/22.3.441. [DOI] [PubMed] [Google Scholar]
- 10.Solera J, Espinosa A, Martinez-Alfaro E, et al. Treatment of human brucellosis with doxycycline and gentamicin. Antimicrob Agents Chemother. 1997;41:80–84. doi: 10.1128/aac.41.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solera J, Geijo P, Largo J, et al. A randomized, double-blind study to assess the optimal duration of doxycycline treatment for human brucellosis. Clin Infect Dis. 2004;39:1776–1782. doi: 10.1086/426024. [DOI] [PubMed] [Google Scholar]
- 12.Solera J, Martinez-Alfaro E, Saez L. Meta-analysis of the efficacy of the combination of +rifampicin and doxycycline in the treatment of human brucellosis. Med Clin (Barc) 1994;102:731–738. [PubMed] [Google Scholar]
- 13.Solera J, Rodriguez-Zapata M, Geijo P, et al. Antimicrob Agents Chemother. 1995;39:2061–2067. doi: 10.1128/aac.39.9.2061. Doxycycline-rifampin versus doxycycline-streptomycin in treatment of human brucellosis due to Brucella melitensis. The GECMEI Group. Grupo de Estudio de Castilla-la Mancha de Enfermedades Infecciosas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troy SB, Rickman LS, Davis CE. Brucellosis in San Diego: epidemiology and species-related differences in acute clinical presentations. Medicine. 2005;84:174–187. doi: 10.1097/01.md.0000165659.20988.25. [DOI] [PubMed] [Google Scholar]
- 15.Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, Zinsstag J. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1929. doi: 10.1371/journal.pntd.0001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 7. Churchill Livingstone/Elsevier; Philadelphia, PA: 2010. [Google Scholar]
- 17.Roop RM, 2nd, Gaines JM, Anderson ES, Caswell CC, Martin DW. Survival of the fittest: how brucella strains adapt to their intracellular niche in the host. Med Microbiol Immunol. 2009;198:221–238. doi: 10.1007/s00430-009-0123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reguera JM, Alarcon A, Miralles F, Pachon J, Juarez C, Colmenero JD. Brucella endocarditis: clinical, diagnostic, and therapeutic approach. Eur J Clin Microbiol Infect Dis. 2003;22:647–650. doi: 10.1007/s10096-003-1026-z. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs F, Abramowicz D, Vereerstraeten P, Le Clerc JL, Zech F, Thys JP. Brucella endocarditis: the role of combined medical and surgical treatment. Rev Infect Dis. 1990;12:740–744. doi: 10.1093/clinids/12.5.740. [DOI] [PubMed] [Google Scholar]
- 20.Cohen N, Golik A, Alon I, et al. Conservative treatment for Brucella endocarditis. Clin Cardiol. 1997;20:291–294. doi: 10.1002/clc.4960200319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mert A, Kocak F, Ozaras R. The role of antibiotic treatment alone for the management of Brucella endocarditis in adults: a case report and literature review. Ann Thorac Cardiovasc Surg. 2002;8:381–385. [PubMed] [Google Scholar]
- 22.Peery TM, Belter LF. Brucellosis and heart disease. II. Fatal brucellosis: a review of the literature and report of new cases. Am J Pathol. 1960;36:673–697. [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez Doval FJ, Ruiz de Erenchun Lasa F, Sola Casas MA, Soto de Delas J, Quintanilla Gutierrez E. Acute brucellosis presenting as leukocytoclastic vasculitis. J Investig Allergol Clin Immunol. 1991;1:411–413. [PubMed] [Google Scholar]
- 24.Ferrero MC, Bregante J, Delpino MV, et al. Proinflammatory response of human endothelial cells to brucella infection. Microbes Infect. 2011;13:852–861. doi: 10.1016/j.micinf.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Aguado JM, Barros C, Gomez Garces JL, Fernandez-Guerrero ML. Infective aortitis due to brucella melitensis. Scand J Infect Dis. 1987;19:483–484. doi: 10.3109/00365548709021683. [DOI] [PubMed] [Google Scholar]
- 26.al Kasab S, al Fagih M, al Rasheed A, et al. Management of brucella endocarditis with aortic root abscess. Chest. 1990;98:1532–1534. doi: 10.1378/chest.98.6.1532. [DOI] [PubMed] [Google Scholar]
- 27.Bakhos CT, Gangadharan SP, Snyder GM, Wong MT, Hagberg RC. Management of aortic brucellosis with infection of a descending thoracic aortic stent graft. Ann Thorac Surg. 2010;89:2038–2040. doi: 10.1016/j.athoracsur.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 28.Bergeron P, Gonzales-Fajardo J, Mangialardi N, Courbier R. False aneurysm of the abdominal aorta due to Brucella suis. Ann Vasc Surg. 1992;6:460–463. doi: 10.1007/BF02007004. [DOI] [PubMed] [Google Scholar]
- 29.Biyik I, Oto O, Ergene O. Brucella pancarditis with dissecting aortic root abscess, left ventricular pseudoaneurysm and ventricular septal defect. J Int Med Res. 2007;35:422–426. doi: 10.1177/147323000703500318. [DOI] [PubMed] [Google Scholar]
- 30.Blain H, Laraki R, Levy-Soussan M, et al. Aneurysm of the thoracic aorta and spondylodiscitis disclosing brucellosis. Rev Med Interne. 1997;18:876–881. doi: 10.1016/s0248-8663(97)81961-3. [DOI] [PubMed] [Google Scholar]
- 31.Cakalagaoglu C, Keser N, Alhan C. Brucella-mediated prosthetic valve endocarditis with brachial artery mycotic aneurysm. J Heart Valve Dis. 1999;8:586–590. [PubMed] [Google Scholar]
- 32.Caylan R, Keske S, Durmaz T, Keles T, Tasyaran MA. A case of brucella endocarditis in association with superficial femoral artery thrombus. Trop Doct. 2009;39:251–252. doi: 10.1258/td.2009.080342. [DOI] [PubMed] [Google Scholar]
- 33.Cueto Garcia L, Cuan M, Gonzalez-Serna JL, Vizcaino A. Diagnosis of mycotic aneurysm of the aorta using bidimensional echocardiography. Arch Inst Cardiol Mex. 1983;53:23–26. [PubMed] [Google Scholar]
- 34.da Gama AD, Rosa A, Martins C, et al. Primary aneurysms of carotid bifurcation: surgical management. Rev Port Cirurgia Cardiotorac Vasc. 2005;12:163–16. [PubMed] [Google Scholar]
- 35.Erbay AR, Turhan H, Dogan M, Erbasi S, Cagli K, Sabah I. Brucella endocarditis complicated with a mycotic aneurysm of the superior mesenteric artery: a case report. Int J Cardiol. 2004;93:317–319. doi: 10.1016/S0167-5273(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 36.Esmailpour N, Borna S, Nejad MR, Badie SM, Badie BM, Hadadi A. Brucella endocarditis: a report from Iran. Trop Doct. 2010;40:47–49. doi: 10.1258/td.2009.090039. [DOI] [PubMed] [Google Scholar]
- 37.Gelfand MS, Kaiser AB, Dale WA. Localized brucellosis: popliteal artery aneurysm, mediastinitis, dementia, and pneumonia. Rev Infect Dis. 1989;11:783–788. doi: 10.1093/clinids/11.5.783. [DOI] [PubMed] [Google Scholar]
- 38.Gillet M, Sava P, Cassou M, Guidet M, Camelot G, Mantion G. Infectious aneurysm of the infrarenal aorta. Comments apropos of 2 cases of brucellar etiology. Chirurgie; memoires de l’Academie de chirurgie. 1983;109(2):168–72. [PubMed] [Google Scholar]
- 39.Gillet M, Sava P, Cassou M, Guidet M, Camelot G, Mantion G. Infectious aneurysm of the infrarenal aorta. Comments apropos of 2 cases of brucellar etiology. Chirurgie. 1983;109:168–172. [PubMed] [Google Scholar]
- 40.Sava P, Camelot G, Miguet JP, et al. Aneurysm of the abdominal aorta and brucellosis. A new case operated on successfully (author’s transl) Ann Chir Thorac Cardiovasc. 1977;16:221–225. [PubMed] [Google Scholar]
- 41.Golden B, Layman TE, Koontz FP, Mergner WJ. Brucella suis endocarditis. South Med J. 1970;63:392–395. doi: 10.1097/00007611-197004000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Harman M, Irmak H, Arslan H, Arslan U, Kayan M. Popliteal artery pseudoaneurysm: a rare complication of brucellosis. J Clin Ultrasound. 2004;32:33–36. doi: 10.1002/jcu.10217. [DOI] [PubMed] [Google Scholar]
- 43.Kumar N, Prabhakar G, Kandeel M, et al. Brucella mycotic aneurysm of ascending aorta complicating discrete subaortic stenosis. Am Heart J. 1993;125:1780–1782. doi: 10.1016/0002-8703(93)90775-5. [DOI] [PubMed] [Google Scholar]
- 44.Kusztal M, Dorobisz A, Kuzniar J, et al. Dissecting aneurysm of the thoracic aorta in a patient with nephrotic syndrome and brucellosis. Int Urol Nephrol. 2007;39:641–645. doi: 10.1007/s11255-006-9090-9. [DOI] [PubMed] [Google Scholar]
- 45.Kwon TW, Kim HK, Moon KM, Cho YP, Park SJ. In situ polytetrafluoroethylene graft bypass for primary infected aneurysm of the infrarenal abdominal aorta. World J Surg. 2010;34:1689–1695. doi: 10.1007/s00268-010-0507-3. [DOI] [PubMed] [Google Scholar]
- 46.McKee MA, Ballard JL. Mycotic aneurysms of the tibioperoneal arteries. Ann Vasc Surg. 1999;13:188–190. doi: 10.1007/s100169900240. [DOI] [PubMed] [Google Scholar]
- 47.Park SJ, Kim MN, Kwon TW. Infected abdominal aortic aneurysm caused by Brucella abortus: a case report. J Vasc Surg. 2007;46:1277–1279. doi: 10.1016/j.jvs.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 48.Piampiano P, McLeary M, Young LW, Janner D. Brucellosis: unusual presentations in two adolescent boys. Pediatr Radiol. 2000;30:355–357. doi: 10.1007/s002470050760. [DOI] [PubMed] [Google Scholar]
- 49.Quaniers J, Durieux R, de Leval L, Limet R. Abdominal aortic aneurysm due to Brucella melitensis. Acta Chir Belg. 2005;105:93–95. [PubMed] [Google Scholar]
- 50.Sanchez-Gonzalez J, Garcia-Delange T, Martos F, Colmenero JD. Thrombosis of the abdominal aorta secondary to Brucella spondylitis. Infection. 1996;24:261–262. doi: 10.1007/BF01781108. [DOI] [PubMed] [Google Scholar]
- 51.Tsioufis K, Stefanadis C, Kallikazaros I. A footprint of brucella infection: enormous saccular aneurysm of the ascending aorta. Heart. 2006;92:1308. doi: 10.1136/hrt.2005.082347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ustuner E, Erden A, Fitoz S, Erden I, Sancak T. Deep femoral artery pseudoaneurysm due to brucellosis. J Ultrasound. 2001;20:1353–1356. doi: 10.7863/jum.2001.20.12.1353. [DOI] [PubMed] [Google Scholar]
- 53.Wolff T, Leupold J, Nuesch R. Bang! The smoker with suprapubic pain. Lancet. 2009;374:174. doi: 10.1016/S0140-6736(09)60869-2. [DOI] [PubMed] [Google Scholar]
- 54.Yee N, Roach DJ. Infected abdominal aortic aneurysm caused by spinal brucellar infection. AJR Am J Roentgenol. 1996;167:1068–1069. doi: 10.2214/ajr.167.4.8819420. [DOI] [PubMed] [Google Scholar]
- 55.Colomba C, Siracusa L, Rubino R, et al. A case of brucella endocarditis in association with subclavian artery thrombosis. Case Rep Infect Dis. 2012;2012:581489. doi: 10.1155/2012/581489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colonna L, Cristallo E. Clinico-anatomical conference: cardiogenic shock with intestinal hemorrhage. Dissecting aortic aneurysm or pulmonary embolism? Spastic tetraparesis due to chronic brucella infection? Cardiol Prat. 1978;29:57–64. [PubMed] [Google Scholar]
- 57.Fudge T, Ochsner J, Ancalmo N, Mills N. Surgical resection of multiple aortic aneurysms due to Brucella suis. Surgery. 1977;81:236–238. [Google Scholar]
- 58.DeGowin E, Carter J, Borts I. A case of infection with Brucella suis, causing endocarditis and nephritis; death fro rupture of mycotic aneurysm. Am Heart J. 1945;30:77–87. [Google Scholar]
- 59.Cano Trigueros E, Carranza Martinez JM, Perez Garcia E, Pobo Ruiz V, Marco Luque MA. A digestive hemorrhage due to an aortoduodenal fistula secondary to an aortic aneurysm of brucellar etiology. Rev Esp Enferm Dig. 1997;89:728–729. [PubMed] [Google Scholar]
- 60.Colmenero JD, Reguera JM, Martos F, et al. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine. 1996;75:195–211. doi: 10.1097/00005792-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Keshtkar-Jahromi M, Razavi SM, Gholamin S, Keshtkar-Jahromi M, Hossain M, Sajadi MM. Medical versus medical and surgical treatment for brucella endocarditis. Ann Thorac Surg. 2012;94:2141–2146. doi: 10.1016/j.athoracsur.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorvel JP. Brucella: a Mr “Hide” converted into Dr Jekyll. Microbes Infect. 2008;10:1010–1013. doi: 10.1016/j.micinf.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Solis Garcia del Pozo J, Solera J. Systematic review and meta-analysis of randomized clinical trials in the treatment of human brucellosis. PloS One. 2012;7:e32090. doi: 10.1371/journal.pone.0032090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yousefi-Nooraie R, Mortaz-Hejri S, Mehrani M, Sadeghipour P. Antibiotics for treating human brucellosis. Cochrane Database Syst Rev. 2012;10:CD007179. doi: 10.1002/14651858.CD007179.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]