Abstract
The tropical disease vector mosquito Anopheles gambiae possesses 11 rhodopsin genes. Three of these, GPROP1, GPROP3, and GPROP4, encode rhodopsins with >99% sequence identity. We created antisera against these rhodopsins and used immunohistology to show that one or more of these rhodopsins are expressed in the major R1-6 photoreceptor class of the adult Anopheles gambiae eye. Under dark conditions, rhodopsin accumulates within the light-sensitive rhabdomere of the photoreceptor. Light treatment, however, causes extensive movement of rhodopsin to the cytoplasmic compartment. Protein electrophoresis showed that the rhodopsin is present in two different forms. The larger form is an immature species that is deglycosylated during the posttranslational maturation process to generate the smaller, mature form. The immature form is maintained at a constant level regardless of lighting conditions. These results indicate that rhodopsin biosynthesis and movement into the rhabdomere occurs at a constant rate. In contrast, the mature form increases in abundance when animals are placed in dark conditions. Light-triggered internalization and protein degradation counteracts this rhodopsin increase and keeps rhabdomeric rhodopsin levels low in light conditions. The interplay of the constant maturation rate with light-triggered degradation causes rhodopsin to accumulate within the rhabdomere only in dark conditions. Thus, Anopheles photoreceptors possess a mechanism for adjusting light sensitivity through light-dependent control of rhodopsin levels and cellular location.
Indexing terms: mosquito vision, photoreceptor, visual pigment, rhodopsin cycling, light adaptation
INTRODUCTION
The photoreceptors of some invertebrates show a remarkable daily cycle in which the photosensitive rhabdomeric membranes are broken down at dawn and then restored at dusk (Blest et al., 1978; Chamberlain and Barlow, 1984; Nässel and Waterman, 1978; Williams and Blest, 1980). This daily renewal process was documented for the mosquito Aedes aegypti (Brammer et al., 1978) and, further, the major visual pigment rhodopsin Aaop1 undergoes dramatic redistribution during this process (Hu et al., 2012). Whereas light causes a 50% reduction in rhabdomeric membrane volume (Brammer et al., 1978), nearly 100% of the Aedes rhodopsin is removed from these membranes (Hu et al., 2012). Loss of rhodopsin from the photosensitive membranes diminishes the efficiency of phototransduction, so this provides a mechanism by which Aedes photoreceptors, and likely photoreceptors of many other invertebrates, are desensitized in bright light and regain high light sensitivity when placed in dim light conditions. However, the best-studied invertebrate model, Drosophila, does not show a daily cycle of robust membrane recycling and rhodopsin movement. Therefore, the development of other invertebrate models is needed to characterize these photoreceptor processes.
The mosquito Anopheles gambiae is a nocturnal organism and, as the vector of malaria, of significant global health importance. The evolutionary lineages of the Aedes and Anopheles mosquito species separated 145–200 Myr ago (Krzywinski et al., 2006), not long after separation from the Drosophila lineage at ~225–250 Myr ago (Wiegmann et al., 2011). Both mosquito species share a common retinal organization (Hu et al., 2009) and retain similar sets of rhodopsin genes (Holt et al., 2002; Nene et al., 2007). In Aedes, the long-wavelength rhodopsin Aaop1 (GPROP1) is the major adult rhodopsin, being expressed in all R1-6 photoreceptors and most R8 photoreceptors (Hu et al., 2012). In this study, we show that a closely related rhodopsin ortholog is similarly expressed in Anopheles photoreceptors. The cellular distribution of the Anopheles rhodopsin is controlled by light in a similar way as shown for Aedes photoreceptors. The Anopheles work further shows that rhodopsin biosynthesis occurs at a steady rate. The light-driven processes, then, acts to remove rhodopsin from the rhabdomeric compartment to trigger rhodopsin degradation.
MATERIALS AND METHODS
Mosquito rearing
The white-eyed M2 strain of Anopheles gambiae (Benedict et al., 1996) was obtained from Malaria Research and Reference Reagent Resource Center (Manassas, VA). The strain was maintained at 27°C and 85% humidity under a 12 hr light/12 hr dark cycle for all stages of development. The light/dark cycle contained transition periods of one hour during which light was gradually increased at dawn and dimmed off at dusk. Time of day is reported as Zeitgeber time (ZT), with ZT0 being the initiation of the light period and ZT12 being the initiation of the dark period. Due to the one-hour dimming period, the mosquitoes are not in daylight until ZT1 or total darkness until ZT13.
Antibody production, immunostaining and protein blot analysis
To create an antiserum recognizing the Agop1 rhodopsin encoded by the closely related Anopheles gambiae rhodopsin genes GPROP1, GPROP3 and GPROP4, a peptide containing the terminal 15-amino acid sequence (QSVASGATQASDEKA) at the C-terminal end of the Agop1 was synthesized, conjugated to keyhole limpet hemocyanin protein, and injected into rabbits. The resulting antibody was then affinity purified using the same peptide. Peptide synthesis and all steps in Agop1 antibody production was carried out by Biomatik (Ontario, Canada).
For imaging of Agop1 in retinal preparations, whole mount immunostaining analysis was carried out as previously described in Aedes aegypti (Hu et al., 2012). In brief, bisected mosquito heads were left overnight in 4% paraformaldehyde/1xPBS at 4 °C, then transferred to PBS. Retinal tissues were manually dissected from the bisected heads and incubated in Agop1 antibody diluted 1:100 in BNT (1X PBS/0.1% BSA/0.1% Triton X-100/250 mM NaCl) for 1 hour. After three washes in PBS, the retina were incubated in Alexa Fluor 488 goat anti- rabbit IgG secondary antibody (Molecular Probes) diluted 1:500 in BNT. To allow visualization of the actin-rich rhabdom, the secondary antibody solution also included Alexa Fluor 594 phalloidin (Molecular Probes) at a concentration of 1:40. After three washes in PBS, the retina was mounted in 7 μL of Vectashield (Vector Labs) and imaged on a Nikon A1R confocal microscope. Micrographs were uniformly manipulated for contrast and brightness using Photoshop CS software.
To assess Agop1 protein levels by protein blots, mosquito heads were isolated from live Anopheles mosquitoes, homogenized in lysis buffer, and fractionated on a gradient SDS-PAGE gel as previously described (Hu et al., 2012). The fractionated proteins were then transferred to low auto-fluorescent Immobilon-FL polyvinyl fluoride transfer membrane (Millipore). This membrane was blocked using Odyssey Blocking Buffer (Li-Cor) and probed simultaneously for Agop1 and tubulin using a 1:7500 dilution of the rabbit anti-Agop1 antibody and a 1:7500 dilution of the mouse anti-tubulin antibody 12G10 (Developmental Studies Hybridoma Bank, Univ. Iowa). In the endoglycosidase-H digestion experiment, a 1:7500 dilution of the mouse anti-actin antibody A1978 (Sigma-Aldrich) was used instead of the anti-tubulin antibody. Secondary antibodies were IRDye 680RD goat anti-rabbit antibody and IRDye 800CW goat anti-rabbit antibody (Li-Cor). Labeling and wash procedures followed Li-Cor IRDye protocols. The labeled membrane was scanned on an Odyssey phosphorimager (Li-Cor), and analysis carried out using Odyssey software. For display of protein blot images, the data from the two infrared channels of the phosphoimager were converted to gray scale images using Adobe Photoshop CS software. Statistical analysis utilized one-way ANOVA followed by Dunnet’s post hoc test, comparing the Agop1 levels at different timepoints to the initial ZT9 timepoint.
RESULTS
Light dependent translocation of Agop1 in R1-6 photoreceptor cells
The long wavelength rhodopsin Aaop1 is the major adult rhodopsin, expressed in all R1-6 photoreceptors, of the Aedes aegypti adult eye. To document rhodopsin movement in Anopheles gambiae, we first needed to identify the corresponding rhodopsin of the Anopheles R1-6 photoreceptors. The eleven rhodopsins of Anopheles are clustered into similar functional groupings as the rhodopsins of the Aedes mosquito (Nene et al., 2007). The closest Anopheles orthologs of the Aedes Aaop1 rhodopsin gene are three genes (GPROP1, GPROP3 and GPROP4) coding for rhodopsins with >99% protein sequence identity. The rhodopsin proteins encoded by these genes are not distinguished by the reagents employed in this report and so will be collectively referred to as Agop1 rhodopsins or Agop1.
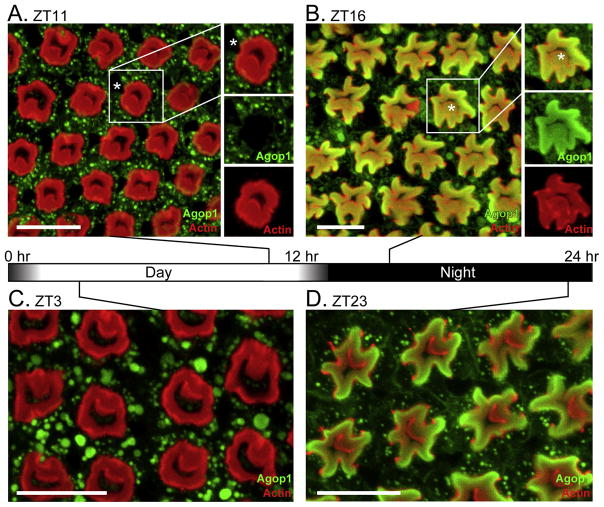
A peptide corresponding to the carboxy terminus of the Agop1 rhodopsin was generated and used to prepare antibodies against Agop1. When the antiserum was used for immunostaining of Anopheles retinal preparations, Agop1 was detected in the R1-6 class of photoreceptors (Figure 1). In these preparations, the actin-rich fused rhabdom was stained red by phalloidin, and Agop1 was detected by a green fluorophore. The images are from retinas prepared at four different time points. Figure 1A shows a cross section view of a retina prepared in the late afternoon (ZT11=zeitgeber time hour 11), one hour before the onset of darkness. A stereotypic retinal pattern is generated by the fused circular rhabdoms centrally located within each ommatidial unit. The cell bodies of the R1-7 photoreceptor cells are located at the periphery of the rhabdom, while the cell body of the R8 cell occupies the central space. In these images, the Agop1 rhodopsin, labeled in green, is largely excluded from the fused rhabdom, instead being localized within vesicular structures within the cytoplasm of the R1-6 cells at the periphery of the fused rhabdoms. In the boxed ommatidium in the image, an asterisk identifies the location of the R7 photoreceptor cell body. R7 photoreceptors express a UV rhodopsin within the central region of the retina (Hu et al., 2009) and therefore predictably fail to contain Agop1.
Figure 1. Light-mediated control of Agop1 cellular localization.
A. An Anopheles retina at ZT11, an hour prior to the initiation of the dark cycle, was probed for Agop1 (green) and actin (red). The location of the R7 photoreceptor cell body, lacking Agop1 expression, is marked by an asterisk in the highlighted ommatidial unit. Scale bars in all images are 20 μm.
B.Anopheles retina at ZT16, four hours after initiation of the dark cycle. After the light-dark transition, Agop1 (green) moves from the cytoplasmic region of photoreceptors surrounding the actin-rich rhabdom (red) to within the rhabdom. Agop1 is expressed in all peripheral cells (R1-6). Agop1 expression in the R8 photoreceptor is also evident in some central R8 rhabdomeres (asterisk in highlighted ommatidial unit at right).
C, D. Prior to dawn (ZT23), Agop1 rhodopsin localization remains within the peripheral rhabdoms (D), and moves completely to the cytoplasm by ZT3 (C), three hours after initiation of the light cycle.
A retina from a mosquito taken four hours after nightfall (ZT16) shows marked changes from the daytime retina (Figure 1B). The rhabdom (red) is larger in size and less organized. Agop1 rhodopsin is now localized within the rhabdom structures. This situation is maintained throughout the night, as evidenced by the retinal organization and rhodopsin localization at ZT23, one hour before dawn (Figure 1D). After the dawn period, Agop1 is lost from the rhabdomere and is now localized to large vesicular structures within the cell body (Figure 1C).
The localization of Agop1 within the rhabdom structure allowed assignment of Agop1 expression to a subset of central R8 photoreceptor (Figure 1B, marked by an asterisk in boxed ommatidial unit) in addition to R1-6 cells. The expression of the same rhodopsin in all R1-6 photoreceptors and a subset of R8 photoreceptors is a property that Anopheles shares with Aedes mosquitoes (Hu et al., 2012).
Post-translational modification of Agop1 occurs during maturation
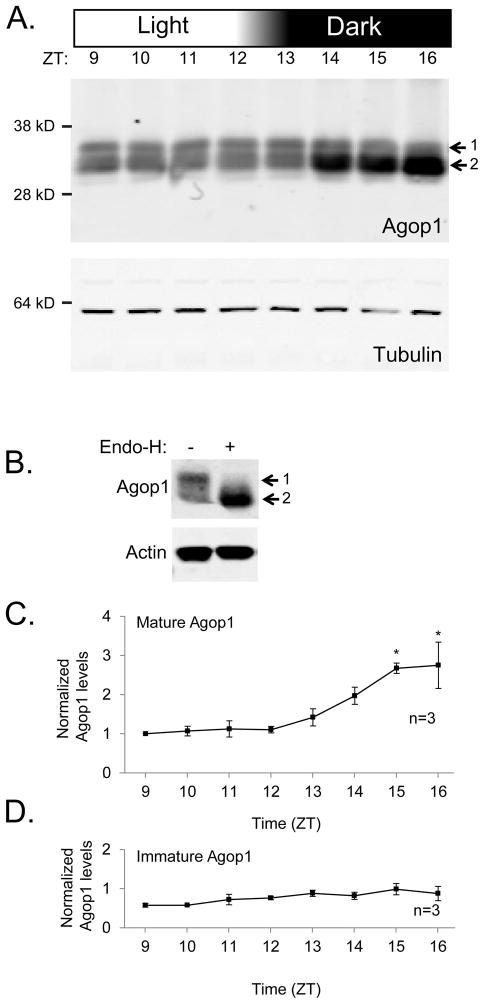
To investigate fluctuations in Agop1 protein levels during the light-dark transition, head proteins were prepared at hourly time points during the light to dark transition (ZT9 to ZT16). Figure 2A shows Agop1 visualized as two different forms on a protein blot following SDS-PAGE separation of these samples. The higher band (labeled 1) shows an apparent molecular weight of 35 kDa, while the lower band (labeled 2) shows an apparent molecular weight of 32 kDa. The observation that these sizes are smaller than the predicted molecular weight of 41.55 kDa is common for other invertebrate rhodopsins (Chou et al., 1996; Hu et al., 2012).
Figure 2. Analysis of mature and immature rhodopsin levels during the light-dark transition.
A. Protein blot assesses Agop1 rhodopsin levels before and after the normal light-dark transition from ZT12 to ZT13. Agop1 is found in a higher MW form (1) and a lower MW form (2). Tubulin protein levels were determined to control for variations in sample and loading amounts.
B. Protein blot shows detection of both immature (1) and mature (2) rhodopsin in absence of endoglycosidase H treatment, and loss of the immature rhodopsin upon endoglycosidase H treatment. Rhodopsin extract is from ZT11 time point. Detection of actin confirms similar amounts of proteins were analyzed.
C. Graph displays the quantitative assessment of the observed changes in mature Agop1 levels. The increase in Agop1 levels was statistically significant for time points three hours after initiation of the light-to-dark transition (ZT15, ZT16, * denotes p < 0.05, n=3). All values are specified as levels relative to the mature Agop1 at ZT9, and the amount of mature Agop1 present at each time point was normalized to tubulin levels.
D. Graph displays the quantitative assessment of the observed changes in immature Agop1 levels. Levels of Agop1 showed no significant changes through the time points sampled. Other details of the graph presentation are as specified in the legend text under C.
Drosophila’s Rh1 rhodopsin transiently exists as a high molecular weight glycosylated form during the posttranslational maturation process before being deglycosylated to reach the mature form (Katanosaka et al., 1998; Kurada et al., 1998; Webel et al., 2000). To determine if glycosylation is responsible for the presence of the higher molecular weight species of Agop1, Agop1 rhodopsin was extracted from animals at ZT11, a time point in which the higher molecular weight form represents approximately 50% of the detected rhodopsin (Figure 2A). Treatment with endoglycosidase H alters the mobility of the higher molecular weight form such that it now migrates similarly to the lower form (Figure 2B). These results show that the higher molecular weight form of Agop1 is a glycosylated form. This finding indicates that Agop1, like Drosophila Rh1, is deglycosylated before being moved to the rhabdomere. The analysis described below supports this view, so we will refer to the higher molecular weight form of Agop1 as immature Agop1, and the lower molecular weight form as mature Agop1.
The level of mature Agop1, but not immature Agop1, increases in dark
To characterize the expression of the immature and mature forms of Agop1, we determined their protein levels every hour through the light to dark transition (ZT9 to ZT16). Three independent replicates of this experiment were carried out, and each provided profiles similar to the protein blot shown in Figure 2A. To provide quantitative analysis, the intensity of the Agop1 signal was determined by direct infrared phosphoimaging, and the Agop1 signal was adjusted for sample loading and other technical variances by the simultaneous detection of tubulin content in the same head samples. These results, with levels normalized to the Agop1 levels at the first time point (ZT9), are shown in Figure 2C and D. The mature form of Agop1 (Figure 2C) shows statistically significant increase in levels at the ZT15 and ZT16 time points. These results document a 2.5 fold increase in Agop1 content within the first three hours of the dark cycle. In contrast, no statistically relevant changes in the immature Agop1 form were found (Figure 2D).
To investigate the possibility that the circadian cycle was responsible for the increase in mature Agop1 rather than the light-dark transition, we entrained animals to a 12 hr light/12 hr dark photoperiod, then subjected them to a 10 hr light period such that darkness occurred 2 hr earlier than normal. Agop1 content in samples collected before and after this early dark treatment were analyzed by protein blotting (Figure 3). The mosquitoes exposed to the dark treatment two hours early showed significant increase in mature Agop1 level at ZT13, two hours earlier than those on the 12hr light/12 hr dark cycle. These results indicate that the level of Agop1 in Anopheles increased significantly two hours after introduction to dark treatment regardless of the actual time of day, showing that the increase in Agop1 levels is directly under light exposure, not the circadian entrainment.
Figure 3. Analysis of mature and immature rhodopsin levels during an early transition to dark conditions.
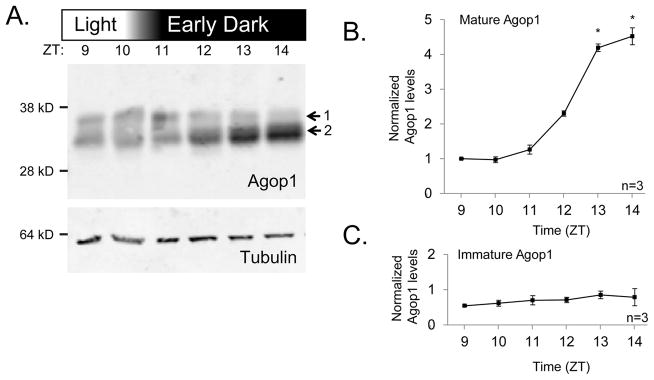
A. Protein blot to assess the level of Agop1 protein levels in mosquitoes subjected to a light-dark transition, two hours earlier than normal, starting at ZT10. Agop1 is found in a higher MW form (1) and a lower MW form (2). Tubulin was detected as a control.
B. Graph displays the quantitative assessment of the observed changes in mature Agop1 levels. The increase in Agop1 levels was statistically significant for time points three hours after initiation of the light-to-dark transition (ZT13, ZT14, * denotes p < 0.05, n=3). This is two hours earlier than in the experiment in which the light-dark transition occurred at ZT12 (Figure 2).
C. Graph displays the quantitative assessment of the level of immature Agop1. No statistically significant variations were detected. For B and C, parameters for the graph presentation are as specified in the Figure 2C legend.
We further investigated the effect of light treatment on Agop1 levels by maintaining the mosquitoes under light conditions past the expected dusk transition. One Agop1 protein blot from this experiment is displayed in Figure 4A. In this case, the samples were contained within the same SDS-PAGE, immunoblot, and membrane detection experiment that generated the data displayed in Figure 2A. This allowed the two experiments to share the daytime time points (ZT9-12) and allowed direct comparison of all samples. The results show a marked increase in the mature Agop1 levels only in the samples moved to dark conditions. The analysis of data collected from three independent runs of this experiment (Figure 4B,C) supports this conclusion. In continuous light conditions, there is no significant increase for both the immature and the mature Agop1 levels.
Figure 4. Analysis of mature and immature Agop1 under constant light conditions.
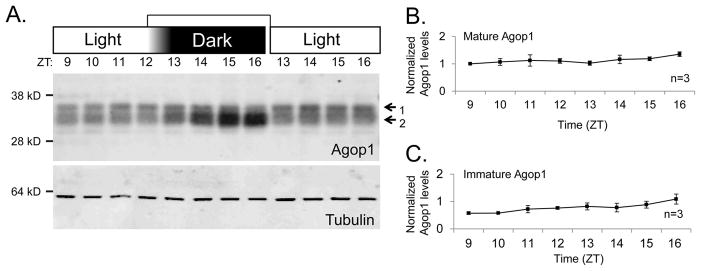
A. Protein blots assess the level of mature and immature Agop1 protein level in both light/dark conditions and constant light conditions. Mosquitoes for all time points were reared together, and groups were split at ZT12 to create both the light and dark samples. Tubulin levels are assessed to control for sample preparation and loading.
B, C. Graphs display the quantitative assessments of the level of mature and immature Agop1 when mosquitoes were maintained in constant light conditions. No statistically significant variations were detected. Details of the graph presentations are as specified in Figure 2C legend.
DISCUSSION
The R1-6 photoreceptors of the crepuscular mosquito Aedes aegypti possess a striking mechanism for modulation of light sensitivity in which rhodopsin is completely removed from the light-sensitive rhabdomeric membranes during light treatment, and moved back into these membranes when animals are placed in dark conditions (Hu et al., 2012). The study reported here was carried out to determine if the R1-6 photoreceptors of the nocturnal Anopheles gambiae mosquito, the primary vector for malaria, possess this same capability.
To identify the rhodopsin expressed in the Anopheles R1-6 photoreceptors, we reasoned that the rhodopsin member closest in sequence to the Aedes aegypti Aaop1, the R1-6 rhodopsin in this mosquito species, was the most likely candidate. Phylogenetic analysis of gene families in mosquitoes (Nene et al., 2007) indicated that three family members of Anopheles (GPROP1, GPROP3 and GPROP4) shared >99% identify with Aaop1. This high level of sequence identity precluded the preparation of antibodies capable of uniquely recognizing each of these rhodopsins. Given the high likelihood that one or more of these rhodopsin genes are active in R1-6 cells, we adopted the strategy of collectively referring to them as the Agop1 rhodopsin family, and created an antibody capable of recognizing all three rhodopsin proteins. Application of this reagent on adult Anopheles retina revealed that one or more members of the Agop1 rhodopsin family are indeed expressed in the R1-6 major class of photoreceptors.
The identification of Agop1 as the R1-6 rhodopsin allowed us to investigate if Anopheles exhibits rhodopsin redistributions during the daily light-dark cycle. The results are very similar to those observed previously for the Aedes mosquito (Hu et al., 2012). For animals collected during daylight timepoints, rhodopsin was present within the cytoplasmic compartment, and moved to the rhabdomeres only when placed under dark conditions. Rhodopsin movement into the rhabdomeres, the photosensitive organelle of the photoreceptor, is expected to increase light sensitivity and thereby enhance visual capability in low light environment. An increase in rhodopsin content and accompanying rhabdomere volume under dark conditions also occurs in Limulus polyphemus, the horseshoe crab (Sacunas et al., 2002). Even in the absence of direct knowledge of rhodopsin movement, earlier work in several other arthropods documented large changes in rhabdom volume during a day/night cycle (Blest, 1978; Nässel and Waterman, 1978; Williams and Blest, 1980). Thus, rhodopsin movement in and out of the rhabdomere is likely a common strategy to adjust light sensitivity in invertebrate photoreceptors.
We used protein blots to investigate changes in overall levels of Agop1 during the daily light/dark cycle. The analysis revealed that Agop1 migrates as two distinct forms in SDS-PAGE. Earlier analysis of Drosophila Rh1 rhodopsin showed that a larger rhodopsin form corresponds to newly synthesized rhodopsin, due to the presence of N-linked glycosylation on the protein during transit through the secretory pathway (Katanosaka et al., 1998) that is removed prior to rhodopsin movement into the rhabdomere. By using endoglycosidase treatment to remove the N-linked polysaccharides from Agop1, we confirmed that the larger Agop1 form is the glycosylated form. Thus, the same mechanism discovered for Drosophila rhodopsin results in the two distinct forms of Anopheles Agop1 rhodopsin.
Gene array analysis carried out previously on Anopheles heads indicated that Agop1 mRNA transcripts do not fluctuate during a 12 hr light/12 hr dark cycle, as well as under constant dark treatment (Rund et al., 2011). Further, we show here that the levels of the larger, immature Agop1 protein remain relatively constant in all light/dark conditions. These results suggest that the rates of immature Agop1 transcription and translation, and the conversion to the mature form, do not change during a daily cycle. In contrast, mature Agop1 remains at a constant low level in the light, but will accumulate to higher levels in the dark. The increase in the dark is accompanied by movement of Agop1 from the cytoplasm to the rhabdomere.
Our hypothesis is that during the day, immature Agop1 is constantly produced, converted to the mature form, and trafficked into the rhabdomere. However, it is quickly removed by light-triggered endocytosis and degraded through the endocytic pathway. The endocytosis of activated receptors is a commonly used mechanism for attenuating signaling of G protein-coupled receptors (Moore et al., 2007). In invertebrate photoreceptors, light converts rhodopsin to a stable photoproduct, metarhodopsin, While in the metarhodopsin state, the binding of arrestin initiates the endocytic process that removes rhodopsin from the rhabdomeric membrane (Alloway et al., 2000; Orem et al., 2006; Satoh and Ready, 2005). This reduces the rhodopsin content of the rhabdomeres and hence photoreceptor sensitivity, thus providing a mechanism for photoreceptor light adaptation. It is also the case that excessive light triggers photoreceptor degeneration in many species (Blest, 1980; Meyer-Rochow, 2001; Organisciak et al., 1998; Thomas et al., 2012), so rhodopsin removal may further serve to protect the photoreceptor from damage.
In the dark, in the absence of light driven endocytosis, the continuous synthesis results in the accumulation of the mature form of Agop1 in the rhabdomere where it is not subjected to degradation. The increased levels of Agop1 will enhance visual sensitivity in low light conditions. We have shown here that the nocturnal Anopheles gambiae mosquito exhibits extensive light-driven rhodopsin redistribution. This property likely reflects an adaptation for enhancement of vision at night. Interventions that limit this capability have the potential to reduce mosquito fitness and thereby provide novel approaches for fighting mosquito-borne diseases.
Acknowledgments
Confocal microscopy was conducted within the Notre Dame Integrated Imaging Facility.
Grant sponsor: National Institutes of Health; Grant number: EY006808 (to J.E.O.)
Abbreviations
- GPROP1
G-Protein Receptor Opsin 1
- GPROP3
G-Protein Receptor Opsin 3
- GPROP4
G-Protein Receptor Opsin 4
- Agop1
Anopheles gambiae opsin 1
- Aaop1
Aedes aegypti opsin 1
- Myr
Million Years
- ZT
Zeitgeber time
- PBS
phosphate buffered saline
References
- Alloway PG, Howard L, Dolph PJ. The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron. 2000;28:129–138. doi: 10.1016/s0896-6273(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Benedict M, Besansky N, Chang H, Mukabayire O, Collins F. Mutations in the Anopheles gambiae pink-eye and white genes define distinct, tightly linked eye-color loci. J Hered. 1996;87:48–53. [Google Scholar]
- Blest A. The rapid synthesis and destruction of photoreceptor membrane by a Dinopid spider: a daily cycle. Proc R Soc Lond B. 1978;200:463–483. [Google Scholar]
- Blest AD. Photoreceptor membrane turnover in arthropods: comparative studies of breakdown processes and their implications. In: Williams TP, Baker BN, editors. The Effects of Constant Light on Visual Processes. Springer; US: 1980. pp. 217–245. [Google Scholar]
- Blest AD, Kao L, Powell K. Photoreceptor membrane breakdown in the spider Dinopis: the fate of rhabdomere products. Cell Tissue Res. 1978;195:425–444. doi: 10.1007/BF00233887. [DOI] [PubMed] [Google Scholar]
- Brammer J, Stein P, Anderson R. Effect of light and dark adaptation upon the rhabdom in the compound eye of the mosquito. J Exp Zool. 1978;206:151–156. [Google Scholar]
- Chamberlain SC, Barlow RB., Jr Transient membrane shedding in Limulus photoreceptors: control mechanisms under natural lighting. J Neurosci. 1984;4:2792–2810. doi: 10.1523/JNEUROSCI.04-11-02792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WH, Hall KJ, Wilson DB, Wideman CL, Townson SM, Chadwell LV, Britt SG. Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron. 1996;17:1101–1115. doi: 10.1016/s0896-6273(00)80243-3. [DOI] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JC, Wides R. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Hu X, England JH, Lani AC, Tung JJ, Ward NJ, Adams SM, Barber KA, Whaley MA, O’Tousa JE. Patterned rhodopsin expression in R7 photoreceptors of mosquito retina: Implications for species-specific behavior. J Comp Neurol. 2009;516:334–342. doi: 10.1002/cne.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Leming MT, Metoxen AJ, Whaley MA, O’Tousa JE. Light-mediated control of rhodopsin movement in mosquito photoreceptors. J Neurosci. 2012;32:13661–13667. doi: 10.1523/JNEUROSCI.1816-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanosaka K, Tokunaga F, Kawamura S, Ozaki K. N-Linked glycosylation of Drosophila rhodopsin occurs exclusively in the amino-terminal domain and functions in rhodopsin maturation. FEBS Lett. 1998;424:149–154. doi: 10.1016/s0014-5793(98)00160-4. [DOI] [PubMed] [Google Scholar]
- Krzywinski J, Grushko OG, Besansky NJ. Analysis of the complete mitochondrial DNA from Anopheles funestus: an improved dipteran mitochondrial genome annotation and a temporal dimension of mosquito evolution. Mol Phylogenet Evol. 2006;39:417–423. doi: 10.1016/j.ympev.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Kurada P, Tonini TD, Serikaku MA, Piccini JP, O’Tousa JE. Rhodopsin maturation antagonized by dominant rhodopsin mutants. Vis Neurosci. 1998;15:693–700. doi: 10.1017/s0952523898154093. [DOI] [PubMed] [Google Scholar]
- Meyer-Rochow VB. The crustacean eye: dark/light adaptation, polarization sensitivity, flicker fusion frequency, and photoreceptor damage. Zoolog Sci. 2001;18:1175–1197. doi: 10.2108/zsj.18.1175. [DOI] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Nässel M, Waterman T. Massive diurnally modulated photoreceptor membrane turnover in crab light and dark adaptation. J Comp Physiol. 1978;131:205–216. [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orem NR, Xia L, Dolph PJ. An essential role for endocytosis of rhodopsin through interaction of visual arrestin with the AP-2 adaptor. J Cell Sci. 2006;119:3141–3148. doi: 10.1242/jcs.03052. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, Darrow RA, Kutty RK, Kutty G, Wiggert B. Light history and age-related changes in retinal light damage. Invest Ophthalmol Vis Sci. 1998;39:1107–1116. [PubMed] [Google Scholar]
- Rund SS, Hou TY, Ward SM, Collins FH, Duffield GE. Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 2011;108:E421–E430. doi: 10.1073/pnas.1100584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacunas RB, Papuga MO, Malone MA, Pearson AC, Marjanovic M, Stroope DG, Weiner WW, Chamberlain SC, Battelle BA. Multiple mechanisms of rhabdom shedding in the lateral eye of Limulus polyphemus. J Comp Neurol. 2002;449:26–42. doi: 10.1002/cne.10263. [DOI] [PubMed] [Google Scholar]
- Satoh AK, Ready DF. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr Biol. 2005;15:1722–1733. doi: 10.1016/j.cub.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Nelson CM, Luo X, Hyde DR, Thummel R. Characterization of multiple light damage paradigms reveals regional differences in photoreceptor loss. Exp Eye Res. 2012;97:105–116. doi: 10.1016/j.exer.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webel R, Menon I, O’Tousa JE, Colley NJ. Role of asparagine-linked oligosaccharides in rhodopsin maturation and association with its molecular chaperone, NinaA. J Biol Chem. 2000;275:24752–24759. doi: 10.1074/jbc.M002668200. [DOI] [PubMed] [Google Scholar]
- Wiegmann BM, Trautwein MD, Winkler IS, Barr NB, Kim JW, Lambkin C, Bertone MA, Cassel BK, Bayless KM, Heimberg AM, et al. Episodic radiations in the fly tree of life. Proc Natl Acad Sci U S A. 2011;108:5690–5695. doi: 10.1073/pnas.1012675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Blest AD. Extracellular shedding of photoreceptor membrane in the open rhabdom of a tipulid fly. Cell Tissue Res. 1980;205:423–438. doi: 10.1007/BF00232283. [DOI] [PubMed] [Google Scholar]