Abstract
Background
Kawasaki disease (KD) is a systemic pediatric vasculitis. Its main complication is the development of coronary arterial aneurysms (CAA), causing an increased risk for ischemia and myocardial infarction. It is unclear whether KD patients, apart from the presence of CAA, have an increased cardiovascular disease (CVD) risk due to the previous systemic vasculitis. The aim of this study was to systematically review and meta-analyse the literature regarding surrogate markers for CVD risk in KD patients.
Methods
Medline and Embase were searched for articles comparing endothelial dysfunction (flow-mediated dilation, nitroglycerin-mediated dilation and peripheral arterial tonometry), vascular stiffness (stiffness index, pulse wave velocity) and carotid intima-media thickness (cIMT) between patients and controls. Two investigators assessed the articles for eligibility and evaluated quality.
Results
Thirty studies were included. For all outcomes, moderate to high heterogeneity between studies was found. Most studies reported a decreased flow-mediated dilation in the whole KD- and CAA-positive group compared to controls, while data on CAA-negative patients were conflicting. The stiffness index was increased in the majority of studies evaluating the whole KD- and CAA-positive group, but not in most studies on CAA-negative patients. Mean cIMT was neither significantly increased in the whole KD-group nor in the CAA-positive group nor in most studies studying CAA-negative patients. Studies measuring maximum cIMT were conflicting.
Conclusion
Literature suggests that surrogate markers for CVD risk in KD patients are increased in CAA-positive but not in CAA-negative patients. This may indicate that CAA-positive patients should be monitored for CVD in later life. The results of this review have to be interpreted with care due to substantial heterogeneity between studies and methodological limitations, as well as the lack of long-term follow-up studies.
Introduction
Kawasaki disease (KD) is a pediatric vasculitis mainly affecting children under the age of 5[1]. Coronary artery aneurysms (CAA) develop in 25% of untreated and 5–15% of patients treated with intravenous immunoglobulins, making it the most common cause of pediatric acquired heart disease in the Western world.
It can be hypothesized that, due to the previous systemic vasculitis, patients with KD have an increased risk for cardiovascular disease (CVD) at a later age, apart from the presence or absence of CAA. This hypothesis is difficult to test since KD was first described less than 50 years ago and therefore most of the KD patients are too young to have experienced cardiovascular events.
In recent years, several non-invasive surrogate markers of CVD risk have become available.
Endothelial dysfunction can be measured by flow-mediated dilatation (FMD), nitroglycerin-mediated dilation (NMD) or peripheral arterial tonometry (PAT) [2,3]. Peripheral arterial stiffness can also be an indicator of increased CVD risk. It can be measured by pulse wave velocity (PWV) or by the beta stiffness index (SI) [4]. Furthermore, structural changes in the arterial wall can be found by measuring the carotid intima-media thickness (cIMT), well-established surrogate marker of atherosclerosis and subsequent predictor of cardiovascular events [5,6].
The aim of this study was to systematically review and meta-analyze the existing literature regarding CVD risk after KD, as measured by surrogate markers.
Methods
Search strategies
We conducted a systematic literature search of Medline (1966-September 2014) and Embase (1980-September 2014) for studies addressing KD and surrogate markers of cardiovascular risk (i.e. endothelial dysfunction, peripheral arterial stiffness and cIMT). We used two domains of MeSH terms and free text words combined by ‘AND’, and in each domain the terms were combined by ‘OR’. The first domain contained terms of KD (including all synonyms and abbreviations), and the second contained terms of surrogate markers of cardiovascular risk (including all synonyms, abbreviations and free word text such as ‘carotid intima-media thickness’, ‘vascular stiffness’, ‘endothelial dysfunction’, ‘flow-mediated dilatation’, ‘pulse wave velocity’, ‘peripheral arterial tonometry’). The complete protocol is registered in the Prospero database under CRD42014005706, the PRISMA checklist and Medline electronic search strategy are added as S1 PRISMA Checklist and S1 File.
Study selection and quality assessment
We selected those original studies that reported on surrogate markers of cardiovascular risk (i.e. endothelial dysfunction [FMD, NMD, PAT], vascular stiffness [PWV, SI] and cIMT) in KD patients. Studies were excluded if healthy control groups were not available within the same studies, if lipid-lowering medication was used when measuring subjects, or if data contained preliminary results. Furthermore, because of the possible influence of the acute inflammation, studies measuring patients within 6 months after the acute phase were excluded. Language restrictions were not imposed. The selection process was divided into three successive stages: title-, abstract- and manuscript selection. Two investigators (SD and CT) independently determined eligibility of the retrieved studies, according to predefined criteria. Using an adjusted version of the Newcastle-Ottawa scale for observational studies (S1 Table: Quality assessment criteria), the same investigators assessed the methodological quality of the eligible studies. Selection of patients and controls, comparability, and outcome measurements were evaluated. Disagreements were solved by discussion, and if necessary, by the opinion of a third reviewer.
Data extraction
Using a predetermined form, two investigators (SD and CT) independently extracted data of the eligible articles. Information was collected on study characteristics (study design, country and sample size). The CAA-classification used was retrieved and the number of CAA-positive patients was noted based on whether patients ever had CAA (worst-ever CAA-score). In addition, the following characteristics of participants were extracted: gender, age, blood pressure, BMI, and treatment during the acute KD phase. Outcome measurements were, if possible, collected for the control- and the whole KD group, as well for the CAA-negative and CAA-positive group. When data were missing, the corresponding authors were emailed to request the information.
Statistical analysis
When studies described multiple CAA-positive groups based on severity, we calculated pooled estimates of the mean and standard deviation (SD) values for the overall CAA positive group. The same was done when cIMT was described separately for the left and the right carotid artery. When p-values were not provided, but mean, SD and numbers were reported, we calculated whether there was a significant difference between patients and controls using review manager software, version 5.2 (Cochrane Collaboration).
We used the same software to create forest plots for cIMT, FMD and SI in KD patient compared to controls and in CAA positive patients compared to controls, with study-level effect sizes calculated as absolute mean differences. We measured the proportion of between-study differences not attributable to chance with the I2 statistic. We considered values of 25–50%, 50–75% and ≥75% to indicate low, moderate and high heterogeneity, respectively. Only when heterogeneity was low or moderate (≤75%), pooled estimates of the summary mean difference were computed using the random-effects model according to the method of DerSimonian and Laird. [7]. A Z-test was performed to test the overall effect.
When studies with overlapping inclusions were present, the largest study was used for the forest plot and if applicable, the pooled estimate.
Heterogeneity was explored by a sensitivity analyses for all analyses. Furthermore, for the whole KD group, a meta-regression analysis using the random effects, methods of moments approach was performed, with study characteristics as covariates. We looked at ‘time since KD’, ‘percentage of IVIG-treated patients’ and ‘percentage of CAA-positive patients’. We did not perform meta-regression analyses on the CAA-positive groups because covariables were usually not described separately for CAA-positive groups in most studies. We used comprehensive meta-analyses software (version 2) to execute the meta-regression analyses.
We did not perform meta-analyses on the data of CAA-negative patients because not all studies used the same CAA-criteria. This implies that a child might have no enlargement according to one classification but does have an aneurysm according to the other. All CAA-positive patients have enlargement according to at least one classification system.
Results
Description of studies
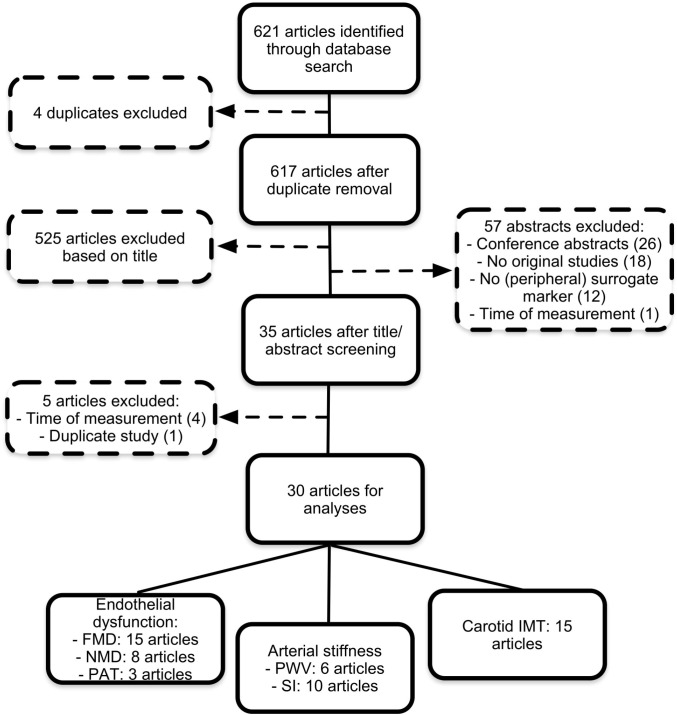
Our search retrieved 621 articles. After scanning titles and/or abstracts, we excluded 586 studies. Of the remaining 35 articles, five were excluded based on the whole article. Hence, 30 studies remained for final inclusion (Fig 1). Table 1 shows the characteristics of these studies.
Fig 1. Flow diagram of selected studies.
Table 1. Characteristics of studies.
| First author, year | Country | Kawasaki patients | Controls | Reported outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % IVIG | No; CAA+/CAA- | Age (yrs.) | % ♂ | Time since KD (yrs.) | No. | Age (yrs.) | % ♂ | |||
| Dhillon, 1996 [8] | UK | 15 | 20 (3/17) | 13 (11–19)* | 12 (60) | 11.3 (5.3–17.1)* | 20 | 15 (10–16)* | NA | FMD, NMD |
| Silva, 2001 [9] | Canada | 33 | 24 (13/11) | 14.3±1.8 | 18 (75) | 11.3±1.8 | 11 | 14.1±1.5 | 6 (55) | FMD, NMD |
| Noto, 2001 [10] | Japan | 58 | 20 (20/0) | 16.6±4.1 | 12 (60) | 9.8±4.0 | 20 | 16.3±4.7 | 12 (60) | cIMT, SI |
| Deng, 2002 [11] | China | 87 | 39 (6/33) | 7.1±2.7 | 28 (72) | 3.4±2.1 | 17 | 7.0±3.1 | 13 (76) | FMD, NMD |
| Cheung, 2004 [12] | China | 93 | 71 (43/28) | CAA+: 10.2±4.1 | 47 (66) | 7.8 (1–16.6)† | 35 | 10.3±3.6 | 25 (71) | SI |
| CAA-: 9.7±3.2 | 6.9 (1.1–13.2)† | |||||||||
| Cheung, 2004 [13] | China | 92 | 66 (37/29) | CAA+: 9.0±3.1 | 43 (65) | 7.8±3.7 | 36 | 9.1±2.6 | 24 (67) | PWV |
| CAA-: 8.9±3.2 | 6.2±2.4 | |||||||||
| Cheung, 2004 [14] | China | 93 | 71 (42/29) | 9.5±3.7 | 48 (68) | 7.7±3.9 | 41 | 10.5±3.7 | 28 (68) | PWV |
| Ikemoto, 2005 [15] | Japan | 100‡ | 65 (34/31) | 13 (9–22)* | 38 (58) | 12 (5–21)* | 20 | 15 (9–23)* | 11 (55) | cIMT, SI, FMD |
| Kadono, 2005 [16] | Japan | NA | 24 (15/9) | 8.3±4.1 | 13 (54) | 5.8±4.6 | 41 | 10.7±4.4 | 29 (71) | cIMT, FMD |
| Borzutzky, 2007 [17] | Chili | 100 | 11 (1/10) | 10.6±2 | 7 (64) | 8.1±3.6 | 11 | 10.4±1.8 | 7 (64) | FMD |
| McCrindle, 2007 [18] | Canada | 64 | 52 (19/33§) | 15.5±2.3 | 35 (67) | 11.2±3.7 | 60 | 14.9±2.4 | 30 (50) | FMD, NMD |
| Cheung, 2007 [19] | China | 90 | 50 (26/24) | CAA+:8.6±2.8 | 33 (66) | 7.4±3.5 | 22 | 9.5±2.5 | 14 (64) | CIMT, PWV, SI |
| CAA-:8.6±3.3 | 5.8±2.1 | |||||||||
| Huang, 2008 [20] | Taiwan | 100 | 11 (11/0) | 12.9±2.5 | 8 (73) | 10.77±3.01 | 11 | 12.97±2.42 | 8 (73) | FMD |
| Niboshi, 2008 [21] | Japan | 6 | 35 (15/20) | 27±4.2 | 16 (46) | 24.1±4.5 | 36 | 25.5±3.9 | 19 (53) | FMD, PWV |
| Cheung, 2008 [22] | China | 75 | 51 (32/19) | 13.4±0.6 | 40 (78) | 10.5±0.68 | 32 | 14.6±0.6 | 26 (81) | cIMT, SI |
| Liu, 2009 [23] | China | 100 | 41 (21/20) | CAA+: 7.0 (3–11)† | 25 (61) | 5.0 (1.6–10.0)† | 22 | 8.4 (3.2–14.0)† | 13 (59) | FMD, SI |
| CAA-: 7.3 (4.9–11.0)† | 3.8 (1.5–8.0)† | |||||||||
| Gupta-Malhotra, 2009 [24] | USA | 36 | 28 (9/19) | 20.9±6 | 19 (68) | 16±6 | 27 | 21.3±7.5 | 16 (59) | cIMT, SI |
| Lee, 2009 [25] | Korea | 100 | 25 (25/0) | 12.6±2.0 | 11 (44) | > 8 | 55 | 14.5±0.7¶ | 29 (53) | cIMT, PWV |
| Noto, 2009 [26] | Japan | 52 | 35 (35/0) | 20.5±9.3 | 28 (80) | 18.6±8.4 | 35 | 19.6±7.2 | 28 (80) | cIMT, FMD, NMD, SI |
| Duan, 2011 [27] | China | 97 | 31 (31/0) | 6.2±3.4 | 22 (71) | 2.53 (1–12.5)* | 21 | 5.7±2.5 | 14 (67) | cIMT, FMD, NMD, SI |
| Noto, 2012 [28] | Japan | 64 | 18 (18/0) | 17.2±5.3 | 13 (72) | 14.1±6.9 | 15 | 15.3±2.0 | 12 (80) | cIMT, SI |
| Pinto, 2013 [29] | Portugal | 100 | 19 (0/19) | 21±6 | 12 (63) | >5 | 16 | 21±6 | 9 (56) | PAT |
| Tobayama, 2013 [30] | Japan | 21 | 14 (11/3) | 31.5±5.5 | 8 (57) | 28.6±5.6 | 41 | 32.6±5.3 | 21 (51) | PAT |
| Ishikawa, 2013 [31] | Japan | 100 | 24 (9/15) | 6.5±1.7 | 14 (58) | 3.3 (2.0–4.4)|| | 22 | 7.9±2.8 | 13 (59) | cIMT, FMD, NMD |
| Selamet Tierney, 2013 [32] | USA | 93 | 203 (47/156§) | 16.73±4.21 | 122 (60) | 11.6 (1.2–26)* | 50 | 17.57±4.33 | 29 (58) | cIMT, PAT |
| Duan, 2014 [33] | China | 100 | 13 (13/0) | 5.8±2.1 | 13 (100) | 3.1 ±1.7 | 14 | 5.5 ± 2.3 | 14 (100) | FMD, SI |
| Cho, 2014 [34] | Korea | 91 | 68 (19/49) | CAA+: 8.00±1.89 | 40 (59) | 5.75±2.65 | 30 | 7.65 ± 0.78 | 16 (53) | PWV |
| CAA-: 7.22±1.49 | 4.36±2.21 | |||||||||
| Laurito, 2014 [35] | Italy | 0 | 14 (7/7) | 10.0±3.7 | 9 (64) | 6.3±4.8 | 14 | 10.2±2.4 | 7 (50) | cIMT, FMD |
| Oguri, 2014 [36] | Japan | 81 | 75 (11/64) | 8.2±2.8 | 49 (65) | 5.68±2.48 | 50 | 8.3±3.5 | 25 (46)¶ | cIMT, SI |
| Singh-Meena, 2014 [37] | India | 100 | 27 (10/17) | 8.22±2.6 | 20 (74) | 2.45 ± 1.20 | 23 | 8.46 ± 2.9 | 12 (52) | cIMT |
Values represent mean ± SD unless otherwise indicated.
* Median (range).
† mean (range).
‡ 400mg for 5 days
§ McCrindle et al: Ectasia in 8 patients. Selamet Tierney et al: Ectasia in 20 patients.
|| Median (IQR)
¶ p<0.05 for age or sex when KD group is compared to control group
IVIG, Intravenous immunoglobulin; CAA, Coronary arterial aneurysm; FMD, Flow-mediated dilation; NA, not available; NMD, Nitroglycerin-mediated dilation; cIMT, Carotid intima-media thickness; SI, stiffness index; PWV, Pulse wave velocity
Four studies were cohort studies with a maximal follow-up of 6 months [11,20,33,37] and the remainder had a cross-sectional study design. The total number of subjects per study varied from 22 to 253. The mean age of KD patients ranged from 6.5 to 31.5 years. Although the criteria used were not clearly stated in all manuscripts, most studies defined CAA according to Japanese criteria or by z-scores [38,39]. Two articles used deviating classifications [11,23]. Most studies reported the worst-ever CAA-score, two studies reported the CAA-status 30 days after beginning of the disease [15,36] and in three studies it was not clear which status was reported on [16,17,30]. Some research groups invited the same patients for different studies, which created overlap of inclusions between studies [10,12–14,19,22,26–28,33]. Table 2 shows a summary of findings.
Table 2. Summary of findings.
| Site, unit | Mean controls | Mean CAA- and CAA+ pt. | P * | Mean CAA- | P † | Mean CAA+ | P ‡ | ||
|---|---|---|---|---|---|---|---|---|---|
| FMD and NMD | |||||||||
| Dhillon, 1996 [8] | BA, side unclear | % FMD | 9.40±3.5 | 3.1±3.5 | <0.001 | - | - | - | - |
| % NMD | 21.7±5.4 | 23.0±9.5 | 0.58 | - | - | - | - | ||
| Silva, 2001 [9] | BA, side unclear | % FMD | 6.20±2.8 | 4.6±3.3 | 0.17 | - | - | - | - |
| % NMD | 15.1±3.6 | 14.4±6.9 | 0.73 | - | - | - | - | ||
| Deng, 2002 [11] | Right BA | % FMD | 14.1±6.8 | 6.2±3.9 | <0.0001 | 6.3±4.3 | - | 5.7±1.4 | - |
| % NMD | 33.2±13.7 | 30.6±9.2 | 0.49 | 30.2±9.7 | - | 33.3±5.0 | - | ||
| Ikemoto, 2005 [15] | Right BA | % FMD | 18.8±2.8 | - | - | 19.4±3.9 | NS | 12.5±7.6 | § |
| Kadono, 2005 [16] | Right BA | % FMD | 11.7±14.7 | 3±11.0 | <0.05 | 8.3±9.1 | - | -0.5±9.2 | - |
| Borzutzky, 2008 [17] | BA, n-d arm | % FMD | 8.00±2.9 | 11.1±5.7 | 0.12 | - | - | - | - |
| McCrindle, 2007 [18] | BA, side unclear | % FMD | 9.4±9.0 | 8.9±11.6 | 0.6 | - | - | - | - |
| % NMD | - | - | 0.93 | - | - | - | - | ||
| Huang, 2008 [20] | Right BA | % FMD | 13.11±1.00 | - | - | - | - | 6.12±1.61 | <0.001 |
| Niboshi, 2008 [21] | Right BA | % FMD | 14.4±3.2 | 10.4±2.6 | <0.01 | 11.5±2.8 | < 0.05 | 9.1±2.1 | < 0.05 |
| Liu, 2009 [23] | Left BA | % FMD | 12.1±2.3 | - | - | 9.5±2.8 | <0.01 | 4.5±1.5 | <0.01 |
| Noto, 2009 [26] | BA, side unclear | % FMD | 13.30±4.8 | - | - | - | - | 9.1±2.7 | <0.001 |
| % NMD | 20.6±7.0 | - | - | - | - | 20.5±6.2 | 0.96 | ||
| Duan, 2011 [27] | Left BA | % FMD | 12±8 | - | - | - | - | 4±8 | 0.001 |
| % NMD | 29±12 | - | - | - | - | 23±10 | 0.075 | ||
| Ishikawa, 2013 [31] | Left BA | % FMD | 11.10 (10.1–13.9)|| | - | - | 9.1 (6.6–10.7)|| | <0.05 | 4.4 (2.6–5.7)|| | <0.01 |
| % NMD | 25.1±4.5 | - | - | 24.0±8.2 | NS | 21.7±5.0 | NS | ||
| Duan, 2014 [33] | Left BA | % FMD | 11.1 (8.8–16.4)|| | - | - | - | - | 4.2 (-1.71–8.16)|| | 0.001 |
| % NMD | 24.13 (18.62–30.86)|| | - | - | - | - | 24.4 (11.2–30.65)|| | 0.545 | ||
| Laurito, 2014 [35] | Right BA | % FMD | 9.54±1.8 | 9.38 ± 1.4 | 0.79 | 9.5±1.2 | - | 9.3±1.6 | - |
| PAT | |||||||||
| Pinto, 2013 [29] | Left and right index fingers | 2.31±0.53 | - | - | 1.67±0.49 | 0.001 | - | - | |
| Tobayama, 2013 [30] | 1 finger of each hand | 1.89±0.51 | 2.03 ± 0.44 | 0.19 | - | - | - | - | |
| Selamet Tierney, 2014 [32] | Left and right index fingers | 1.70±0.53 | 1.78 ± 0.46 | 0.35 | 1.79±0.44 | 0.55|| | 1.71±0.51 | 0.55|| | |
| SI | |||||||||
| Noto, 2001 [10] | Right CA | 2.94±0.91 | - | - | - | - | 4.11±0.86 | < 0.001 | |
| Cheung, 2004 [12] | Right CA | 4.24±0.86 | - | - | 4.27±0.83 | - | 5.07±1.11 | 0.001 | |
| Ikemoto, 2005 [15] | CA, side unclear | 2.47±0.79 | - | - | 2.54±0.9 | NS | 2.59±1.06 | NS | |
| Cheung, 2007 [19] | Right CA | 3.77±0.92 | - | - | 4.22±0.64 | - | 4.72±1.20 | 0.003 | |
| Cheung, 2008 [22] | Right CA | 3.39±0.76 | - | - | 3.79±1.00 | - | 4.38±1.10 | < 0.001 | |
| Liu, 2009 [23] | Right CA | 3.59±0.46 | - | - | 3.81±0.50 | 0.142 | 4.10±0.44 | <0.01 | |
| Gupta-Malhotra, 2009 [24] | Left and right CA | 1.93±0.48 | 1.9±0.1 | 0.912# | 2.0±0.7 | 0.912# | 1.8±0.3 | 0.912# | |
| Duan, 2011 [27] | Right CA | 2.8±0.6 | - | - | - | - | 3.6±0.8 | 0.001 | |
| Oguri, 2014 [36] | Right CA | 2.89±0.59 | 3.03±0.61 | 0.2 | - | - | - | - | |
| Duan, 2014 [33] | Right CA | 2.93 (2.67–3.15) | - | - | - | - | 3.12 (3.02–3.37) | 0.032 | |
| PWV | |||||||||
| Cheung, 2004 [13] | Right brachial-radial, m/s | 5.89±1.35 | - | - | 6.71±1.82 | 0.042 | 7.17±1.79 | 0.001 | |
| Cheung, 2004 [14] | Right brachial-radial, m/s | 6.2±1.5 | 7.2 ± 2.8 | 0.034 | - | - | - | - | |
| Cheung, 2007 [19] | Right brachial-radial, m/s | 5.77±1.25 | - | - | 6.73±1.88 | - | 7.22±1.67 | 0.009 | |
| Niboshi, 2008 [21] | L+R brachial-ankle, cm/s | 1103±139 | 1146 ± 147 | ¶ | - | - | - | - | |
| Lee, 2009 [25] | Left brachial-ankle, cm/s | 984.0±96.5 | - | - | - | - | 1020.6±146.5 | <0.05 | |
| Right brachial-ankle, cm/s | 976.3±93.5 | - | - | - | - | 979.7±154.8 | NS | ||
| Cho, 2014 [34] | L+R brachial-radial, cm/s** | 966.71±88.7 | - | - | 1076.86±164.10 | < 0.05 | 1181.50±7.78 | <0.05 | |
| cIMT | |||||||||
| Noto, 2001 [10] | Max RCCA, mm | 0.48±0.08 | - | - | - | - | 0.54±0.09 | <0.05 | |
| Ikemoto, 2005 [15] | Max RLCCA, mm | 0.5±0.04 | - | - | 0.47±0.06 | NS | 0.50±0.05 | NS | |
| Kadono, 2005 [16] | Max RCCA, mm | 0.46±0.06 | 0.45±0.07 | 0.77 | - | - | - | - | |
| Cheung, 2007 [19] | Mean RCCA, mm | 0.36±0.04 | - | - | 0.39±0.04 | 0.008 | 0.41±0.04 | <0.001 | |
| Cheung, 2008 [22] | Mean RCCA, mm | 0.42±0.016 | - | - | 0.42±0.019 | NS | 0.44±0.023 | 0.006 | |
| Gupta-Malhotra, 2009 [24] | Mean RLCCA, mm | 0.48±0.06 | 0.49±0.07 | 0.905# | 0.5±0.01 | 0.905# | 0.47±0.01 | 0.905# | |
| Lee, 2009 [25] | Max RCCA, mm | 0.50±0.01 | - | - | - | - | 0.41±0.19 | NS | |
| Noto, 2009 [26] | Max RCCA, mm | 0.46±0.05 | - | - | - | - | 0.57±0.15 | <0.001 | |
| Duan, 2011 [27] | Mean RCCA, mm | 0.44±0.04 | - | - | - | - | 0.49±0.07 | 0.025 | |
| Noto, 2012 [28] | Max, RCCA, mm | 0.42±0.04 | - | - | - | - | 0.54±0.08 | 0.005 | |
| Ishikawa, 2013 [31] | Mean RCCA, mm | 0.43±0.04 | - | - | 0.43±0.02 | NS | 0.45±0.03 | NS | |
| Selamet Tierney, 2013 [32] | Mean RCCA, mm | 0.432±0.029 | 0.428±0.024 | 0.35 | 0.429±0.02 | 0.41# | 0.426±0.024 †† | 0.41# | |
| Mean LCCA, mm | 0.434±0.028 | 0.438±0.034 | 0.42 | 0.439±0.04 | 0.05# | 0.439±0.035 †† | 0.05# | ||
| Mean LRCCA calculated | 0.433±0.028 | 0.433±0.030 | 0.434±0.032 | 0.433±0.031 | |||||
| Singh-Meena, 2014 [37] | Max LCCA, mm | 0.417±0.065 | 0.500±0.071 | <0.001 | - | - | - | - | |
| Laurito, 2014 [35] | Mean RLCCA, mm | 0.5±0.1 | 0.5±0.1 | 0.93 | 0.4±0.1 | - | 0.5±0.1 | - | |
| Oguri, 2014 [36] | Mean RCCA, mm | 0.39±0.04 | 0.40±0.03 | 0.15 | - | - | - | - | |
* p whole KD group vs controls
† p CAA-negative patients vs controls
‡ p CAA-positive patients vs controls
§ p< 0.0001 moderate and severe aneurysms vs controls
|| Median (IQR)
# based on ANOVA
¶ p<0.05 for male patients, p not significant for female patients
** in original article stated as m/s
†† z-score > 3.
NS, non-significant (as reported in original articles); BA, Brachial artery; cIMT; carotid intima-media thickness; FMD, flow-mediated dilatation; LCCA, Left common carotid artery; n-d; non-dominant; NMD, nitroglycerin-mediated dilation; PAT, peripheral arterial tonometry; PWV, pulse wave velocity; RCCA, Right common carotid artery; SI, stiffness index. Italic numbers are calculated using the data in the original articles
Quality of studies
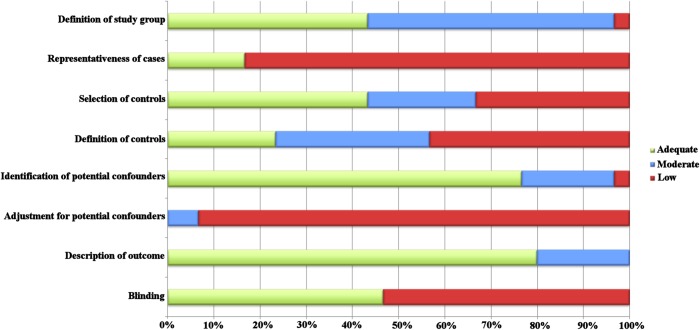
The results of the quality assessments are shown in Fig 2. All studies were found to have methodological limitations; the overall scores ranged from 4 to 11 (maximum of 16, S2 Table: Quality assessment per study). Most limitation arose from representativeness of cases, definition of controls and lack of adjustment for potential confounding factors.
Fig 2. Quality of studies.
Endothelial dysfunction
Flow-mediated dilation
A total of 15 studies reported on FMD (Table 2). Eleven studies showed a significantly decreased FMD in patients after KD as compared to controls, with a mean or median difference ranging from -9.7% to -2.7%. Four studies showed no statistical significant difference.
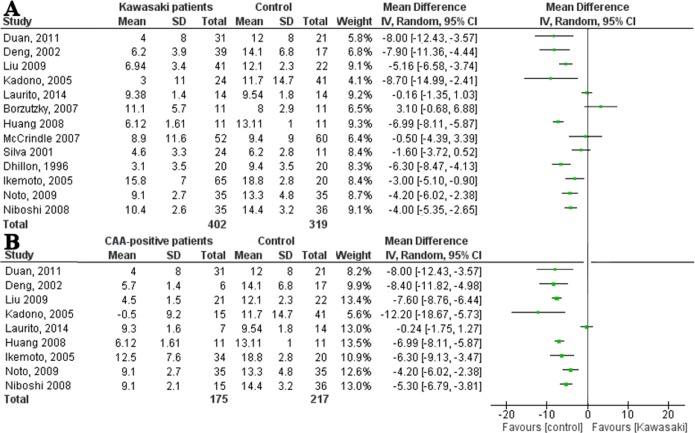
In meta-analyses, after excluding two studies reporting median instead of mean FMD, an extensive heterogeneity between the remaining 13 studies was found (I2 = 89%). Therefore, we did not pool the results (Fig 3A). Subsequently, heterogeneity was explored by sensitivity analyses and by means of meta-regression analysis. None of the three predefined covariables were shown to be of significant influence on heterogeneity.
Fig 3. Forest plots of difference in flow-mediated dilation between patients and controls.
(A) Forest plot: flow-mediated dilation of Kawasaki patients and controls, in order of ‘time since KD’. (B) Forest plot: flow-mediated dilation of CAA-positive patients and controls, in order of ‘time since KD’.
Flow-mediated dilation and CAA status
Seven studies compared CAA-negative patients to controls; four studies found a significantly decreased FMD, whereas three studies did not find any difference (Table 2).
CAA-positive patients were found to have a significantly decreased FMD compared to controls in 10 out of 11 studies. Two studies reported median FMD. When analyzing the nine studies describing mean FMD, a high heterogeneity was found (I2 = 89%, Fig 3B). If the small study by Laurito et al. [35], which included only seven patients with mild transient CAA, was excluded from the analyses, moderate heterogeneity was calculated (I2 = 59%). Combining the remaining eight studies, a statistically significant decreased FMD was observed in patients after KD (mean difference -6.06%, 95%CI: -7.76% to -5.47%).
Niboshi et al. found that, compared to patients without CAA, FMD was significantly decreased in patients with persisting CAA but not in patients with transient CAA [21]. Ikemoto et al found a significant negative correlation between the severity of the coronary artery lesion and FMD [15].
Nitro-glycerine-mediated dilation
In addition to FMD, seven studies measured NMD (Table 2). None of the studies found a significant difference between patients after KD and controls, neither when looking at the groups as a whole nor when looking at CAA-negative or CAA-positive patients.
Peripheral arterial tonometry
All three studies reporting PAT used the Endo-PAT device to measure the reactive hyperemia index (RHI) or Endo-PAT Index. Selamet Tierney et al., studying 203 patients, found no difference in Endo-PAT index between patients and controls [32]. A similar result was reported by Tobayama et al.[30]. Pinto et al. found a significantly lower RHI in their CAA-negative patients (Table 2) [29].
Vascular stiffness
Stiffness index
A total of 10 studies studied the SI of the carotid artery (Table 2). Seven studies found a significant increased SI in patients compared to controls, while three studies found no difference.
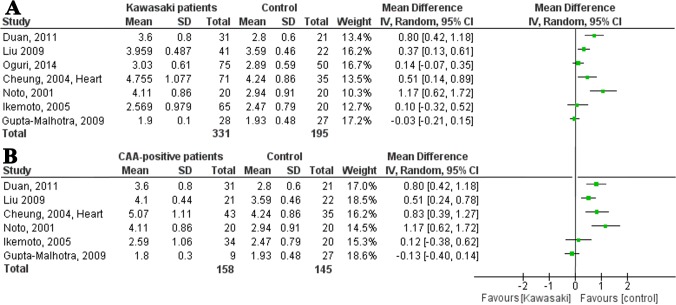
After excluding two studies because of overlapping inclusions in the meta-analysis [19,22] and one study because they reported the median [33], there was high heterogeneity between the remaining studies (I2 = 81%, Fig 4A).
Fig 4. Forest plots of difference in stiffness index between patients and controls.
(A) Forest plot: stiffness index of Kawasaki patients and controls, in order of ‘time since KD’. (B) Forest plot: stiffness index of CAA-positive patients and controls, in order of ‘time since KD’.
A meta-regression analysis showed an independent positive association between the percentage of CAA-positive patients and the mean difference in SI (p<0.0001), which may partly explain the high heterogeneity.
Stiffness index and CAA status
Five out of six studies reporting on CAA-negative patients did not find a significant difference in SI of patients compared to controls.
Nine studies studied CAA-positive patients. Seven studies found a significant difference in SI between CAA-positive patients and controls, while two studies did not. Two studies were not included in the meta-analysis because of overlapping inclusions [19,22] and one because it measured the median instead of mean[33]. High heterogeneity was found between the remaining six studies (I2 = 88%; Fig 4B). After excluding the small study of Gupta-Malhotra et al.[24] with only nine patients with early transient CAA, heterogeneity decreased to 60%. When pooling the data of the remaining studies, a history of CAA was associated with a significantly increased SI of 0.67 (95% CI 0.38–0.96).
Pulse wave velocity
PWV in patients after KD was reported in six studies. Cheung et al. performed three of these, all showing an increased brachial-radial PWV in CAA-positive and CAA-negative patients compared to controls [13,14,19]. These results were similar to the results of Cho et al.[34].
Two studies measured brachial-ankle PWV (baPWV). Lee et al. found an increased PWV in their CAA-positive patients compared to controls [25]. Of note, their patient group was significantly younger than the control group, although one would expect the difference to be greater as PWV increases with age. Niboshi et al. found a significantly faster PWV in adult male but not in female KD patients as compared to controls [21].
Carotid intima-media thickness
A total of 15 studies reported on cIMT in patients after KD (Table 2). The studies reported the cIMT of the right common carotid artery (CCA), the left CCA or the mean of both. Mean cIMT was reported in eight, while maximum cIMT was reported in seven studies.
Seven studies reported a significantly increased cIMT, seven studies showed no significant difference and one study showed a decreased cIMT as compared to controls.
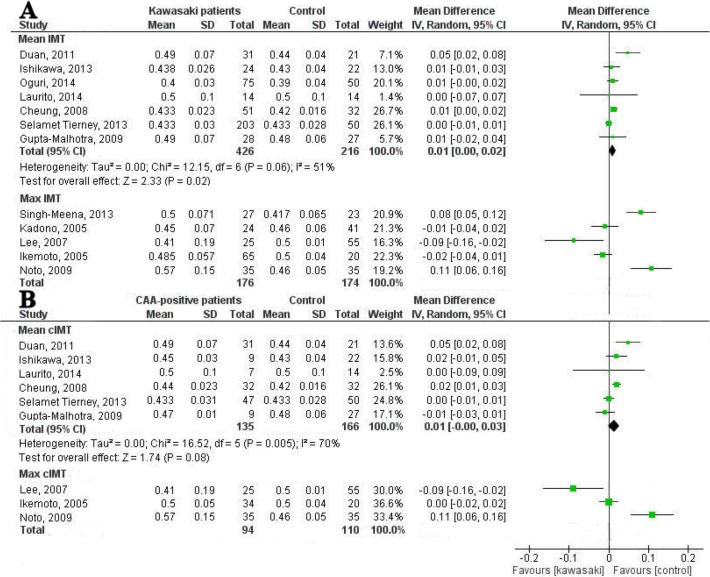
In the meta-analysis, studies measuring mean cIMT and maximum cIMT were analyzed separately (Fig 5A and 5B). When analyzing the studies measuring mean cIMT, one study was excluded because of overlapping inclusions [19]. We found moderate heterogeneity in the remaining seven studies (I2 = 51%). When pooling the data of these studies, a mean difference of 0.01 mm (95% CI 0.00 to 0.02 mm) was found between patients and controls (Fig 5A). It was remarkable that in the two studies showing a thinner cIMT in KD patients, the control group was or seemed to be significantly older [16,25].
Fig 5. Forest plots of difference in carotid intima-media thickness between patients and controls.
(A) Forest plot: Carotid intima-media thickness of Kawasaki patients and controls, in order of ‘time since KD’. (B) Forest plot: Carotid intima-media thickness of CAA-positive patients and controls, in order of ‘time since KD’.
In meta-regression analyses none of the three predefined covariables was of significant influence on the heterogeneity.
Two studies measuring maximum cIMT were not included in the forest plot because of overlapping inclusions (Fig 5A) [10,28]. We did not combine the data of the five remaining studies because of the limited number of studies and the high heterogeneity and conflicting results (I2 = 90%).
Carotid intima-media thickness and CAA-status
Seven studies described CAA-negative patients and only one found an increased cIMT in patients compared to controls (Table 2).
Of the twelve studies measuring cIMT in CAA-positive patients, six reported a significantly increased cIMT and one showed a decreased cIMT (Table 2). One of the seven studies measuring mean cIMT was not included in the forest plot because of overlapping inclusions. The remaining six studies showed a moderate heterogeneity (I2 = 70%; Fig 5B). When pooling the data of these studies, a mean difference of 0.01 mm (95% CI 0.00 to 0.03 mm) was found between patients and controls (Fig 5B).
Maximum cIMT of CAA-positive patients was measured in five studies of which two were not included in the meta-analysis because of overlapping inclusions. As is shown in Fig 5B, the remaining studies had conflicting results.
Two studies divided their CAA-positive patients in subgroups according to diameter or z-score [15,32]. Ikemoto et al. did not find any difference between patients with mild, moderate and giant aneurysms, although these groups consisted of few patients only (16, 8 and 10 patients respectively). Selamet Tierney et al. found a significantly thicker left cIMT in patients with a history of giant aneurysms, whereas patients with a history of ectasia, small or medium CAA did not show this phenomenon.
Discussion
Our systematic review summarizes 30 studies on surrogate markers for CVD risk in patients after KD compared to unaffected controls. FMD and SI were increased in most studies, being more pronounced in CAA-positive patients. Mean CIMT in the whole KD-group and the CAA-positive group did not seem to be increased while data on maximum cIMT were inconclusive. The results of this review have to be interpreted with care due to methodological limitations and substantial heterogeneity between studies.
Quality of studies
Most studies had important methodological limitations. First, CVD risk is dependent on many factors. It has been shown that cIMT and FMD are dependent on life-style factors such as social-economic status and physical activity [40,41]. Therefore, a suitable control group is vital, which many studies failed to include or describe. Secondly, factors such as age, gender, blood pressure and BMI are known to influence surrogate markers [42]. These variables should be identified and adjusted for in the final outcome measurement. None of the studies adjusted for these factors. Moreover, most surrogate markers are highly dependent on the ultrasonographist(s) and/or interpreter(s) of the images. Hence, blinding is required but this was not described in 16 out of the 30 studies. Finally, many studies included a very limited number of patients. Only three studies included ≥50 participants for both groups.
Heterogeneity of studies
Substantial heterogeneity existed between studies. In addition to the methodological limitations, the study populations varied in ‘time since KD’, ‘number of CAA-positive patients’, ‘percentage of IVIG-treated patients’, ‘gender distribution’, ‘age’, ‘ethnicity’ and ‘CAA-criteria’. When exploring heterogeneity by analyzing the first three variables, we could only find a significant covariate for the meta-analyses on SI. It was however, difficult to define some of these variables: the percentage of CAA-positive patients does not necessarily correlate to the severity of the aneurysms; some studies only included patients with transient dilations, while others included patients with severe or persistent aneurysms.
Endothelial (dys)function
FMD in correlation with CVD risk has been researched extensively. Ras et al. found a CVD risk ratio of 0.9 per 1% higher FMD in a systematic review in adults [43]. In children, a significantly lower FMD has been found in sub-populations with an increased cardiovascular risk such as familial hypercholesterolemia [44].
FMD is an endothelium-dependent marker, which is mediated by the release of nitric oxide (NO) [45]. In contrast to FMD, NMD is an endothelium-independent marker, thought to reflect smooth muscle (dys)function. It has shown to be increased in diabetes mellitus and hypertension and is suggested to be a marker of the grade of cardiovascular risk[46]. NMD was not increased in any of the studies in this review, suggesting that the patients are at-risk but the endothelial dysfunction is at an early stage when smooth muscle function is not (yet) affected.
PAT is thought to correlate with coronary endothelial dysfunction. Studies in adults and children have shown a correlation between a lower PAT and coronary atherosclerosis and cardiovascular events or risk factors [47,48]. An earlier, large cohort study did not show correlation between FMD and PAT, indicating that they might reflect distinct aspects of endothelial function and possibly explaining the difference in PAT and FMD in our review [49].
Arterial stiffness
Aortic PWV is a known predictor of cardiovascular events [50]. In contrast, studies included in this review measured brachial-radial PWV or baPWV. Earlier studies found a significant correlation between baPWV and cardiovascular events or risk factors, but large prognostic studies are missing [51,52]. Brachioradial PWV is less common in use and to our knowledge, no large studies looking at the association between brachioradial PWV and cardiovascular events have been performed.
SI has shown to be increased in children with obesity and in adults after myocardial infarction, although no large studies have investigated the exact correlation between CVD event and SI [53,54].
Carotid IMT
CIMT is a validated measure of cardiovascular risk. Lorenz et al. found a hazard ratio (HR) of 1.15 for myocardial infarction (MI) and 1.18 for stroke with every 0.1 mm increase in cIMT in their systematic review [6]. In addition, they showed that people <50 years of age are at higher relative risk with increasing cIMT compared to people >50 years [55]. Also, hazards increase significantly faster for cIMT values of 0.6–1 mm compared to higher IMT values [56].
Although cIMT is validated, it is important to realize that a distance of 0.5 mm is measured using a device with an axial resolution of around 0.04–0.05 mm, implicating a large standard deviation by default, hence not suitable for research in small groups. In our review, 13 out of the 15 studies measuring cIMT included less than 50 participants per group.
Cardiovascular disease risk
Even though most surrogate markers for CVD risk showed a significant difference between one of the KD-groups and controls in most studies, the pathophysiological mechanism behind these changes following KD is still unclear. In fact, post-mortem studies have failed to show atherosclerotic changes, even in affected coronary arteries, [57]. In this post-mortem study, active remodeling of the coronary arterial wall could be identified years after the acute stage of the disease, potentially indicating that a distinct cardiovascular process, other than atherosclerosis, may be held responsible for an increased CVD risk following the early period of acute vascular inflammation in KD. Prolonged (low-grade) inflammation as suggested by the presence of increased levels of inflammatory markers such as (high-sensitivity) CRP are believed to be associated with the occurrence of cardiovascular events in adults [58,59]. However, controversy exists as to whether patient with KD have a continued low-grade inflammation years after the disease [12,21,24,32].
A pathophysiological mechanism responsible for the changes in the vasculature in KD, both in the coronary and the peripheral arteries, has yet to be elucidated. Whether persistent low-grade inflammation or genetic factors may play a role in this “KD-vasculopathy” and in remodelling of the arterial wall is as yet unclear.
Limitations
Some limitations of our study have to be mentioned. First, we found substantial heterogeneity between studies. Because of this heterogeneity, we could not pool most of the results from the original studies. Hence, conclusions can only be drawn from a summary of these studies without a statistical finding.
Although we tried to find the source of heterogeneity by performing meta-regression analyses, we could not find factors for all surrogate markers. For both cIMT and SI, we could include less than 10 studies in the meta-regression analyses; it is questionable whether such numbers are large enough because of a lack of power.
For this review we considered patients who ever had CAA as CAA-positive. However, CAA range from small to giant, reflecting the severity of the original vasculitis and it may thus not be appropriate to combine all CAA-positive patients into one group.
None of the included studies reported on long-term longitudinal data as they were all cross-sectional or very short-term cohort studies. Long-term follow-up is necessary to investigate the course of the surrogate markers over time as well as the natural course of the disease and to predict CVD risk at a later age.
Conclusion
This systematic review and meta-analyses suggests that surrogate markers for CVD risk in patients after KD are increased in CAA-positive but not in CAA-negative patients. The results have to be interpreted with care due to methodological limitations and high heterogeneity between studies which prevents the possibility of data pooling. However, these findings might indicate that CAA-positive patients should be monitored and counselled for CVD in later life. Long-term follow-up of former KD patients is needed to confirm our results.
Supporting Information
(DOC)
(DOC)
(DOCX)
(DOCX)
Acknowledgments
We thank Arnold Leenders, clinical librarian, for his help with conducting the search in the different databases, and Junfeng Wang for his help with the translation of one article.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Stinafo Foundation (The Hague, The Netherlands). The sponsor had no role in the study design, the data collection and analysis, the writing of the report, or the decision to submit the manuscript for publication.
References
- 1. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110: 2747–71. [DOI] [PubMed] [Google Scholar]
- 2. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39: 257–65. [DOI] [PubMed] [Google Scholar]
- 3. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146: 168–74. [DOI] [PubMed] [Google Scholar]
- 4. O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15: 426–44. [DOI] [PubMed] [Google Scholar]
- 5. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34: 290–6. 10.1159/000343145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115: 459–67. [DOI] [PubMed] [Google Scholar]
- 7. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7: 177–88. [DOI] [PubMed] [Google Scholar]
- 8. Dhillon R, Clarkson P, Donald AE, Powe AJ, Nash M, Novelli V, et al. Endothelial dysfunction late after Kawasaki disease. Circulation. 1996;94: 2103–6. [DOI] [PubMed] [Google Scholar]
- 9. Silva AA, Maeno Y, Hashmi A, Smallhorn JF, Silverman ED, McCrindle BW. Cardiovascular risk factors after Kawasaki disease: a case-control study. J Pediatr. 2001;138: 400–5. [DOI] [PubMed] [Google Scholar]
- 10. Noto N, Okada T, Yamasuge M, Taniguchi K, Karasawa K, Ayusawa M, et al. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesions. Pediatrics. 2001;107: 1095–9. [DOI] [PubMed] [Google Scholar]
- 11. Deng YB, Xiang HJ, Chang Q, Li CL. Evaluation by high-resolution ultrasonography of endothelial function in brachial artery after Kawasaki disease and the effects of intravenous administration of vitamin C. Circ J. 2002;66: 908–12. [DOI] [PubMed] [Google Scholar]
- 12. Cheung YF, Ho MH, Tam SC, Yung TC. Increased high sensitivity C reactive protein concentrations and increased arterial stiffness in children with a history of Kawasaki disease. Heart. 2004;90: 1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheung YF, Yung TC, Tam SC, Ho MH, Chau AK. Novel and traditional cardiovascular risk factors in children after Kawasaki disease: implications for premature atherosclerosis. J Am Coll Cardiol. 2004;43: 120–4. [DOI] [PubMed] [Google Scholar]
- 14. Cheung YF, Ho MH, Ip WK, Fok SF, Yung TC, Lau YL. Modulating effects of mannose binding lectin genotype on arterial stiffness in children after Kawasaki disease. Pediatr Res. 2004;56: 591–6. [DOI] [PubMed] [Google Scholar]
- 15. Ikemoto Y, Ogino H, Teraguchi M, Kobayashi Y. Evaluation of preclinical atherosclerosis by flow-mediated dilatation of the brachial artery and carotid artery analysis in patients with a history of Kawasaki disease. Pediatr Cardiol. 2005;26: 782–6. [DOI] [PubMed] [Google Scholar]
- 16. Kadono T, Sugiyama H, Hoshiai M, Osada M, Tan T, Naitoh A, et al. Endothelial function evaluated by flow-mediated dilatation in pediatric vascular disease. Pediatr Cardiol. 2005;26: 385–90. [DOI] [PubMed] [Google Scholar]
- 17. Borzutzky A, Gutierrez M, Talesnik E, Godoy I, Kraus J, Hoyos R, et al. High sensitivity C-reactive protein and endothelial function in Chilean patients with history of Kawasaki disease. Clin Rheumatol. 2008;27: 845–50. [DOI] [PubMed] [Google Scholar]
- 18. McCrindle BW, McIntyre S, Kim C, Lin T, Adeli K. Are patients after Kawasaki disease at increased risk for accelerated atherosclerosis? J Pediatr. 2007;151: 244–8. [DOI] [PubMed] [Google Scholar]
- 19. Cheung YF, Wong SJ, Ho MH. Relationship between carotid intima-media thickness and arterial stiffness in children after Kawasaki disease. Arch Dis Child. 2007;92: 43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang SM, Weng KP, Chang JS, Lee WY, Huang SH, Hsieh KS. Effects of statin therapy in children complicated with coronary arterial abnormality late after Kawasaki disease: a pilot study. Circ J. 2008;72: 1583–7. [DOI] [PubMed] [Google Scholar]
- 21. Niboshi A, Hamaoka K, Sakata K, Yamaguchi N. Endothelial dysfunction in adult patients with a history of Kawasaki disease. Eur J Pediatr. 2008;167: 189–96. [DOI] [PubMed] [Google Scholar]
- 22. Cheung YF O K, Woo CW, Armstrong S, Siow YL, Chow PC, et al. Oxidative stress in children late after Kawasaki disease: relationship with carotid atherosclerosis and stiffness. BMC Pediatr. 2008;8: 20 10.1186/1471-2431-8-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu XQ, Huang GY, Liang XV, Ma XJ. Endothelial progenitor cells and arterial functions in the late convalescence period of Kawasaki disease. Acta Paediatr. 2009;98: 1355–9. 10.1111/j.1651-2227.2009.01334.x [DOI] [PubMed] [Google Scholar]
- 24. Gupta-Malhotra M, Gruber D, Abraham SS, Roman MJ, Zabriskie JB, Hudgins LC, et al. Atherosclerosis in survivors of Kawasaki disease. J Pediatr. 2009;155: 572–7. 10.1016/j.jpeds.2009.04.054 [DOI] [PubMed] [Google Scholar]
- 25. Lee SJ, Ahn HM, You JH, Hong YM. Carotid intima-media thickness and pulse wave velocity after recovery from kawasaki disease. Korean Circ J. 2009;39: 264–9. 10.4070/kcj.2009.39.7.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noto N, Okada T, Karasawa K, Ayusawa M, Sumitomo N, Harada K, et al. Age-related acceleration of endothelial dysfunction and subclinical atherosclerosis in subjects with coronary artery lesions after Kawasaki disease. Pediatr Cardiol. 2009;30: 262–8. 10.1007/s00246-008-9329-6 [DOI] [PubMed] [Google Scholar]
- 27. Duan C, Du ZD, Wang Y, Jia LQ. [Late endothelial function in children with coronary aneurysm due to Kawasaki disease]. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13: 373–6. [PubMed] [Google Scholar]
- 28. Noto N, Okada T, Abe Y, Miyashita M, Kanamaru H, Karasawa K, et al. Characteristics of earlier atherosclerotic involvement in adolescent patients with Kawasaki disease and coronary artery lesions: significance of gray scale median on B-mode ultrasound. Atherosclerosis. 2012;222: 106–9. 10.1016/j.atherosclerosis.2012.01.049 [DOI] [PubMed] [Google Scholar]
- 29. Pinto FF, Laranjo S, Parames F, Freitas I, Mota-Carmo M. Long-term evaluation of endothelial function in Kawasaki disease patients. Cardiol Young. 2013;23: 517–22. 10.1017/S1047951112001357 [DOI] [PubMed] [Google Scholar]
- 30. Tobayama H, Takahashi K, Fukunaga H, Matsui K, Tanaka N, Harada M, et al. Analysis of arterial function in adults with a history of Kawasaki disease. J Cardiol. 2013;61: 330–5. 10.1016/j.jjcc.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 31. Ishikawa T, Iwashima S. Endothelial dysfunction in children within 5 years after onset of Kawasaki disease. J Pediatr. 2013;163: 1117–21. 10.1016/j.jpeds.2013.04.046 [DOI] [PubMed] [Google Scholar]
- 32. Selamet Tierney ES, Gal D, Gauvreau K, Baker AL, Trevey S, O'Neill SR, et al. Vascular health in Kawasaki disease. J Am Coll Cardiol. 2013;62: 1114–21. 10.1016/j.jacc.2013.04.090 [DOI] [PubMed] [Google Scholar]
- 33. Duan C, Du ZD, Wang Y, Jia LQ. Effect of pravastatin on endothelial dysfunction in children with medium to giant coronary aneurysms due to Kawasaki disease. World J Pediatr. 2014;10: 323–237. [DOI] [PubMed] [Google Scholar]
- 34. Cho HJ, Yang SI, Kim KH, Kim JN, Kil HR. Cardiovascular risk factors of early atherosclerosis in school-aged children after Kawasaki disease. Korean J Pediatr. 2014;57: 217–21. 10.3345/kjp.2014.57.5.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laurito M, Stazi A, Delogu AB, Milo M, Battipaglia I, Scalone G, et al. Endothelial and platelet function in children with previous Kawasaki disease. Angiology. 2014;65: 716–22. 10.1177/0003319713502392 [DOI] [PubMed] [Google Scholar]
- 36. Oguri M, Nakamura T, Tamanuki K, Akita C, Kitaoka C, Saikawa Y, et al. Subclinical arterial stiffness in young children after Kawasaki disease. Cardiol Young. 2014;24: 87–94. 10.1017/S1047951112002302 [DOI] [PubMed] [Google Scholar]
- 37. Meena RS, Rohit M, Gupta A, Singh S. Carotid intima-media thickness in children with Kawasaki disease. Rheumatol Int. 2014;34: 1117–21. 10.1007/s00296-013-2820-2 [DOI] [PubMed] [Google Scholar]
- 38. Group JCSJW. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2008)—digest version. Circ J. 2010;74: 1989–2020. [DOI] [PubMed] [Google Scholar]
- 39. Manlhiot C, Millar K, Golding F, McCrindle BW. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol. 2010;31: 242–9. 10.1007/s00246-009-9599-7 [DOI] [PubMed] [Google Scholar]
- 40. Lamotte C, Iliescu C, Beghin L, Salleron J, Gonzalez-Gross M, Marcos A, et al. Association of socioeconomic status, truncal fat and sICAM-1 with carotid intima-media thickness in adolescents: the HELENA study. Atherosclerosis. 2013;228: 460–5. 10.1016/j.atherosclerosis.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 41. Pahkala K, Heinonen OJ, Simell O, Viikari JS, Ronnemaa T, Niinikoski H, et al. Association of physical activity with vascular endothelial function and intima-media thickness. Circulation. 2011;124: 1956–63. 10.1161/CIRCULATIONAHA.111.043851 [DOI] [PubMed] [Google Scholar]
- 42. Dawson JD, Sonka M, Blecha MB, Lin W, Davis PH. Risk factors associated with aortic and carotid intima-media thickness in adolescents and young adults: the Muscatine Offspring Study. J Am Coll Cardiol. 2009;53: 2273–9. 10.1016/j.jacc.2009.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168: 344–51. 10.1016/j.ijcard.2012.09.047 [DOI] [PubMed] [Google Scholar]
- 44. de Jongh S, Lilien MR, op't Roodt J, Stroes ES, Bakker HD, Kastelein JJ. Early statin therapy restores endothelial function in children with familial hypercholesterolemia. J Am Coll Cardiol. 2002;40: 2117–21. [DOI] [PubMed] [Google Scholar]
- 45. Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension. 2014;63: 376–82. 10.1161/HYPERTENSIONAHA.113.02044 [DOI] [PubMed] [Google Scholar]
- 46. Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, et al. Nitroglycerine-induced vasodilation for assessment of vascular function: a comparison with flow-mediated vasodilation. Arterioscler Thromb Vasc Biol. 2013;33: 1401–8. 10.1161/ATVBAHA.112.300934 [DOI] [PubMed] [Google Scholar]
- 47. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31: 1142–8. 10.1093/eurheartj/ehq010 [DOI] [PubMed] [Google Scholar]
- 48. Hedetoft M, Olsen NV. Evaluation of endothelial function by peripheral arterial tonometry and relation with the nitric oxide pathway. Nitric Oxide. 2014;42C: 1–8. [DOI] [PubMed] [Google Scholar]
- 49. Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57: 390–6. 10.1161/HYPERTENSIONAHA.110.160812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55: 1318–27. 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- 51. Han JY, Choi DH, Choi SW, Kim BB, Ki YJ, Chung JW, et al. Predictive value of brachial-ankle pulse wave velocity for cardiovascular events. Am J Med Sci. 2013;346: 92–7. 10.1097/MAJ.0b013e318268c05a [DOI] [PubMed] [Google Scholar]
- 52. Im JA, Lee JW, Shim JY, Lee HR, Lee DC. Association between brachial-ankle pulse wave velocity and cardiovascular risk factors in healthy adolescents. J Pediatr. 2007;150: 247–51. [DOI] [PubMed] [Google Scholar]
- 53. Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989;80: 78–86. [DOI] [PubMed] [Google Scholar]
- 54. Nunez F, Martinez-Costa C, Sanchez-Zahonero J, Morata J, Chorro FJ, Brines J. Carotid artery stiffness as an early marker of vascular lesions in children and adolescents with cardiovascular risk factors. Rev Esp Cardiol. 2010;63: 1253–60. [DOI] [PubMed] [Google Scholar]
- 55. Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke. 2006;37: 87–92. [DOI] [PubMed] [Google Scholar]
- 56. Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146: 483–94. [DOI] [PubMed] [Google Scholar]
- 57. Suzuki A, Miyagawa-Tomita S, Komatsu K, Nishikawa T, Sakomura Y, Horie T, et al. Active remodeling of the coronary arterial lesions in the late phase of Kawasaki disease: immunohistochemical study. Circulation. 2000;101: 2935–41. [DOI] [PubMed] [Google Scholar]
- 58. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342: 836–43. [DOI] [PubMed] [Google Scholar]
- 59. Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151: 483–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.