Abstract
Over the past several decades the traditional view of cancer being a homogeneous mass of rapid proliferating malignant cells is being replaced by a model of ever increasing complexity, which points out that cancers are complex tissues composed of multiple cell types. A large variety of immune and other host cells constitute the tumor microenvironment, which supports the growth and progression of the tumor where individual cancer cells evolve with increasing phenotypic and genetic heterogeneity. Furthermore, it has also become clear that, in addition to this cellular and genetic heterogeneity, most tumors exhibit a hierarchical organization composed of tumor cells displaying divergent lineage markers and at the apex of this hierarchy are cells capable of self-renewal. These “cancer stem cells” not only drive tumor growth, but also mediate metastasis and contribute to treatment resistance. Besides displaying remarkable genetic and phenotypic heterogeneity, cancer stem cells maintain plasticity to transition between mesenchymal-like (EMT) and epithelial-like (MET) states in a process regulated by the tumor microenvironment. These stem cell state transitions may play a fundamental role in the process of tumor metastasis. In this review, we will discuss emerging knowledge regarding the plasticity of cancer stem cells and the role that this plasticity plays in tumor metastasis. We also discuss the implications of these findings for the development of cancer stem cell targeted therapeutics.
Keywords: Breast cancer stem cells, MET, EMT, metastasis, therapeutic resistance
1. INTRODUCTION
Breast cancer remains a major health issue in women world-wide. Over the past two decades, the development of diagnostic technologies for early detection and the advent of targeted therapies for ER- (estrogen receptor) and HER2- (epidermal growth factor receptor 2) positive cancers have driven the mortality rates of breast cancer steadily downward in a large member of countries especially the wealthy ones [1]. However, despite recent advances in breast cancer therapies, many patients still succumb to metastasis due to therapeutic resistance and disease recurrence, which is the main culprit of breast cancer deaths. Thus, the ultimate goal in combating breast cancer, especially those in advanced stages, is to overcome therapeutic resistance and to prevent disease recurrence.
Developmentally, tumorigenesis can be viewed as tissue repair or organogenesis gone awry. There is now substantial evidence that breast cancers are hierarchically organized and driven by a small fraction of tumor cells displaying stem cell properties [2–4]. This small population of breast cancer stem cells (BCSCs), also termed breast cancer initiating cells, were the first identified in any solid tumors. They were characterized by expression of specific cell surface markers including EpCAM+, CD24− and CD44+ [5]. As few as 100 cells beating this phenotype were able to produce tumors in immune deficient NOD/SCID mice whereas over 100-fold greater cells that did not bear this phenotype were non-tumorigenic. Furthermore, tumors generated from EpCAM+CD24−CD44+ BCSCs recapitulated the cell type heterogeneity of the primary tumor. More recently, it has been shown that both normal and malignant mammary stem/progenitor cells express high level of enzyme aldehyde dehydrogenase (ALDH) [6], which can be assessed by the Aldefluor assay (Stem Cell Technologies).
Interestingly, we have recently reported that the EpCAM+CD24− CD44+ and ALDH expressing CSCs identify anatomically distinct BCSCs within breast cancers. Furthermore, gene expression profiling suggests that the EpCAM+CD24−CD44+ CSCs express genes associated with epithelial-mesenchymal transition (EMT) and are relatively quiescent, whereas the ALDH+ stem cells have an epithelial phenotype and are associated with a self-renewal state [7]. Furthermore, we find that the EMT and MET states of CSCs are not fixed, but rather that CSCs maintain plasticity to transition between EMT and MET states in a process regulated in the tumor microenvironment. Emerging evidence suggests that the plasticity of CSCs that enables them to transition between the EMT and MET states may play a crucial role in the ability of these cells to metastasize. In this review, we assess the current state of knowledge of how EMT and MET developmental programs are reactivated in cancer and are linked to breast cancer metastasis. We highlight the implications of cellular plasticity in driving metastasis, treatment resistance and tumor recurrence.
2. EMT AND MET: DEVELOPMENTAL PROGRAMS REACTIVATED DURING CANCER PROGRESSION
2.1. EMT and Tumor Cell Invasion, Dissemination and Micro-metastasis
The cellular process converting adherent epithelial cells into individual mesenchymal cells with the ability to migrate and invade adjacent tissues is known as epithelial-mesnechymal transition or EMT. During embryogenesis, EMT enables embryonic epithelial cells to become mesenchymal-like and travel to distant sites where new tissues and organs form. EMT is a multi-step process manifested by the loss of cell junctions and the reorganization of the cytoskeletal network, resulting in the loss of epithelial polarity and acquisition of a mesenchymal-like phenotype [8]. During tumor progression, this EMT process is thought to be reactivated and ultimately facilitates tumor cell migration through the basement membrane, invasion into adjacent tissues, and penetrating into the circulation. Indeed, many studies using cell culture and mouse models have documented that epithelial tumor cells can acquire a mesenchymal morphology associated with the expression of mesenchymal markers including vimentin, N-cadherin, fibronectin, α smooth muscle actin (α-SMA), and fibroblast specific protein 1 [9–12]. These phenotypic changes are induced by a wide variety of extracellular signals that subsequently activate one or several transcription factors of different families, including the zinc-finger proteins Snail, Slug, Zebl, and Zeb2, the bHLH proteins Twist and TCF3, the forkhead box proteins FOXC1 and FOXC2, as well as the homeobox protein goosecoid [13, 14].
In epithelial derived cancers, tumors at the primary and metastatic sites frequently have a similar heterogeneous organization manifested by regions of dedifferentiation [15]. EMT-like tumor cells are typically seen at the invasive edge of primary tumors. These cells are most probably the cells that eventually enter into the next steps of tumor metastasis cascade, including intravasation, extravasation, and formation of microscopic and macroscopic metastases in distant organs [15, 16]. The roles of EMT to promote tumor cell dissemination are well supported by recent studies on circulating tumor cells (CTCs) and disseminated bone marrow tumor cells, both of which exhibited EMT and sternness characteristics [17–19]. The subset of CTCs able to generate metastatic growth with clonal capacity in distant organs has been termed as metastasis-initiating cells (MICs) [20, 21]. Clinically, detection of five or more CTCs in 7.5 ml of peripheral blood serves as an indicator of breast cancer progression and the number of CTCs in patients with metastatic breast cancer tends to be a better indicator of tumor prognosis compared to other diagnostic means [22–24]. A recent study on CTCs from breast cancer patients has further implicated an association of mesenchymal CTCs with cancer progression [25]. This study also showed that CTCs, in forms of single cells or multicellular clusters, express known EMT regulators such as TGF-β pathway components and the transcription factor FOXC1 [25]. Moreover, a high degree of epithelial-mesenchymal plasticity in the CTCs appeared to associate with treatment cycle [25], which may reflect a cell state plasticity in these cells.
2.2. MET and Metastatic Colonization
Although migratory tumor cells in primary tumors and CTCs have been shown to present typical EMT features, distant metastases in a majority of epithelial cancers are generally characterized as having epithelial type morphology. In some cases, metastatic tumors even have a greater degree of cellular differentiation as compared to the primary tumor [15]. Such seemingly paradoxical observations suggest that the EMT program activated during tumor dissemination must have been suppressed upon arrival at the site of metastasis and the reciprocal program, MET, is subsequently induced to help disseminated tumor cells form sizable macro-metastatic colonies at distal organs. Such dynamic EMT/MET state transitions for metastatic tumor cells may serve as the underlying driving force of metastasis.
Accumulating evidence supports this epithelial-mesenchymal-epithelial plasticity in establishing carcinoma metastasis [26–31]. In breast cancer, the content of CD24−CD44+ BCSCs in the primary tumor correlates with increased risk of distant metastasis. However, distant metastases formed from these tumors frequently show a higher differentiation rate as manifested by increased expression of the luminal epithelial marker CD24 [26]. Similarly, in a mouse model of breast cancer driven by MMTV-PyMT oncogene, it has been shown that CD90+ CTCs are responsible for lung metastasis. However, the portion of CD90+ tumor cells decreases in differentiating and growing metastatic nodules [27]. Using mouse skin cancer model, Tsai et al. recently demonstrated that the reversion of EMT by turning off Twist1 is required for disseminated tumor cells to proliferate and develop metastases [28]. Similarly, Ocaña et al. demonstrated that temporal loss of the EMT inducer Prrx1 is required for cancer cells to form lung macrometastasis [29]. Recent studies also documented that induction of MET by miRNA regulatory networks especially the miR-200 family is able to promote breast cancer metastatic colonization [30]. In another study, specific expression of the Id1 gene in breast cancer cells that have undergone EMT induces MET through antagonism of Twist1 and this phenotypic switching is required for metastatic colonization in the lung [31]. Together, these studies indicate that a reversible EMT appears to be necessary for the formation of macrometastasis. This mesenchymal-epithelial plasticity of cancer cells may thus be harnessed for therapeutic intervention to prevent metastatic colonization.
3. BCSCS: KEY PLAYERS OF BREAST CANCER METASTASIS AND TREATMENT RESISTANCE
3.1. BCSCs Mediate Tumor Metastasis
Tumor metastasis is a complex process requiring the disseminated cancer cells to survive the long periods of shear stress in the circulation, to escape out of the blood vessels, and to invade the foreign microenvironment and proliferate in distant organs following extravasation. Indeed, even though primary tumors release large amount of cancer cells into the circulation, only a small fraction of these cells (~2%) are able to initiate growth as micrometastases and only ~0.02% of CTCs are estimated to form sizeable macrometastases in distal organs [32–36]. Therefore, metastatic colonization, the last step of metastasis, appears to be the rate-limiting step of distant metastasis. An increasing body of evidence has indicated that, BCSCs, although initially identified as a subset of tumor cells with high tumorigenic properties when transplanted into immune deficient mice, are the critical cells that mediate tumor metastasis, treatment resistance and disease recurrence.
An early gene profiling study revealed that BCSCs possess an invasive gene signature which correlates with increased metastasis and poor overall survival [37]. The association of BCSCs and cancer metastasis is further supported by observation that disseminated bone marrow cancer cells of breast cancer patients have a BCSC phenotype [17]. In a mouse xenograft model of human triple negative breast cancer, spontaneous lung metastasis was examined using noninvasive optical imaging and metastatic tumor cells were collected and analyzed. This study revealed that metastatic cancer cells from the lungs highly express BCSC marker CD44 and are able to regenerate tumors following transplantation in immune suppressed mice [38]. This study strongly suggests a metastatic role for BCSCs.
The relationship between BCSCs and MICs in CTCs of patients with metastatic breast cancer has been further documented in a recent study by showing that functional MIC-containing CTCs highly express BCSC markers [24]. Moreover, the number of CTCs with the EpCAM+CD44+MET+CD47+ signature increased with the clinical progression while no significant change was found in the number of CTCs representing the bulk tumor population [24]. In another study, a subset of breast cancer cells (Oct4hi/CD44hi/med/CD24−/+) demonstrating BCSC properties including self-renewal, cycling quiescence, asymmetric division, high metastatic and invasive capability was also found in the circulation of breast cancer patients who were undergoing or had completed treatment [39]. Together, these studies implicate that BCSCs have the ability to metastasize to distal organs where they serve as the seeds of metastatic lesions.
BCSCs Mediate Treatment Resistance
Besides a causal role in metastasis, a plethora of studies have also indicated that BCSCs are relatively resistant to traditional cancer therapies including chemotherapy and ionizing radiation in cultured breast cancer cell lines [40–43], in primary mammary tumor cells derived from mouse models of human breast cancer [44–46] and in patient-derived tumor xenografts [41, 47, 48]. The intrinsic resistance of BCSCs to neoadjuvant chemotherapy in the clinical setting has also been shown in a number of studies. For example, breast cancer cells isolated from tumors treated with neoadjuvant chemotherapy compared to those rescued from chemotherapy-naive patients exhibited increased mammosphere forming activity and CD24−CD44+ BCSC content [47]. In another study, the percentage of CD24−CD44+ BCSCs and tumorsphere forming activity in the residual tumor tissues were also significantly increased after twelve weeks of treatment with docetaxel or doxonibicin/eyelophosphamide [49]. Interestingly, in a separate group of patients with HER2 amplification, treatment with HER2 and EGFR inhibitor Lapatinib following chemotherapy did not increase, but rather slightly decreased the content of CD24−CD44+ BCSCs and tumorsphere forming activity [49]. Since HER2 overexpression has been show to drive BCSC activity [50], this clinical study suggests that strategies combining BCSC targeting agents (e.g., Trastuzumab and Lapatinib) with conventional chemotherapy hold the potential to overcome BCSC associated treatment resistance and achieve better therapeutic outcomes.
Previous studies have shown that ALDH1 expression in human breast tumors is associated with poor prognosis, suggesting that ALDH+ MET-like BCSCs share properties with EMT type of BCSCs in terms of metastasis/recurrence and treatment resistance [6]. Consistent with this observation, a clinical study examining ALDH1 expression in a cohort of primary breast cancer samples treated with sequential paclitaxel and epirubicin-based chemotherapy revealed that ALDH1 positivity was significantly associated with a low pathological complete response (pCR) rate and resistance to the therapy [51]. Furthermore, presence of residual ALDH+ cells following new adjuvant chemotherapy was found to associate with a high recurrence rate [52]. Thus, ALDH+ MET type BCSCs, similar to CD24−CD44+ EMT type BCSCs, also play a role in resistance to conventional chemotherapy.
The association of different BCSC states with tumor metastasis and therapeutic resistance as discussed above is dictated by unique properties of CSCs. Both ALDH+ and EpCAM+CD24−CD44+ BCSCs are endowed with enhanced migration/invasion capacity [7, 53], intrinsic ding detoxifying abilities [54, 55] or efflux activities [56–59], increased DNA-damage repair responses [60, 61] and anti-oxidant defense [46]. BCSCs especially EMT BCSCs express higher levels of inflammatory cytokines and proteins associated with invasion and bone metastasis, including IL-1α, IL-6, IL-8 and urokinase plasminogen activator [62]. CD44, the functional marker of EMT BCSCs, serves as a major adhesion molecule and receptor for extracellular glycosaminoglycan hyaluronic acid [63]. CD44 and its alternative splicing variants form co-receptor complexes with various receptor tyrosine kinases to modulate diverse cellular signaling events and regulate cell proliferation, migration and invasion [63–67]. Expression of CD44 has been demonstrated to potentiate the adherence of breast cancer cells to bone marrow endothelial cells [68] and promote bone metastasis by enhancing hyaluronan expression, cell motility, and tumorigenicity [69]. In addition to promote BCSC motility, CD44 has been implicated in EMT and a shift of CD44 expression from the variant (CD44v) to the standard (CD44s) isoform is essential for mammary epithelial cells to undergo EMT, and to promote the formation of mammary tumors displaying EMT characteristics [70]. The role of CD44 to enhance EMT and breast cancer formation is in agreement with the studies showing that acquisition of EMT promote stem cell properties [71]. The role of EMT in promoting BCSC traits is discussed in the following section.
4. EMT AND CANCER STEM CELL TRAITS
4.1. EMT Induced Formation of BCSCs
In general, the induction of EMT tends to cause an increase in expression of genes associated with “stemness” and CSC numbers in some tumor types. This has been particularly well studied in normal breast tissue and breast cancer. One such study showed that induction of EMT in immortalized human mammary epithelial cells was sufficient to induce the expression of stem cell markers. This was accompanied by increases in the formation of mammosplieres, colonies in a soft agar assay and tumorigenicity in immune deficient mice, which are all properties associated with CSCs [71]. As well as being experimentally induced, natural increases in EMT caused by endothelial cells have also been shown to increase CSCs [72]. Finally, in normal breast tissue overexpression of the transcription factors Slug and Sox9 were enough to push luminal line-age cells into a more stem-like state, while only Sox9 was required in basal cells that already expressed the EMT associated transcription factor Slug [73].
4.2. The Breast Cancer Cell of Origin
In discussions of the effect EMT can have on CSCs, it is important to discuss the possible models for the cell of origin in breast cancer. Some models propose that the cell of origin should be the most stem-like cell of the natural cell hierarchy since CSCs have stem-like properties including self-renewal capacity and this would require the shortest path to tumorigenesis. This also would require no induction of EMT or dedifferentiation to create the CSC as it would already be formed from the cell of origin. Other models hypothesize that the cell of origin in breast cancer is most likely a luminal progenitor cell or a unipotent luminal stem cell [74, 75]. This is a reasonable model since almost all breast carcinomas tend to have mostly luminal cells with few myoepithelial cell components. If a bipotent stem cell was the cell of origin for breast cancer, one might expect similar numbers of the two cell types derived from the bipotent stem cell of the normal hierarchy. Of course, if a luminal type of cell is the most common cell of origin for breast cancer, then it suggests that EMT could play an important role is transitioning the luminal cell of origin back to a more mesenchymal stem-like CSC. Another alternative model suggests that the different molecular subtypes of breast cancer originate from distinct cellular compartment in the normal mammary epithelial hierarchy [76]. According to this model, claudin-low breast cancers originate from the most primitive mammary stem cells, while basal breast cancers originate from a luminal progenitor. This model also envisions that luminal breast cancers are derived from more differentiated luminal cells.
5. CANCER STEM CELL PLASTICITY IN BREAST CANCER
5.1. BCSCs Transition between EMT- and MET-like States
Two of the most widely used methods of enriching for CSCs are sorting cells that are CD44+/CD24− [5] or using Aldefluor positivity [6]. Recently it was shown that these two populations of BCSCs are plastic and have the capacity to transition between these states [7]. While both of these populations show characteristics of CSCs, they also have properties unique to each particular type. The CD44+/CD24− population has signatures of EMT such as low expression of E-Cadherin, high levels of vimentin, and tends to be quiescent. Therefore this population was labeled as EMT-CSCs. The ALDH+ population, on the other hand, had a relatively opposite phenotype with high expression of E-Cadherin and low expression of vimentin. These cells were also much more proliferative, which pointed towards a more epithelial signature and was therefore labeled as MET-CSCs. The transition between these two states is likely to be critical for tumor expansion. The EMT-CSCs sit at the invasive edge of the tumor, their mesenchymal features allowing them to quickly move into the surrounding tissue. While the EMT-CSCs allow the tumor to expand into new territory, the proliferative MET-CSCs likely drive tumor cell growth in the tumor interior. When tumor conditions change or the invasive edge becomes the interior of the tumor, the two CSCs can change states. This is because of the extreme plasticity of tumor cells that are able to rapidly switch the transcriptional machinery to undergo MET or EMT when needed. Apparently, more research is required to more conclusively identify the cells of origin of these CSC states in human breast cancers.
5.2. Tumor Microenvironment in Dictating BCSC Plasticity
The tumor microenvironment can have very large effects on CSCs in breast cancer. In breast cancer, the tumor microenvironment is primarily composed of endothelial cells, fibroblasts, macrophages, plus a variety of other infiltrating immune cells. Each has their own specific function related to their normal, non-tumor associated, counterparts, but also has extended functions that the tumor has co-opted to promote tumor growth and metastasis.
Endothelial cells in the solid tumor microenvironment were at first thought to mainly provide nutrients and oxygen for the rapidly growing tumor. While this is certainly part of their function, recent evidence perhaps points towards their more important function being niche space for various CSCs. This was originally discovered in glioblastoma where it was shown that microvascular endothelial cells in the tumor microenvironment are home to the brain CSCs and greatly enhances their tumorigenicity when glioblastoma cells were xenografted into immunocompromised mice [77]. Similarly, when breast epithelial cells were co-cultured in 3D with endothelial cells similar effects were seen such an increase in CD44+/CD24− cells, reduction of E-Cadherin, and increased expression of N-Cadherin [70]. An increase in EMT and CSCs was also recently shown in head and neck cancer in response to EGF secreted by endothelial cells [78]. Endothelial cells are a main component of a number of normal stem cell niches so it follows that they constitute an important component of the CSC niche. However, the exact mechanism by which endothelial cells cause this increase in EMT remains to be determined but endothelial cells are a source of TGF-β as well as 1L-6 [79] which have been shown to increase EMT [80].
Fibroblasts are the major cell type in connective tissue whose main function is to secrete the proteins and fibers that form the extracellular matrix (ECM). They are found in most tissues in the body and therefore it is not surprising that they are one of the most common tumor microenvironment cells across tumor types. They can have a multitude of effects on the tumor including changing the ECM and secreting angiogenic compounds, chemokines and cytokines [81–83]. For example, lactate in the tumor microenvironment can stimulate fibroblasts to secrete higher levels of hyaluronic acid, also leading to increases in levels of CD44 which is the hyaluronic acid receptor and one of the markers for BCSCs [84]. Tumor associated fibroblasts (TAFs) can also secrete powerful chemokines such as SDF-1/CXCL12 [85] which can both attract stem cells through its interaction with its receptor CXCR4, but which can also attract further endothelial cells causing an increase in angiogenesis and which would, as mentioned above, contribute to even more niche space for CSCs. Fibroblasts, similarly to endothelial cells, have been shown to secrete TGF-β that can cause an increase in EMT and CSC properties in breast cancer cell lines [86].
5.3. Models of BCSC Plasticity
In a normal stem cell hierarchy, maintaining the stem cell population and preventing aberrant differentiation is a very tightly controlled process primarily mediated by epigenetic mechanisms such as methylation and histone modifications [87]. On the other hand, cancer is characterized by its loss of epigenetic control [88, 89]. It is likely that this loss of epigenetic control facilitates the increased plasticity of CSC transitions and allows for greater flexibility in transitioning between states and the rare dedifferentiation event back to a CSC state, something that is very uncommon in normal tissue biology. One example of this is a recent study showing that the chromatin surrounding the EMT transcription factor ZEB1 is poised to be rapidly switched on or off depending on the microenvironmental signals [90]. It has also been shown that the expression of polycomb complex (PRC1 and PRC2) proteins such as EZH2 is associated with the maintenance of CSCs and disease progression in a number of cancers including breast cancer [91, 92] and these pathological effects are mediated through induction of EMT [93, 94].
6. IMPLICATIONS OF BCSC PLASTICITY IN CANCER METASTASIS AND THERAPEUTIC RESISTANCE
6.1. BCSC Plasticity Complements to Current Model of Metastasis
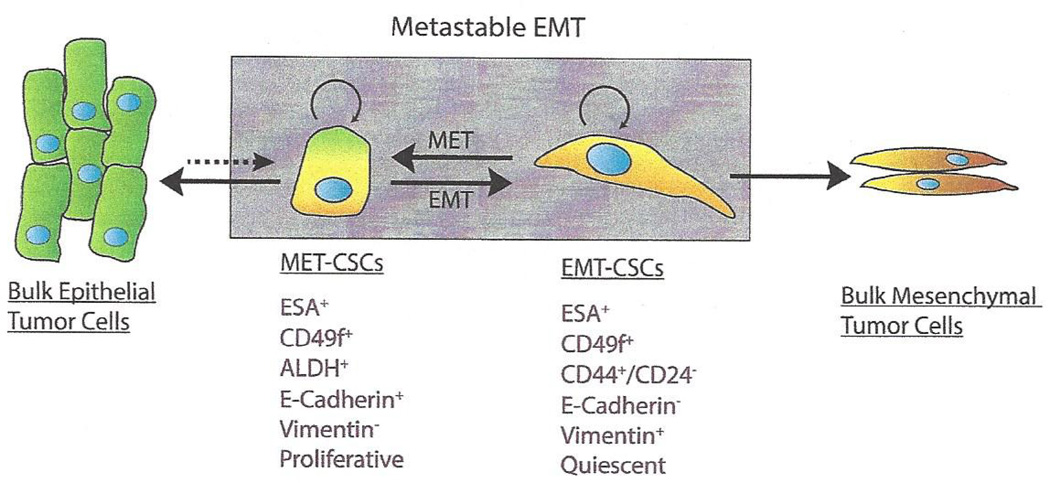
It is worthy to note that, although gene expression profiling of CD24−CD44+ and ALDH+ cell populations across different subtypes of human breast cancers indicate that they are distinct cancer cells with respective EMT and MET gene expression signatures, both cell populations share overlapping gene signature associated with stemness [7]. Together with experimental evidence demonstrating that both CD24−CD44+ and ALDH+ cell populations enrich functional BCSC activities [5, 6], it is evident that functional BCSCs exist in inter-convertible EMT and MET states. The CD24−CD44+ BCSCs exist in an EMT-like state that are E-cadherin and EpCAM negative, vimentin positive, and relatively quiescent, whereas the MET-like ALDH+ BCSCs are cycling, E-cadherin and EpCAM positive, and vimentin negative (Fig. 1). This reversible, metastable epithelial-mesenchymal plasticity of BCSCs is closely connected to current model of cancer metastasis postulating that EMT drives tumor cell dissemination and a consecutive MET drives metastatic colonization. In the case of breast cancer, the CD24−CD44+ EMT-like BCSCs mediate tumor invasion toward the basal membrane and neighboring tissues and into the blood, where they survive due to their intrinsic quiescence and anoikis resistance. After extravasation of the circulation, these mesenchymal-like BCSCs form micrometastasis in distant organs, where metastatic niche or specific microenvironment in distal sites induces MET, which drives BCSC self-renewal and generation of macrometastasis. An exception to this may be the claudin-low breast cancers which are characterized by a mesenchymal phenotype [95]. These tumors may contain CSCs that simultaneously display EMT and MET properties. The existence of CSCs that are simultaneously invasive and proliferate might contribute to the very aggressive nature of this breast cancer subtype.
Fig. (1). Model of EMT and MET in driving the plasticity of breast cancer stem cells (BCSCs).
BCSCs can exist in two inter-convertible states: MET-like (ESA+CD49f+ALDH+) and EMT-like (ESA+CD24−CD44+). The reversible, metastable state change between BCSCs is induced by the tumor microenvironment. Permanent EMT processes induced by constitutive EMT inducing signals will promote differentiated mesenchymal-like tumor cells, leading to loss of BCSC properties. Similarly, permanent MET induced by constitutive MET inducing signals will promote luminal differentiation, leading to loss of BCSC properties. On the other hand, differentiated bulk epithelial tumor cells may undergo dedifferentiation (dashed arrow) and enter into the MET BCSC state. CSCs may be permanently locked into a proliferating mesenchymal state in claudin-low tumors.
Recent studies suggest a relationship between CSCs and the acquisition of an EMT state [71, 96]. However, other studies have suggested that induction of constitutive EMT in subpopulations of tumor cells displaying CSC/TIC properties suppressed major attributes of CSCs/TICs, including anchorage-independent growth and metastatic potential [97]. These seemingly contradictory results could be reconciled by the model that BCSCs exist in a dynamic equilibrium of MET- and EMT-like states (Fig. 1). Based on this model, induction of a metastable EMT program in proliferating ALDH+ MET-like BCSCs will promote an imbalanced equilibrium from the MET toward the EMT state, leading to amplification of EMT BCSCs through epithelial-mesenchymal transition (EMT BCSCs themselves are non-cycling cells due to their relative quiescent nature). However, induction of a permanent EMT program in ALDH+ MET BCSCs by constitutive expression of EMT transcription factors such as Snail or Twist will break the epithelial-mesenchymal plasticity of BCSCs, leading to the formation of cells permanently locked into the mesenchymal state. In such a case as reported by Celia-Terrassa and colleagues [97], the properties of CSCs including anchorage-independent growth and metastatic potential will be lost. In this regard, the studies of Celia-Terrassa et al. also highlight the critical role of epithelial-mesenchymal plasticity in maintaining CSC characteristics.
6.2. BCSC Plasticity, Treatment Resistance and Therapeutic Interventions
The CSC model suggests that tumor re-growth following traditional resection and/or chemo/radio approaches can be arrested if the re-populating cells are destroyed with a selective CSC targeting agent. As CSCs are more aggressive, invasive and prone to promote metastasis than the bulk tumorigenic cells [98], targeting the CSC pool, which remains a most challenging task, could lead to a better clinical outcome especially in the reduction of treatment resistance, metastasis and tumor recurrence. The identification of epithelial-mesenchymal plasticity of BCSCs provides another level of complexity regarding development of strategies to eliminate these lethal seeds of breast cancer. As BCSCs frequently transition between their MET and EMT states, future treatment strategies designed to target BCSCs have to consider this epithelial-mesenchymal plasticity and target both states of BCSCs in order to eliminate them more thoroughly.
6.2.1. Epithelial-mesenchymal Plasticity of BCSC as the Origin of Therapeutic Resistance
A plethora of experimental and clinical studies have implicated that the accumulation of CD24−CD44+ EMT BCSCs in the residual tumor of breast cancer is associated with treatment resistance. For example, previous studies have suggested that the relatively quiescent EMT BCSCs are particularly resistant to cytotoxic chemotherapy and radiation therapy [49]. This therapeutic resistance of CD24− CD44+ EMT BCSCs is further supported by a neo-adjuvant clinical trial which demonstrated that residual tumor cells in triple-negative breast cancer following chemotherapy or in luminal breast cancer following hormonal therapy express an EMT-like CSC profile [99], suggesting that these divergent molecular subtypes of breast cancer contain similar therapy resistant CSC populations. Previous studies suggested a potential common cell origin for breast cancer cells that are resistant to chemotherapy [100]. In agreement with this notion, recent gene expression profiling of CD24−CD44+ (and ALDH+) BCSC subsets isolated from different subtypes of primary breast cancers exhibited a remarkable similarity in their patterns of gene expression, although whole-tumor gene expression profiles are distinct across different subtypes [7]. These studies, together with the fact that BCSCs transit between the EMT and MET states, suggest a potential common cell of origin for BCSCs across different breast cancer subtypes.
The cell origin of EMT BCSCs (CD24−CD44+) in different subtypes of breast cancer is currently a hot area of breast cancer research and remains to be fully characterized. In women carrying germ line BRCA1 mutations, aberrant luminal progenitor cell population (EpCAM+CD49f+) are proposed as the cell origin of BRCA1 associated basal breast cancer [101]. Genome-wide transcriptome analyses of different subtypes of breast cancers and different mammary epithelial subpopulations in human BRCA1 mutation carriers revealed that the luminal progenitor cell gene signature is associated with the basal subtype of breast cancer while the basal/mammary stem cell signature is correlated to tumors of normal-like and claudin-low subtypes [101]. Using a mouse model carrying conditional BRCA1 alleles, Molyneux et al. have further demonstrated that BRCA1 basal-like breast cancer originates from luminal epithelial progenitors, but not from basal mammary stem cells [102]. Several other studies using mouse models and isolated mammary epithelial cell populations also indicated that basal stem cells and mature luminal epithelial cells are not important targets for tumor initiation, rather, luminal progenitor cells are emerging as key players in breast tumorigenesis [103, 104].
Recent lineage tracing studies of the mouse mammary gland suggested that distinct basal and luminal stem cells give rise to cells restricted to the basal and luminal lineage respectively under normal developmental conditions [105]. Studies by Liu et al. further demonstrated that human mammary epithelial cells display a similar hierarchy organization, containing functional luminal stem cells located in the terminal lobules that are EpCAM+CD49f+ALDH+, and basal stem cells located in the mammary ducts that are CD24−CD44+EpCAM−CD49f+ [7]. Interestingly, in the luminal compartment, only EpCAM+CD49f+ALDH+ luminal stem cells, which constitute 6% of total EpCAM+CD49f+ population, have high colony-forming activity and generate ductal/alveolar structures in 3D Matrigel [7]. These studies suggest that ALDH serves as a functional marker of the luminal stem cells and the self-renewing/proliferative state of ALDH+ luminal stem cells may increase their susceptibility to carcinogenic mutations, making them a logical cell of origin for breast cancer, especially basal subtype of breast cancer (Fig. 2). This notion was supported by studies showing that expansion of lobules containing ALDH1-expressing cells is associated with loss of heterozygosity in BRCA1 mutation carriers [106]. Therefore, it may be the case that luminal stem cells rather than luminal progenitors are involved in the tumorigenesis of BRCA1 basal breast cancer and this ALDH+ luminal stem cell population may serve as a common cell origin of MET-like BCSCs. The cell origin of EMT-like BCSCs is more elusive. Recent gene profiling studies have indicated the similarity between the claudin-low subtype of breast cancer, EMT BCSCs and the bipotent mammary stem cells (EpCAM-loCD49fhiCD29hiER−PR−) [75, 95], suggesting that EMT-like BCSCs may directly originate from their normal counterpart in the mammary epithelial hierarchy (Fig. 2). Despite such gene expression similarity, oncogenic mutation(s) in claudin-low breast cancer may still hit the more abundant luminal stem/progenitor cells, leading them permanently locked into a proliferating EMT-like state. Such alternative routes of tumorigenesis for claudin-low breast cancer need to be further investigated.
Fig. (2). Cell origins of EMT and MET BCSCs.
The EMT BCSCs may directly originate from their normal counterpart, bipotent stem cells (EpCAM-loCD49fhiCD29hiER−PR−) in the mammary epithelial hierarchy, which generate claudin-low subtype of breast cancer. The MET BCSCs are likely derived from the EpCAM+CD49f+ALDH+ER−PR−unipotent luminal stem cells, which drive tumor initiation and growth of basal subtype of breast cancer. As tumor grows, EMT signals produced in the tumor microenvironment will promote an EMT-like state transition in MET BCSCs, facilitating tumor invasion, dissemination and metastasis.
Several lines of evidence suggest that both normal and non-stem tumor cells can spontaneously dedifferentiate into a stem-like state [107]. A recent study demonstrated that non-CSCs of human basal breast cancers are plastic cell populations readily switching from a non-CSC to CSC state [108]. In non-CSCs, the ZEB1 promoter is maintained in a poised chromatin configuration, which allows the tumor cells to respond rapidly to microenvironment signals (e.g., TGFβ) that triggers the conversion of ZEB1 promoter from a bivalent to active chromatin configuration, leading to formation of CD44hi EMT-like BCSCs [108]. It is worthy to note that the non-CSC population defined by the study of chaffer et at [108] was CD44low cells that may contain a population of ALDH+ MET-like BCSCs. Therefore, the observed de novo formation of CD44hi BCSCs from CD44low non-CSCs may originate from the well differentiated ALDH−CD44low cells or ALDH+ MET-like BCSCs. In the latter case, the formation of CD44hi BCSCs may again reflect state transition of BCSCs from a MET to EMT state. Future studies will need to evaluate the frequency of de novo CD44hi BCSC formation from well-differentiated cells and ALDH+ MET-like cancer cell population. We predict that the frequency of CD44hi BCSC formation from well-differentiated cancer cells will be much lower than that of ALDH+ MET-like cancer cell population.
6.2.2. Implications of BCSC Plasticity in Therapeutic Interventions
As discussed above, a reversible, metastable EMT induced by the tumor microenvironment plays a critical role in the formation of quiescent and therapeutic-resistant BCSCs (CD24−CD44+) that are responsible for metastatic diseases and tumor recurrence. Targeting this EMT process is therefore a promising approach to treat breast cancers, especially those with high metastatic potential. Since targeting EMT core transcription factors remains technically challenging, current treatment strategies and compounds targeting EMT are mainly aimed at various EMT-inducing signals [109]. In this regard, rapamycin and 17-AGG have been used as inhibitors of TGFβ-induced EMT [110], while inhibitors of ALK5, MEK, and SRC have potential roles to prevent EMT in response to EGF, HGF, and IGF-1 [110, 111]. Notably, although chronic activation of EGFR [112, 113] and IGF1R [114] has been reported to promote EMT-like transitions, cells that have undergone EMT show relative resistance to selective EGFR [115–117] and IGF1R/insulin receptor [118] inhibition. These studies suggest that alternate pathways other than EMT inducing signals are engaged in the maintenance of EMT-derived cells. Therefore, targeting these alternative pathways holds the potential to eliminate EMT-like BCSCs.
A recent proteomics-based study using distinct epithelial, metastable EMT and “epigenetically-fixed” mesenchymal tumor cells in an isogenic background provided a system view of EMT signaling states [119]. Associated with EMT, EGFR, IGF1R, and MET signaling was decreased, while the pro-survival IL11/IL6-JAK2-STAT and Ax1/Tyro3/PDGER/FGER RTK signaling were increased. This study also revealed a coordinated metabolic reduction in seventeen free-radical stress pathway related components, together with reduced glycolytic and increased oxidative phosphorylation enzyme capacity [119]. These newly emerged signaling states associated with metastable EMT provide novel venues for future targeting strategies against EMT-derived cancer cells such as EMT BCSCs.
One potential therapeutic target of EMT BCSCs is the inflammatory cytokines including IL6 and IL8. High levels of IL-6, by promoting tumorigenesis, angiogenesis and metastasis, are associated with poor clinical outcome in cancer patients [120]. IL-6 has been shown to act as a direct regulator of the self-renewal of BCSCs through IL-6R/GP130 mediated Stat3 activation [121]. The activation of Stat3 in turn results in transcriptional activation of NF-kB in inflammatory cells, which promotes additional release of IL-6 (and IL-8). Thus, a positive feedback loop between immune cells and tumor cells through IL-6 signaling is generated that further stimulates CSC self-renewal, metastasis and therapeutic resistance. Indeed, recent studies in our laboratory have shown that activation of an IL6 inflammatory loop plays an important role for trastuzumab resistance of HER2+ breast cancer by expanding EMT BCSCs [122]. Conversely, through blockade of the IL-8 receptor CXCR1, we have successfully depleted BCSCs in vitro and in NOD/SCID xenograft models which is mediated by the FAK/AKT/FOX03A pathway [123]. This strategy is currently being evaluated in an early phase clinical trial utilizing the CXCR1 inhibitor, reparaxin, in combination with the chemotherapeutic agent taxol.
Notably, although approaches targeting EMT in breast cancer and other tissue malignancies may prove to be effective by reducing EMT-like CSC component, this strategy may be counterproductive once tumor cells have disseminated from the primary site. As formation of macrometastases from disseminated tumor cells in distant organs need the tumor cells revert to a MET state, inhibition of EMT at this late stage may actually stimulate metastasis by promoting MET. Thus, for breast cancers with existing metastasis, specific strategies designed to target the metastatic niche that allows dormant disseminated tumor cells to revive into a self-renewal MET state may prevent new metastasis formation. In this regard, the BMP inhibitor, Coco, a secreted antagonist of TGF-β ligands, has been found to mediate breast cancer colonization in the lungs [124]. Future therapeutics that selectively activates BMP signaling will have the potential to inhibit cancer stem cell traits and lung colonization.
7. FUTURE PERSPECTIVES
The critical roles of BCSCs in breast cancer initiation, progression and recurrence highlight the pressing need for developing novel therapeutic strategies to eradicate these cells in order to cure this deadly disease. As BCSCs mediate tumor metastasis and relapse by nature of their therapeutic resistance [41, 49, 51], targeting essential mechanisms underlying BCSC therapeutic resistance is urgently needed in the combat of breast cancer. The demonstration that BCSCs exist in inter-convertible EMT and MET states that can be readily identified by expression of distinct CSC markers [7] provides a novel model to understand how BCSCs contribute to breast cancer metastasis and therapeutic resistance. We believe that the plasticity of BCSCs to transition between an EMT and MET state endows them with the capacity for tissue invasion, dissemination and metastatic growth at distal organs. This plasticity of BCSCs also suggest that targeting either state alone may not be sufficient since the targeted cell populations would be rapidly regenerated by BCSCs in the alternative state. If this is the case, future studies will be necessary to simultaneously target both BCSC states to achieve maximum efficacy. In HER2+ breast cancer, experimental evidence in our laboratory indeed suggested that simultaneously targeting MET-like BCSCs by trastuzumab, a HER2 blocking antibody and EMT-like BCSCs by tociluzumab, an IL-6R inhibitor, resulted in maximum reduction of the BCSC population [122]. Future studies will be required to investigate other combinatory strategies to target both EMT- and MET-like BCSCs in other subtypes of breast cancer.
ACKNOWLEDGEMENTS
We would like to acknowledge the assistance of Dr. Shawn G. Clouthier in proofreading this article. This work was supported by NIH grants CA101860 and CA66233 (to MSW).
MSW holds equity in OncoMed Pharmaceuticals and currently receives research support from Verastem, MedImmune and Dompe Pharmaceuticals. MSW is a scientific advisor to MedImmune, Verastem, Cerulean and Paganini.
Biography

Max S. Wicha
Footnotes
CONFLICT OF INTEREST
All other authors have no competing interests to disclose.
REFERENCES
- 1.Servick K. Breast cancer. Breast cancer: a world of differences. Science. 2014;343(6178):1452–1453. doi: 10.1126/science.343.6178.1452. [DOI] [PubMed] [Google Scholar]
- 2.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66(4):1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 95–6. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Wicha MS. Targeting breast cancer stem cells. J clinical oncology : official J the American Society of Clinical Oncology. 2010;28(25):4006–4012. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charafe-Jauffret E, Monville F, Ginestier C, Dontu G, Birnbaum D, Wicha MS. Cancer stem cells in breast: current opinion and future challenges. Pathobiology : J Immunopathol, Mol Cellular Biol. 2008;75(2):75–84. doi: 10.1159/000123845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDHI is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Cong Y, Wang D, et al. Breast Cancer Stem Cells Transition between Epithelial and Mesenchymal States Reflective of their Normal Counterparts. Stem Cell Reports. 2014;2(1):78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342(6159):1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 9.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 10.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J cell biology. 2006;172(7):973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hugo H, Ackland ML, Blick T, et al. Epithelial—mesenchymal and mesenchymal—epithelial transitions in carcinoma progression. Cellular Physiol. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 12.Thiery JP, Sleeman IP. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Rev Mol Cell Biel. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 13.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27(55):6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 15.Brabletz T. To differentiate or not--routes towards metastasis. Nat Rev Cancer. 2012;12(6):425–436. doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- 16.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investigation. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clinical cancer research : an official J Am Assoc Cancer Res. 2006;12(19):5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 18.Watson MA, Ylagan LR, Trinkaus KM, Gillanders WE, Naughton MI, Weilbaecher ICN, et al. Isolation and molecular profiling of bone marrow micrometastases identifies TWIST1 as a marker of early tumor relapse in breast cancer patients. Clinical cancer research : an official J Am Assoc Cancer Res. 2007;13(17):5001–5009. doi: 10.1158/1078-0432.CCR-07-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raimondi C, Gradilone A, Naso G, et al. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treatment. 2011;130(2):449–455. doi: 10.1007/s10549-011-1373-x. [DOI] [PubMed] [Google Scholar]
- 20.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6(6):339–351. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 21.Yu M, Stott S, Toner M, Maheswaran S, Haber DA, et al. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192(3):373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Eng J Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 23.Budd GT, Cristofanilli M, Ellis MI, et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clinical cancer research : an official J Am Assoc Cancer Res. 2006;12(21):6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 24.Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31(6):539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 25.Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shipitsin M, Campbell LL, Argani P, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11(3):259–271. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Malanchi I, Santamaria-Martinez A, Susanto E, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481(7379):85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 28.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22(6):725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ocana OH, Corcoles R, Fabra A, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22(6):709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Korpal M, Ell BJ, Buffa FM, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nature Med. 2011;17(9):1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stankic M, Pavlovic S, Chin Y, et al. TGF-beta-Id1 Signaling Opposes Twist1 and Promotes Metastatic Colonization via a Mesenchymal-to-Epithelial Transition. Cell reports. 2013;5(5):1228–1242. doi: 10.1016/j.celrep.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron MD, Schmidt EE, Kerkvliet N, et al. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 2000;60(9):2541–2546. [PubMed] [Google Scholar]
- 33.Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC. Critical steps in hematogenous metastasis: an overview. Surgical Oncol Clin North America. 2001;10(2):243–255. [PubMed] [Google Scholar]
- 34.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 35.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 36.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Eng J Med. 2007;356(3):217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Patel MR, Prescher IA, et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci USA. 2010;107(42):18115–18120. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel SA, Ramkissoon SH, Bryan M, et al. Delineation of breast cancer cell hierarchy identifies the subset responsible for dormancy. Scientific Reports. 2012;2:906. doi: 10.1038/srep00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagadec C, Vlashi E, Della Donna L, et al. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast cancer research : BCR. 2010;12(1):R13. doi: 10.1186/bcr2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Institute. 2006;98(24):1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 42.Karimi-Busheri F, Rasouli-Nia A, Mackey JR, Weinfeld M. Senescence evasion by MCF-7 human breast tumor-initiating cells. Breast Cancer Research : BCR. 2010;12(3):R31. doi: 10.1186/bcr2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Research : BCR. 2008;10(2):R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shafee N, Smith CR, Wei S, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68(9):3243–3250. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen TM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104(2):618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu F, Yao H, Zhu P, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 48.Zielske SP, Spalding AC, Wicha MS, Lawrence TS. Ablation of breast cancer stem cells with radiation. Translational Oncol. 2011;4(4):227–233. doi: 10.1593/tlo.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Institute. 2008;100(9):672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 50.Korkaya H, Paulson A, Iovino F, Wicha MS. ITER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27(47):6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clinical cancer research : an official J Am Assoc Cancer Res. 2009;15(12):4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 52.Alamgeer M, Ganju F, Kumar B, Fox J, Hart S, White M, Harris M, Stuckey J, Prodanovic Z, Schneider-Kolsky ME, Watkins DN. Changes in aldehyde dehydrogenase-1 expression during neoadjuvant chemotherapy predict outcome in locally advanced breast cancer. Breast Cancer Res. 2014;16:R44. doi: 10.1186/bcr3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71(2):614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreb JS, Maccow C, Schweder M, Hecomovich J. Expression of antisense RNA to aldehyde dehydrogenase class-1 sensitizes tumor cells to 4-hydroperoxycyclophosphamide in vitro. J Pharmacol Experimental Therapeutics. 2000;293(2):390–396. [PubMed] [Google Scholar]
- 55.Magni M, Shammah S, Schiro R, Mellado W, Dalla-Favera R, Gianni AM. Induction of cyclophosphamide-resistance by aldehyde-dehydrogenase gene transfer. Blood. 1996;87(3):1097–1103. [PubMed] [Google Scholar]
- 56.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct "side population" of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101(39):14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65(14):6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 58.Britton KM, Eyre R, Harvey IJ, et al. Breast cancer, side population cells and ABCG2 expression. Cancer Letters. 2012;323(1):97–105. doi: 10.1016/j.canlet.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakanishi T, Chumsri S, Khakpour N, et al. Side-population cells in luminal-type breast cancer have tumour-initiating cell properties, and are regulated by HER2 expression and signalling. Br J Cancer. 2010;102(5):815–826. doi: 10.1038/sj.bjc.6605553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin H, Glass J. The phenotypic radiation resistance of CD44+/CD24(−or low) breast cancer cells is mediated through the enhanced activation of ATM signaling. PloS one. 2011;6(9):e24080. doi: 10.1371/journal.pone.0024080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang M, Atkinson RL, Rosen JM. Selective targeting of radiation-resistant tumor-initiating cells. Proc Natl Acad Sci USA. 2010;107(8):3522–3527. doi: 10.1073/pnas.0910179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheridan C, Kishimoto H, Fuchs RK, et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res : BCR. 2006;8(5):R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 64.Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Development. 2006;20(13):1715–1720. doi: 10.1101/gad.1430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Development. 2002;16(23):3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherman LS, Rizvi TA, Karyala S, Ratner N. CD44 enhances neuregulin signaling by Schwann cells. J Cell Biol. 2000;150(5):1071–1084. doi: 10.1083/jcb.150.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC. Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biological Chem. 1997;272(44):27913–27918. doi: 10.1074/jbc.272.44.27913. [DOI] [PubMed] [Google Scholar]
- 68.Draffin SE, McFarlane S, Hill A, Johnston PG, Waugh DJ. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res. 2004;64(16):5702–5711. doi: 10.1158/0008-5472.CAN-04-0389. [DOI] [PubMed] [Google Scholar]
- 69.Hiraga T, Ito S, Nakamura H. Cancer stem-like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and hyaluronan production. Cancer Res. 2013;73(13):4112–4122. doi: 10.1158/0008-5472.CAN-12-3801. [DOI] [PubMed] [Google Scholar]
- 70.Brown RL, Reinke LM, Damerow MS, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Investigation. 2011;121(3):1064–1074. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stern cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sigurdsson V, Hilmarsdottir B, Sigmundsdottir H, et al. Endothelial induced EMT in breast epithelial cells with stem cell properties. PloS one. 2011;6(9):e23833. doi: 10.1371/journal.pone.0023833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo W, Keckesova Z, Donaher JL, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148(5):1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lindeman GJ, Visvader SE. Insights into the cell of origin in breast cancer and breast cancer stem cells. Asia-Pacific J Clin Oncol. 2010;6(2):89–97. doi: 10.1111/j.1743-7563.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 75.Lim E, Valliant F, Wu D, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nature Med. 2009;15(8):907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 76.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Development. 2009;23(22):2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Z, Dong Z, Lauxen IS, Filho MS, Nor JE. Endothelial Cell-Secreted EGF Induces Epithelial to Mesenchymal Transition and Endows Head and Neck Cancer Cells with Stern-like Phenotype. Cancer Res. 2014;74(10):2869–2881. doi: 10.1158/0008-5472.CAN-13-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merrilees MJ, Sodek J. Synthesis of TGF-beta 1 by vascular endo thelial cells is correlated with cell spreading. J Vascular Res. 1992;29(5):376–384. doi: 10.1159/000158954. [DOI] [PubMed] [Google Scholar]
- 80.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biological Chem. 2008;283(28):19864–19871. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- 82.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5(15):1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 83.Tyan SW, Kuo WH, Huang CK, et al. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PloS one. 2011;6(1):e15313. doi: 10.1371/journal.pone.0015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Experimental cell Res. 2002;276(1):24–31. doi: 10.1006/excr.2002.5508. [DOI] [PubMed] [Google Scholar]
- 85.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 86.Yu Y, Xiao C, Tan L, Wang Q, Li X, Fang Y. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. British J cancer. 2013 doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Y, Kim J, Yuan X, Braun T. Epigenetic modifications of stem cells: a paradigm for the control of cardiac progenitor cells. Circulation Res. 2011;109(9):1067–1081. doi: 10.1161/CIRCRESAHA.111.243709. [DOI] [PubMed] [Google Scholar]
- 88.Lund AH, van Lohuizen M. Epigenetics and cancer. Genes Development. 2004;18(19):2315–2335. doi: 10.1101/gad.1232504. [DOI] [PubMed] [Google Scholar]
- 89.Herceg Z, Vaissiere T. Epigenetic mechanisms and cancer: an interface between the environment and the genome. doi: 10.4161/epi.6.7.16262. (1559–2308 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 90.Chaffer Christine L, Marjanovic Nemanja D, Lee T, et al. Poised Chromatin at the ZEB1 Promoter Enables Breast Cancer Cell Plasticity and Enhances Tumorigenicity. Cell. 154(1):61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suva ML, Riggi N, Janiszewska M, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69(24):9211–9218. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- 92.Jene-Sanz A, Varaljai R, Vilkova AV, et al. Expression of polycomb targets predicts breast cancer prognosis. Mol Cellular Biol. 2013;33(19):3951–3961. doi: 10.1128/MCB.00426-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cao Q, Yu J, Dhanasekaran SM, et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27(58):7274–7284. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to poly-comb-mediated repression required for the formation and maintenance of cancer stem cells. Molecular Cell. 2010;39(5):761–772. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast cancer research : BCR. 2010;12(5):R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PloS one. 2008;3(8):e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Celia-Terrassa T, Meca-Cortes O, Mateo F, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Investigation. 2012;122(5):1849–1868. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu S, Clouthier SG, Wicha MS. Role of microRNAs in the regulation of breast cancer stem cells. J Mammary Gland Biol Neoplasia. 2012;17(1):15–21. doi: 10.1007/s10911-012-9242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Nati Acad Sci USA. 2009;106(33):13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang JC, Wooten EC, Tsimelzon A, et al. Patterns of resistance and incomplete response to docetaxel by gene expression profiling in breast cancer patients. J clinical oncology: official J Am Soc Clin Oncoi. 2005;23(6):1169–1177. doi: 10.1200/JCO.2005.03.156. [DOI] [PubMed] [Google Scholar]
- 101.Lim E, Vailliant F, Wu D, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15(8):907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 102.Molyneux G, Geyer FC, Magnay FA, et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell stem cell. 2010;7(3):403–417. doi: 10.1016/j.stem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 103.Proia TA, Keller PS, Gupta PB, et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell stem cell. 2011;8(2):149–163. doi: 10.1016/j.stem.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keller PJ, Arendt LM, Skibinski A, et al. Defining the cellular precursors to human breast cancer. Proc Nati Acad Sci USA. 2012;109(8):2772–2777. doi: 10.1073/pnas.1017626108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Keymeulen A, Rocha AS, Ousset M, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479(7372):189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- 106.Liu S, Ginestier C, Charafe-Jauffret E, et al. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci USA. 2008;105(5):1680–1685. doi: 10.1073/pnas.0711613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaffer CL, Brueckmann I, Scheel C, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA. 2011;108(19):7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chaffer CL, Marjanovic ND, Lee T, et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154(1):61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Development. 2013;27(20):2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reka AK, Kuick R, Kurapati H, Standiford TJ, Omenn GS, Keshamouni VG. Identifying inhibitors of epithelial-mesenchymal transition by connectivity map-based systems approach. J thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6(11):1784–1792. doi: 10.1097/JTO.0b013e31822adfb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chua KN, Sim WJ, Racine V, Lee SY, Goh BC, Thiery JP. A cell-based small molecule screening method for identifying inhibitors of epithelial-mesenchymal transition in carcinoma. PloS one. 2012;7(3):e33183. doi: 10.1371/journal.pone.0033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Angelucci A, Gravina GL, Rucci N, et al. Suppression of EGF-R signaling reduces the incidence of prostate cancer metastasis in nude mice. Endocrine-related Cancer. 2006;13(1):197–210. doi: 10.1677/erc.1.01100. [DOI] [PubMed] [Google Scholar]
- 113.Lo HW, Hsu SC, Xia W, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67(19):9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim HJ, Litzenburger BC, Cui X, et al. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol and Cellular Biol. 2007;27(8):3165–3175. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomson S, Buck E, Petri F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65(20):9455–9462. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 116.Fuchs BC, Fujii T, Dorfman JD, et al. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68(7):2391–2399. doi: 10.1158/0008-5472.CAN-07-2460. [DOI] [PubMed] [Google Scholar]
- 117.Frederick BA, Helfrich BA, Coldren CD, et al. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol Cancer Therapeutics. 2007;6(6):1683–1691. doi: 10.1158/1535-7163.MCT-07-0138. [DOI] [PubMed] [Google Scholar]
- 118.Buck E, Eyzaguirre A, Rosenfeld-Franklin M, et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68(20):8322–8332. doi: 10.1158/0008-5472.CAN-07-6720. [DOI] [PubMed] [Google Scholar]
- 119.Thomson S, Petti F, Sujka-Kwok I, et al. A systems view of epithelial-mesenchymal transition signaling states. Clin Experimental Metastasis. 2011;28(2):137–155. doi: 10.1007/s10585-010-9367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer's inflammatory roots. Clinical cancer research : an official J Am Assoc Cancer Res. 2011;17(19):6125–6129. doi: 10.1158/1078-0432.CCR-10-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sansone P, Storci G, Tavolari S, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Korkaya H, Kim GI, Davis A, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mot Cell. 2012;47(4):570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ginestier C, Liu S, Diebel ME, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Investigation. 2010;120(2):485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gao H, Chakraborty G, Lee-Lim AP, et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150(4):764–779. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]