Abstract
Vitamin D deficiency in mothers and infants is a global health disorder despite recognition that it is preventable. Recent data support the theory that vitamin D deficiency in adults and children may increase the risk of infections and auto-immune diseases. In most cases, vitamin D deficiency is caused by sunlight deprivation and inadequate corrective vitamin D intake. There is a strong mother/infant vitamin D relationship that affects vitamin D status both in utero and in infancy. Recognition that vitamin D deficiency is a worldwide mother/infant health problem is a basis on which to modify public health strategies to reduce the burden of disease and improve maternal and child vitamin D nutrition. This review provides an update on vitamin D function and the global scope and implications of vitamin D deficiency as it relates to pregnancy and infancy. It also addresses a combined strategy to prevent vitamin D deficiency during pregnancy, lactation and infancy.
Keywords: vitamin D, mother, infant, deficiency
Introduction
Vitamin D deficiency (VDD) and rickets in infancy and childhood are increasingly reported as public health problems in many parts of the world, especially Asia, the Middle East and North Africa, and among immigrant populations in Europe, Australia and New Zealand, as well as minority groups in the UK and US.1–4 Many infants worldwide are born with low vitamin D stores because of the high prevalence of maternal VDD and, hence, are at risk of rickets.5 This is despite recognition that vitamin D is effective in preventing VDD in adults and children.
In addition to its classical action of maintaining calcium homeostasis and bone health, vitamin D is involved in immune modulation, cell growth and cell metabolism.6,7 A recent review discussed the association between VDD and the risk of multiple non-skeletal adverse health effects such as auto-immune diseases, cardiovascular diseases, diabetes and certain types of cancer.6 In pregnancy, low vitamin D status or intake is detrimental to mother and fetus and predisposes to VDD in early infancy.8 VDD in infancy and childhood has been linked to increased risk of lower respiratory tract infections9,10 and low vitamin D levels in cord blood is associated with increased risk of acute respiratory infections and wheezing in childhood.11 In addition, inadequate vitamin D intake in infancy has been associated with increased risk of type I diabetes mellitus.12 A recent publication by the United States’ Institute of Medicine (IOM), however, concluded that the association between vitamin D and non-skeletal outcomes does not prove causality and recommended more large-scale, randomised controlled trials to test the effects of vitamin D supplementation on non-skeletal conditions.13
There is growing concern that VDD during pregnancy and from birth through childhood is a global public health issue, but the magnitude is not well described. In view of new knowledge of possible multiple adverse health effects of low vitamin D status, it is appropriate and timely to review the scale of VDD in mothers and their infants and re-examine the strategies for prevention. The IOM’s 2010 report recommends serum 25-hydroxyvitamin D [25(OH)D] concentrations <50 nmol/L as low vitamin D status in adults and children.13
This review addresses (i) new information on vitamin D functions; (ii) global magnitude and emerging consequences of VDD during pregnancy, lactation and infancy; and (iii) strategies to shift the focus of prevention to mothers as well as infants.
Vitamin D Synthesis and Functions
Vitamin D3 is produced from epidermal 7-dehydrocholesterol after skin exposure to ultraviolet B (UVB) light with a wavelength of 290–310 nm, forming pre-vitamin D3 which is then transformed into vitamin D3 by thermal conversion through the skin. Subsequently, vitamin D, either made in the skin or ingested in the diet (either from animal sources as cholecalciferol or vitamin D3 or from plant sources such as ergocalciferol or vitamin D2), is bound to vitamin D protein and albumin and transferred via the bloodstream to the liver to be hydroxylated to form 25(OH)D.14 The blood concentration of 25(OH)D is considered the best measure of vitamin D status in the body. Exposure to sunlight is the major source of vitamin D and, at best, only an average 10% of the body’s vitamin D stores are provided by diet.15 Full exposure to sunlight, causing slight pinkness to the skin in lighter-pigmented adults, would generate an equivalent of between 10,000 and 20,000 IU of vitamin D within 24 hours of exposure.14 Individuals with darker pigmentation require about ten times more exposure to generate similar levels of vitamin D3.16,17
In addition to skin pigmentation, the amount of vitamin D synthesis following UVB exposure depends on other factors which include amount of time spent outdoors and the degree of exposure, body mass index, latitude, season, the degree to which UV light is blocked by air pollution and the level of protection against UVB including use of sunscreens.6,16 In many parts of the world, lifestyle changes are resulting in inadequate endogenous vitamin D synthesis. In the Middle East and many Arab countries, for example, little time is spent outside and women’s outdoor clothing prevents skin being exposed to sunlight.18–20 In western countries, the amount of time spent indoors has increased significantly. In the US alone, an average 93% of time is spent indoors.21 In addition, concern about the increased risk of cancer, especially in those with lighter skin exposed in high concentrations for a sustained amount of time, has led to recommendations to limit sun exposure.22 Since sunlight is the major source of vitamin D, the current lifestyle of some populations, associated with inadequate sun exposure and endogenous vitamin D synthesis, contributes to the increasing risk and prevalence of VDD, especially among women of reproductive age.1,5,23
After vitamin D synthesis, a second hydroxylation in the kidney leads to formation of the most important active metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D].16,24 The major physiological function of 1,25(OH)2D is to maintain serum calcium and phosphate at physiologically acceptable levels to maximize a wide variety of metabolic functions. This includes increasing the efficiency of intestinal calcium transport and dietary phosphate absorption, especially from the small intestine, and calcium reabsorption in the renal tubules. When calcium and phosphate products are maximized, the result is mineralisation of osteoid bone laid down by the osteoblast.16
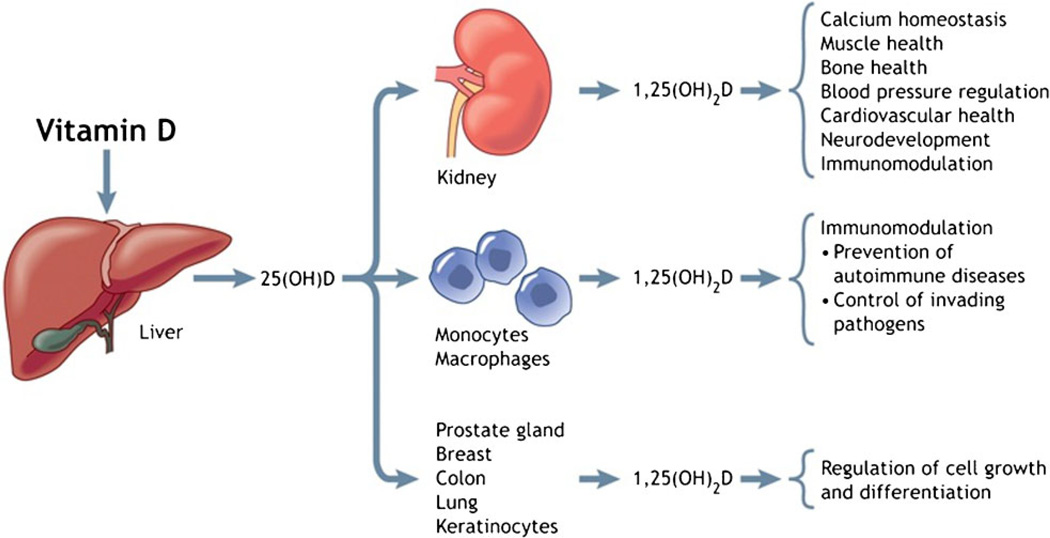
It is now known that 1,25(OH)2D is also locally produced in extra-renal tissues and directly or indirectly controls multiple genes including those responsible for the regulation of cellular proliferation and differentiation, apoptosis and angiogenesis.6 1,25(OH)2D is also important in the regulation of innate and adaptive immune responses. When produced in monocytes and macrophages, 1,25(OH)2D inhibits pro-inflammatory and auto-immune cytokine synthesis and promotes anti-inflammation.25 In addition, activation of macrophages by mycobacteria or lipopolysaccharide enhances production of 1,25(OH)2D, which stimulates the synthesis of cathelicidin, an antimicrobial peptide that enhances the killing of mycobacteria and other infectious agents.26,27 These non-skeletal functions28 are summarised in Fig. 1 and have been discussed in recent reviews.6,7,27 VDD is now thought to be associated with increased risk of multiple adverse health effects, including malignancy, cardiovascular and auto-immune diseases in adults and lower respiratory infections and type 1 diabetes in children,6 but intervention studies are needed to show the effect of vitamin D supplementation on the burden of these conditions.13 While such investigations are awaited, the reported high prevalence of childhood rickets and VDD in mothers and their infants5 worldwide is unacceptable and needs urgent preventive action.
Figure 1.
Biological functions of vitamin D. Metabolism of 25-hydroxyvitamin D [25(OH)D] to 1,25(OH)2D in the kidney and a variety of other organs and tissues and the biological functions of 1,25(OH)2D. (Reproduced from Hollis and Wagner28 with permission from the Canadian Medical Association Journal.)
Vitamin D Homeostasis During Pregnancy and Lactation
Early in pregnancy, 25(OH)D crosses the placenta from mother to fetus. Vitamin D stores in the fetus and infant at birth, as measured by serum 25(OH)D concentrations in cord blood, depend on maternal vitamin D status.29 Hence, maternal vitamin D sufficiency will ensure adequate vitamin D status at birth. Many studies have reported that the umbilical cord blood 25(OH)D level is maintained at 60–85% of the maternal value.29–33 Therefore, maternal VDD will expose the fetus to hypovitaminosis D and predispose infants to VDD at birth.
Vitamin D status in infancy depends on vitamin D stores at birth, dietary intake (human milk and formula), vitamin D supplementation and sunlight exposure. The anti-rachitic activities (vitamin D content) in human milk depend on mothers’ vitamin D intake or UVB exposure.29,34–36 Hence, mothers with low vitamin D status or intake will produce milk with low anti-rachitic activities and their breastfeeding infants will have low vitamin D status, unless supplemented by vitamin D or exposed to sunlight.29,37,38 A study from The Netherlands showed that, even when serum 25(OH)D concentration was normal at birth, levels in breastfed infants became very low within 8 weeks because of inadequate vitamin D in breast-milk and limited sun exposure.39 A study of 35 exclusively breastfed infants in Greece showed that mean maternal serum 25(OH)D concentration 1 week after delivery was low (32.3 nmol/L).40 Subsequent mean serum 25(OH)D concentrations in the infants remained low, viz 25.3 nmol/L at 1 week, 24.5 nmol/L at 3 months and 33.3 nmol/L at 6 months.40 In contrast, previous studies from New Zealand41 and the US42 demonstrated that, when a mother’s vitamin D status is normal and there is no history of limited sun exposure, the vitamin D status of unsupplemented, exclusively breastfed infants is close to normal43 in the 1st 6 months of life. In the New Zealand study,41 mean maternal serum 25(OH)D concentrations at delivery, 3 months and 6 months were 86.9 nmol/L, 60.9 nmol/L and 64.9 nmol/L, respectively. Infants’ mean serum 25(OH)D at birth (cord‘ blood), 3 months and 6 months was 74.3, 49.5 and 45.9 nmol/L, respectively. In the US study,42 mean maternal serum 25(OH)D at 2 weeks, 4 months and 6 months after delivery was 60, 50 and 47.5 nmol/L, respectively, and mean serum 25(OH)D concentrations in unsupplemented, exclusively breastfed infants at 2 weeks, 4 months and 6 months after birth were 50, 42.5 and 47.5 nmol/L, respectively; bone mineral content was similar to that in breastfed infants who were supplemented with daily 400 IU vitamin D or were formula-fed. Taken together, these studies indicate that when mothers’ vitamin D stores and infants’ vitamin D status are normal at birth and sun exposure is not limited, exclusively breastfed infants can maintain normal vitamin D status adequate to support bone health43 in the 1st 6 months of life. Studies in Nigeria and Gambia support this premise and have reported that when mothers’ and infants’ exposure to sunlight is unrestricted, VDD is rare in infants at birth44 and in childhood.45 However, calcium deficiency has been also implicated in rickets in Nigerian children.46 Adequate maternal vitamin D stores in pregnancy and lactation combined with modest sun exposure after birth is the natural means to prevent VDD in infants. However, if a mother’s vitamin D status is low and sunlight exposure is limited, vitamin D supplementation is essential to ensure vitamin D sufficiency in infants.47
Vitamin D Functions In Utero
The human fetus accumulates 30 g of calcium, mostly in the 3rd trimester,48,49 the result of active transplacental transfer from mother to fetus. Fetal calcium demand is met by increased maternal intestinal calcium absorption and resorption from bone. There is considerable debate about the role of vitamin D in fetal bone development.48,49 Data mostly from animal studies indicate that fetal calcium homeostasis and skeletal development are not related to maternal vitamin D status.49
There are no published randomised controlled trials (RCTs) on the effect of prenatal vitamin D supplementation and maternal vitamin D status during pregnancy on fetal skeletal development. However, observational studies link maternal vitamin D status to fetal bone development and childhood bone health. A UK study50 found that low maternal vitamin D status (serum 25(OH)D <27 nmol/L) compared with serum 25(OH)D >50 nmol/L during late pregnancy is associated with lower bone mass in their children at 9 years of age. The authors suggested that intra-uterine vitamin D status may determine bone health in childhood. A study from Cincinnati, USA51 found an association between season of birth and infant bone mass, indicating a relationship between fetal bone health and seasonal variation in maternal vitamin D status. A recent study of 125 pregnant Finnish women who participated in a cross-sectional study at 8–10 weeks gestation with postnatal follow-up found an association between maternal vitamin D status and infants’ bone health after birth.52 The tibia bone mineral content after birth was significantly greater and the cross-sectional area was larger in infants whose mothers’ serum 25(OH)D levels were above the median (54.4 nmol/L) than in those whose mothers’ serum was below the median (35.6 nmol/L). This significant relationship was maintained after adjustment for newborn Z-score birthweight, maternal height and newborn age at the time of measurement. No differences were detected in bone mineral density.
In the first study of fetal skeletal morphology related to maternal vitamin D status, Mahon et al.53 performed high resolution 3-D ultrasound analysis in pregnant women and showed that inadequate vitamin D status (serum 25(OH)D <50 nmol/L) is associated with increased fetal femur metaphyseal cross-sectional area and femur splaying index at 19 and 34 weeks of gestation, while femur lengths showed no variability. These changes are similar to radiological findings in rickets. Taken together, observational studies indicate that maternal vitamin D status during pregnancy may enhance fetal bone development.
The Magnitude and Emerging Implications of Worldwide Vitamin D Deficiency During Pregnancy and Infancy
Different stages of vitamin D status in adults have been categorised in the current literature as deficiency, insufficiency and sufficiency and are based on physiological and functional outcome measures.6 More specifically, categorisation is based on the serum 25(OH)D level’s negative correlation with serum parathyroid hormone concentration and positive correlation with maximum calcium absorption, optimal bone mineral density and prevention of fractures in the elderly. Using this categorisation, serum 25(OH)D >75 nmol/L is considered to be vitamin D sufficiency. Values of 50–75 nmol/L are considered insufficient, and values <50 nmol/L are considered deficient.6,54 On the basis of this classification, it is estimated that one billion people worldwide have VDD or insufficiency.6 However, the recent IOM report questions this categorisation and considers that serum 25(OH)D >50 nmol/L meets the needs of 97.5% of the North American population and that using current classification in the literature might lead to over-estimation of the burden of low vitamin D status.43 It is generally accepted that serum 25(OH)D <25 nmol/L is associated with increased risk of rickets and osteomalacia.13 In view of the relationship between maternal vitamin D status and infant vitamin D status at birth and in early infancy, a review of the magnitude of low vitamin D status as measured by serum 25(OH)D concentrations of <25 nmol/L and <50 nmol/L in pregnancy and infancy would be appropriate.
Vitamin D deficiency in pregnancy
There are now many reports worldwide of the high prevalence of low circulating serum 25(OH)D concentrations in pregnant women which increase the risk of adverse health consequences for the mother and fetus (see Table 1). Low vitamin D status is linked to a lack of exposure to sunlight and inadequate intake of corrective vitamin D supplements.5 In most recent reports,50,52,55–66 15–84% of pregnant women worldwide have very low vitamin D status (serum 25(OH)D <25 nmol/L) which in mothers is associated with an increased risk of osteomalacia. In many of these reports, 40–98% of the women have serum 25(OH)D <50 nmol/L, thus exposing the infants to low vitamin D stores in utero and at birth and the risk of VDD and rickets in early infancy. This level of global VDD in pregnancy is of major concern and needs to be addressed urgently as part of the strategy to improve the health of mothers and prevent VDD in infants.
Table 1.
Recent studies of vitamin D deficiency in women during pregnancy or at delivery: international comparison.
| Population | % Serum 25(OH)D, nmol/L | ||||||
|---|---|---|---|---|---|---|---|
| Author | Country/Yr | No. studied | Race/ ethnicity |
Gestation, wks |
Season | <25 | <50 |
| Javaid50 | UK/2006 | 596 | Caucasian | 34 | All | 18 | 49 |
| Holmes55 | UK/2009 | 99 | Caucasian | 35 | All | 17 | 75 |
| Newhook56 | Canada/2009 | 50 | Caucasian | NR | All | 2 | 42 |
| Hamilton57 | USA/2010 | 559 | Mixed | 18 | All | 15.8* | 48 |
| Ginde58 | USA/2010 | 928 | Mixed | NR | All | 7 | 33 |
| Judkins59 | New Zealand/2006 | 90 | Mixed | 13 | All | 61 | 85 |
| Bowyer60 | Australia/2009 | 971 | Mixed | 28 | All | 15† | 48 |
| Viljakainen52 | Finland/2010 | 124 | Caucasian | 8–10 | Winter | NR | 77 |
| Van der | The Netherlands/2006 | 79 | Turkish | 12 | All | 84 | NR |
| Meer61 | 69 | Moroccan | 12 | All | 81 | NR | |
| 105 | Other non-Western | 12 | All | 59 | NR | ||
| 105 | Western | 12 | All | 8 | NR | ||
| Sahu62 | India/2009 | 139 | Indian | 28 | All | 32 | 74 |
| Molla63 | Kuwait/2005 | 128 | Kuwaiti | Term | All | 41 | 83 |
| Bassir64 | Iran/2001 | 50 | Iranian | 38–41 | All | 80 | NR |
| Dawodu65 | UAE/2010 | 105 | Arab | 12 | All | 75 | 98 |
| Narchi66 | UAE/2010 | 75 | Multi-ethnic | 10 | Warm‡ | 26 | 69 |
<30 nmol/L;
≤25 nmol/L;
warm but sunny – September to November; NR, not reported.
Vitamin D deficiency in infants and lactating mothers
In view of reports of a high prevalence of VDD in pregnancy, it is not surprising that it is also of growing concern for both nursing mothers and their infants. Studies of vitamin D status of exclusively breastfed infants are few and recent studies33,40,67–71 report a high prevalence of VDD in mothers and their infants (Table 2). In a recent study in a predominantly white population in Iowa, USA, 70% of unsupplemented breastfed infants had serum 25(OH)D <27.5 nmol/L at 4 months of age.69 The prevalence of the low vitamin D levels was 50% in summer and 79% in winter. Twenty (57%) of 35 infants who were followed longitudinally still had VDD at 6 months. In another study from Cincinnati, USA, 18% of exclusively breastfed infants aged 1 month had vitamin D levels <25 nmol/L; 76% of the infants and 17% of their mothers had serum 25(OH)D <50 nmol/L. The prevalence of serum 25(OH)D <50 nmol/L was five times higher in infants born to African American mothers than to non-Hispanic white mothers.68 A study from Greece (mentioned above) found that 27% of exclusively breastfed infants had serum 25(OH)D <25 nmol/L.40 Seasonal variation in the prevalence of VDD were documented in all three studies, supporting the contribution of sunlight and endogenous vitamin D synthesis to vitamin D status.
Table 2.
Vitamin D deficiency in unsupplemented breastfed infants and mothers
| Infants | Mothers | |||||||
|---|---|---|---|---|---|---|---|---|
| Author/Yr | Location | No. studied |
Infants’ ages |
Season | Prevalence of deficiency, % |
Cut-off for 25(OH)D concentration, nmol/L |
Prevalence of deficiency, % |
Cut-off for 25(OH)D concentration, nmol/L |
| Challa/200540 | Greece (Ioannina) | 66 | 6 m | All | 27 | <25 | NR | NR |
| Bhalala/200733 | India (Mumbai) | 35 | 3 m | NA | 51 | <37.5 | NR | NR |
| Dawodu/200367 | UAE (Al Ain) | 78 | 1–4 m | All | 82 | <25 | 61 | <25 |
| Dawodu/201068 | USA (Cincinnati) | 87 | 1 m | All | 18 | <25 | 17 | <50 |
| 76 | <50 | |||||||
| Ziegler/200669 | USA (Iowa) | 35 | 4 m | All | 70 | <27.5 | NR | NR |
| Seth/200970 | India (New Delhi) | 180 | 2–24 w | All | 43 | <25 | 48 | <25 |
| Wagner/201071 | USA (SC – Rochester) | 33 | 1 m | All | 72 | <50 | NR | NR |
Studies from India and United Arab Emirates (UAE) indicate that serum 25(OH)D <25 nmol/L is of epidemic proportions in mothers and infants.67,70 In a study of 180 breastfeeding mother–infant pairs in New Delhi, almost half of the infants and their mothers had serum 25(OH)D <25 nmol/L.70 The mean (SD) PTH level in mothers with serum 25(OH)D <25 nmol/L was 78.7 (65.5) pg/ml compared with 28.6 (13.6) pg/ml in mothers with serum 25(OH)D ≥25 nmol/L (P = 0.001). A significant negative correlation was found between serum 25(OH)D and serum alkaline phosphatase (ALP) in the infants (r = 0.24, P = 0.001). The findings support the expected inverse relationship between low vitamin D status and PTH and ALP levels.
In the UAE study, the median baseline serum 25(OH)D concentration at a median age of 6 weeks was 21.5 nmol/L (IQR 14.8–34.1) in mothers and 11.5 nmol/L (IQR 6.3–19.8) in their infants.67 Mothers with low serum 25(OH)D concentrations (<25 nmol/L) had higher median PTH levels (40.8 pg/ml, IQR 26.8–56.7) than those with serum 25(OH)D >25 nmol/L (29.4 pg/mL, IQR 19–39.1) (P = 0.02). Infants with serum 25(OH)D >25 nmol/L also had higher median ALP levels (323 IU/L, IQR 253–425) compared with those with serum 25(OH)D <25 nmol/L (260 IU/L, IQR 229–303) (P = 0.02). In addition, 12 (19%) of 64 infants with serum 25(OH)D <25 nmol/L had ALP above the 75th percentile compared with none of the infants with serum 25(OH)D ≥25 nmol/L. These studies in populations at risk of very low vitamin D status indicate that a large proportion of unsupplemented, breastfed infants have biochemical markers of rickets. If low vitamin D status is sustained, the risk of ALRTI is increased9,10 and some infants could later develop clinical evidence of VDD rickets. For example, a recent longitudinal study from India found that 55% of exclusively breastfed infants had serum 25(OH)D <27.5 nmol/L at 10 weeks of age and at 6 months 43% still had serum 25(OH)D <27.5 nmol/L and 16% had developed clinical and radiological evidence of rickets.72 Knowledge of baseline vitamin D status and the high prevalence of VDD and rickets is important in determining the extent of the public health issue of vitamin D requirements in high-risk populations.
Implications of the High Prevalence of Low Vitamin D Status in Pregnancy, Infancy and Childhood
VDD in pregnancy is detrimental to the health of the mother and the developing fetus. In many countries, a serum 25(OH)D concentration <25 nmol/L, which is known to be associated with an increased risk of osteomalacia,13 is widely reported in pregnant women (Table 1). In addition, large studies from the USA73 and Norway74 link low vitamin D intake or status in pregnancy with an increased risk of pre-eclampsia, which is an important cause of perinatal morbidity and mortality. Therefore, urgent action is required worldwide to prevent epidemics of VDD in pregnancy.
Although the role of maternal vitamin D status in fetal skeletal development, as mentioned earlier, has generated considerable controversy,48,49,51,52 studies in humans suggest that maternal VDD during pregnancy is associated with a risk of reduced fetal bone mineral content52,53 and occasionally presents with rickets at birth or in early infancy in populations in which severe VDD is common.75–78
Severe hypocalcaemia with or without seizures is a common complication of VDD in the neonatal period or in early infancy owing to maternal VDD during pregnancy, coupled with inadequate vitamin D intake from human milk or supplements.79,80 It generally responds to vitamin D and calcium supplementation.79,81,82 This life-threatening condition can be prevented by ensuring that maternal vitamin D status is adequate during pregnancy.
Rickets, the end-stage of VDD, is a public health problem in many countries and has re-emerged in minority groups in industrialised countries because of inadequate exposure to sunlight and a lack of appropriate vitamin D supplementation.1,2,83,84 VDD is also common in mothers of children with VDD rickets, suggesting a common major risk factor, probably sunshine deprivation.23 Other serious complications such as cardiomyopathy and heart failure secondary to hypocalcaemia can complicate severe VDD.1,85,86
More recent data mostly from observational studies indicate that low maternal vitamin D intake during pregnancy and VDD in infants at birth and in early childhood are associated with an increased risk of extra-skeletal disorders. Case-control studies from Ethiopia,87 India,9 Turkey88 and Bangladesh10 have linked subclinical VDD in childhood to ALRTI. Other studies indicate an inverse relationship between vitamin D intake during pregnancy and cord blood vitamin D status and the risk of recurrent wheezing and respiratory infections in the offspring in the 1st 5 years of life.11,89 A recent RCT in school children in Japan reported that daily supplementation with 1200 IU vitamin D is associated with a 42% reduction in the incidence of seasonal influenza A.90 In a birth cohort study from Finland, daily vitamin D intake of 2000 IU during the 1st year of life was associated with an 80% reduction in the incidence of type 1 diabetes during a follow-up period of 30 years.12
These findings support the premise that maintaining adequate vitamin D status in fetal life, early infancy and childhood has the potential not only to prevent rickets and calcium biochemical disorders but also to reduce the burden of respiratory illness in childhood, and even auto-immune disease such as type 1 diabetes.
Strategies to Prevent Vitamin D Deficiency in Mothers and Their Infants
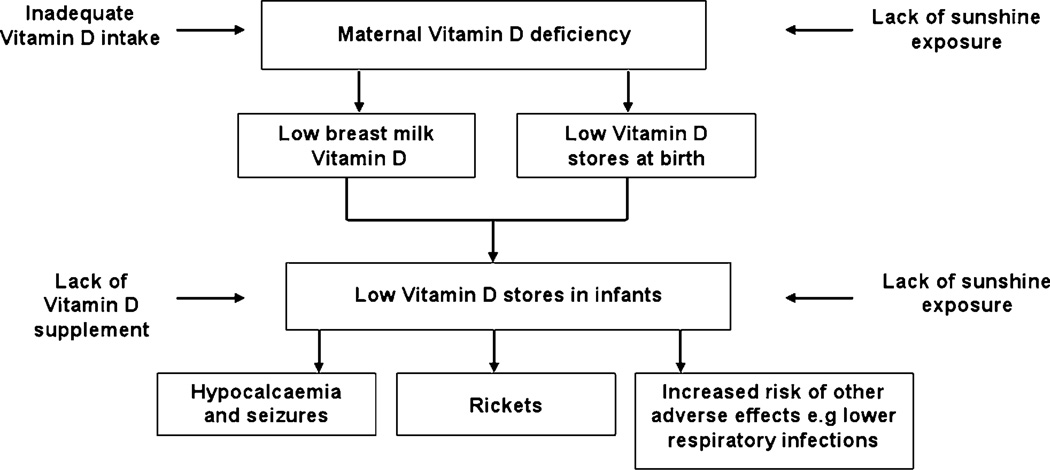
The inter-relationship between maternal vitamin D nutrition and vitamin D nutrition in utero and in breastfeeding infants is illustrated in Fig. 2.91 This could form the basis of a combined mother–infant strategy to prevent VDD in infants. The strategy, which should commence in pregnancy, is based on the principle of a continuum of vitamin D sufficiency through fetal life, infancy and childhood. Current recommendations for preventing VDD in infants and children focus on supplementing with 400 IU/day formula-fed and exclusively breastfed infants whose vitamin D intake is likely to be inadequate.47 In view of the high prevalence of VDD at birth, prevention in infancy should start by ensuring maternal vitamin D sufficiency during pregnancy and lactation. This would reduce intra-uterine exposure to hypovitaminosis D and improve vitamin D status at birth and in early infancy.
Figure 2.
Maternal and infant vitamin D inter-relationship during breastfeeding. Maternal vitamin D deficiency from lack of sunshine exposure and inadequate vitamin D intake results in low infant vitamin D stores at birth and intake from breast-milk, resulting in low vitamin D status in the infant. Lack of infant vitamin D supplementation and sun exposure further lowers infant vitamin D status and results in vitamin D deficiency and complications such as hypocalcaemia, rickets and increased risk of other adverse effects including lower respiratory tract infections. (Adapted from Dawodu and Tsang91.)
Ensure vitamin D sufficiency in pregnant and lactating women
When exposure to sunlight is insufficient,1,13,18,84 how much vitamin D supplementation is required to prevent maternal VDD? In November 2010, the IOM, assuming minimal exposure to sunlight, recommended 600 IU/day as a dietary reference intake for pregnant and lactating women in the USA and Canada.13,43 This intake is considered to correspond with a serum 25(OH)D concentration of at least 50 nmol/L in 97.5% of the North American population. The tolerable upper intake level was set at 4000 IU/day for adults including pregnant and lactating women.43 The response to vitamin D supplementation depends on baseline vitamin D status92,93 and the recommendation is based on studies in which the baseline vitamin D level is generally higher5,71 than in populations with a high prevalence of severe VDD.
A study of vitamin D supplementation was recently undertaken in 180 women of multi-ethnicity in the UK who were at risk of VDD.32 The subjects were randomised at 27 weeks gestation to three treatment groups of 60 subjects per group: (i) a single oral dose of 200,000 IU vitamin D, (ii) daily supplementation of 800 IU vitamin D, and (iii) no treatment. In the daily 800 IU group, the median 25(OH)D concentration at 27 weeks gestation was 26 nmol/L (IQR 20– 37) and serum 25(OH)D was <25 nmol/L in 27 (45%). After supplementation with 800 IU/day from 27 weeks to delivery, median serum 25(OH)D concentrations at delivery had risen to 42 nmol/L (IQR 31–76) but in 7 of the 60 (12%) it remained at <25 nmol/L.32 Only 30% of the women treated with 800 IU daily achieved serum 25(OH)D >50 nmol/L. In an earlier UK study, 80 consecutive pregnant women from ethnic minority groups whose serum 25 (OH)D was <20 nmol/L were started on vitamin D supplementation at the first antenatal visit. The serum 25(OH)D concentrations increased from 14.4 nmol/L at the first antenatal visit to 28.5 nmol/L at delivery with supplementation of 800–1600 IU vitamin D/day.94 Twenty-three (40%) of 58 mothers tested at delivery still had serum 25(OH)D levels <20 nmol/L. It seems, therefore, that 800–1600 IU/day vitamin D is insufficient to prevent VDD in these high-risk populations.
A study of vitamin D supplementation was undertaken in 90 healthy lactating and 88 nulliparous women in UAE where VDD from lack of exposure to sunshine is common.95 The study compared the efficacy of 2000 IU/day vitamin D2 with 60,000 IU/month vitamin D2 in women with vitamin D deficiency. Vitamin D2 was given because it was the only high-dose oral vitamin D available. Mean (SD) baseline serum 25(OH)D concentration in the lactating women was 27.3 (10.4) nmol/L. All had serum 25(OH)D levels <50 nmol/L and 33% had serum 25(OH)D levels <20 nmol/L. After 3 months of supplementation, mean (SD) serum 25 (OH)D concentrations in the group who received 2000 IU/day had increased to 42.2 (13.9) nmol/L. Only 35% of the lactating women achieved serum 25(OH)D levels >50 nmol/L, which is considered acceptable by the IOM43 but not by some other groups.6,54 It is clear that the amount of vitamin D supplementation necessary to prevent VDD in pregnant and lactating women in populations with a high prevalence of vitamin D deficiency is not known and needs to be investigated using higher doses of vitamin D3 than currently recommended by the IOM.43
Some have advocated a daily intake for adults of ≥1000 IU vitamin D to achieve optimal vitamin D status associated with improved BMD and reduction in the risk of fractures and colorectal cancer.6,54 Critical review of published studies suggests that an additional intake of 100 IU/day would increase serum 25(OH)D concentration by 1–2 nmol/L.13,96,97 Therefore, in a population with a mean baseline 25(OH)D concentration of approximately 25 nmol/L, daily vitamin D3 intake of 1250–2500 IU may be expected to build the body stores and achieve a mean serum 25(OH)D concentration of 50 nmol/L to prevent VDD. In high-risk populations in whom severe VDD is prevalent and adequate exposure to sunlight cannot be assured, a dose of at least 2000 IU/day might well be required in pregnancy and during lactation to prevent maternal VDD and enhance fetal and infant vitamin D status.98 Meanwhile, it is important to alert paediatricians, other healthcare providers and the public at large to the need for adequate maternal vitamin D intake during pregnancy and lactation as part of a strategy to ensure vitamin D sufficiency in mothers and breastfeeding infants,
Improve vitamin D status of breastfeeding infants
There is a positive correlation between maternal vitamin D intake, vitamin D status and breast-milk vitamin D anti-rachitic activities, which reflect vitamin D intake by unsupplemented breastfed infants.29,34,36 When the vitamin D content of breast-milk is low, vitamin D status correlates with childhood exposure to sunlight.38,99 In view of the recommendation to restrict exposure of infants aged <6 months to direct sunlight22 and the high prevalence of VDD in lactating women, vitamin D supplementation of lactating mothers and their exclusively breastfed infants is necessary to prevent VDD. The American Academy of Pediatrics currently recommends a minimum of 400 IU/day vitamin D for all breastfeeding infants and for non-breastfed infants who ingest less than 1 litre of fortified formula per day.47 In the UK, vitamin D supplementation of 400 IU/day is recommended for all breastfeeding infants.100 The most recent IOM report recommends 400 IU/day for infants <1 year and 600 IU/day for children aged 1–8 years.13 In populations at risk of very low baseline vitamin D status, it is not known whether such doses would achieve a serum 25(OH)D level of 50 nmol/L in the majority of breastfed infants.
In a study of the effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants in the UAE, 90 healthy breastfeeding mothers were randomly assigned to 2000 IU/day or 60,000 IU/month vitamin D2 and all their infants received 400 IU/day vitamin D2 for 3 months.37 In 45 mothers assigned to daily 2000 IU vitamin D, mean (SD) baseline serum 25(OH)D concentration at a mean (SD) postnatal age of 19 days was 27.3 (10.4) nmol/L in the mothers and 13.1 (7.3) nmol/L in their infants. At 19 days post-partum, 89% of mothers and 98% of infants were considered vitamin D-deficient as defined by serum 25(OH)D concentration <37.5 nmol/L. After 3 months of combined maternal and infant supplementation, mean (SD) serum 25(OH)D in 22 infants who completed the 3-month study was 49.6 (18.5) nmol/L. Despite the substantial increase in serum 25(OH)D levels, 23% still had serum 25(OH)D <37.5 nmol/L. Based on the IOM guidelines for low vitamin D status, 50% of the infants still had serum 25(OH)D <50 nmol/L. A dose of 400 IU vitamin D2 to the infants combined with daily maternal supplementation of 2000 IU was therefore insufficient to elevate serum 25(OH)D to 50 nmol/L in the majority of infants. In contrast, in a North American study of exclusively breastfed infants, 400 IU vitamin D3/day was sufficient to elevate mean serum 25(OH)D from 40.0 (23.3) at 1 month of age to 109.0 (35.3) nmol/L at 4 months, and only 5% had serum 25(OH)D <50 nmol/L after 3 months supplementation.71 Where VDD is highly prevalent because of low maternal vitamin D status and a lack of infant exposure to sunlight, supplementation of 600–800 IU/day vitamin D3 for breastfeeding infants and 2000 IU/day for their mothers might be appropriate.98
Maternal supplementation alone for prevention of vitamin D deficiency in breastfeeding mothers and their infants
Studies in the 1980s found that supplementing lactating mothers with 2000 IU/day vitamin D had a substantial effect on the vitamin D status of their breastfeeding infants as measured by serum 25(OH)D levels.101 Two recent small pilot studies of high-dose vitamin D supplementation in nursing mothers given 2000 and 4000 IU/day vitamin D2 demonstrated that the mean (SD) milk anti-rachitic activity during 3 months of treatment increased from 35.5 (3.5) to 69.7 (3.0) IU/L in the 2000 IU group while the milk anti-rachitic activity in the 4000 IU/day group increased from 40.4 (3.7) to 134.6 (48.3) IU/L. The mean (SD) infant serum 25(OH)D level increased from 19.8 (2.8) to 69.5 (7.3) nmol/L in the 2000 group and from 33.5 (8.3) to 77.0 (12.5) nmol/L in the 4000 group.102 In another study by the same group, maternal supplementation with 6400 IU/day vitamin D3 for a period of 6 months increased the mean anti-rachitic activity in breast-milk from 82 to 873 IU/L; the serum 25 (OH)D concentrations in the infants (32.5–115 nmol/L) were similar to those (35–108 nmol/L) in infants supplemented with 300 IU/day vitamin D3.103 Serum and urinary calcium to creatinine ratios remained in the normal range for mothers and infants during the study period. Therefore, vitamin D concentration can be raised to adequate levels in breast-milk by high-dose vitamin D supplementation. Such high doses, however, must be validated and demonstrated to be safe in larger sample-size studies and in diverse geographical areas. If maternal supplementation alone is proven to be a safe and effective method to prevent VDD, it would achieve the double effect of preventing VDD in mothers and their breastfeeding infants. It would also support the view that ensuring adequate maternal vitamin D nutrition provides adequate vitamin D for her breastfeeding infant and combat the notion that human milk is deficient in vitamin D.
Conclusion
VDD in mothers and their infants appears to be a major public health problem with potentially serious adverse health consequences worldwide. The strategy for prevention must ensure vitamin D sufficiency in women during pregnancy and lactation. If maternal supplementation alone is safe and effective in preventing VDD in breastfeeding mothers and their infants, this will be a step forward. Areas for future research include RCTs to determine (i) optimal vitamin D requirements in women and breastfeeding infants in high-risk populations in whom adequate exposure to sunlight cannot be assured; (ii) whether ensuring vitamin D sufficiency will improve fetal skeletal development and reduce pregnancy-induced hypertension; and (iii) the effect of vitamin D supplementation on other non-skeletal conditions such as respiratory infections, wheezing and type 1 diabetes associated with low vitamin D in mothers and infants.13
Acknowledgments
This work is supported in part by grants from the Thrasher Research fund (Dawodu A and Wagner CL) and from the National Institute of Health R01HD043921 and R01HD047511 and NIH/NCRR UL1 RR029882 (Wagner CL).
References
- 1.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Ann Trop Paediatr. 2006;26:1–16. doi: 10.1179/146532806X90556. [DOI] [PubMed] [Google Scholar]
- 3.Lazol JP, Cakan N, Kamat D. 10-year case review of nutritional rickets in Children’s Hospital of Michigan. Clin Pediatr. 2008;47:379–384. doi: 10.1177/0009922807311397. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed SF, Franey C, McDevitt H, et al. Recent trends and clinical features of childhood vitamin D deficiency presenting to a children’s hospital in Glasgow. [doi:10.1136/adc.2009.173195];Arch Dis Child. 2010 doi: 10.1136/adc.2009.173195. [DOI] [PubMed] [Google Scholar]
- 5.Dawodu A, Wagner CL. Mother-child vitamin D deficiency: an international perspective. Arch Dis Child. 2007;92:737–740. doi: 10.1136/adc.2007.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2009;202:e1–e9. doi: 10.1016/j.ajog.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 10.Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;99:389–393. doi: 10.1111/j.1651-2227.2009.01594.x. [DOI] [PubMed] [Google Scholar]
- 11.Camargo CA, Jr, Ingham T, Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 12.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. McCollum Award Lecture, 1994. Vitamin D – new horizons for the 21st century. Am J Clin Nutr. 1994;60:619–630. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(suppl):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka LY, Wortsman J, Haddad JG, Kolm P, Hollis BW. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatol. 1991;127:536–538. [PubMed] [Google Scholar]
- 18.Dawodu A, Absood G, Patel M, et al. Biosocial factors affecting vitamin D status of women of childbearing age in the United Arab Emirates. J Biosoc Sci. 1998;30:431–437. doi: 10.1017/s0021932098004313. [DOI] [PubMed] [Google Scholar]
- 19.Gannage-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone Min Res. 2000;15:1856–1862. doi: 10.1359/jbmr.2000.15.9.1856. [DOI] [PubMed] [Google Scholar]
- 20.Alagol F, Shihadeh Y, Boztepe H, et al. Sunlight exposure and vitamin D deficiency in Turkish women. J Endocrinol Invest. 2000;23:173–177. doi: 10.1007/BF03343702. [DOI] [PubMed] [Google Scholar]
- 21.US Environmental Protection Agency. Washington, DC: EPA; 1989. Report to Congress on Indoor Air Quality, Volume II: Assessment and Control of Indoor Air Pollution. Report No. 400-1-89-001C. [Google Scholar]
- 22.American Academy of Pediatrics, Committee on Environmental Health. Ultraviolet light: a hazard to children. Pediatrics. 1999;104(2 pt 1):328–333. [PubMed] [Google Scholar]
- 23.Dawodu A, Agarwal M, Sankarankutty M, Hardy D, Kochiyil J, Badrinath P. Higher prevalence of vitamin D deficiency in mothers of rachitic than nonrachitic children. J Pediatr. 2005;147:109–111. doi: 10.1016/j.jpeds.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 24.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxy vitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 26.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 27.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr Res. 2009;65:106R–113R. doi: 10.1203/PDR.0b013e31819dba91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollis BW, Wagner CL. Nutritional vitamin D status during pregnancy: reasons for concern. CMAJ. 2006;174:1287–1290. doi: 10.1503/cmaj.060149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 30.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81:1060–1064. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- 32.Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol. 2009;70:685–690. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- 33.Bhalala U, Desai M, Parekh P, Mokal R, Chheda B. Subclinical hypovitaminosis D among exclusively breastfed young infants. Indian Pediatr. 2007;44:897–901. [PubMed] [Google Scholar]
- 34.Greer FR, Hollis BW, Cripps DJ, Tsang RC. Effects of maternal ultraviolet B irradiation on vitamin D content of human milk. J Pediatr. 1984;105:431–433. doi: 10.1016/s0022-3476(84)80021-9. [DOI] [PubMed] [Google Scholar]
- 35.Hollis BW, Roos BA, Draper HH, Lambert PW. Vitamin D and its metabolites in human and bovine milk. J Nutr. 1981;111:1240–1248. doi: 10.1093/jn/111.7.1240. [DOI] [PubMed] [Google Scholar]
- 36.Specker BL, Tsang RC, Hollis BW. Effect of race and diet on human-milk vitamin D and 25-hydroxyvitamin D. Am J Dis Child. 1985;139:1134–1137. doi: 10.1001/archpedi.1985.02140130072032. [DOI] [PubMed] [Google Scholar]
- 37.Saadi HF, Dawodu A, Afandi B, et al. Effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants. Matern Child Nutr. 2009;5:25–32. doi: 10.1111/j.1740-8709.2008.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hillman LS. Mineral and vitamin D adequacy in infants fed human milk or formula between 6 and 12 months of age. J Pediatr. 1990;117(2 pt 2):S134–S142. doi: 10.1016/s0022-3476(05)80011-3. [DOI] [PubMed] [Google Scholar]
- 39.Hoogenboezem T, Degenhart HJ, de Muinck Keizer-Schrama SM, et al. Vitamin D metabolism in breast-fed infants and their mothers. Pediatr Res. 1989;25:623–628. doi: 10.1203/00006450-198906000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Challa A, Ntourntoufi A, Cholevas V, Bitsori M, Galanakis E, Andronikou S. Breastfeeding and vitamin D status in Greece during the first 6 months of life. Eur J Pediatr. 2005;164:724–729. doi: 10.1007/s00431-005-1757-1. [DOI] [PubMed] [Google Scholar]
- 41.Birkbeck JA, Scott HF. 25-Hydroxycholecalciferol serum levels in breast-fed infants. Arch Dis Child. 1980;55:691–695. doi: 10.1136/adc.55.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan GM, Roberts CC, Folland D, Jackson R. Growth and bone mineralization of normal breast-fed infants and the effects of lactation on maternal bone mineral status. An J Clin Nutr. 1982;36:438–443. doi: 10.1093/ajcn/36.3.438. [DOI] [PubMed] [Google Scholar]
- 43.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okonofua F, Houlder S, Bell J, Dandona P. Vitamin D nutrition in pregnant Nigerian women at term and their newborn infants. J Clin Pathol. 1986;39:650–653. doi: 10.1136/jcp.39.6.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66(10) suppl 2:S153–S164. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 46.Okonofua F, Gill DS, Alabi ZO, Thomas M, Bell JL, Dandona P. Rickets in Nigerian children: a consequence of calcium malnutrition. Metabolism. 1991;40:209–213. doi: 10.1016/0026-0495(91)90177-x. [DOI] [PubMed] [Google Scholar]
- 47.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 48.Abrams SA. In utero physiology: role in nutrient delivery and fetal development for calcium, phosphorus, and vitamin D. Am J Clin Nutr. 2007;85:604S–607S. doi: 10.1093/ajcn/85.2.604S. [DOI] [PubMed] [Google Scholar]
- 49.Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. Am J Clin Nutr. 2008;88:520S–528S. doi: 10.1093/ajcn/88.2.520S. [DOI] [PubMed] [Google Scholar]
- 50.Javaid MK, Crozier SR, Harvey NC, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 51.Namgung R, Tsang RC. Bone in the pregnant mother and newborn at birth. Clin Chim Acta. 2003;333:1–11. doi: 10.1016/s0009-8981(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 52.Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–1757. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 53.Mahon P, Harvey N, Crozier S, et al. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Min Res. 2010;25:14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 55.Holmes VA, Barnes MS, Alexander HD, McFaul P, Wallace JM. Vitamin D deficiency and insufficiency in pregnant women: a longitudinal study. Br J Nutr. 2009;102:876–881. doi: 10.1017/S0007114509297236. [DOI] [PubMed] [Google Scholar]
- 56.Newhook LA, Sloka S, Grant M, Randell E, Kovacs CS, Twells LK. Vitamin D insufficiency common in newborns, children and pregnant women living in Newfoundland and Labrador, Canada. Matern Child Nutr. 2009;5:186–191. doi: 10.1111/j.1740-8709.2008.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamilton SA, McNeil R, Hollis BW, et al. Profound vitamin D deficiency in a diverse group of women during pregnancy living in a sun-rich environment at latitude 32 degrees N. Int J Endocrinol. 2010 doi: 10.1155/2010/917428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ginde AA, Sullivan AF, Mansbach JM, Camargo CA., Jr Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202:e1–e8. doi: 10.1016/j.ajog.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Judkins A, Eagleton C. Vitamin D deficiency in pregnant New Zealand women. NZ Med J. 2006;119:1–6. [PubMed] [Google Scholar]
- 60.Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clin Endocrinol. 2009;70:372–377. doi: 10.1111/j.1365-2265.2008.03316.x. [DOI] [PubMed] [Google Scholar]
- 61.van der Meer IM, Karamali NS, Boeke AJ, et al. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr. 2006;84:350–353. doi: 10.1093/ajcn/84.1.350. [DOI] [PubMed] [Google Scholar]
- 62.Sahu M, Bhatia V, Aggarwal A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol. 2009;70:680–684. doi: 10.1111/j.1365-2265.2008.03360.x. [DOI] [PubMed] [Google Scholar]
- 63.Molla AM, Al Badawi M, Hammoud MS, et al. Vitamin D status of mothers and their neonates in Kuwait. Pediatr Int. 2005;47:649–652. doi: 10.1111/j.1442-200x.2005.02141.x. [DOI] [PubMed] [Google Scholar]
- 64.Bassir M, Laborie S, Lapillonne A, Claris O, Chappuis MC, Salle BL. Vitamin D deficiency in Iranian mothers and their neonates: a pilot study. Acta Paediatr. 2001;90:577–579. [PubMed] [Google Scholar]
- 65.Dawodu A, Saadi HF, Bakdache G, Altaye M, Hollis BW. Extraordinarily high prevalence and lack of seasonal variation of vitamin D deficiency in pregnant Arab women. Pediatric Academic Societies Annual Meeting; 1–4 May 2010; Vancouver. 2010. p. 1451. E-PAS. [Google Scholar]
- 66.Narchi H, Kochiyil J, Zayed R, Abdulrazzak W, Agarwal M. Maternal vitamin D status throughout and after pregnancy. J Obstet Gynecol. 2010;30:137–142. doi: 10.3109/01443610903315652. [DOI] [PubMed] [Google Scholar]
- 67.Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142:169–173. doi: 10.1067/mpd.2003.63. [DOI] [PubMed] [Google Scholar]
- 68.Dawodu A, Zalla L, Woo J, Herbers P, Heubi J, Morrow A. Vitamin D deficiency in breast feeding mothers and their infants. Pediatric Academic Societies Annual Meeting; 1–4 May 2010; Vancouver. 2010. p. 1355. E-PAS. [Google Scholar]
- 69.Ziegler EE, Hollis BW, Nelson SE, Jeter JM. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics. 2006;118:603–610. doi: 10.1542/peds.2006-0108. [DOI] [PubMed] [Google Scholar]
- 70.Seth A, Marwaha RK, Singla B, et al. Vitamin D nutritional status of exclusively breast fed infants and their mothers. J Pediatr Endocrinol Metab. 2009;22:241–246. doi: 10.1515/jpem.2009.22.3.241. [DOI] [PubMed] [Google Scholar]
- 71.Wagner CL, Howard C, Hulsey TC, et al. Circulating 25-hydroxyvitamin D levels in fully breastfed infants on oral vitamin D supplementation. Int J Endocrinol. 2010 doi: 10.1155/2010/235035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agarwal N, Faridi MM, Aggarwal A, Singh O. Vitamin D status of term exclusively breastfed infants and their mothers from India. Acta Paediatr. 2010;99:1671–1674. doi: 10.1111/j.1651-2227.2010.01912.x. [DOI] [PubMed] [Google Scholar]
- 73.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haugen M, Brantsaeter AL, Trogstad L, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20:720–726. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- 75.Anatoliotaki M, Tsilimigaki A, Tsekoura T, Schinaki A, Stefanaki S, Nicolaidou P. Congenital rickets due to maternal vitamin D deficiency in a sunny island of Greece. Acta Paediatr. 2003;92:389–391. doi: 10.1080/08035250310009347. [DOI] [PubMed] [Google Scholar]
- 76.Maiyegun SO, Malek AH, Devarajan LV, Dahniya MH. Severe congenital rickets secondary to maternal hypovitaminosis D: a case report. Ann Trop Paediatr. 2002;22:191–195. doi: 10.1179/027249302125000940. [DOI] [PubMed] [Google Scholar]
- 77.Mohapatra A, Sankaranarayanan K, Kadam SS, Binoy S, Kanbur WA, Mondkar JA. Congenital rickets. J Trop Pediatr. 2003;49:126–127. doi: 10.1093/tropej/49.2.126. [DOI] [PubMed] [Google Scholar]
- 78.Moncrieff M, Fadahunsi TO. Congenital rickets due to maternal vitamin D deficiency. Arch Dis Child. 1974;49:810–811. doi: 10.1136/adc.49.10.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ladhani S, Srinivasan L, Buchanan C, Allgrove J. Presentation of vitamin D deficiency. Arch Dis Child. 2004;89:781–784. doi: 10.1136/adc.2003.031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 81.Camadoo L, Tibbott R, Isaza F. Maternal vitamin D deficiency associated with neonatal hypocalcaemic convulsions. Nutr J. 2007;6:23. doi: 10.1186/1475-2891-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teaema FH, Al Ansari K. Nineteen cases of symptomatic neonatal hypocalcemia secondary to vitamin D deficiency: a 2- year study. J Trop Pediatr. 2010;56:108–110. doi: 10.1093/tropej/fmp063. [DOI] [PubMed] [Google Scholar]
- 83.Molla AM, Badawi MH, al-Yaish S, Sharma P, el-Salam RS, Molla AM. Risk factors for nutritional rickets among children in Kuwait. Pediatr Int. 2000;42:280–284. doi: 10.1046/j.1442-200x.2000.01230.x. [DOI] [PubMed] [Google Scholar]
- 84.Wharton B, Bishop N. Rickets. Lancet. 2003;362:1389–1400. doi: 10.1016/S0140-6736(03)14636-3. [DOI] [PubMed] [Google Scholar]
- 85.Chesney RW. Vitamin D deficiency and rickets. Rev Endocr Metab Disord. 2001;2:145–151. doi: 10.1023/a:1010071426415. [DOI] [PubMed] [Google Scholar]
- 86.Davies JH, Shaw NJ. Preventable but no strategy: vitamin D deficiency in the UK. Arch Dis Child. doi: 10.1136/adc.2010.191627. [DOI] [PubMed] [Google Scholar]
- 87.Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 88.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;63:473–477. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 89.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 91.Dawodu A, Tsang R. Sunshine deprivation rickets: a maternal-infant health problem. Middle East Paediatr. 2005;10:102–104. [Google Scholar]
- 92.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporosis Int. 1998;8:222–230. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 93.Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 2009;183:1–420. [PMC free article] [PubMed] [Google Scholar]
- 94.Datta S, Alfaham M, Davies DP, et al. Vitamin D deficiency in pregnant women from a non-European ethnic minority population — an interventional study. BJOG. 2002;109:905–908. doi: 10.1111/j.1471-0528.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- 95.Saadi HF, Dawodu A, Afandi BO, Zayed R, Benedict S, Nagelkerke N. Efficacy of daily and monthly high-dose calciferol in vitamin D-deficient nulliparous and lactating women. Am J Clin Nutr. 2007;85:1565–1571. doi: 10.1093/ajcn/85.6.1565. [DOI] [PubMed] [Google Scholar]
- 96.Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007;158:1–235. [PMC free article] [PubMed] [Google Scholar]
- 97.Cranney A, Weiler HA, O’Donnell S, Puil L. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008;88:513S–519S. doi: 10.1093/ajcn/88.2.513S. [DOI] [PubMed] [Google Scholar]
- 98.Canadian Paediatric Society. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12:583–598. [PMC free article] [PubMed] [Google Scholar]
- 99.Specker BL, Valanis B, Hertzberg V, Edwards N, Tsang RC. Sunshine exposure and serum 25-hydroxyvitamin D concentrations in exclusively breast-fed infants. J Pediatr. 1985;107:372–376. doi: 10.1016/s0022-3476(85)80509-6. [DOI] [PubMed] [Google Scholar]
- 100.Hypponen E, Boucher BJ. Avoidance of vitamin D deficiency in pregnancy in the United Kingdom: the case for a unified approach in national policy. Br J Nutr. 2010;104:309–314. doi: 10.1017/S0007114510002436. [DOI] [PubMed] [Google Scholar]
- 101.Ala-Houhala M, Koskinen T, Terho A, Koivula T, Visakorpi J. Maternal compared with infant vitamin D supplementation. Arch Dis Child. 1986;61:1159–1163. doi: 10.1136/adc.61.12.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80(6 suppl):1752S–1758S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 103.Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1:59–70. doi: 10.1089/bfm.2006.1.59. [DOI] [PubMed] [Google Scholar]