Abstract
Introduction
The pattern and duration of breastfeeding (BF) and the age at onset of complementary feeding, as well as its quality, have been associated with the prevalence of overweight in childhood.
Objective
To assess the effect of a pro-BF and healthy complementary feeding intervention, targeted to adolescent mothers and maternal grandmothers, on growth and prevalence of overweight and obesity in children at preschool age. This intervention had a positive impact on duration of BF and timing of onset of complementary feeding.
Methods
This randomized clinical trial involved 323 adolescent mothers, their infants, and the infants’ maternal grandmothers, when they cohabited. Mothers and grandmothers in the intervention group received counseling sessions on BF and healthy complementary feeding at the maternity ward and at home (7, 15, 30, 60, and 120 days after delivery). When children were aged 4 to 7 years, they underwent anthropometric assessment and collection of data on dietary habits. Multivariable Poisson regression with robust estimation was used for analysis.
Results
BMI-for-age and height-for-age were similar in the intervention and control groups, as was the prevalence of overweight (39% vs. 31% respectively; p=0.318). There were no significant between-group differences in dietary habits.
Conclusion
Although the intervention prolonged the duration of exclusive BF and delayed the onset of complementary feeding, it had no impact on growth or prevalence of overweight at age 4 to 7 years.
Trial Registration
ClinicalTrials.gov NCT00910377
Introduction
Human dietary habits are undergoing significant changes, with increased intake of industrialized products, a reduction in fresh fruit and vegetable consumption, and more frequent meals outside the home. These changes have led to an inversion in the nutritional pattern of the population, i.e., the nutrition transition. Currently, the increasing prevalence of overweight in many parts of the world, including in children and adolescents, is a big concern [1–3]. According to the World Health Organization (WHO), the prevalence of obesity in under-fives had increased from 4.2% in 1990 to 6.7% in 2010. If the trend continues, this prevalence is expected to rise to 9.1% by 2020 –a relative increase of 36% from 2010 levels [4].
In Brazil, the prevalence of obesity in under-fives is 7.3% [5]. In the 5-to-9 age range, the prevalence of overweight and obesity is 33.5% and 14.3% respectively. The prevalence of overweight among boys more than doubled between 1989 and 2009, from 15% to 34.8%, and that of obesity rose even more markedly in the same period, from 4.1% in 1989 to 16.6% in 2008–2009. The prevalence of overweight and obesity in girls, in turn, rose from 11.9% to 32% and from 2.4% to 11.8% respectively, over the same period [6].
Overweight in childhood is a result of multiple factors [7–9], including absence or short duration of breastfeeding (BF) and exclusive BF (EBF) [10–16], early introduction of complementary foods, and inadequate dietary practices [17–21].
Despite the recommendation of the World Health Organization—EBF in the first six months of life and maintenance of BF for 2 years or more [22]-, in Brazil, the prevalence of EBF in infants under 6 months is low, particularly in children of adolescent mothers (35.8%); the duration of BF is less than one year (median = 11.2 months); and 21% of infants aged 4–6 months eat salty foods, including cereal grains, vegetables, meats and eggs, and 24% eat fruit regularly [23]. Moreover, inadequate dietary practices are common in under-fives [24]. This situation prompted the development of a pro-BF and healthy complementary feeding intervention geared to adolescent mothers and their own mothers (i.e., the infants’ maternal grandmothers), when they cohabited. Grandmothers were included in the intervention due to their potential for negative influence on child feeding practices [25–30]. This intervention, which was tested through a randomized controlled trial, proved to be effective in prolonging the duration of EBF [31] and increasing the prevalence of BF in the first year of life [32], and had a positive impact against early introduction of complementary feeding [33]. In view of the positive results of this intervention and presuming that mode and duration of BF and timing of onset of complementary feeding can influence the future nutritional status of children [10–20], the present study sought to assess the medium-term impact of the same intervention on child growth and prevalence of overweight at age 4–7 years.
Methods
This randomized clinical trial enrolled 323 adolescent mothers, their infants, and, when living in the same household, their own mothers (that is, the maternal grandmothers of the infants) from May 2006 to January 2008.
All participants were recruited from the rooming-in facility of Hospital de Clínicas de Porto Alegre (HCPA). HCPA is a public general hospital in Porto Alegre, Brazil, and a Baby-friendly Hospital accredited facility where approximately 3,000 deliveries take place per year. On a daily basis, investigators identified all mothers who met the inclusion criteria: age younger than 20 years, lived within Porto Alegre municipal limits, had given birth to a healthy singleton infant with a birth weight of 2,500 g or greater, and had begun BF. Mothers of multiple infants, those who could not room in with their infants due to maternal or neonatal complications, and those who lived with their mothers-in-law (i.e., the child’s paternal grandmother) were excluded from the study. Once identified, adolescent mothers were randomly allocated in blocks of two into the control or intervention groups, i.e., if one mother was randomly allocated to the intervention group, the next eligible mother was automatically allocated to the control group. To ensure the estimated required number of adolescents living with their mothers, it was predetermined that adolescent mothers cohabiting with their own mothers would compose half of the study sample.
Intervention sessions took place in the maternity ward and at each mother’s household, at 7, 15, 30, 60, and 120 days post-delivery. The first session always took place in the maternity ward, 24 to 72 hours after delivery, and consisted of a pro-BF counseling intervention using the communication skills advocated by WHO [34]. In the no-cohabitation group, adolescent mothers alone received the intervention. In the cohabitation group, both mother and grandmother received initial counseling; the initial session was held separately for mothers and grandmothers, on a one-on-one basis. The sessions were led by members of a team composed of two nurses, a dietitian, and a pediatrician, three of whom were International Board Certified Lactation Consultants (IBCLCs). During the first session, the consultant and the mother or grandmother had an informal conversation about several aspects related to BF, with an emphasis on EBF. Supporting material for sessions included booklets and flipcharts designed specifically for the study intervention. All mothers, regardless of group allocation, received standard care as provided at the maternity ward.
When mothers and grandmothers lived in the same household, joint counseling sessions were held. These sessions were used to reinforce messages originally conveyed during initial counseling and to discuss any challenges related to child feeding. The sessions held at 120 days placed emphasis on the introduction of healthy complementary feeding starting at age 6 months, as advocated by the guidelines provided in the Guia de Alimentação para Crianças Brasileiras Menores de 2 Anos [35]. During this session, participants were also given brochures with guidance on healthy and timely introduction of complementary feeding.
Data were collected at several time points. At the maternity ward, after agreeing to take part in the study and providing written informed consent, signed by the guardians/caretakers, adolescent mothers and their own mothers (when they cohabited) were interviewed separately to collect sociodemographic data and information on prenatal care, delivery, and prior experience with BF. Data on child feeding during the first year of life were obtained once monthly during the first 6 months and every two months thereafter until 12 months, by means of telephone interviews with the mother or house visits when telephone contact could not be established. Final assessment took place from September 2012 to July 2013, when children were aged 4 to 7 years, at the HCPA Clinical Research Center (or at home when mothers and children failed to attend the center). At this moment, after providing an updated written informed consent, mothers were interviewed to obtain information on current sociodemographic characteristics and child feeding, and duration of breastfeeding. The children were weighed and measured. For the assessment of the dietary intake, we used a not validated food frequency questionnaire, created especially for the study, containing all the food customarily consumed by the studied population, such as vegetables, cereal grains, leguminous, meats, eggs and dairy products.
The consumption of these foods was evaluated in weekly frequency from none to more than five days a week.
For anthropometric assessment, two weight and height measurements were obtained from each child, using the techniques recommended by the Brazilian Ministry of Health [36]. For classification of children by BMI-for-age and height-for-age, the WHO reference populations and cutoff points were used as a standard [37–41]. Data collection and anthropometric assessment were always performed by investigators blinded to group allocation.
Since the original clinical trial was planned to evaluate another question (rates of EBF and BF in the first year of life), we calculated the effect size that can be detected with the sample available at the follow up assessment (n = 207), considering the new question. Thus, estimating a prevalence of overweight of 30% in children aged 4–7 years not exposed to the intervention group, this sample size is sufficient to detect a difference of 20% or more in overweight prevalence among the exposed and unexposed intervention, adopting α = 5% and β = 20%.
All statistical analyses were carried out in SPSS 21.0 for Windows, using the intention-to-treat principle.
Initially, we compared the characteristics of children who were lost to follow-up to those who completed the trial. We then compared the characteristics of the control and intervention groups. The Student t or Mann–Whitney U tests were used as appropriate for comparison of means, and the Pearson chi-square or Fisher’s exact tests for comparison of proportions. As a result of losses to follow-up, an imbalance between the control and intervention groups was detected for some variables. To make up for this heterogeneity, we used multivariable Poisson regression model with robust variance. We first tested the unadjusted model, and then constructed a series of cumulative models (by sequential addition of new variables) as a result of comparative analysis between groups. The sequential model included those variables with a p-value <0.20, in addition to the propensity score [42, 43]. The propensity scores were estimated using logistic regression, modeling the probability of an individual being allocated to the intervention group and considering the following predictors: maternal age, educational attainment, skin color, and parity; infant weight and mode of delivery; and parental cohabitation. The significance level was set at 5% (p≤0.05).
The study was approved by the HCPA Research Ethics Committee and by Plataforma Brasil (no. 120249), and registered at ClinicalTrials.gov with accession number NCT00910377. The authors confirm that all ongoing and related trials for this drug/intervention are registered.
Results
Fig 1 shows a flow diagram of the study from recruitment to final assessment, which took place when children were aged 4 to 7 years. Of the 323 mothers/children who started the trial, 207 (64.1%) were located and took part in final assessment.
Fig 1. Flow chart of the randomized clinical trial phases from sample selection to the latest assessment, at 4–7 years of age.
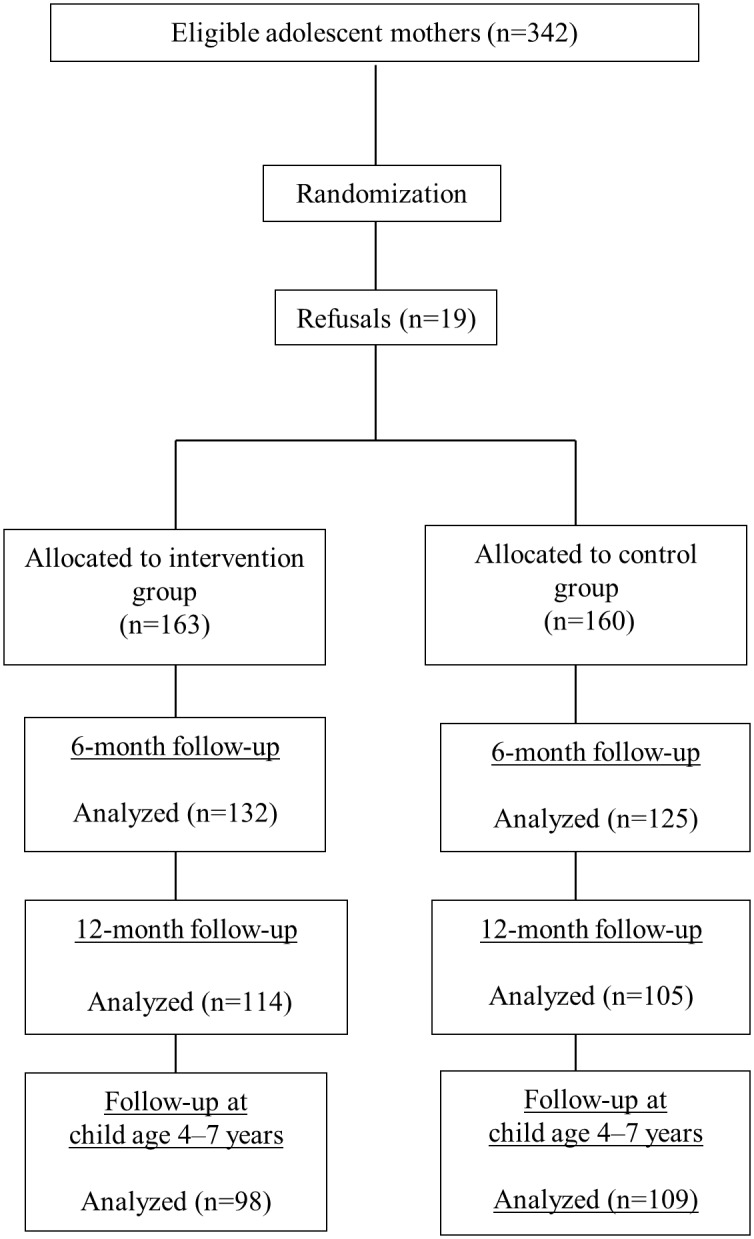
Table 1 shows that loss to follow-up occurred predominantly in the intervention and no-cohabitation groups, although the differences were not significant. Nevertheless, the intervention and control groups were imbalanced in terms of proportion of cohabitation, current age of the child, and maternal educational attainment (Table 2). The intervention group had a greater proportion of adolescent mothers who lived with their own mothers during the intervention period, a greater proportion of adolescent mothers with ≥8 years of formal schooling and a lower mean child age at final assessment.
Table 1. Characteristics of participants who completed the study and of those lost to follow-up.
| Variable | Completers (n = 207) | Lost to follow-up (n = 116) | P |
|---|---|---|---|
| Group—n (%) | 0.167 | ||
| Intervention | 98 (47.3) | 65 (56.0) | |
| Control | 109 (52.7) | 51 (44.0) | |
| Maternal age (years)—mean ± SD | 17.5 ± 1.5 | 17.5 ± 1.5 | 0.968 |
| Maternal educational attainment, ≥ 8 years—n (%) | 110 (53.1) | 60 (51.7) | 0.898 |
| Infant birth weight (g)—mean ± SD | 3.252 ± 424 | 3.214 ± 385 | 0.428 |
| Per capita income (MW*)—median (interquartile range) | 0.4 (0.3–0.6) | 0.4 (0.3–0.7) | 0.921 |
| Infant sex, male—n (%) | 101 (48.8) | 63 (54.3) | 0.403 |
| Maternal skin color, white—n (%) | 129 (62.3) | 74 (63.8) | 0.932 |
| Mode of delivery, vaginal—n (%) | 154 (74.4) | 87 (75.0) | 1.000 |
| Primiparity at the time of intervention—n (%) | 177 (85.5) | 99 (85.3) | 1.000 |
| Cohabiting with partner at the time of intervention—n (%) | 125 (60.4) | 76 (65.5) | 0.428 |
| Cohabiting with maternal grandmother at the time of intervention—n (%) | 117 (56.5) | 52 (44.8) | 0.057 |
SD = standard deviation
*MW: minimum wage (US$195.00 at the time of the study).
Table 2. Characteristics of participants who completed the study stratified by group allocation.
| Variable | Intervention (n = 98) | Control (n = 109) | P |
|---|---|---|---|
| At the time of intervention | |||
| Maternal age (years)—mean ± SD | 17.4 ± 1.5 | 17.5 ± 1.4 | 0.675 |
| Maternal educational attainment, ≥ 8 years—n (%) | 55 (56.1) | 55 (50.5) | 0.499 |
| Infant birth weight (g)—mean ± SD | 3252 ± 421 | 3252 ± 428 | 0.995 |
| Per capita income (MW*)—median (interquartile range) | 0.5 (0.3–0.6) | 0.4 (0.2–0.6) | 0.685 |
| Infant sex, male–n (%) | 45 (45.9) | 56 (51.4) | 0.519 |
| Maternal skin color, white—n (%) | 62 (63.3) | 67 (61.5) | 0.902 |
| Mode of delivery, vaginal—n (%) | 73 (74.5) | 81 (74.3) | 1.000 |
| Primiparity—n (%) | 88 (89.8) | 89 (81.7) | 0.143 |
| Cohabiting with partner—n (%) | 57 (58.2) | 68 (62.4) | 0.633 |
| Cohabiting with maternal grandmother—n (%) | 64 (65.3) | 53 (48.6) | 0.023 |
| At the time of last assessment | |||
| Maternal age (years)—mean ± SD | 23.9 ± 4.0 | 24.4 ± 1.7 | 0.305 |
| Child age (years)—mean ± SD | 5.82 ± 0.52 | 6.30 ± 0.36 | <0.001 |
| Per capita income (MW)—median (interquartile range) | 0.6 (0.4–0.9) | 0.6 (0.4–0.8) | 0.814 |
| Other children born—n (%) | 36 (36.7) | 42 (38.9) | 0.861 |
| Bolsa Família recipient** —n (%) | 31 (32.0) | 30 (27.8) | 0.617 |
| Maternal educational attainment, ≥ 8 years—n (%) | 76 (80.9) | 72 (67.3) | 0.044 |
| Maternal employment outside the home—n (%) | 49 (52.1) | 63 (58.3) | 0.457 |
| Cohabitation with maternal grandmother—n (%) | 30 (31.3) | 25 (23.4) | 0.270 |
| Cohabitation with paternal grandmother—n (%) | 3 (3.2) | 10 (9.3) | 0.138 |
| Cohabitation with partner—n (%) | 57 (60.6) | 76 (71.0) | 0.160 |
SD = standard deviation
* MW: minimum wage (US$195.00 at the time of the study).
**Bolsa Família is a conditional cash transfer program of the Brazilian federal government whereby benefits are provided to families living in poverty and extreme poverty across the country.
The results of anthropometric assessment are shown in Table 3. The height-for-age and BMI-for-age Z scores were similar between groups. Overall, 38.8% of children in the intervention group and 31.2% of those in the control group had overweight (including obesity), with no significant between-group difference.
Table 3. Anthropometric indicators of children at age 4 to 7 years, stratified by group allocation.
| Variable | Intervention (n = 98) | Control (n = 109) | P |
|---|---|---|---|
| BMI-for-age–z score | 0.87 ± 1.37 | 0.73 ± 1.33 | 0.461 |
| Excessive weight (overweight + obesity)—n (%) | 38 (38.8) | 34 (31.2) | 0.318 |
| Overweight* | 21 (21.4) | 19 (17.4) | |
| Obesity** | 17 (17.3) | 15 (13.8) | |
| Height-for-age—z score | 0.12 ± 0.93 | -0.01 ± 1.04 | 0.331 |
| Stunting*** | 0 (0.0) | 3 (2.8) | 0.248 |
BMI = body mass index
*defined as BMI-for-age > +2 z-score and ≤ +3 z-score for children under five; and > +1 z-score and ≤ +2 z-score for older children, according WHO standards
** defined as BMI-for-age > +3 z-score for children under five; and > +2 z-score for older children, according WHO standards
*** defined as length-for-age < -2 z-score, according WHO standards
Data on the child feeding patterns at final assessment are shown in Table 4. The duration of EBF and the age at onset of complementary feeding were significantly greater in the intervention group than in the control group. However, there was no significant difference in the median duration of BF. Furthermore, there were no between-group differences in intake of vegetables, fruit, soft drinks, processed snack foods, fried foods, candy/sweets, cookies, and artificial fruit juices.
Table 4. Data on child feeding, stratified by group allocation.
| Variable | Intervention (n = 98) | Control (n = 109) | P |
|---|---|---|---|
| *Duration of EBF (months)—median (interquartilerange) | 2.9 (1.0–4.7) | 1.3 (0.6–3.0) | 0.001 |
| *Age at onset of complementary feeding (months)–median (interquartile range) | 5 (4–6) | 4 (4–6) | 0.004 |
| **Duration of BF (months)–median (interquartile range) | 12 (4.5–24) | 12 (4–24) | 0.649 |
| ***Food intake vegetables ≥ 5×/week—n (%) | 47 (48.0) | 46 (42.2) | 0.489 |
| fruit ≥ 5×/week—n (%) | 55 (56.1) | 69 (63.3) | 0.363 |
| processed snack foods < 1×/week—n (%) | 19 (19.4) | 23 (21.1) | 0.894 |
| fried foods < 1×/week—n (%) | 19 (19.4) | 18 (16.5) | 0.721 |
| candy/sweets < 1×/week—n (%) | 15 (15.3) | 15 (13.8) | 0.906 |
| cookies < 1×/week—n (%) | 26 (26.5) | 27 (24.8) | 0.896 |
| soft drinks < 1×/week—n (%) | 7 (7.1) | 8 (7.3) | 1.000 |
| ****artificial fruit drinks—n (%) | 72 (73.5) | 93 (85.3) | 0.052 |
EBF = exclusive breastfeeding
BF = breastfeeding
* variables measured through monthly interviews during the first six months of children’s life.
** variable collected through interviews when children were aged 4 to 7 years.
*** variables collected through food frequency questionnaire when children were aged 4 to 7 years.
**** variable collected when children were aged 4 to 7 years and analyzed in order to determine the consumption between groups. The frequency of intake wasn’t measured.
The crude and adjusted effects of intervention on the prevalence of overweight and obesity showed that the study intervention had no impact on overweight and obesity in this sample of children (Table 5).
Table 5. Poisson regression model with robust estimation for the effect of intervention on overweight/obesity.
| Model | RR (95%CI) | P |
|---|---|---|
| 1—Intervention group | 1.24 (0.86–1.81) | 0.254 |
| 2—Model 1 + propensity score | 1.16 (0.80–1.69) | 0.442 |
| 3—Model 2 + cohabitation with maternal grandmother at time of intervention | 1.16 (0.80–1.70) | 0.428 |
| 4—Model 3 + maternal educational attainment at final intervention | 1.09 (0.74–1.61) | 0.675 |
| 5—Model 4 + child age | 1.11 (0.72–1.70) | 0.645 |
| 6—Model 5 + cohabitation with paternal grandmother at final intervention | 1.11 (0.72–1.70) | 0.648 |
| 7—Model 6 + cohabitation with partner at final intervention | 1.09 (0.70–1.68) | 0.703 |
RR = relative risk
Discussion
The tested intervention had no effect on nutritional status of children at age 4–7 years, contradicting our initial expectations, which were based on studies showing that increased duration of EBF and BF [13, 16, 44, 45] and later introduction of complementary feeding [9, 17–19, 35, 46–48] are associated with a lower risk of overweight and obesity in childhood. In the present study, children in the intervention group had double the median duration of EBF and a later introduction of complementary feeding than those in the control group; however, this was not enough to influence their nutritional status at age 4–7 years. Although they failed to prove our hypothesis, the results of this study are consistent with those of previous investigations that assessed BF and healthy complementary feeding promotion interventions and had overweight and obesity at preschool age as outcomes of interest [49–52]. Particularly worthy of note is a study conducted in Belarus, by Kramer et al. [49], which was the largest randomized clinical trial to date to test the effect of a BF promotion intervention conducted during the first year of life on a variety of outcomes, including child weight, height, and adiposity at age 12 months and 6.5 years. The BMIs of children in the experiment and control groups at age 6.5 years were similar, as were the proportions of children with overweight (13.4% and 12.2% respectively) and obesity (5.9% and 5.0% respectively). Similar results were found by studies conducted in Bangladesh [52] and London [50], which respectively addressed the impact of a pro-BF intervention in the first 6 months of life and the impact of a dietary practices intervention in the first year of life on nutritional status during preschool age (4–5 years). Furthermore, a previous Brazilian study found no significant differences in the proportions of overweight and obesity between 7- and 8-year-olds whose mothers had received BF counseling during the first year of life and children whose mothers had received no such intervention. The prevalence of overweight and obesity was 31.6% and 15.8% in boys and 29.1% and 12.7% in girls, respectively, in the intervention group, vs. 26.3% and 9.1% in boys and 24.4% and 10.3% in girls, respectively, in the control group [51].
On the basis of some studies that showed that breastfed infants—particularly those breastfed for longer—exhibited healthier dietary habits both in the first year of life and during preschool age as compared with children who had not been breastfed or who had been breastfed for shorter periods [53–57], we expected that children in the present sample would have higher-quality diets after the trial intervention. Conversely, there were no between-group differences in intake of healthy or unhealthy foods.
The absence of any effect of the study intervention on child nutritional status during the preschool years may be attributed to the multitude of factors involved in the genesis of overweight and obesity, such as: maternal obesity in the pre-gestational, gestational, and post-gestational periods; high birth weight; rapid weight gain during the first year of life; maternal smoking; sleep deprivation; TV time; among others [7–9]. Therefore, any intervention seeking to reduce overweight and obesity in children must take these factors into account, and must be sustained, as these factors are dynamic and may change at any point in time. The intervention tested herein took place during the first 4 months of life, and no later “booster sessions” were held. This brief intervention period was sufficient to have an impact on the rates of BF and EBF during the first year of life and on the timing of onset of complementary feeding [31–33], but did not influence later nutritional status.
The merits of this study include its pioneering nature as the first randomized clinical trial to test a pro-BF and healthy complementary feeding intervention in a sample of adolescent mothers and maternal grandmothers, with child nutritional status during preschool age as the outcome. However, some limitations must be noted. Despite exhaustive attempts to locate all families enrolled in the trial, there was a significant rate of loss to follow-up, especially due to participants who moved to unknown locations. High follow-up loss rates are common in population-based prospective studies, particularly those involving young adults living in the peripheral areas of large urban centers in developing countries. To minimize the possibility of selection bias due to attrition, the effect of the intervention on the outcome of interest was adjusted for variables that exhibited between-group differences at the p<0.20 level. It is noteworthy that the losses to follow up during the period between 12 months and the last assessment at 4–7 years were relatively smaller than during the first year, especially if we consider that this is a longer period. In fact, the number of children at the 4–7 years follow-up stage in the control group was higher than the number at 12 months. We believe that this could be possible due to the inclusion of social networks as a search tool of the families for the last assessment, which allowed us to find some families that had been lost before the 12 months arm.
Another possible limitation is the large age range (4 to 7 years) at follow up assessment. It took almost two years to recruit the sample and ten months to locate all families for the follow up assessment. As we did not determine a specific age for the follow up evaluation, we ended up having this wide age range. Yet, we believe that this fact has not affected the results, especially as the child's age was considered in the multivariable analyses.
Regarding the duration of BF, we can not rule out recall bias, as the information was collected retrospectively for mothers breastfeeding for over 12 months (53.2% of the sample). However, this type of bias is more relevant when investigating duration of exclusive breastfeeding [58] as mothers tend to recall the duration of BF with relative accuracy. According to a study conducted in the United States, BF duration was only slightly overestimated at 1 to 3.5 years after the outcome [59]. And finally, we can not disregard the fact that this study did not provide for collection of data that might assist in interpretation of results, such as parental weight and height, infant weight and length during the first year of life, and child physical activity patterns, sleep duration, and TV time.
We conclude that a pro-BF and healthy complementary feeding intervention geared to adolescent mothers and their own mothers (i.e., the maternal grandmothers of the infants) was not effective in preventing child overweight or obesity at preschool age. The multifactorial nature of overweight and obesity in children and the brief intervention period may be implicated in these findings. Nevertheless, even if a longer duration of BF/EBF is not associated with lower prevalence of overweight and obesity later in childhood, other benefits of prolonged BF still stand, such as better metabolic patterns with lower risk of developing heart disease, hypertension, and diabetes [44, 60–63]; superior cognitive development [44]; and benefits for the mother, such as lower risk of breast cancer and type 2 diabetes [64–67]. These benefits mean that promotion of BF should be a priority among health promotion strategies for all nations.
Supporting Information
(DOC)
(DOC)
Acknowledgments
Financial support was provided by FIPE-HCPA (Research and Events Support Fund at Hospital de Clínicas de Porto Alegre) and CNPq (National Council for Scientific and Technological Development).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Financial support was provided by FIPE-HCPA (Research and Events Support Fund at Hospital de Clínicas de Porto Alegre) and CNPq (National Council for Scientific and Technological Development). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kac G, Velasquez-Melendez G (2003) [The nutritional transition and the epidemiology of obesity in Latin America]. Cad Saude Publica 19 Suppl 1: S5, S4 [PubMed] [Google Scholar]
- 2. Triches RM, Giugliani ER (2005) Obesity, eating habits and nutritional knowledge among school children. Rev Saude Publica 39: 541–547. [DOI] [PubMed] [Google Scholar]
- 3. Cai W (2014) Nutritional challenges for children in societies in transition. Curr Opin Clin Nutr Metab Care 17: 278–284. 10.1097/MCO.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 4. de Onis M, Blössner M, Borghi E (2010) Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 92: 1257–1264. 10.3945/ajcn.2010.29786 [DOI] [PubMed] [Google Scholar]
- 5. BRASIL (2009) Pesquisa Nacional de Demografia e Saúde da Criança e da Mulher—PNDS 2006 Brasília: pp. 300p. [Google Scholar]
- 6. IBGE (2010) POF-2008-2009. Pesquisa de Orçamentos Familiares 2008–2009 Antropometria e estado nutricional de crianças, adolescentes e adultos no Brasil. Instituto Brasileiro de Geografia e Estatística—Ministério da Saúde; Ministério do Planejamento, editors. [Google Scholar]
- 7. Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. (2005) Early life risk factors for obesity in childhood: cohort study. BMJ 330: 1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monasta L, Batty GD, Cattaneo A, Lutje V, Ronfani L, Van Lenthe FJ, et al. (2010) Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev 11: 695–708. 10.1111/j.1467-789X.2010.00735.x [DOI] [PubMed] [Google Scholar]
- 9. Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP (2012) Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child 97: 1019–1026. 10.1136/archdischild-2012-302263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arenz S, Rückerl R, Koletzko B, von Kries R (2004) Breast-feeding and childhood obesity-a systematic review. Int J Obes Relat Metab Disord 28: 1247–1256. [DOI] [PubMed] [Google Scholar]
- 11. Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG (2005) Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 115: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 12. Harder T, Bergmann R, Kallischnigg G, Plagemann A (2005) Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol 162: 397–403. [DOI] [PubMed] [Google Scholar]
- 13. Griffiths LJ, Smeeth L, Hawkins SS, Cole TJ, Dezateux C (2009) Effects of infant feeding practice on weight gain from birth to 3 years. Arch Dis Child 94: 577–582. 10.1136/adc.2008.137554 [DOI] [PubMed] [Google Scholar]
- 14. Liese AD, Hirsch T, von Mutius E, Keil U, Leupold W, Weiland SK (2001) Inverse association of overweight and breast feeding in 9 to 10-y-old children in Germany. Int J Obes Relat Metab Disord 25: 1644–1650. [DOI] [PubMed] [Google Scholar]
- 15. Scanferla de Siqueira R, Monteiro CA (2007) Breastfeeding and obesity in school-age children from families of high socioeconomic status. Rev Saude Publica 41: 5–12. [DOI] [PubMed] [Google Scholar]
- 16. Simon VG, Souza JM, Souza SB (2009) Breastfeeding, complementary feeding, overweight and obesity in pre-school children. Rev Saude Publica 43: 60–69. [DOI] [PubMed] [Google Scholar]
- 17. Wilson AC, Forsyth JS, Greene SA, Irvine L, Hau C, Howie PW (1998) Relation of infant diet to childhood health: seven year follow up of cohort of children in Dundee infant feeding study. BMJ 316: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu TC, Chen PH (2009) Health consequences of nutrition in childhood and early infancy. Pediatr Neonatol 50: 135–142. 10.1016/S1875-9572(09)60051-6 [DOI] [PubMed] [Google Scholar]
- 19. Huh SY, Rifas-Shiman SL, Taveras EM, Oken E, Gillman MW (2011) Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics 127: e544–551. 10.1542/peds.2010-0740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abraham EC, Godwin J, Sherriff A, Armstrong J (2012) Infant feeding in relation to eating patterns in the second year of life and weight status in the fourth year. Public Health Nutr 15: 1705–1714. 10.1017/S1368980012002686 [DOI] [PubMed] [Google Scholar]
- 21. Pearce J, Langley-Evans SC (2013) The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond) 37: 477–485. [DOI] [PubMed] [Google Scholar]
- 22. WHO (2003) Global strategy for infant and young child feeding. Geneva: WHO—World Health Organization. [Google Scholar]
- 23. BRASIL (2009) II Pesquisa de Prevalência de Aleitamento Materno nas Capitais Brasileiras e Distrito Federal / Ministério da Saúde, Secretaria de Atenção à Saude, Departamento de Ações Programáticas e Estratégicas. Ministério da Saúde; Brasília: pp. 108. [Google Scholar]
- 24. Bortolini GA, Gubert MB, Santos LM (2012) Food consumption of Brazilian children by 6 to 59 months of age. Cad Saude Publica 28: 1759–1771. [DOI] [PubMed] [Google Scholar]
- 25. Bentley M, Gavin L, Black MM, Teti L (1999) Infant feeding practices of low-income, African-American, adolescent mothers: an ecological, multigenerational perspective. Soc Sci Med 49: 1085–1100. [DOI] [PubMed] [Google Scholar]
- 26. Susin LR, Giugliani ER, Kummer SC (2005) Influence of grandmothers on breastfeeding practices. Rev Saude Publica 39: 141–147. [DOI] [PubMed] [Google Scholar]
- 27. Giugliani ER, do Espírito Santo LC, de Oliveira LD, Aerts D (2008) Intake of water, herbal teas and non-breast milks during the first month of life: associated factors and impact on breastfeeding duration. Early Hum Dev 84: 305–310. [DOI] [PubMed] [Google Scholar]
- 28. Grassley J, Eschiti V (2008) Grandmother breastfeeding support: what do mothers need and want? Birth 35: 329–335. 10.1111/j.1523-536X.2008.00260.x [DOI] [PubMed] [Google Scholar]
- 29. Nesbitt SA, Campbell KA, Jack SM, Robinson H, Piehl K, Bogdan JC (2012) Canadian adolescent mothers' perceptions of influences on breastfeeding decisions: a qualitative descriptive study. BMC Pregnancy Childbirth 12: 149 10.1186/1471-2393-12-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pilkauskas NV (2014) Breastfeeding initiation and duration in coresident grandparent, mother and infant households. Matern Child Health J 18: 1955–1963. 10.1007/s10995-014-1441-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oliveira LD, Giugliani ER, Santo LC, Nunes LM (2014) Counselling sessions increased duration of exclusive breastfeeding: a randomized clinical trial with adolescent mothers and grandmothers. Nutr J 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bica OC, Giugliani ER (2014) Influence of counseling sessions on the prevalence of breastfeeding in the first year of life: a randomized clinical trial with adolescent mothers and grandmothers. Birth 41: 39–45. 10.1111/birt.12097 [DOI] [PubMed] [Google Scholar]
- 33. Oliveira LD, Giugliani ER, Santo LC, Nunes LM (2012) Impact of a strategy to prevent the introduction of non-breast milk and complementary foods during the first 6 months of life: a randomized clinical trial with adolescent mothers and grandmothers. Early Hum Dev 88: 357–361. 10.1016/j.earlhumdev.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 34. WHO (2006) Infant and young child feeding counselling: an integrated course. Geneva: WHO—World Health Organization. [Google Scholar]
- 35. BRASIL (2005) Guia Alimentar para Crianças Menores de 2 Anos. Ministério da Saúde; Brasília: pp. 152. [Google Scholar]
- 36. BRASIL (2011) Orientações para a coleta e análise de dados antropométricos em serviços de saúde: Norma Técnica do Sistema de Vigilância Alimentar e Nutricional. Ministério da Saúde; Brasília: pp. 76. [Google Scholar]
- 37. de Onis M (2006) Dept. of Nutrition for Health and Development: WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization. [Google Scholar]
- 38. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. WHO (2006) WHO Anthro 2005, Beta version Feb 17th, 2006: Software for assessing growth and development of the world’s children. Geneva: WHO—World Health Organization. [Google Scholar]
- 40. WHO (2009) WHO AnthroPlus for personal computers manual: software for assessing growth of the world’s children and adolescents. Geneva: WHO—World Health Organization. [Google Scholar]
- 41. BRASIL (2011) Caderneta de Saúde da Criança. Ministério da Saúde; Brasília: pp. 94p. [Google Scholar]
- 42. Xu Z, Kalbfleisch JD (2010) Propensity score matching in randomized clinical trials. Biometrics 66(3): 813–823. 10.1111/j.1541-0420.2009.01364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. D'Agostino RB (1998) Tutorial in Biostatistics—Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17(19):2265–2281. [DOI] [PubMed] [Google Scholar]
- 44. Horta BL, Victora CG (2013) Long-term effects of breastfeeding A systematic review. Geneva: WHO—World Health Organization. [Google Scholar]
- 45. Balaban G, Silva GA (2004) Protective effect of breastfeeding against childhood obesity. J Pediatr (Rio J) 80: 7–16. [PubMed] [Google Scholar]
- 46. Agostoni C, Decsi T, Fewtrell M, Goulet O, Kolacek S, Koletzko B, et al. (2008) Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 46: 99–110. [DOI] [PubMed] [Google Scholar]
- 47. Kramer MS, Kakuma R (2012) Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev 8: CD003517 10.1002/14651858.CD003517.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. WHO (1998) Complementary feeding of young children in developing countries: a review of current scientific knowledge. Geneva: WHO—World Health Organization. [Google Scholar]
- 49. Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, et al. (2007) Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr 86: 1717–1721. [DOI] [PubMed] [Google Scholar]
- 50. Scheiwe A, Hardy R, Watt RG (2010) Four-year follow-up of a randomized controlled trial of a social support intervention on infant feeding practices. Matern Child Nutr 6: 328–337. 10.1111/j.1740-8709.2009.00231.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Louzada ML, Campagnolo PD, Rauber F, Vitolo MR (2012) Long-term effectiveness of maternal dietary counseling in a low-income population: a randomized field trial. Pediatrics 129: e1477–1484. 10.1542/peds.2011-3063 [DOI] [PubMed] [Google Scholar]
- 52. Khan AI, Hawkesworth S, Ekström EC, Arifeen S, Moore SE, Frongillo EA, et al. (2013) Effects of exclusive breastfeeding intervention on child growth and body composition: the MINIMat trial, Bangladesh. Acta Paediatr 102: 815–823. 10.1111/apa.12282 [DOI] [PubMed] [Google Scholar]
- 53. Noble S, Emmett P (2006) Differences in weaning practice, food and nutrient intake between breast- and formula-fed 4-month-old infants in England. J Hum Nutr Diet 19: 303–313. [DOI] [PubMed] [Google Scholar]
- 54. Burnier D, Dubois L, Girard M (2011) Exclusive breastfeeding duration and later intake of vegetables in preschool children. Eur J Clin Nutr 65: 196–202. 10.1038/ejcn.2010.238 [DOI] [PubMed] [Google Scholar]
- 55. Vitolo MR, Bortolini GA, Campagnolo PD, Hoffman DJ (2012) Maternal dietary counseling reduces consumption of energy-dense foods among infants: a randomized controlled trial. J Nutr Educ Behav 44: 140–147. 10.1016/j.jneb.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 56. Armstrong J, Abraham EC, Squair M, Brogan Y, Merewood A (2014) Exclusive breastfeeding, complementary feeding, and food choices in UK infants. J Hum Lact 30: 201–208. 10.1177/0890334413516383 [DOI] [PubMed] [Google Scholar]
- 57. Galloway AT, Lee Y, Birch LL (2003) Predictors and consequences of food neophobia and pickiness in young girls. J Am Diet Assoc 103: 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bland RM, Rollins NC, Solarsh G, Van den Broeck J, Coovadia HM (2003) Maternal recall of exclusive breast feeding duration. Arch Dis Child 88(9): 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gillespie B, d'Arcy H, Schwartz K, Bobo JK, Foxman B (2006) Recall of age of weaning and other breastfeeding variables. Int Breastfeed J 1: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG (2006) Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr 84: 1043–1054. [DOI] [PubMed] [Google Scholar]
- 61. Järvisalo MJ, Hutri-Kähönen N, Juonala M, Mikkilä V, Räsänen L, Lehtimäki T, et al. (2009) Breast feeding in infancy and arterial endothelial function later in life. The Cardiovascular Risk in Young Finns Study. Eur J Clin Nutr 63: 640–645. 10.1038/ejcn.2008.17 [DOI] [PubMed] [Google Scholar]
- 62. Guardamagna O, Abello F, Cagliero P, Lughetti L (2012) Impact of nutrition since early life on cardiovascular prevention. Ital J Pediatr 38: 73 10.1186/1824-7288-38-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martin RM, Ebrahim S, Griffin M, Davey Smith G, Nicolaides AN, Georgiou N, et al. (2005) Breastfeeding and atherosclerosis: intima-media thickness and plaques at 65-year follow-up of the Boyd Orr cohort. Arterioscler Thromb Vasc Biol 25: 1482–1488. [DOI] [PubMed] [Google Scholar]
- 64. Bernier MO, Plu-Bureau G, Bossard N, Ayzac L, Thalabard JC (2000) Breastfeeding and risk of breast cancer: a metaanalysis of published studies. Hum Reprod Update 6: 374–386. [DOI] [PubMed] [Google Scholar]
- 65. Lipworth L, Bailey LR, Trichopoulos D (2000) History of breast-feeding in relation to breast cancer risk: a review of the epidemiologic literature. J Natl Cancer Inst 92: 302–312. [DOI] [PubMed] [Google Scholar]
- 66. Möller T, Olsson H, Ranstam J, Cancer CGoHFiB (2002) Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50, 302 women with breast cancer and 96, 973 women without the disease. Lancet 360: 187–195. [DOI] [PubMed] [Google Scholar]
- 67. Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB (2005) Duration of lactation and incidence of type 2 diabetes. JAMA 294: 2601–2610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


