Abstract
Toll-like receptor 4 is a part of the innate immune system and recognizes Helicobacter pylori lipopolysaccharide. The goal of this study was to analyze the role of Toll-like receptor 4 polymorphisms +896 (rs4986790) and +1196 (rs4986791) in the pathogenesis of Helicobacter pylori related gastroduodenal diseases in relation to gastric secretion and inflammation. Toll-like receptor 4 polymorphisms, serum gastrin-17 and pepsinogen I and II concentrations were determined, and gastroscopies with histopathological analyses were performed to 216 dyspeptic patients. As genotype controls, 179 controls and 61 gastric cancer patients were studied. In our study, the Toll-like receptor 4 +896 and +1196 polymorphisms were in total linkage disequilibrium. The homozygous wild types displayed higher gastrin-17 serum concentrations than the mutants (p = 0.001) and this effect was independent of Helicobacter pylori. The homozygous wild types also displayed an increased risk for peptic ulcers (OR: 4.390). Toll-like receptor 4 genotypes did not show any association with Helicobacter pylori positivity or the features of gastric inflammation. Toll-like receptor 4 expression was seen in gastrin and somatostatin expressing cells of antral mucosa by immunohistochemistry. Our results suggest a role for Toll-like receptor 4 in gastric acid regulation and that the Toll-like receptor 4 +896 and +1196 wild type homozygozity increases peptic ulcer risk via gastrin secretion.
Introduction
Toll-like receptor 4 (TLR4) is a part of a pattern recognition molecule family. TLR4 binds several microbial ligands and the subsequent downstream signaling stimulates cytokine production creating a proinflammatory environment. The bacterial lipopolysaccharides (LPS) of Helicobacter pylori and other gram negative bacteria are TLR4 target molecules and thus TLR4 has been thought to have a role in H. pylori related diseases [1,2].
The gene encoding TLR4 in humans is located on chromosome locus 9q32-q33 and contains 4 exons. Two non-synonymous polymorphisms TLR4 +896 adenine/guanine (rs4986790) and TLR4 +1196 cytosine/thymine (rs4986791) have been located in the fourth exon causing amino acid substitutions: glycine for aspartic acid at 299 position and isoleucine for threonine at 399 position respectively [2]. These two polymorphisms are in linkage disequilibrium, and 6 –14% of Indo-European individuals are double heterozygotes for these alleles [3]. Both of these mutations are associated with LPS hyporesponsiveness and the double mutant even more prominently so [2].
Previous reports have shown contradictory associations between the TLR4 +896 and +1196 polymorphisms and H. pylori related gastritis and peptic ulcer and have not presented a clear physiological mechanism for the polymorphisms in their pathogenesis [4–7]. The key mechanism in the pathogenesis of most peptic ulcers is thought to be H. pylori induced excessive gastrin secretion and the following excessive acid secretion, but the mechanisms of such aberration of regulation are unclear [8,9]. However, physiological connections between TLR4 and gastrin secretion has been documented in animal models [10,11] but there are no studies about the possible role of TLR4 polymorphisms in gastrin secretion in humans.
We hypothesized that TLR4 could affect gastrin levels and thereby affect peptic ulcer pathogenesis also in human subjects. We have investigated the relation between the TLR4 +896 and +1196 polymorphisms, and serum gastrin-17 (G17) and pepsinogen I (PGI) and II (PGII) concentrations, H. pylori infection and histopathologic gastric inflammation in dyspeptic patients. We also compared the genotype distribution between peptic ulcer, non-ulcer dyspepsia, gastric cancer and control patients.
Patients and Methods
The patient group comprised of 216 dyspeptic patients collected from three hospitals in the city of Oulu, Finland, all performing outpatient endoscopies. Exclusion criteria were following: treated H. pylori infection, treatment with immunosuppressive drugs, ongoing antibiotic treatment and previous gastric surgery. The patients were inquired for the use of antacids, sucralfate, histamine 2 receptor antagonists or proton pump inhibitors. Upper gastrointestinal endoscopies were performed by experienced endoscopists. Endoscopy findings, including the presence or absence of gastric or duodenal ulcer, were registered. Biopsies form the descending part of duodenum, gastric antrum and gastric body were taken for histological analysis [12].
A series of 61 patients with gastric cancer was collected during the years 1996–2000 in Oulu University Hospital and represents an unselected series of patients treated by surgery. The control group consisted of university staff and students of which no data were collected concerning dyspeptic symptoms or visits to gastroenterologists. All controls and study subjects originated from the homogenic ethnically Finnish population. The groups have been described previously [13,14].
The control and dyspeptic patients’ DNA was extracted from blood leucocytes and, in patients with gastric cancer, from a fresh frozen gastric tissue specimen representing non-neoplastic tissue. Extraction was performed as previously described [13]. PCR tests from the DNA samples were performed to detect the TLR4 +896 and +1196 polymorphisms as previously described [15]. The investigators who performed the genetic analyses were blinded to the clinical data, and the clinical investigators were blinded to the genetic data.
H. pylori status was analyzed in the dyspeptic patients. Positive H. pylori status was based on a positive serology and a positive bacterial culture or a PCR test as previously described [13]. The presence of the pathogenetic H. pylori gene variant, cagA, was detected as previously described [16]. G17, PGI and PGII measurements were performed with GastroPanel assays by Biohit (Helsinki, Finland) laboratory from the patients’ serum samples.
For immunohistochemical stainings, formalin fixed and paraffin embedded sections were pretreated by heating with microwaves in ethylenediaminetetraacetic acid (for single stains) or sodium citrate (pH 6, for double stains) with 850W for 10 minutes for antigen retrieval. The immunohistochemical stainings were performed with mouse monoclonal antibody against human TLR4 (1:1000, H00007099-M02, Abnova, Taipei, Taiwan), rabbit polyclonal antibody against human gastrin-17 (1:250, A 0568, Dako, Glostrup, Denmark) and rabbit polyclonal antibody against human somatostatin (1:600, A0566, Dako). The incubation time was 60 minutes for TLR4 and somatostatin antibodies and 30 minutes for gastrin antibody at room temperature. For the detection of the antibody reaction we used EnVision detection kit and the double stains of TLR4, gastrin and somatostatin were performed by EnVision G|2 Doublestain kit (Dako) with related protocols. The validation of immunohistochemical analysis was performed with negative controls including buffer solution or irrelevant antibodies instead of gastrin and TLR4 antibodies. Duodenal epithelial cells were used as a positive control for TLR4 [17]. Expression intensity of TLR4 in different cell populations of gastric mucosa was assessed by using single stained sections and a scale from negative to weak, moderate and strong expression.
In the dyspeptic patients’ group, 129 patients had no signs of active or inactive peptic ulcer and had no previously diagnosed ulcer. In the group, 50 dyspeptic patients had an active duodenal ulcer seen in the endoscopy, 23 patients had an active gastric ulcer, 5 patients had a previously diagnosed duodenal ulcer or duodenal scarring consistent with a duodenal ulcer and 9 patients had a previously diagnosed gastric ulcer or scarring in the stomach consistent with a gastric ulcer. In the ulcer risk tests with the control group, where we used only permanent cofactors, we included all the previously diagnosed patients into the ulcer group. When we did ulcer risk analyses between the ulcer and non-ulcer patients’ group and also used non-permanent risk factors as H. pylori positivity and smoking, we excluded all the patients with inactive ulcers from the analysis to prevent them from confounding the results.
The gastric cancers were re-classified by two pathologists (JMM and TJK). Other histopathologic changes in the gastric mucosa were analyzed according to the Sydney system [18] by one pathologist (TJK) blinded for clinical and genetic data. The amount of duodenal lamina propria mononuclear cells was evaluated by using a four point scale (0–3) as described previously [12].
Medians and interquartile ranges (IQR) are reported for skewed continuous variables (G17, PGI and PGII). The effects of the risk factors on the serum levels of G17, PGI and PGII were analyzed with Mann-Whitney U test and binary logistic regression models. The risks of diseases associated with the genetic polymorphisms were analyzed with binary logistic regression models. Forward likelihood ratio criteria were used in stepwise multifactorial regression models. The correlations between the genetic polymorphisms and the histological variables were analyzed with Mann-Whitney U test. P-value of less than 0.05 was considered statistically significant. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were calculated from the regression models. Missing data were excluded pairwise from the analyses. The data were analyzed using the SPSS software version 19 (IBM, Armonk, New York, United States).
The study followed the guidelines of the declaration of Helsinki and was approved by the regional ethics committee of the Northern Ostrobothnia Hospital District. Dyspeptic patients gave written informed consent and control patients gave verbal informed consent. The cancer patient data and tissue samples were obtain from the archives of Oulu University Hospital. A permission to use the data after de-identification procedure by a third party was obtained from the ethics committee.
Results
In all of our study populations, the TLR4 +896 and +1196 polymorphisms were in total linkage disequilibrium so all subjects who had the mutant allele for TLR4 +896 had also the mutant allele for TLR4 +1196 and vice versa. The genotype frequencies in the control and non-ulcer dyspepsia groups are consistent with the Hardy-Weinberg equilibrium. Subject group characteristics and genotype frequencies are displayed in Table 1. For the analyses, the TLR4 +896/+1196 heterozygous and homozygous double mutants were combined into a single group. H. pylori positivity did not associate with the TLR4 genotypes in the ulcer or non-ulcer dyspepsia patients’ groups or in the combined group; 62.1% (110/177) of the TLR4 homozygous wild type patients and 51.3% (20/39) of TLR4 heterozygous or homozygous mutant patients were H. pylori positive (p = 0.210).
Table 1. Characteristics of the subject groups.
| Subject group | |||||
|---|---|---|---|---|---|
| Control | Non-ulcer dyspepsia | Active peptic ulcer | Inactive peptic ulcer | Gastric cancer | |
| N | 179 | 129 | 73 | 14 | 61 |
| Male | 56 (31.3%) | 48 (37.2%) | 34 (46.6%) | 7 (50.0%) | 31 (50.8%) |
| Female | 123 (68.7%) | 81 (62.8%) | 39 (53.4%) | 7 (50.0%) | 30 (49.2%) |
| Mean age (SD 1 ) | 39.3 (13.4) | 52.0 (14.0) | 56.6 (13.6) | 55.2 (16.0) | 65.9 (12.6) |
| H. pylori positive | No data | 51/129 (39.5%) | 71/73 (97.3%) | 8/14 (57.1%) | No data |
| cagA positive | No data | 39/47 (83.0%) | 50/52 (96.2%) | 8/8 (100%) | No data |
| Smoking | No data | 21/129 (16.3%) | 29/73 (39.7%) | 3/14 (21.4%) | No data |
| On medication 2 | No data | 21/129 (16.3%) | 33/73 (45.2%) | 4/13 (30.8%) | No data |
| TLR4 wild type | 143 (79.9%) | 100 (77.5%) | 65 (89.0%) | 12 (85.7%) | 47 (77.0%) |
| TLR4 heterozygous mutant | 35 (19.6%) | 28 (21.7%) | 7 (9.6%) | 2 (14.3%) | 12 (19.7%) |
| TLR4 homozygous mutant | 1 (0.6%) | 1 (0.8%) | 1 (1.4%) | 0 (0.0%) | 2 (3.3%) |
1Standard deviation
2Proton pump inhibitor, histamine receptor 2 antagonist, sucralfate or antacid
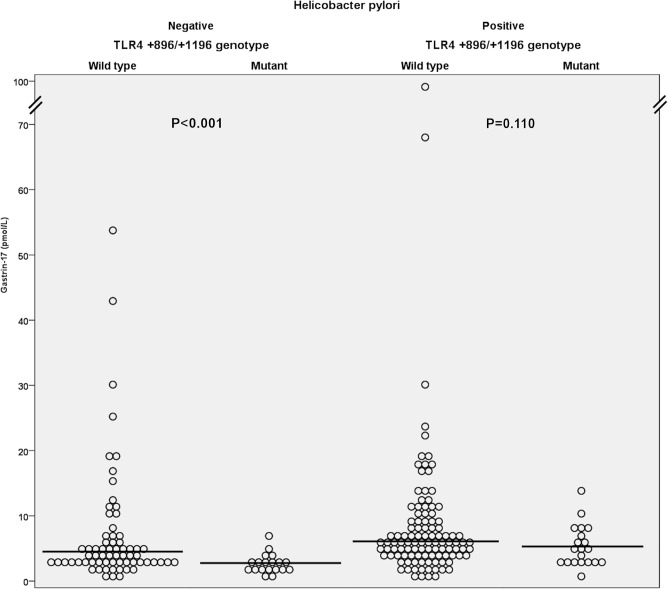
Serum G17 serum levels were higher in the TLR4 wild type patients (median 5.0 pmol/l; IQR: 5.3 pmol/l; n = 174) compared to the heterozygous and homozygous mutants (median 3.1 pmol/l; IQR: 3.2 pmol/l; n = 39; p = 0.001). This difference was also evident in the H. pylori negative subgroup (respectively, median 4.1 pmol/l; IQR: 3.7 pmol/l; n = 67 and median 2.4 pmol/l; IQR: 1.8 pmol/l; n = 19; p = 0.001), and a similar trend was seen in the H. pylori positive patients (Fig 1). The TLR4 homozygous wild types had also higher PGI (respectively, median 93.1 μg/l; IQR: 43.3 μg/l; n = 171 and median 80.2 μg/l; IQR: 45.8 μg/l; n = 39; p = 0.035) and PGII (respectively, median 11.2 μg/l; IQR: 9.8 μg/l; n = 177 and median 7.8 μg/l; IQR: 7.8 μg/l; n = 39; p = 0.005) serum levels compared to the heterozygous and homozygous mutants.
Fig 1. Dot diagram of gastrin-17 serum level distributions in the TLR4 +896/+1196 genotypes with median value lines.
P-values are calculated with Mann-Whitney U test.
As expected [8], serum G17 concentrations were higher in the H. pylori positive patients than in the negative ones (respectively, median 5.7 pmol/l; IQR: 5.2 pmol/l; n = 127 and median 3.3 pmol/l; IQR: 3.0 pmol/l; n = 86; p = 0.001). The use of proton pump inhibitor medication (n = 15) or other dyspepsia medication (n = 43) did not affect G17 serum levels significantly. To take account of the effects of multiple variables on the G17 levels, we performed a stepwise logistic regression model. In the model, the TLR4 wild type genotypes were more common in the patients who surpassed the upper reference limit for serum gastrin concentration provided by the test manufacturer (Biohit) of 7.0 pmol/l (27.2%; 58/213) with an OR of 2.835 (CI: 1.040–7.728; p = 0.042). This model also recognized H. pylori positivity as a risk factor for high gastrin levels (OR: 2.297; CI: 1.170–4.510; p = 0.016), but indicated the absence of role for age, use of medication, smoking and sex.
To assess if the observed effect of TLR4 +896 and +1196 polymorphisms on G17 levels affect the incidence of peptic ulcer and gastric cancer, we compared the genotype distributions between the subject groups. The ulcer group including both active and inactive ulcers was compared to the control group by a binary logistic regression analysis: the homozygous wild types had a significant association with peptic ulcers (OR: 2.423; CI: 1.022–5.746; p = 0.045) against heterozygous and homozygous mutants with age (per year OR: 1.098; CI: 1.070–1.126; p<0.001) and sex (male OR: 1.910; CI: 1.028–3.550; p = 0.041) as covariates. The duodenal and gastric ulcer groups displayed similar genotype distributions compared to each other but the separate tests were not statistically significant obviously due to low amount of patients. The TLR4 genotype distribution of the gastric cancer group did not differ significantly from the control subjects’ genotype distribution in a similar model.
To take the H. pylori and cagA status into account, we compared the active ulcer group to the non-ulcer dyspepsia group in crude and adjusted logistic regression models. In the crude model, the homozygous wild types displayed an increased risk for ulcers with an OR of 2.356 (Table 2). In the stepwise models, we used TLR4 polymorphisms, H. pylori or cagA positivity, sex, age and smoking as covariates. The first multifactorial model with H. pylori positivity as a cofactor recognized only H. pylori (OR: 55.79; CI: 12.84–242.3; p<0.001) and smoking (OR: 3.614; CI: 1.535–0.508; p = 0.003) as risk factors, which was unexpected based on the other results. However, on the second model, where we accounted for cagA positivity, we saw a statistically significant risk increase associated also with the TLR4 homozygous wild types (OR: 4.390).
Table 2. Peptic ulcer risk.
| Crude model | Adjusted model 4 | |||||
|---|---|---|---|---|---|---|
| P-value | OR 2 | CI 3 | P-value | OR | CI | |
| TLR4 homozygous wild type 1 | 0.046 | 2.356 | 1.014–5.473 | 0.032 | 4.390 | 1.134–16.998 |
| H. pylori positivity | <0.001 | 54.294 | 12.749–231.215 | - | - | - |
| cagA positivity | 0.046 | 5.128 | 1.030–25.529 | 0.037 | 6.221 | 1.117–34.644 |
| Smoking | <0.001 | 3.390 | 1.748–6.571 | 0.001 | 5.491 | 1.959–15.388 |
| Age, per year | 0.028 | 1.024 | 1.003–1.046 | - | - | - |
| Male | 0.194 | 1.471 | 0.822–2.633 | - | - | - |
Active peptic ulcer risk in comparison with the non-ulcer dyspepsia patients in logistic regression models.
1Versus +896/+1196 heterozygous and homozygous mutants
2Odds ratio
395% confidence interval
4Stepwise forward (likelihood ratio criteria) model with TLR4 polymorphisms, cagA positivity, smoking, age and sex as covariates.
We also analyzed the association of the TLR4 polymorphisms and the histological findings of the dyspepsia and ulcer patients’ gastric and duodenal biopsies. The TLR4 polymorphisms did not correlate with the Sydney system based variables of chronic or active gastritis, atrophy, intestinal metaplasia or H. pylori score (Table 3). The only difference was that the TLR4 homozygous wild types displayed slightly higher scores of duodenal lymphocytes (mean score 1.04 versus 0.92; p = 0.013).
Table 3. TLR4 genotypes and gastric histology.
| Helicobacter pylori negative | Helicobacter pylori positive | |||||
|---|---|---|---|---|---|---|
| TLR4 +896/+1196 genotype | Wild type | Mutant 1 | P-value 2 | Wild type | Mutant | P-value |
| Antrum mononuclear cells (0–3; n = 193) | 0.19 | 0.17 | 0.884 | 2.32 | 2.30 | 0.797 |
| Antrum neutrophils (0–3; n = 212) | 0.04 | 0.00 | 0.604 | 1.47 | 1.45 | 0.952 |
| Antrum atrophy (0–3; n = 209) | 0.05 | 0.00 | 0.373 | 0.95 | 1.25 | 0.162 |
| Body mononuclear cells (0–3; n = 204) | 0.21 | 0.16 | 0.706 | 1.89 | 1.89 | 0.864 |
| Body neutrophils (0–3; n = 207) | 0.00 | 0.00 | 1.000 | 0.66 | 0.78 | 0.626 |
| Body atrophy (0–3; n = 207) | 0.09 | 0.00 | 0.449 | 0.17 | 0.11 | 0.648 |
Variables are based on the Sydney system. Both non-ulcer dyspepsia and ulcer patients were included in analysis. Mean values are indicated.
1Heterozygotes and homozygotes
2Mann-Whitney U test
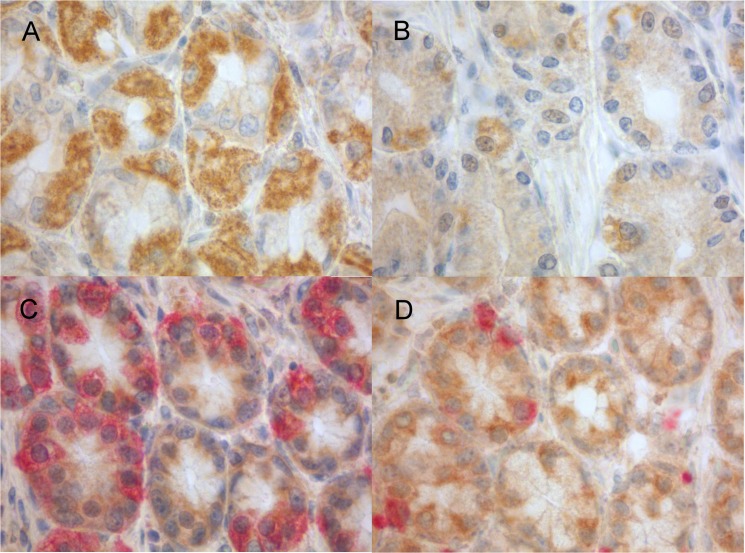
To assess the expression of TLR4 in relation to gastrin secreting G cells and somatostatin secreting D cells in the antral mucosa and expression patterns in the body mucosa, we used immunohistochemical single and double stainings for samples representing normal human antral and body mucosa (Fig 2). Strong TLR4 expression was seen in the cytoplasm of epithelial cells in gastric surface and the upper parts of the foveolar epithelium. In the antrum glandular neck zone, the epithelial cells were positive with weak to moderate expression levels. However, some cells were moderately to strongly positive and some cells were weakly stained or negative as were majority of cells in the antral glands. The double stained slides showed that majority of the gastrin expressing cells present in the glandular neck were TLR4 positive, and that the majority of somatostatin expressing cells were similarly TLR4 positive. These expression patterns suggest that the majority of moderately or strongly TLR4 positive cells in the antral glandular neck region are G cells and D cells. In the body glands TLR4 immunopositivity was present in the parietal cells, where the expression varied from mild to moderate but some individual cells were strongly positive.
Fig 2. Microphotographs demonstrating TLR4 expression in gastric mucosa.
The immunohistochemical staining of TLR4 expression in the body glands show expression mainly in the parietal cells (A), while in the glandular neck zone of the antrum (B) of stomach only occasional cells are positive. Double stainings (C, D) show TLR4 positivity (brown) in gastrin positive cells (red, C) and in somatostatin positive cells (red, D) in the antrum.
Discussion
To our knowledge, our study is the first one to document that the TLR4 +896/+1196 polymorphisms affect gastrin levels. The TLR4 homozygous wild type patients had higher G17 serum levels and had more reference limit surpassing scores in the multifactorial model. As an indirect evidence of possible physiological link between TLR4 and gastric secretion, we demonstrate TLR4 expression in the gastrin and somatostatin expressing cells of the antral mucosa. Additionally, we report an increased risk for peptic ulcer for the TLR4 +896/+1196 homozygous wild type patients over the double mutant polymorphism carriers, which is physiologically appropriate considering the G17 results. Since the ulcer risk genotype of TLR4 associated with high serum gastrin but not with gastric inflammation, we suggest that the role of TLR4 in the regulation of gastric secretion is more important in mediating the ulcer risk than its direct proinflammatory effects.
Hypergastrinemia and increased gastric acid output are well known abnormalities associated especially to the duodenal ulcer phenotype of H. pylori related gastroduodenal disease, but the specific mechanisms of H. pylori related hypergastrinemia are not known [8,9]. Animal models have previously documented a connection between activation of innate immunity and increased gastrin secretion [10]. Interestingly, murine G cells express TLR4, and in vitro experiments have shown that LPS, a major ligand of TLR4, induces secretion of gastrin from G cells presumably by TLR4 mediated mechanisms [11]. Somatostatin is the primary inhibitor of gastrin stimulated acid secretion with effects on the gastrin secretion of G cells and directly on parietal cells. In physiological conditions the secretion of somatostatin by D cells is induced by the acidity sensed by the cells. Gastric infection has been associated with the suppression of somatostatin secretion suggesting that either direct or indirect recognition of bacterial products is important in the regulation of D cells [19].
Our immunohistochemical studies of gastric antral mucosa indicate, that in addition to the expression in the surface and foveolar epithelium, TLR4 positivity is seen in the glandular neck zone and it is located predominantly in the gastrin and somatostatin expressing cells, i.e. G and D cells. In humans, the presence of TLR4 in G and D cells has not been previously reported. Schmausser et al. detected TLR4 in human foveolar epithelium but did not comment on G or D cells [20]. We speculate that TLR4–ligand interaction in G and D cells could have a direct effect in the gastrin and somatostatin secretion, respectively, and potentially explain the associations between the TLR4 polymorphisms and increased serum gastrin and the peptic ulcer risk. However, evaluation of the physiological role of TLR4 in human G and D cells needs additional studies. We observed also heterogeneous expression of TLR4 in parietal cells in the body mucosa. This has not been previously documented, and the physiological role of TLR4 expression in parietal cells needs further studies. However, TLR4 in parietal cells could also be involved in the innate immune defense by gastric acid regulation.
Physiologically, enhanced gastrin secretion in response to TLR4 activation can be considered appropriate as TLR4 is essential for the innate immunity against gram negative bacteria and gastric acid secretion is a part of the immunological surveillance mechanism in the gastrointestinal tract. The TLR4 +896 and +1196 wild type receptors have been documented to be more responsive to LPS than the mutant receptors [2]. So in cases where H. pylori is able to avoid or locally neutralize the low pH, the constant activation of wild type TLR4 +896/+1196 receptors could be hypothesized to lead to hypergastrinemia and increased acid load leading to an ulcer.
In our study, the higher gastrin levels in the TLR4 homozygous wild types could not be explained by atrophic gastritis as no association between atrophic gastritis and TLR4 genotypes was found. Also the PGI and PGII levels were similarly higher in the TLR4 homozygous wild types indicating functional secretion in relation to the increased gastrin levels both in the body and antrum of the stomach. According to our multivariate analyses, the G17 increase was only explained by the differences in the TLR4 genotypes and H. pylori positivity and, for example, not by the use of acid secretion affecting drugs including proton pump inhibitors.
The TLR4 +896/+1196 homozygous wild types and high G17 levels were linked also in the H. pylori negative subjects. This could result from TLR4 recognizing the LPS of other gram negative microbial flora present within the gastric contents. Interestingly, the Kidd et al. murine G cell model showed higher gastrin secretion stimulating potency for Salmonella enteritidis and Escherichia coli LPS than for H. pylori LPS [11].
TLR4 polymorphisms have been proposed to affect the probability of disease either through increased susceptibility for bacterial infections or through altered inflammatory states [1,2]. Defective TLR4 function has been suggested to lead to a pro-inflammatory response due to a deficiency in the anti-inflammatory interleukin 10 [1]. Previous studies on TLR4 polymorphisms and gastric inflammation have provided conflicting results: Achuyt et al. reported higher neutrophil scores for TLR4 +896 mutants and higher plasma cell scores for +1196 mutants [4]. Rigoli et al. associated +896 mutants to both antrum and body predominant gastritis and +1196 mutants to body predominant gastritis [6]. Bagheri et al. reported higher mononuclear cell scores for +896 mutants, no significant difference in polymorphonuclear cell scores, but still the wild types were more prevalent in active chronic gastritis group when compared to the chronic gastritis group [7]. There are also several studies where no associations between these TLR4 polymorphisms and gastric inflammation were seen [21–23]. In our study, we saw no difference in the prevalence of H. pylori infection or Sydney system based histological inflammatory markers between the TLR4 genotypes. The TLR4 homozygous wild types were only associated with a slightly increased lymphocyte count in duodenum, which could also be a secondary effect, related with the increased gastric acid secretion. However, since specimens of the actual ulcers were not studied, we cannot exclude the possibility of pro-inflammatory effects manifesting within or in the vicinity the ulcer.
There is only one study reporting an association between these TLR4 polymorphisms and peptic ulcer. The TLR4 +896 mutants were associated with duodenal ulcers in a study, which, however, had technical problems, as in over 40% of the duodenal ulcer patients the TLR4 +896 polymorphism could not be analyzed [5]. In our study, the risk of peptic ulcer was increased in the TLR4 homozygous wild types and it persisted also after accounting for other risk factors with multivariate analyses. Our ulcer group was heterogeneous in respect of the location of the ulcers and the significance of non-steroidal anti-inflammatory drug (NSAID) use cannot be excluded. However, it should be noted that in addition of having a key role in H. pylori related ulcers, gastric acid secretion also has a major role in NSAID related ulcers [24]. Thus TLR4 activation and its effects on gastrin and acid secretion might also have a role in the pathogenesis of NSAID ulcers.
We did not observe associations between the TLR4 genotypes and gastric cancer. TLR4 +896 and +1196 mutant polymorphisms have both been associated with gastric cancer risk in a meta-analysis [25]. The lack of association in our study might be due to the small amount of subjects or the composition of the cancer group.
In conclusion, we document here that the TLR4 +896/+1196 homozygous wild types have increased G17 serum levels. We also show that antral G and D cells express TLR4 suggesting a potential physiological link between TLR4 and the regulation of gastrin secretion. In line with the association with increased gastrin levels, we show that the TLR4 +896/+1196 homozygous wild types have an increased risk for peptic ulcers over the double mutant +896/+1196 allele carriers and the ulcer risk association was seen against the control group and the non-ulcer dyspepsia group. We suggest that the effects of TLR4 polymorphisms on gastrin could lead to the ulcer risk by enhanced TLR4 activation in G and D cells and the potentially following activation on gastric acid secretion. Confirmation of this pathophysiological link needs additional studies, but might provide possibilities for new treatment strategies in gastric acid related diseases, as many compounds able to activate or inhibit TLR4 have been recently recognized [26].
Supporting Information
(XLSX)
Acknowledgments
The authors thank Biohit Oyj, Finland, for generously performing the G17, PGI and PGII measurements from our samples.
The authors thank Mr. Manu Tuovinen for performing the immunohistochemical stainings.
The authors thank Professor Karl-Heinz Herzig for advice on the regulatory mechanisms of gastrin.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. El-Omar EM, Ng MT, Hold GL. (2008) Polymorphisms in toll-like receptor genes and risk of cancer. Oncogene 27: 244–252. 10.1038/sj.onc.1210912 [DOI] [PubMed] [Google Scholar]
- 2. Kutikhin AG. (2011) Impact of toll-like receptor 4 polymorphisms on risk of cancer. Hum Immunol 72: 193–206. 10.1016/j.humimm.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 3. Ferwerda B, McCall MB, Alonso S, Giamarellos-Bourboulis EJ, Mouktaroudi M, Izagirre N, et al. (2007) TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc Natl Acad Sci U S A 104: 16645–16650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Achyut BR, Ghoshal UC, Moorchung N, Mittal B. (2007) Association of toll-like receptor-4 (Asp299Gly and Thr399Ileu) gene polymorphisms with gastritis and precancerous lesions. Hum Immunol 68: 901–907. [DOI] [PubMed] [Google Scholar]
- 5. Trejo-de la OA, Torres J, Perez-Rodriguez M, Camorlinga-Ponce M, Luna LF, Abdo-Francis JM, et al. (2008) TLR4 single-nucleotide polymorphisms alter mucosal cytokine and chemokine patterns in mexican patients with helicobacter pylori-associated gastroduodenal diseases. Clin Immunol 129: 333–340. 10.1016/j.clim.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 6. Rigoli L, Di Bella C, Fedele F, Procopio V, Amorini M, Lo Giudice G, et al. (2010) TLR4 and NOD2/CARD15 genetic polymorphisms and their possible role in gastric carcinogenesis. Anticancer Res 30: 513–517. [PubMed] [Google Scholar]
- 7. Bagheri N, Azadegan-Dehkordi F, Sanei H, Taghikhani A, Rahimian G, Salimzadeh L, et al. (2014) Associations of a TLR4 single-nucleotide polymorphism with H. pylori associated gastric diseases in iranian patients. Clin Res Hepatol Gastroenterol 38: 366–371. 10.1016/j.clinre.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 8. McColl KE, Gillen D, El-Omar E. (2000) The role of gastrin in ulcer pathogenesis. Baillieres Best Pract Res Clin Gastroenterol 14: 13–26. [DOI] [PubMed] [Google Scholar]
- 9. Chu S, Schubert ML. (2013) Gastric secretion. Curr Opin Gastroenterol 29: 636–641. 10.1097/MOG.0b013e328365efc7 [DOI] [PubMed] [Google Scholar]
- 10. Fukui T, Nishio A, Okazaki K, Uza N, Ueno S, Kido M, et al. (2006) Gastric mucosal hyperplasia via upregulation of gastrin induced by persistent activation of gastric innate immunity in major histocompatibility complex class II deficient mice. Gut 55: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kidd M, Hauso O, Drozdov I, Gustafsson BI, Modlin IM. (2009) Delineation of the chemomechanosensory regulation of gastrin secretion using pure rodent G cells. Gastroenterology 137: 231–41, 241.e1-10. 10.1053/j.gastro.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 12. Koskela RM, Niemela SE, Lehtola JK, Bloigu RS, Karttunen TJ. (2011) Gastroduodenal mucosa in microscopic colitis. Scand J Gastroenterol 46: 567–576. 10.3109/00365521.2011.551889 [DOI] [PubMed] [Google Scholar]
- 13. Koivurova OP, Karhukorpi JM, Joensuu ET, Koistinen PO, Valtonen JM, Karttunen TJ, et al. (2003) IL-1 RN 2/2 genotype and simultaneous carriage of genotypes IL-1 RN 2/2 and IL-1beta-511 T/T associated with oesophagitis in helicobacter pylori-negative patients. Scand J Gastroenterol 38: 1217–1222. [DOI] [PubMed] [Google Scholar]
- 14. Pohjanen VM, Koivurova OP, Makinen JM, Karhukorpi JM, Joensuu T, Koistinen PO, et al. (2013) Interleukin 6 gene polymorphism -174 is associated with the diffuse type gastric carcinoma. Genes Chromosomes Cancer 52: 976–982. 10.1002/gcc.22093 [DOI] [PubMed] [Google Scholar]
- 15. Lorenz E, Frees KL, Schwartz DA. (2001) Determination of the TLR4 genotype using allele-specific PCR. BioTechniques 31: 22–24. [DOI] [PubMed] [Google Scholar]
- 16. Karhukorpi J, Yan Y, Kolho KL, Rautelin H, Lahti M, Sirvio A, et al. (2000) cagA, vacA and iceA virulence genes of helicobacter pylori isolates of children in finland. Eur J Clin Microbiol Infect Dis 19: 790–793. [DOI] [PubMed] [Google Scholar]
- 17. Eiro N, Gonzalez-Reyes S, Gonzalez L, Gonzalez LO, Altadill A, Andicoechea A et al. (2012) Duodenal expression of toll-like receptors and interleukins are increased in both children and adult celiac patients. Dig Dis Sci 57: 2278–2285. 10.1007/s10620-012-2184-6 [DOI] [PubMed] [Google Scholar]
- 18. Misiewicz JJ. (1991) The sydney system: A new classification of gastritis. introduction. J Gastroenterol Hepatol 6: 207–208. [DOI] [PubMed] [Google Scholar]
- 19. Zavros Y, Merchant JL. (2005) Modulating the cytokine response to treat helicobacter gastritis. Biochem Pharmacol 69: 365–371. [DOI] [PubMed] [Google Scholar]
- 20. Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Muller-Hermelink HK, et al. (2004) Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in helicobacter pylori infection. Clin Exp Immunol 136: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy G, Thornton J, McManus R, Swan N, Ryan B, Hughes DJ, et al. (2009) Association of gastric disease with polymorphisms in the inflammatory-related genes IL-1B, IL-1RN, IL-10, TNF and TLR4. Eur J Gastroenterol Hepatol 21: 630–635. 10.1097/MEG.0b013e3283140eea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hofner P, Gyulai Z, Kiss ZF, Tiszai A, Tiszlavicz L, Toth G, et al. (2007) Genetic polymorphisms of NOD1 and IL-8, but not polymorphisms of TLR4 genes, are associated with helicobacter pylori-induced duodenal ulcer and gastritis. Helicobacter 12: 124–131. [DOI] [PubMed] [Google Scholar]
- 23. Kato I, Canzian F, Plummer M, Franceschi S, van Doorn LJ, Vivas J, et al. (2007) Polymorphisms in genes related to bacterial lipopolysaccharide/peptidoglycan signaling and gastric precancerous lesions in a population at high risk for gastric cancer. Dig Dis Sci 52: 254–261. [DOI] [PubMed] [Google Scholar]
- 24. Wallace JL. (2012) NSAID gastropathy and enteropathy: Distinct pathogenesis likely necessitates distinct prevention strategies. Br J Pharmacol 165: 67–74. 10.1111/j.1476-5381.2011.01509.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen J, Hu S, Liang S, Chen Q, Yang Q, Zheng W, et al. (2013) Associations between the four toll-like receptor polymorphisms and the risk of gastric cancer: A meta-analysis. Cancer Biother Radiopharm 28: 674–681. 10.1089/cbr.2012.1395 [DOI] [PubMed] [Google Scholar]
- 26. Peri F, Calabrese V. (2013) Toll-like receptor 4 (TLR4) modulation by synthetic and natural compounds: An update. J Med Chem./References [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.