Increasing evidence shows that different ethnic groups respond differently to educational, psychosocial, and pharmacological interventions. If diverse communities are to benefit from the implementation of appropriately derived evidence then it is imperative that the ethnic diversity of populations under study are reflected in clinical trials. In the United States, since 1993, the National Institutes of Health have instituted policy insisting that minority groups are represented in study samples unless there is a compelling reason not to do so.1 However, no comparable legislation exists in Europe. We sought to compare reporting of ethnicity in published reports of US and European randomised controlled studies.
Methods and results
We searched Medline for reports of trials published in 2002 using the Cochrane optimal search strategy.2 We downloaded titles and abstracts of study reports into the reference manager database and randomly selected 200 reports for further scrutiny. We identified trials done in the United States and Europe and got full text reports. We assessed any description of the ethnicity of participants in detail. We used a broad definition of ethnicity that included any reference to race, ethnic origin, language, or nationality. We categorised studies as either detailing the ethnicity of subjects or not. Two reviewers independently extracted data on to a prepiloted sheet; they resolved disagreements through discussion.
We used descriptive statistics to find the proportion of studies reporting information on the ethnicity of subjects and used the χ2 test to compare reporting of ethnicity in United States and European published reports.
For 80% power at the 5% significance level (two tailed test) of detecting a difference from 20% to 40% in the proportion of studies reporting on ethnicity, assuming that one in five European trials detailed this information, we needed to identify a total of 182 trials.
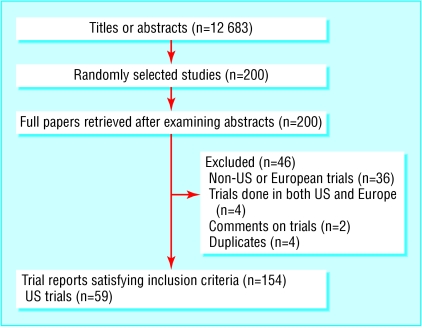
Our searches retrieved 12 683 titles and abstracts, from which we selected 200 for further scrutiny. Of these, 154 studies satisfied our inclusion criteria (figure). A total of 59 (38%) of these trials were based in the United States and 95 (62%) in Europe. Overall, 30 (19%) reports included information on the ethnic profile of participants. American studies were significantly more likely to report on ethnicity than European studies (23 v 7; 39% v 7%; relative risk 5.3, 95% confidence interval 2.4 to 11.6; P < 0.0001).
Figure 1.
Selection of trial reports
Comment
American studies are five times more likely than European trials to report information on the ethnicity of participants. The random selection procedures adopted and the standardised and independent extraction of data with an initially agreed approach to handle disagreements should have minimised the risk of selection bias or information bias accounting for these findings.
Our results seem likely to reflect active policies in the United States. For example, all federally supported programmes with sufficient sample size are required to report statistics according to race or ethnicity.3 None the less, it is still concerning that only two fifths of recently published trials from the United States report on the ethnicity of participants. Possible explanations include known difficulties in identifying, enrolling, and following up minority ethnic populations in trials. Another possible factor is the argument that ethnicity reporting is only important in specific disease areas with known ethnic disparities. Relevant here are recent data showing that in the study of such conditions 59% of US trials report on the ethnicity of participants.4
Mechanisms to facilitate inclusion and standards to ensure reporting of minority ethnic communities in studies are needed. These reporting standards are absent in current CONSORT requirements and we suggest that the merits of insisting on presentation of such data, where appropriate, should be debated.5 In particular, European governments should consider the US model for promoting inclusion of ethnic minority participants in research.
This article was posted on bmj.com on 11 May 2004: http://bmj.com/cgi/doi/10.1136/bmj.38061.593935.F7
Contributors: AS conceived this study, formulated the study protocol, and led the writing of the manuscript. GN and SSP extracted data. GN analysed the data. JK interpreted the results and wrote the paper. AS is guarantor.
Funding: None.
Competing interests: None declared.
Ethical approval: Not needed.
References
- 1.National Institutes of Health. Monitoring adherence to the NIH policy on the inclusion of women and minorities as subjects in clinical research. Bethesda: NIH, 2001. www4.od.nih.gov/orwh/salmonrpt.pdf (accessed 15 Mar 2004).
- 2.Mulrow CD, Oxman AD, eds. Cochrane Collaboration handbook. In: Cochrane Library. Issue 4. Oxford: Update Software, 1997.
- 3.Office of Management and Budget. Recommendations from the interagency committee for the review of the racial and ethnic standards to the Office of Management and Budget concerning changes to the standards for the classification of federal data on race and ethnicity. Washington, DC: OoMaB, 1997. (Directive number 15.) www.whitehouse.gov/omb/fedreg/directive_15.html (accessed 15 Mar 2004).
- 4.Corbie-Smith G, St George DMM, Moody-Ayers S, Ransohoff DF. Adequacy of reporting race/ethnicity in clinical trials in areas of health disparities. J Clin Epidemiol 2003;56: 416-20. [DOI] [PubMed] [Google Scholar]
- 5.Revised consort statement. www.consort-statement.org (accessed 15 Mar 2004).