Abstract
A commonly activated signaling cascade in many human malignancies including glioblastoma multiforme (GBM) is the Akt pathway. This pathway can be activated via numerous upstream alterations including genomic amplification of EGFR, PTEN deletion, or PIK3CA mutations. In this study, we screened PI3K/Akt small molecule inhibitors in an isogenic cell culture system with an activated Akt pathway secondary to a PIK3CA mutation. One small molecule, A-443654, demonstrated the greatest selective inhibition of cells with the mutant phenotype. Based on these findings, this inhibitor was screened in vitro against a panel of GBM cell lines. All cell lines tested were sensitive to A-443654 with a mean IC50 of approximately 150 nM. An analogue of A-443654, methylated at a region that blocks Akt binding, decreased activity by an average of 36 fold. Caspase assays and dual flow cytometric analysis demonstrated an apoptotic mechanism of cell death. A-443654 was further tested in a rat intracranial model of GBM. Animals treated intracranially with polymers containing A-443654 had significantly extended survival compared to control animals; animals survived 79% and 43% longer than controls when A-443654-containing polymers were implanted simultaneously or in a delayed fashion, respectively. This small molecule also inhibited GBM stem-like cells with similar efficacy compared to traditionally cultured GBM cell lines. These results suggest that local delivery of an Akt small molecule inhibitor is effective against experimental intracranial glioma, with no observed resistance to GBM cells grown in stem cell conditions.
INTRODUCTION
Advances over the past few decades have improved the understanding of glioma tumorigenesis, proliferation, and invasion. The serine/threonine kinase Akt/PKB pathway is a nodal point regulating numerous tumor-associated processes including cell growth, cell cycle progression, survival, migration and angiogenesis, and has been shown to be important in many malignancies including glioblastoma. More specifically, he Akt pathway has been shown to be activated in the majority of GBMs (1, 2). In other studies, activation of the Akt pathway in a human astrocytic model of glioma resulted in conversion of anaplastic astrocytoma to GBM (3), and the combined activation of Akt and Ras in neural progenitors induced GBM formation in a murine model (1). Recently, activation of PIK3CA and Akt pathway members has been shown to be associated with reduced patient survival times (4, 5). In addition to PTEN deletion or genomic amplification of growth factor receptors such as EGFR, activating mutations in PIK3CA (a PI3 kinase gene) have been identified in many cancers, including adult and pediatric GBMs and that these mutations also activate the Akt pathway (6–8).
In this study, we screened inhibitors of the PI3K/Akt pathway in a genetically controlled cell culture system in which the Akt pathway was activated. Based on the data obtained from our initial screen, we further tested a small molecule Akt inhibitor in vitro against traditionally cultured GBM cell lines and GBM stem-like cell (GSLC) lines and in vivo in a rat intracranial gliosarcoma model.
METHODS
Cell lines and culture conditions
D-PIK3CA #127 (WT D-PIK-Ex1–2) and D-PIK3CA #129 (MUT D-PIK Ex 1–7) (7) were grown in McCoy’s 5A medium supplemented with 10% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. The human GBM cell lines D 54-MG, H80, H247, H263, H392, H397, H502, H542, H566, H1477, U 87-MG, and 1028S and the rat 9L gliosarcoma cell line were grown in DMEM supplemented with 10% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. The human GBM cell lines SK-15-MG, SK-17-MG, SK-21-MG, and SK-26-MG were grown in RPMI 1640 supplemented with 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 100 μM MEM non-essential amino acids. The human GSLC lines HSR-1a, 040622, 050509, and 060919 were grown in NeuroCult NS-A basal medium (StemCell Technologies) containing NeuroCult NS-A proliferation supplements (StemCell Technologies), 20 ng/ml hEGF (PeproTech), 10 ng/ml hFGF2 (PeproTech), and 2 μg/ml heparin (StemCell Technologies). All cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Drugs
Akt inhibitor III (SH-6), Akt inhibitor IV, Akt inhibitor V (Triciribine, API-2, NSC 154020, TCN), Akt inhibitor VIII (Akti-1/2), Ly 294002, Naltrindole hydrochloride (NTI), and Wortmannin were purchased from Calbiochem. A-443654 and its methylated analogue 2-methyl A-443654 (A-739985) were obtained from Abbott Laboratories. All compounds were dissolved in DMSO except for NTI, which was dissolved in water.
Protein extract preparation and immunoblotting
Cytoplasmic protein lysates were made from cells during exponential growth using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biochemicals) containing Halt Protease and Phosphatase inhibitors cocktails (Pierce) according to the recommendations of the manufacturer. Forty micrograms of the lysates were heated to 95°C in Laemmli sample buffer for 10 min and separated on SDS-polyacrylamide gels. Proteins were transferred to PVDF membranes (Bio-Rad) in Western transfer buffer (25 mM Tris, pH 8.3, 192 mM glycine, and 20% methanol). For Western blot analysis, membranes were blocked for 1 hour at room temperature in 5% non-fat dry milk in TBST (1 × TBS, 0.1% Tween-20) and incubated overnight with at 4°C with antibodies against Akt, phospho-Akt (Thr308), phospho-Akt (Ser473) (Cell Signaling Technology), or GAPDH (Santa Cruz Biotechnology). After washing, membranes were incubated with a horseradish peroxidase-linked goat anti-rabbit antibody for 1 hour at room temperature. Antibody detection was achieved by chemiluminescence according to the manufacturer’s recommendations (Pierce).
Cytotoxicity assays
For the initial PI3K/Akt small molecule inhibitor screen, 2000–3000 cells containing either a wild type (#127) or mutant (#129) PIK3CA gene were plated in 200 ul in 96 well plates. The following day, cells were treated with vehicle (DMSO or water) or 1 uM and 10 uM of Akt inhibitors III, IV, V, VIII, A-443654, NTI, and Wortmannin for 48 hours. Ly294002 was used at 50 μM as a positive control (7). For determination of IC50 values, 1500–4000 GBM cells grown in serum or 5000–8000 GSLC lines grown in NeuroCult media were plated in 96 well plates in 200 μl of respective media and treated the following day with vehicle (DMSO) or various concentrations of A-443654 (1 nM, 5 nM, 10 nM, 50 nM, 100 nM, 500 nM, 1 μM, 5 μM, and 10 μM) and A-739985 (1 nM, 5 nM, 10 nM, 50 nM, 100 nM, 500 nM, 1 μM, 5 μM, 10 μM, and 12.5 μM) for 48 hours. CCK-8 assays were performed according the recommendation of the manufacturer (Alexis Biochemicals). Briefly, 20 μl of CCK-8 were added to each well and incubated at 37°C. Absorbance at 450 nM was measured using the Victor3 microplate reader (Perkin-Elmer). IC50 values were calculated by GraphPad Prism (GraphPad Software, San Diego, CA).
Caspase activity assays
Caspase -3 and -7 activities were detected in cell lysates after various treatments using the Caspase-Glo 3/7 Assay according to the recommendations of the manufacturer (Promega). Luminescence was measured on a Victor3 microplate reader (Perkin Elmer). Experiments were performed in triplicate, with each experiment containing five or six replicates.
Polymer preparation
Polymers were synthesized by incorporating A-443654 into the polyanhydride polymer poly[1,3-bis(carboxyphenoxy)propane-co-sebacic-acid] (CPP:SA). Both substances were mixed, dissolved in N-N dimethyl formamide (Sigma), briefly sonicated, and dried in a vacuum dessicator overnight. The polymers were molded into disks weighing 10 mg by a stainless steel molding press. Control CPP:SA polymers containing no drug were made in a similar fashion.
Animals
Female Fisher 344 rats weighing 150–200 g were used for this study. The animals were housed in standard animal facilities with up to 4 rats per cage and allowed free access to Baltimore city water and rodent chow. All animal protocols were approved by the Animal Care and Use Committee of The Johns Hopkins University School of Medicine.
Surgical technique
Intracranial tumors and polymers were implanted in an analogous fashion. Female Fischer 344 rats were anesthetized and their heads shaved and prepared with povidone-iodine. A midline scalp incision was made and the coronal and sagittal sutures were identified. Using an electric drill, a 3 mm burr hole was made 3 mm lateral to the sagittal suture and 5 mm posterior to the coronal suture. The dura was incised sharply and a small amount of cortex and white matter was removed with gentle suction. To determine the maximally tolerated A-443654 dose, rats underwent intracerebral implantation of wafers containing 0 (n=8), 5% (n=3), 20% (n=3), 30% (n=8), 40% (n=7), and 50% (n=8) A-443654 CPP:SA wafers. For efficacy studies, 9L gliosarcoma grown in the flank of female carrier Fischer rats was excised, cut into 1-mm3 pieces, and placed intracranially. For animals receiving simultaneous implantation of tumor and polymers, the tumor piece was implanted first and the polymer was placed next to it. After implantation of tumor and/or polymers, the skin was closed with surgical staples. The delayed treatment group received tumor alone on day 0 and then underwent a second operation on day 4. On day 4, the animals were anesthetized and prepped as above. The midline scalp incision was reopened and the burr hole identified. Control or A-443654 CPP:SA polymers were placed into the original defect and the scalp incision was closed with surgical staples. Animals were closely monitored for signs of toxicity, including failure to thrive, weight loss, and neurological deficits.
Statistical analysis
For the intracranial efficacy studies, death was the primary endpoint. Statistical analysis was performed using the Kaplan-Meier method and the significance of survival differences was evaluated with the log-rank test using the software GraphPad Prism 4. P-values of less than 0.01 were considered statistically significant.
RESULTS
In vitro isogenic cell line screen of PIK3CA/AKT inhibitors
The effects of several PI3K/Akt inhibitors were initially investigated in an isogenic cell line culture system. In this isogenic system, cells contained either a wild-type (#127) or mutant (#129) PIK3CA with more Akt pathway activation in the mutant PIK3CA-containing cells (7). We tested a panel of known PI3K/Akt inhibitors in this system, seeking selective inhibition of the mutant lines relative to the wild-type control. Cells were plated in 96 well plates and treated with either vehicle, 1 uM or 10 uM of Akt inhibitors III, IV, V, VIII, A-443654, NTI, and Wortmannin and assayed for cell proliferation over the course of four days (Figure 1). Ly294002 was used at 50 μM as this concentration has previously been shown to result in reduced cell proliferation to a greater extent in the mutant cells than in the WT cells (7). Our results with this concentration (Figure 1H) were similar to those previously published (7).
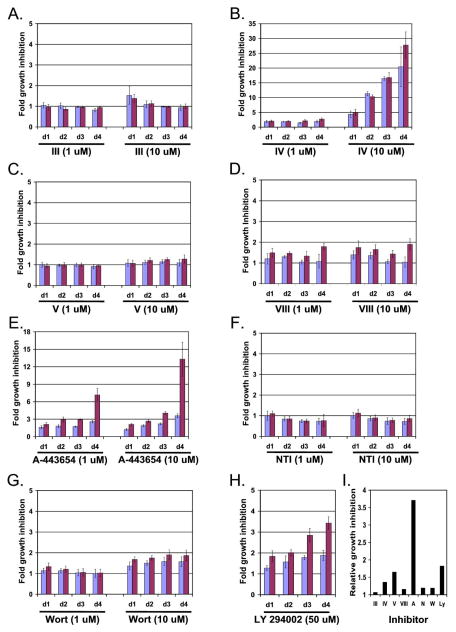
Figure 1.
Among various known Akt inhibitors, A-443654 shows the greatest relative activity against PIK3CA mutant cells, compared to isogenic cells without the mutation. (A–G) Cells containing either a WT (blue bars) or mutant (red bars) PIK3CA gene were treated with 1 or 10 uM of Akt inhibitors III, IV, V, VIII, A-443654, NTI, and Wortmannin for 24 (d1), 48 (d2), 72 (d3) or 72 (d4) hours. (H) Cells containing either a WT (blue bars) or mutant (red bars) PIK3CA gene were treated with 50 μM LY 294002 for 24 (d1), 48 (d2), 72 (d3), or 96 (d4) hours. For (A)–(H), CCK-8 proliferation assays were performed and the results were normalized to vehicle-treated cells. Data represents the average of 5 or 6 replicates. (I) Relative growth inhibition of cells containing a mutant vs a WT PIK3CA treated with 10 μM of Akt inhibitors III, IV, V, VIII, A-443654, NTI, and Wortmannin, and 50 μM LY 294002 at 96 hours.
At the concentrations tested, Akt inhibitors III and V, NTI, and Wortmannin did not demonstrate any significant selective effect between the mutant and WT cells (Figure 1A, C, F, and G). Akt inhibitor IV resulted in reduced cell proliferation in both the mutant and WT cells at 10 μM (Figure 1B). Akt inhibitor VIII demonstrated some selectivity for the mutant cells at both concentrations especially at day 4 (Figure 1D). A-443654 demonstrated the greatest selective effect on the mutant cells compared to the WT cells with greater than 3.5 fold relative growth inhibition of the mutant cells (Figure 1E and I). Based on these findings, this small molecule was selected for further analysis.
Akt is activated in the majority of GBM cell lines
To determine if Akt signaling was active in the GBM cell lines used to test growth inhibition, a series of 16 traditionally cultured GBM cell lines was evaluated using Western blotting with antibodies specific for Akt and phosphorylated Akt. Moderate to very strong phoshorylation at one or both known phosphorylation sites of Akt was observed in 15 of 16 GBM lines (Figure 2A). One cell line, H502, had, in comparison to the other lines, a very small amount of phosphorylated Akt, which was observed only with longer exposure.
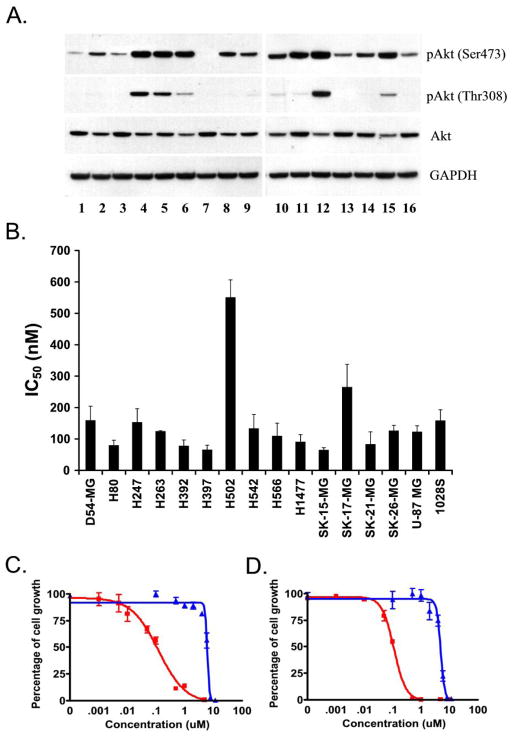
Figure 2.
(A) Akt activity is elevated in human GBM cell lines. Whole cell extracts from human GBM cell lines cultured in 10% serum were analyzed by Western blot analysis using an anti-Akt, anti-phosho Akt (Thr 308), and anti-phospho Akt (Ser 473) antibodies. Blots were probed with an anti-GAPDH antibody as a loading control. (B) A-443654 inhibits growth of GBM cell lines. GBM cell lines cultured in 10% serum were treated with increasing concentrations of A-443654 or an equal volume of DMSO as a control and 48 hour IC50s were calculated. Data represents at least three independent experiments with each containing five or six replicates. (C and D) Cell proliferation assays of D54-MG (C) and H566 (D) treated for 48 hours with A-443654 (red lines) and A-739985, a methylated control that does not bind Akt (blue lines).
A-443654 inhibits GBM growth in vitro
The growth inhibitory effect of A-443654 was evaluated in the 16 GBM cell lines using a cell proliferation assay. Cells were plated in 96 well plates and treated for 48 hours with increasing concentrations of A-443654. All cell lines tested were sensitive to A-443654 with a mean IC50 of 147 nM (range: 64–550 nM). Interestingly, the cell line with the lowest amount of phosphorylated Akt, H502, had the highest IC50 (Figure 2A and 2B), suggesting that A-443654 efficacy is indeed related to interfering with activated Akt signaling. A-443654 is a pan-Akt inhibitor which binds to the ATP site of Akt and inhibits Akt-dependent signal transduction (9). To provide further evidence that A-443654’s mechanism of action involves binding and inhibition of Akt, all sixteen GBM cell lines tested above were plated in 96 well plates and treated for 48 hours with increasing concentrations of either A-443654 or its methylated analogue 2-methyl A-443654 (A-739985), which has a significantly reduced binding affinity for Akt. Cells were less sensitive to the methylated analogue by an average of 36 fold. Cell line H502 had the least difference with 4 fold reduced potency; cell line SK-15-MG had the greatest difference between A-443654 and the methylated analog (76 fold). Two examples are shown in Figure 2C (cell line D54-MG) and 2D (cell line H247). This reduced potency of the methylated analogue supports the Akt-mediated activity of A-443654.
A-443654 induces apoptosis in vitro
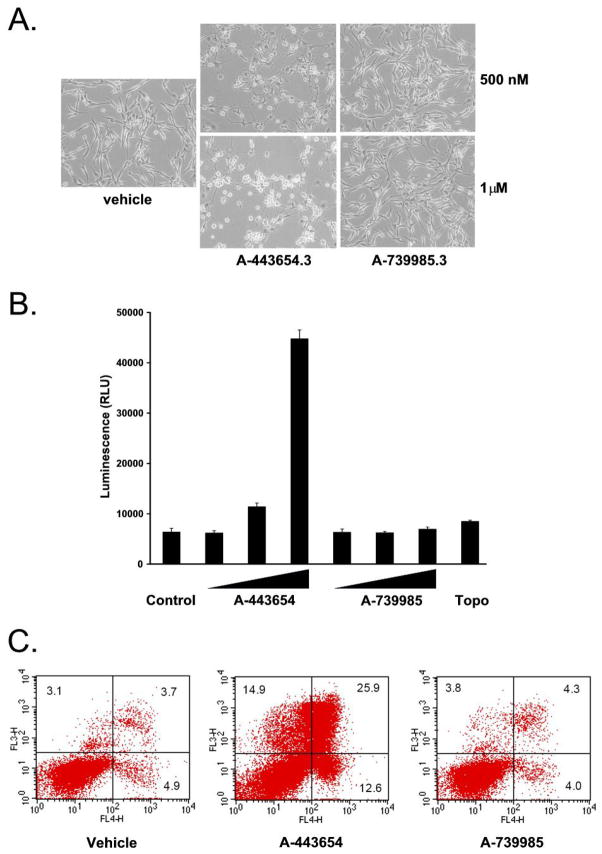
Akt inhibitors including A-443654 have been shown to induce apoptosis (9–11). To investigate the mechanism of cell death in GBM, U-87 MG cells were treated with A-443654 and A-739985. After 24 hours, cells treated with A-443654 lost their astrocytic appearance and were rounder in comparison to control and A-739985 treated cells (Figure 3A). To further determine if the inhibition of GBM cell lines were secondary to an apoptotic mechanism, caspase and dual annexin V/7-amino-actinomycin D flow cytometric assays were performed. U-87 MG cells were treated for 24 hours with increasing concentrations of A-443654 and A-739985. As shown in Figure 3B, caspase activity was induced after exposure to A-443654 but not after exposure to A-739985. In the next series of experiments, U-87 MG cells were treated with vehicle, A-443654, or A-739985 for 24 hours and then stained with annexin V to measure phosphatidyl-serine translocation to the extracellular membrane (early apoptosis) and 7-amino-actinomycin D to measure the loss of phospholipid membrane integrity (late apoptosis/necrosis). Treatment of U-87 MG cells with A-443654 resulted in an increase in both the early apoptotic and late apoptotic/necrotic cells compared to the vehicle-treated cells and cells treated with A-739985 (Figure 3C). Together, these data suggest that the inhibition of GBM cell lines by A-443654 may, at least in part, occur via an apoptotic mechanism.
Figure 3.
A-443654 induces apoptosis in vitro. (A) U87-MG cells were treated for 24 hours with either vehicle (DMSO), 500 nM, or 1 μM of either A-443543 or 739985 and photographed. (B) U87-MG cells were treated with vehicle (DMSO), A-443654 or A-739985 at 500 nM, 1 μM or 5 μM or Topotecan at 20 μM. After 24 hours of treatment, caspase activity was determined. (C) U87-MG cells were treated with 5 μM of either A-443654 or A-739985 for 24 hours. Two color flow cytometric analysis was performed using FITC annexin V and 7-amino-actinomycin D. The percent of viable (lower left), early apoptotic (lower right), and both late apoptotic and necrotic cells (upper right) is indicated. Data shown in all panels is representative of at least three independent experiments.
Rat glioma cell line 9L has activated Akt
Our next major goal was to test the ability to inhibit tumor growth in vivo using a well-defined system. The rat gliosarcoma line 9L is commonly used as a high-grade glioma model and forms aggressive, invasive intracranial tumors. To first determine whether the cell line in this model has the presumed target, cells were examined by Western blot analysis for phosphorylated Akt. As shown in Figure 4A, 9L cells express phosphorylated Akt. Cell proliferation assays with 9L cells demonstrated similar IC50 values for A-443654 (IC50 = 100 nM) and A-739985 (IC50 2.8 μM) (Figure 4B) to the panel of human traditionally cultured GBM cell lines tested earlier. Together, these data suggest that this cell line represents an appropriate model for in vivo testing of A-443654.
Figure 4.
A-443654 increases survival in a syngeneic rat glioma model. (A) Akt activity is elevated in the 9L rat gliosarcoma cell line. Whole cell extracts from 9L cells (lane 1) were analyzed by Western blot analysis using an anti-Akt, anti-phosho Akt (Thr 308), and anti-phospho Akt (Ser 473) antibodies. U87-MG (lane 2) and normal Fischer rat brain (lane 3) were included as positive and negative controls, respectively. Blots were probed with an anti-GAPDH antibody as a loading control. (B) Cell proliferation assays of 9L cells treated for 48 hours with A-443654 (red lines) and A-739985 (blue lines) showing sensitivity to the A-443654. (C) Intracranial toxicity of A-443654 delivered in concentrations of 0% drug to 50% drug in CPP:SA polymer. A-443654-loaded CPP:SA polymers containing 0%, 20%, 30%, 40%, and 50% A-443654 were implanted intracranially in non-tumor bearing animals. Animals receiving the 50% and 40% A-443654 CPP:SA polymers began dying 7 and 9 days after implantation, respectively, and 50% or more of the animals were dead 21 days after implantation. Concentrations below 30% were well tolerated. There was one late death in the 30% A-443654 polymer group at day 65. (D) Kaplan-Meier survival curves for control F344 rats (no polymer), animals that received simultaneous tumor implantation (Day 0) of either empty or A-443654 containing polymers, and animals treated 4 days after tumor implantation (Day 4) with either empty or A-443654 containing polymers 4 days after tumor implantation. Animals treated with polymers containing A-443654 on day 0 or 4 had significantly extended survival compared to control animals. The median survival for the control animals (no polymer), control animals treated with empty polymers on day 0, and control treatment animals treated with empty polymers on day 4 were each 14 days vs 20 days for the A-443654 treated animals on day 4 (p<0.0001) and 25 days for the A-443654 treated animals on day 0 (p=0.0006).
A-443654 polymer CNS toxicity studies
The next question addressed was how best to deliver A-443654 for animal testing. Since systemic administration of A-443654 in animals has resulted in toxicities such as abnormal glucose metabolism and weight loss (9), we evaluated local delivery of A-443654 to the rat central nervous system (CNS). The polymer employed was poly[1,3-bis(carboxyphenoxy)propane-co-sebacic-acid] (CPP:SA), the same polymer used for the manufacture of the clinically approved Gliadel® wafer used to deliver carmustine (BCNU) to high-grade gliomas. A-443654-loaded CPP:SA polymers containing 0%, 20%, 30%, 40%, and 50% A-443654 were implanted intracranially in non-tumor bearing animals. A dose-dependent toxicity was observed. Animals receiving the 50% and 40% A-443654 CPP:SA polymers began dying at days 7 and 9, respectively, and 50% or more of the animals were dead 21 days after implantation in these two treatment groups. Concentrations below 30% were well tolerated. There was one late death in the 30% A-443654 polymer group at day 65. This animal had a cystic cavity under the implantation site. Based on these findings, an efficacy study was performed utilizing 30% A-443654 CPP:SA polymers.
A-443654 extends survival in an intracranial glioma animal model
To determine the effectiveness of local delivery of A-443654, tumor bearing animals underwent implantation of blank or 30% A-443654 polymers either at day 0 or at day 4. Rats that received blank polymers either on day 0 or day 4 had a median survival of 14 days, similar to untreated control rats (Figure 4C). The median survival of rats (n=8) that received 30% polymers implanted on the same day as the tumor increased to 25 days (p=0.0006), with one animal living longer than 400 days (LTS-long term survivor). This represents a 79% increase in survival compared to controls. This LTS was euthanized on day 450 and had no histopathological evidence of tumor (data not shown). The median survival of rats (n=8) that received tumor followed by 30% A-443654 polymers four days later increased to 20 days (p<0.0001). Although there were no LTS in this group, survival was increased by 43%.
A-443654 inhibits growth of GBM stem-like cells
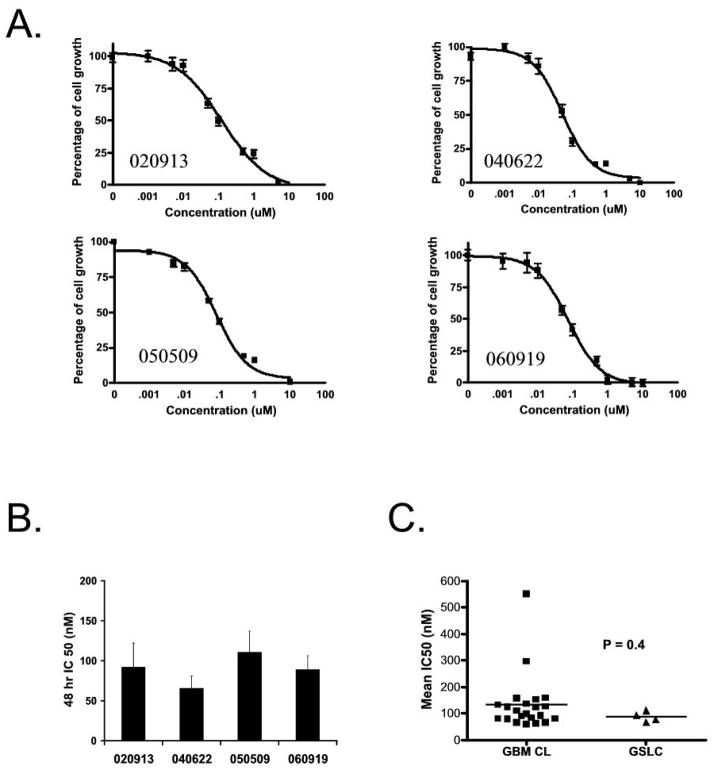
Accumulating evidence has suggested that stem-like precursors exist in GBMs. These cells display extensive self-renewal in vitro and in vivo and are multipotent (12, 13). We next examined the effect of A-443654 on GSLC lines, which are neurosphere cultures of GBMs that are thought to maintain a stem-like cell component. GBM neurosphere-forming cells were isolated from human GBM samples as previously described (14). Flow cytometric analysis was performed on four of these GSLC lines and four traditionally cultured adherent GBM cell lines for the cell surface marker CD133, the best characterized marker to date of the GBM stem-like population. The majority of cells in the neurosphere cultures were positive for CD133 compared to the traditionally cultured GBM cell lines, which had no or little CD133 expression (less than 5%) (data not shown). Proliferation assays were then performed in the four GSLC lines with increasing concentrations of A-443654 (Figure 5A). The 48 hour IC50s ranged from 66–110 nM (Figure 5B). There was no statistical difference between the 48 hour IC50s of the four GSLC compared to the sixteen traditionally cultured cell lines (Figure 5C), suggesting that inhibiting this pathway can kill both cells grown in serum as well as cells grown serum free media containing EGF and FGF2.
Figure 5.
A-443654 inhibits GSLC. (A) Cell proliferation assays of four GSLC lines treated with increasing concentrations of A-443654 for 48 hours. (B) IC50s of GSLC lines at 48 hours treated with A-443654. Data represents at least three independent experiments with each containing five or six replicates. (C) The IC50s at 48 hours of four GSLC lines cultured in serum-free media containing EGF and FGF2 have low IC50s and no significant difference in sensitivity compared to GBM lines grown in serum containing media (P = 0.4).
DISCUSSION
Targeting kinases in human malignancies including GBM represents an active area of research and there are numerous kinase inhibitors currently under investigation in human clinical trials. In this study we screened a panel of PI3K and Akt inhibitors using a PIK3CA isogenic cell culture knockout system in which one cell line possessed a wild-type PIK3CA and the other, a mutant PIK3CA and an activated Akt pathway. One small molecule, A-443654, possessed superior activity against the cells containing the mutant PIK3CA compared to the cells with the wild-type PIK3CA. This molecule was further tested against a panel of traditionally cultured GBM cell lines and displayed activity against all lines. Consistent with previous studies (10), the activity of this compound against this panel of GBM cell lines was, at least in part, due to induction of apoptosis. Our data also demonstrate that local delivery of this inhibitor is efficacious against an intracranial model of GBM. Furthermore, we were able to demonstrate that neurosphere cultures with a large CD133 population were equally sensitive to this Akt inhibitor compared to GBM cell lines cultured in serum.
The Akt signaling pathway is very important in GBM. Previous studies have demonstrated that this pathway is activated in the majority of primary GBM samples (1, 2) as well as in xenografts derived from GBM tumor samples (15). We observed a similar rate of Akt pathway activation in the panel of sixteen GBM cell lines examined in this study (Figure 2A) as well as in the GSLC lines (data not shown). Additional experimental evidence for the importance of this pathway includes the formation of GBM in several animal models (1, 3) as well as the poorer survival of patients in whom this pathway is activated (4, 5). Akt represents a nodal point in cell signaling and can be activated by several upstream events including EGFR amplification or mutation, loss of PTEN, and PIK3CA mutation. As such, targeting this pathway may be able to block GBM proliferation secondary to a variety of upstream etiologies.
There are several Akt inhibitors which have been described in GBM model systems (16, 17). Koul et al. reported two Akt-targeting small molecules, KP-372-1 and KP-372-2, which inhibited the in vitro growth of six GBM cell lines and found that this inhibition was due to the induction of apoptosis. Another study by Momota et al. demonstrated that perifosine, an oral Akt inhibitor, inhibited Akt and Ras-Erk 1/2 pathways in murine glial progenitors engineered to have activated Akt and Ras pathways and induced a G1 and G2 cell cycle arrest. Additionally, this group demonstrated that perifosine reduced proliferation of a murine PDGF-driven glioma model in vivo. Taken together, these observations along with our current study suggest that pharmacologic targeting of Akt represents a potential therapeutic strategy for GBM. Moreover, the synergy observed between A-443654 and chemotherapeutic agents in non-glial models (9, 10) as well as the increased radiosensitivity observed with targeting the PI3K/Akt pathway in GBM (18, 19) raise interesting possibilities regarding treatment regimens which combine A-443654 with other therapeutic modalities such as temozolomide and cranial radiotherapy.
Because of the reported systemic toxicity or A-443654 and other inhibitors of the Akt/PKB pathway, we chose to utilize local intracranial delivery. More specifically, A-4435654 was incorporated into a biodegradable polymer matrix. Results from our in vivo study demonstrate that these A-443654 containing polymers delivered locally either at the time of tumor implantation or in a delayed fashion prolonged survival in an experimental intracranial rodent GBM model.
There is emerging evidence for the existence of GSLCs and that these cell lines may better represent primary human tumors (20, 21). Additionally, there are numerous studies suggesting that these cells may be chemo- and radio-resistant (22–24). Interestingly, in our study, we did not find any difference in the IC50s of traditionally cultured glioma lines compares to GSLCs. Although further investigations will be necessary to understand this effect, it is of note that activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells (25), suggesting that targeting the Akt pathway may be efficacious in both stem and non-stem cell populations of GBMs.
Acknowledgments
PIK3CA knockout cell lines (#127 and #129) were kindly provided by Bert Vogelstein (Johns Hopkins University). This work was supported by the National Institutes of Health (R01 NS052507 to GJR), Virginia and DK Ludwig Fund for Cancer Research (GJR), Irving J. Sherman Research Professorship (GJR) and the Johns Hopkins University Department of Neurosurgery (GLG).
References
- 1.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000 May;25(1):55–7. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 2.Rajasekhar VK, Viale A, Socci ND, Wiedmann M, Hu X, Holland EC. Oncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol Cell. 2003 Oct;12(4):889–901. doi: 10.1016/s1097-2765(03)00395-2. [DOI] [PubMed] [Google Scholar]
- 3.Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001 Sep 15;61(18):6674–8. [PubMed] [Google Scholar]
- 4.Chakravarti A, Zhai G, Suzuki Y, et al. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004 May 15;22(10):1926–33. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 5.Pelloski CE, Lin E, Zhang L, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006 Jul 1;12(13):3935–41. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]
- 6.Gallia GL, Rand V, Siu IM, et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res. 2006 Oct;4(10):709–14. doi: 10.1158/1541-7786.MCR-06-0172. [DOI] [PubMed] [Google Scholar]
- 7.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005 Jun;7(6):561–73. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004 Apr 23;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y, Shoemaker AR, Liu X, et al. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005 Jun;4(6):977–86. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Liu X, Han EK, et al. Optimal classes of chemotherapeutic agents sensitized by specific small-molecule inhibitors of akt in vitro and in vivo. Neoplasia. 2005 Nov;7(11):992–1000. doi: 10.1593/neo.05355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004 Nov;9(6):667–76. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 12.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006 Jun;6(6):425–36. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 13.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005 Aug 25;353(8):811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 14.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004 Oct 1;64(19):7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 15.Sarkaria JN, Yang L, Grogan PT, et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007 Mar;6(3):1167–74. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- 16.Koul D, Shen R, Bergh S, et al. Inhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastoma. Mol Cancer Ther. 2006 Mar;5(3):637–44. doi: 10.1158/1535-7163.MCT-05-0453. [DOI] [PubMed] [Google Scholar]
- 17.Momota H, Nerio E, Holland EC. Perifosine inhibits multiple signaling pathways in glial progenitors and cooperates with temozolomide to arrest cell proliferation in gliomas in vivo. Cancer Res. 2005 Aug 15;65(16):7429–35. doi: 10.1158/0008-5472.CAN-05-1042. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura JL, Karlsson A, Arvold ND, et al. PKB/Akt mediates radiosensitization by the signaling inhibitor LY294002 in human malignant gliomas. J Neurooncol. 2005 Feb;71(3):215–22. doi: 10.1007/s11060-004-1718-y. [DOI] [PubMed] [Google Scholar]
- 19.Kao GD, Jiang Z, Fernandes AM, Gupta AK, Maity A. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem. 2007 Jul 20;282(29):21206–12. doi: 10.1074/jbc.M703042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006 May;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Li A, Walling J, Kotliarov Y, et al. Genomic changes and gene expression profiles reveal that established glioma cell lines are poorly representative of primary human gliomas. Mol Cancer Res. 2008 Jan;6(1):21–30. doi: 10.1158/1541-7786.MCR-07-0280. [DOI] [PubMed] [Google Scholar]
- 22.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006 Dec 7;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 23.Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007 Oct;16(5):837–47. doi: 10.1089/scd.2007.0006. [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006 May 4;25(19):2697–707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]