Abstract
We investigated whether intensive computerized cognitive training in schizophrenia could improve working memory performance and increase signal efficiency of associated middle frontal gyri (MFG) circuits in a functionally meaningful manner. Thirty schizophrenia participants and 13 healthy comparison participants underwent fMRI scanning during a letter N-back working memory task. Schizophrenia participants were then randomly assigned to either 80 hours (16 weeks) of cognitive training or a computer games control condition. After this intervention, participants completed a second fMRI N-back scanning session. At baseline, during 2-back working memory trials, healthy participants showed the largest and most significant activation in bilateral MFG, which correlated with task performance. Schizophrenia participants showed impaired working memory, hypoactivation in left MFG, and no correlation between bilateral MFG signal and task performance. After training, schizophrenia participants improved their 2-back working memory performance and showed increased activation in left MFG. They also demonstrated a significant association between enhanced task performance and right MFG signal, similar to healthy participants. Both task performance and brain activity in right MFG after training predicted better generalized working memory at 6-month follow-up. Furthermore, task performance and brain activity within bilateral MFG predicted better occupational functioning at 6-month follow-up. No such findings were observed in the computer games control participants. Working memory impairments in schizophrenia and its underlying neural correlates in MFG can be improved by intensive computerized cognitive training; these improvements generalize beyond the trained task and are associated with enduring effects on cognition and functioning 6 months after the intervention.
Keywords: working memory, fMRI, prefrontal cortex, schizophrenia, cognitive training
1. Introduction
Individuals with schizophrenia experience prominent working memory deficits that have deleterious functional consequences (Green et al., 2000; Takahashi et al., 2005; Tan, 2000). Compared to healthy participants, they show both hypoactivation (Barch et al., 2001; Carter et al., 1998; Perlstein et al., 2003; Stone et al., 1998; Weinberger et al., 1996) and hyperactivation (Callicott et al., 2000) of dorsolateral prefrontal cortex (i.e., bilateral middle frontal gyri) when performing different working memory tasks, indicating abnormal and inefficient recruitment of prefrontal neural resources as working memory demands increase (Callicott et al., 2000; Minzenberg et al., 2009). In a meta-analytic review across 124 studies, working memory deficits were consistently found in schizophrenia patients regardless of stimulus modality, indicating that they may represent a cardinal cognitive endophenotype of the illness (Lee and Park, 2005). Enhancement of working memory is now seen as a critical treatment target, but has thus far not been amenable to psychopharmacologic interventions (Buchanan et al., 2005; Goldberg et al., 2007; Mishara et al., 2004; Vinogradov et al., 2013)
Four prior studies have examined both the behavioral and prefrontal cortical activation effects of several forms of cognitive remediation for working memory dysfunction in participants with schizophrenia (Bor et al., 2011; Haut et al., 2010; Wexler et al., 2000; Wykes et al., 2002): Wexler et al (2000) studied 8 patients before and after 10 weeks of computerized verbal working memory exercises; Wykes et al (2002) studied 6 patients receiving 40 hours of therapist coaching in executive functioning versus 6 patients in occupational therapy; Bor et al (2011) studied 8 patients receiving 28 hours of computerized cognitive remediation therapy on a verbal and a spatial N-back task versus 9 patients receiving no additional treatment; and Haut et al (2010) studied 9 patients receiving 25 hours of computerized training on a word N-back task versus 9 patients receiving group-based social skills training. All four studies showed increased prefrontal activation as a result of cognitive remediation; additionally, Haut et al (2010) showed that the 9 patients who received word N-back training also improved performance on an animal picture N-back task which correlated with increased activation in bilateral prefrontal regions. However, increased prefrontal activation may not necessarily always be indicative of increased prefrontal efficiency (Koch et al., 2006), particularly in light of mixed findings of both prefrontal hypoactivation and hyperactivation in schizophrenia patients compared with healthy participants (Callicott et al., 2000; Perlstein et al., 2003). Thus, despite the promising findings from earlier cognitive remediation studies, the brain mechanisms underlying critical behavioral changes remain unclear.
In an earlier study, we showed that intensive cognitive training of auditory/verbal, visual and social cognitive processes generalized to improvement in an untrained reality monitoring task and increased medial prefrontal cortical (mPFC) activation during performance of this task (Subramaniam et al., 2012). While these findings showed that the neural system impairments of schizophrenia are not immutable, several questions were unanswered. The use of a reality monitoring task did not allow us to probe lateral prefrontal cortical functioning and enhancement of verbal working memory—which was the target of a significant portion of the cognitive training exercises. Thus, while our findings suggested that medial prefrontal cortical regions came “on-line” after training in support of enhanced reality monitoring performance, we were not able to investigate whether or not training enhanced capacity and/or efficiency in key neural systems that were directly targeted by the exercises, nor whether any such enhancement generalized to improved clinical and functional status.
In the present study, we sought to address these questions. We performed fMRI during an untrained N-back working memory task before and after schizophrenia patients were randomized either to intensive computerized cognitive training of auditory/verbal, visual and social cognitive processes or to a rigorously matched computer games control condition. We hypothesized that, compared to the control condition, subjects who underwent cognitive training would show:
Improvements on the untrained letter N-back task, suggesting generalization of the effects of training.
Restoration of more normal brain-behavior relationships between middle frontal gyrus activation and N-back working memory accuracy, indicating improved lateral prefrontal system efficiency.
An association between working memory gains and lower disorganized symptoms, suggesting an impact of cognitive training on clinical status.
An association between working memory gains and behavioral improvements at 6-month follow up, indicating enduring benefits of intensive cognitive training.
2. Methods and Materials
2.1. Participants and Procedure
Thirty clinically stable volunteer schizophrenia patients (SZ: mean age=41; education=13 years; IQ=103; illness duration=19.4 years) who were willing to undergo fMRI, were recruited from our randomized clinical trial of cognitive training in schizophrenia (ClinicalTrials.gov NCT00312962). This subset of participants who were willing to undertake serial fMRI received the same intervention and all the same behavioral neuropsychological assessments as participants in the parent trial (Fig. 1). These participants who completed fMRI were also matched on demographic variables (i.e., age, education, IQ) to participants in the parent trial. The participants and training procedure are identical to those described in Subramaniam et al. (2012). We report here the results of a working memory fMRI experiment investigating dorsolateral prefrontal cortical systems, in contrast to our prior report on the results of a reality-monitoring experiment investigating medial prefrontal cortical systems (Subramaniam et al., 2012).
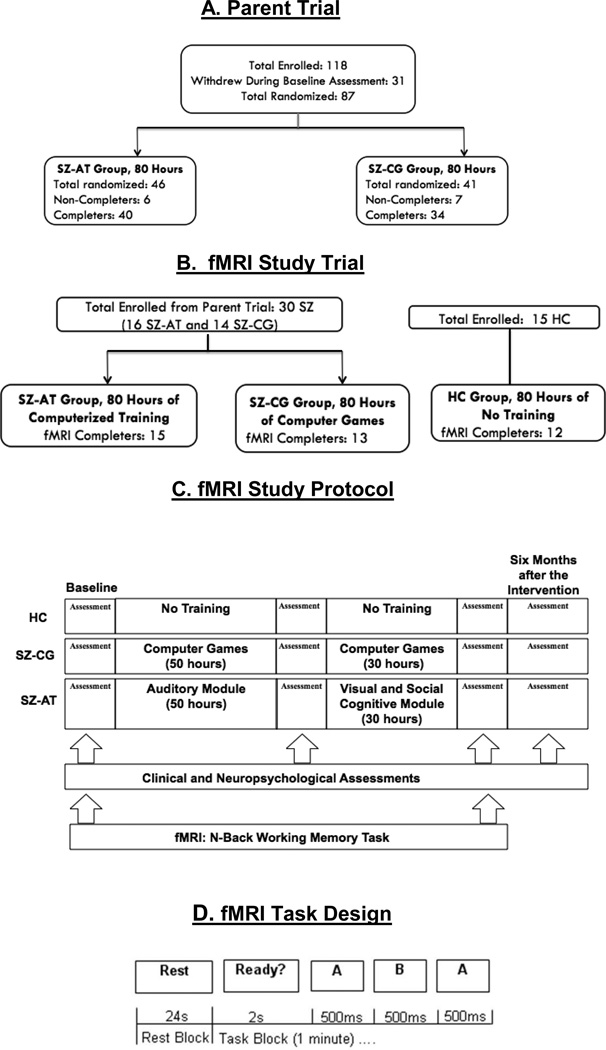
Fig. 1.
Flow chart of fMRI N-back trial and study design in relation to the parent trial. A. Parent trial. B. fMRI Study Trial C. fMRI Study Protocol D. fMRI Task Design.
SZ participants who underwent fMRI were matched to 15 healthy comparison participants (HC) at a group level on age, gender, and education (Table 1). SZ participants were stratified by age, education, gender, and symptom severity and then randomly assigned to either active computerized cognitive training (SZ-AT), or a control condition of commercial computer games (SZ-CG), performed for 80 hours. SZ subjects were blind to group assignment. There were no significant differences in medications between the two patient groups at baseline and no significant medication changes (dosage change<10%) during the study (Table 2). SZ participants also underwent clinical and neuropsychological assessments by personnel blind to group assignment, at baseline, after the intervention, and at 6-month follow-up (Table 3).
Table 1.
Demographics (mean, SD) of Healthy Comparison (HC), Active Training Schizophrenia Subjects (SZ-AT), and Control Condition Computer Games Schizophrenia Subjects (SZ-CG) at Baseline
| Baseline | HC (N=15) | SZ-AT (N=16) | SZ-CG (N=14) | p valuea |
|---|---|---|---|---|
| Age | 44.27 (SD=11.2) | 40.69 (SD=12.7) | 41.21 (SD=9.48) | 0.24 |
| Education | 13.93 (SD=1.44) | 13.19 (SD=2.45) | 13.36 (SD=1.82) | 0.70 |
| Gender | 11M, 4F | 12M, 4F | 10M, 4F |
One-way ANOVAs revealed no significant difference in age or education between the three subject groups at baseline.
Table 2.
Medication Profile of Active Training Schizophrenia Subjects (SZ-AT) and Control Condition Computer Games Schizophrenia Subjects (SZ-CG) at Baseline
| Antipsychotic Medicationa | SZ-AT (N=16) | SZ-CG (N=14) | p value |
|---|---|---|---|
| 1st Generation (N) | 0 | 2 | 0.23 |
| 2nd Generation (N) | 11 | 12 | 0.61 |
| Multiple (N) | 1 | 0 | 0.99 |
| No antipsychotic (N) | 4 | 0 | 0.25 |
| Other Psychiatric Medication | |||
| Antidepressants or Mood Stabilizers (N) | 9 | 5 | 0.48 |
| Benzodiazepines (N) | 4 | 6 | 0.46 |
| Anticholinergics (N) | 2 | 3 | 0.65 |
| Mean Chlorpromazine (CPZ) Equivalentsb | 478 (SD =380) | 410 (SD=445) | 0.61 |
| Mean Cogentin Equivalentsc | 0.73 (SD=0.82) | 1.33 (SD=2.89) | 0.42 |
Fisher’s Exact test (2-tailed) revealed no significant differences in the number of Antipsychotic Medication or Other Psychiatric Medication between the two patient groups at baseline. 1st generation antipsychotic medication = thioridazine; 2nd generation antipsychotic medication = aripiprazole, clozapine, olanzapine, quetiapine, risperidone, ziprasidone.
Two sample two-tailed t-tests revealed no significant differences in mean CPZ Equivalents between groups (Andreasen et al 2010).
Two sample two-tailed t-tests revealed no significant differences in mean Cogentin Equivalents (Minzenberg et al 2004). All patients were clinically stable, and there was no change in medication at 16 weeks compared to baseline in either group.
Table 3.
Clinical Symptoms, Occupational Functioning and Neuropsychological Working Memory Performance (mean, SD) of Active Training Schizophrenia Subjects (SZ-AT), and Control Condition Computer Games Schizophrenia Subjects (SZ-CG) at Baseline, 16 Weeks and 6 Months after the Intervention.
| Baseline | SZ-AT | SZ-CG | p value |
|---|---|---|---|
| Overall Clinical Symptom Severity (PANSS) | 2.57 (SD=.67) | 2.49 (SD=.53) | 0.63 |
| Positive Symptom Severity (PANSS) | 2.92 (SD=1.02) | 2.81 (SD=1.15) | 0.80 |
| Negative Symptom Severity (PANSS) | 2.25 (SD=.97) | 2.26 (SD=.86) | 0.97 |
| Disorganized Symptom Severity (PANSS) | 2.16 (SD=.61) | 2.59 (SD=.81) | 0.12 |
| Occupational Functioning (QLS) | 2.15 (SD=1.46) | 1.5 (SD=1.51) | 0.28 |
| Verbal Working Memory (MATRICS) | −0.78 (SD=.90) | −0.81 (SD=.98) | 0.94 |
| 16 Weeks | |||
| Overall Clinical Symptom Severity (PANSS) | 2.59 (SD=.68) | 2.30 (SD=.51) | 0.43 |
| Positive Symptom Severity (PANSS) | 2.61 (SD=1.00) | 2.56 (SD=1.18) | 0.87 |
| Negative Symptom Severity (PANSS) | 2.43 (SD=.81) | 2.35 (SD=.61) | 0.74 |
| Disorganized Symptom Severity (PANSS) | 1.74 (SD=.41) | 2.04 (SD=.81) | 0.23 |
| Occupational Functioning (QLS) | 2.25 (SD=.86) | 1.78 (SD=.97) | 0.18 |
| 6 Months After the Intervention | |||
| Overall Clinical Symptom Severity (PANSS) | 2.59 (SD=.82) | 2.38 (SD=.64) | 0.48 |
| Positive Symptom Severity (PANSS) | 2.72 (SD=1.37) | 2.52 (SD=1.42) | 0.39 |
| Negative Symptom Severity (PANSS) | 2.73 (SD=1.38) | 2.37 (SD=.99) | 0.18 |
| Disorganized Symptom Severity (PANSS) | 1.89 (SD=.92) | 2.19 (SD=.82) | 0.44 |
| Occupational Functioning (QLS)a | 3.08 (SD=1.66) | 1.83 (SD=1.3) | 0.049 |
| Verbal Working Memory (MATRICS) | −0.17 (SD=.90) | −0.55 (SD=1.18) | 0.38 |
SZ-AT showed improved real-world occupational functioning 6 months after the intervention when compared to the SZ-CG group.
At baseline, two HC participants felt too claustrophobic to remain in the scanner, and provided only behavioral data. All other participants were scanned using fMRI while performing the N-back task. Sixteen weeks later, 15 SZ-AT, 13 SZ-CG, and 12 HC participants completed a second fMRI N-back session. One SZ-AT and 1 SZ-CG were unavailable/unwilling to perform the fMRI N-back task at the second time point, and fMRI data from 1 HC was later excluded due to poor signal. Six months later, 13 SZ–AT and 12 SZ-CG participants returned for cognitive and clinical re-assessment.
2.2. Assessments
Symptom severity in schizophrenia was assessed with the Positive and Negative Syndrome Scale (PANSS), which rates each symptom on a scale of 1 (absent) to 7 (extreme) (Kay et al., 1987). Verbal working memory was assessed at 6-month follow-up with the Letter-Number Span from the MATRICS neurocognitive battery (Nuechterlein et al., 2008), a verbal working memory test in which respondents hear strings of letters and numbers and repeat these to the administrator in a particular order. Real-life functioning was assessed with the Quality of Life Scale (QLS) (Bilker et al., 2003). The QLS is a semi-structured interview that assesses functioning during the preceding four weeks on a scale of 0 = virtually absent to 6 = adequate functioning, and is used to reference the general well-being of individuals in their day-to-day environment. Research staff who conducted neurocognitive testing or PANSS and QLS interviews first completed extensive training on testing/interviewing and scoring criteria of individual items (e.g., scoring videotaped sessions, observation of sessions conducted by experienced staff, and participating in mock sessions). Intraclass correlation coefficients (ICCs) were greater than 0.85 for the PANSS and QLS total and subscale scores. The MATRICS battery demonstrates an ICC of .79 for working memory (Keefe et al., 2011).
2.3. Computerized Cognitive Training
The SZ-AT participants completed two sequential modules of adaptive computerized cognitive training exercises, 1hr a day for 80 hours over 16 weeks. SZ-AT subjects first participated in a module of auditory/verbal processing exercises (http://www.positscience.com/our-products/brain-fitness-program), for 1 hr a day for a total of 50 hours (10 weeks). Next, SZ-AT participants completed a module of visual processing exercises (http://www.positscience.com/our-products/demo), together with a module of computerized emotion identification exercises, composed of training in facial emotion recognition and theory of mind (MindReading, MicroExpressions Training Tool, Subtle Expressions Training Tool; Baron-Cohen et al., 2003; Eckman, 2003) for 1 hr a day for a total of 30 hours (6 weeks). The auditory and visual exercises were continuously adaptive: they first established the precise parameters within each stimulus set required for an individual subject to maintain 80% correct performance, and once that threshold was determined, task difficulty increased systematically and parametrically as performance improved. The social cognition training was partially adaptive, in that difficulty level increased progressively as participants successfully completed blocks of trials at a given difficulty level. The design and implementation of this approach was informed by research demonstrating impairments in schizophrenia in basic auditory and visual perceptual processes, as well as in higher-order auditory and visual working memory, and social cognitive functions (e.g., Green, 1996; Javitt, 2009; Javitt et al., 2000). (See Supplementary Methods for a complete description of the cognitive training exercises).
In the computer games control condition, SZ-CG participants systematically rotated through 16 different commercially available computer games (e.g. clue-gathering and visual-spatial puzzle games such as Hangman, Tic-Tac-Toe, Tetris, Checkers, Dominoes, Solitaire, etc.) for a total of 80 hours over 16 weeks. In each session, SZ-CG participants completed 4 of the 16 computer games for 15 minutes per computer game. The control condition was designed to allow for non-specific motivation and engagement without providing constrained, intensive, and adaptive training on specific cognitive operations. Subjects rated both conditions as equally entertaining on self-report questionnaires, and subjectively found both conditions to be equally beneficial (Fisher et al. 2009); a prior study found excellent maintenance of the study blind with this protocol (Keefe et al., 2012). (See Supplementary Methods for a complete listing of all of the computer games used in the control condition).
Staff exposure during the intervention was kept to a minimum: staff aided all subjects to start each session and paid them at the end of each session, but did not provide any coaching. All participants received $5 for each day of study participation, a bonus $20 for 5 consecutive days of participation, and a bonus $50 at the completion of each module. Payment was contingent on study participation and not performance.
2.4. N-back Working Memory Task
While in the scanner, participants completed 0-back, 1-back and 2-back tasks consisting of serially presented letters. In the 0-back task, which measures visual attention, participants had to press the left button when the letter on the current trial was an ‘X’ and had to press the right button for all other letters. On the 1-back and 2-back tasks, participants had to press the left button when the letter on the current trial matched the letter 1 or 2 trials back, and had to press the right button for all other letters. The 2-back task has been shown to reliably activate the DLPFC in HC and SZ participants (Haut et al 2010; Royer et al., 2009). In order to specifically measure neural activity related to 2-back working memory demands, we contrasted activity on 2-back working-memory trials with 0-back visual attention trials. For each of the three N-back tasks, participants performed four 25-trial blocks, alternating with blocks of rest. The scanning duration for each N-back task was 6 min 31s. N-back working memory training was not a component of any of the cognitive training exercises.
2.4.1. N-back Task: Behavioral Statistical Analyses
Signal detection theoretic d-prime analyses were performed by calculating the hit rate and the false alarm rate (FA), converting each measure to a z-score, and subtracting the FA from the hit rate in order to differentiate sensitivity during accurate performance from response bias. We performed an outlier correction using a winsorization procedure for values above/below a 2 standard deviation (SD) (Wilcox, 2005). There was one outlier in the SZ-AT participants at 16 weeks, who performed more than 2 SD above the mean on the 2-back task. This outlier was winsorised to ensure that all results were normally distributed.
2.4.2. N-back Task: fMRI Acquisition
Visual stimuli were presented with E-Prime (http://www.pstnet.com/eprime.cfm) and back-projected onto an LCD projector. Participants viewed the screen using a mirror attached to the head coil and made finger-press responses on a fiber-optic response pad. fMRI was acquired on a 3 Tesla General Electric Signa LX 15 scanner and eight channel head coil, using a spiral Echo-planar sequence (TR=1s; TE=30ms; flip angle=60, matrix=64×64, FOV=22cm, 14 slices, 6mm thickness). Image analysis was performed using SPM2 software (www.fil.ion.ucl.ac.uk/spm).
2.4.3. N-back Task: fMRI Statistical Analyses
Images were realigned to correct for motion artifacts using a 6-parameter affine transformation, normalized to a standard stereotaxic space (Montreal Neurological Institute Template) using a 12 parameter affine/non-linear transformation, and spatially smoothed with a 10mm FWHM Gaussian kernel. Data were submitted to a whole brain General Linear Model analysis, fitting a reference canonical hemodynamic response function to each task block and to each rest block. Image intensity was scaled to the mean global intensity of each time series. Reported results for 2-back versus 0-back whole-brain working memory load in our combined sample of HC and SZ participants are significant at a p value of .001 uncorrected for multiple comparisons, and cluster size of at least 200 voxels in volume (Fig. 2). Our whole-brain analysis revealed activation across bilateral frontal clusters in middle frontal gyri (MFG), inferior frontal gyri (IFG) and insula that additionally survived whole-brain multiple comparison correction of FWE p<.05 (see Supplementary Table 1, Fig. 1). Next, we selected the ROIs from a different study (Perlstein et al., 2001) that were confirmed by our whole-brain analysis and that used the same population (HC and SZ participants) and the same task contrast (2-back minus 0-back) as the present study in order to minimize any bias and avoid non-independence issues (rather than picking the peaks from the working-memory load effects from our whole-brain analyses). This procedure is consistent with Poldrack (2007). We selected the following ROI centroids reported in Perlstein et al. (2001): R.MFG (38, 27, 24 Talairach coordinates, BA 46/9), L.MFG (−36, 38, 23 Talairach coordinates, BA 46/9), R.IFG (49, 10, 23, BA 44), L.IFG (−47, 7, 23, BA 44), R.Insula (35, 15, 8) and L.Insula (−40, 10, 10). (We used the centroids from the Perlstein et al. (2001) paper as it has a slightly larger sample size than the Perlstein et al. (2003) paper, but we note that when we use coordinates from the Perlstein et al. (2003) study it does not change any of our reported findings. We chose not to use ROIs from the Glahn et al. (2005) meta-analysis since this paper amalgamates data across a very wide range of n-back tasks using letters, numbers, objects, location, and auditory non-words; the focus of our experiment was specifically on 2-back vs. 0-back in a letter N-back task). We calculated mean beta signals within a 10 mm radius spherical volume around each of the centroids from the Perlstein et al (2001) paper, during 2-back versus 0-back conditions for each group (HC, SZ-CG, SZ-AT) and for each session (baseline, 16 weeks). The mean beta signals for each ROI were then entered into repeated-measures group-by-session ANOVAs in SPSS to compare change in 2-back working-memory signal from baseline to 16 weeks in each group. Signal change from baseline to 16 weeks was correlated with behavioral change in 2-back performance for each group.
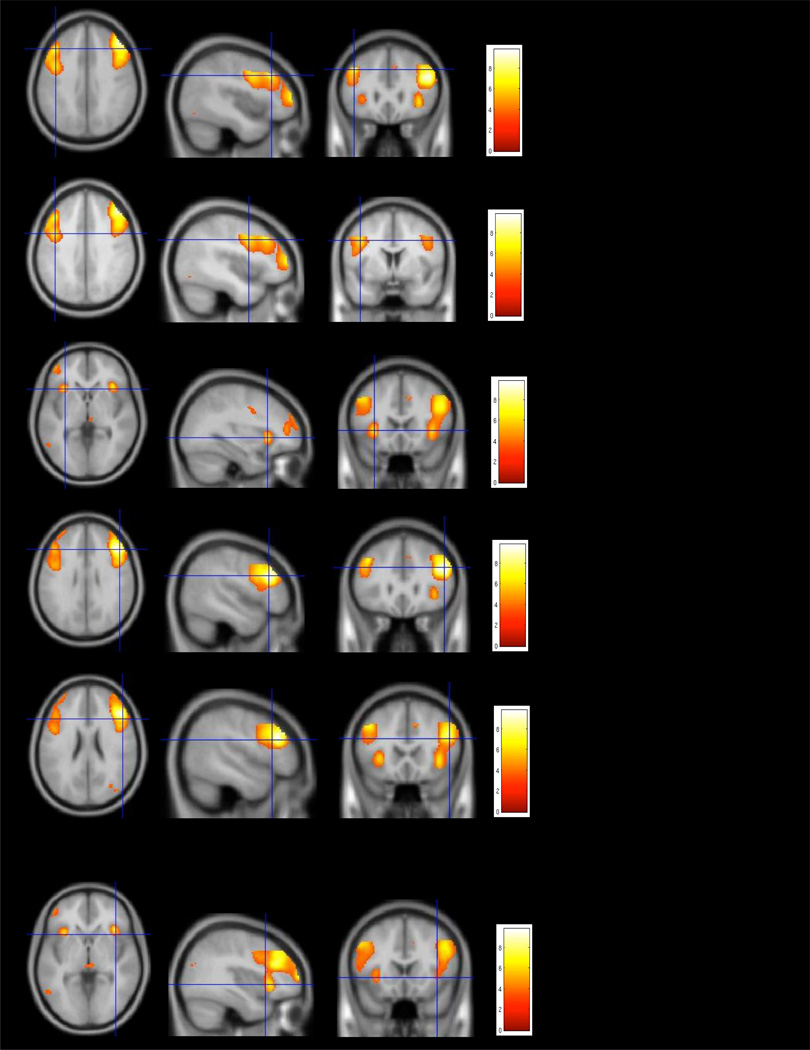
Fig. 2.
Pre-training (baseline) whole brain clusters of activation in our combined sample of Healthy Comparison (HC) and Schizophrenia (SZ) subjects for the 2-back minus 0-back contrast, significant at a p value of .001, and cluster size of at least 200 voxels in volume.
3. Results
3.1. Baseline Behavioral and fMRI Findings
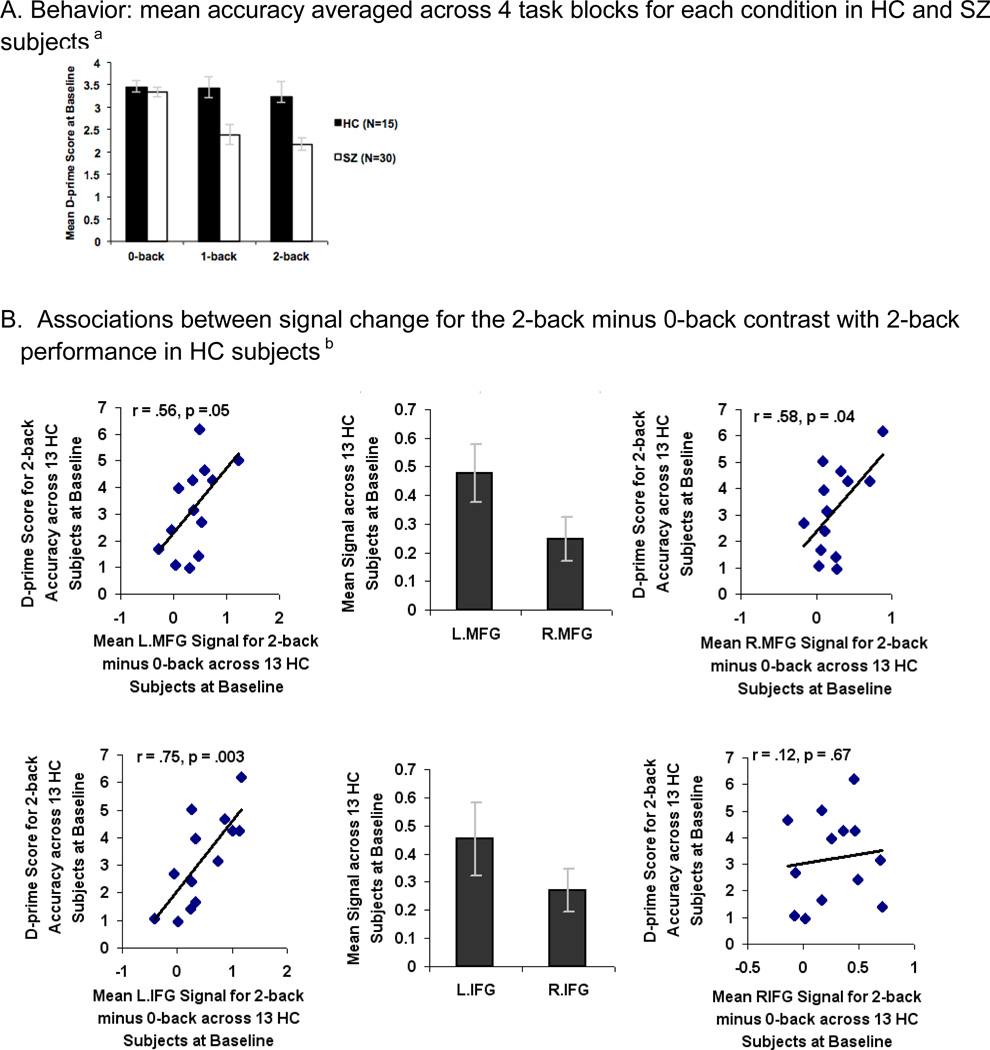
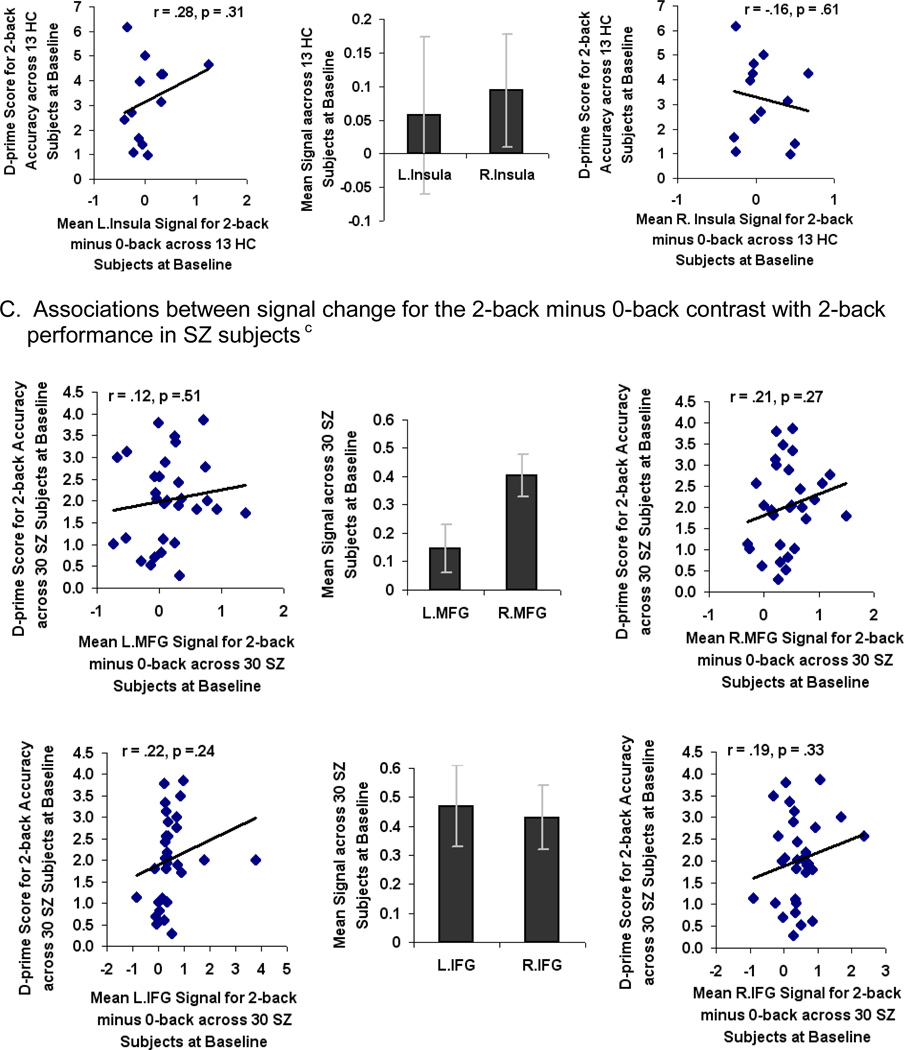
One-way ANOVAs of task accuracy revealed that SZ participants were significantly impaired on 1-back (F=5.04, df=1, p=0.03) and 2-back tasks (F=6.49, df=1, p=0.01) compared to HC participants (Fig. 3A). The effect size of the overall accuracy difference between HC and SZ subjects at baseline was 0.77 for the 1-back and 0.76 for the 2-back task.
Fig. 3.
Pre-training (baseline) performance and brain signal differences between Healthy Comparison (HC) and Schizophrenia (SZ) subjects during a Letter N-back task. A. Behavior: mean accuracy averaged across 4 task blocks for each condition in HC and SZ subjectsa B. Associations between signal change for the 2-back minus 0-back contrast with 2-back performance in HC subjectsb C. Associations between signal change for the 2-back minus 0-back contrast with 2-back performance in SZ subjectsc
aSignificant difference between HC and SZ subjects on the 1-back (F=5.04, df=1, p=.03) and 2-back (F=6.49, df=1, p=.01) tasks at baseline.
bMean signal in bilateral MFG correlated with 2-back accuracy in HC subjects.
cThere was no association between signal change in any of the ROIs with 2-back accuracy in SZ subjects.
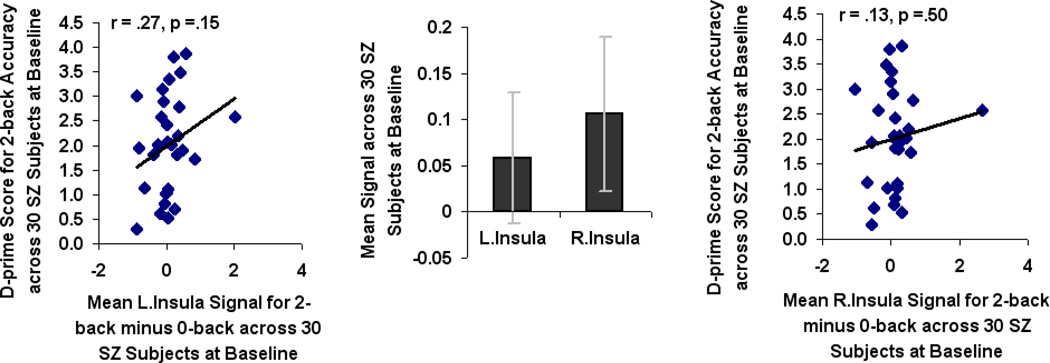
When compared to HC participants, SZ participants revealed hypoactivation in the L.MFG only (F=4.61, df=1, p=. 04). Mean beta signal calculated across all voxels within bilateral MFG and L.IFG ROIs correlated with accurate 2-back performance in HC participants (Fig. 3B). There was no correlation in HC participants between accurate 2-back performance and mean beta signal in R.IFG or bilateral insula (Fig. 3B). None of the 6 ROIs were associated with 2-back performance in SZ participants (Fig. 3C). This suggests a pattern of prefrontal cortical inefficiency in which activation in bilateral MFG does not support 2-back working memory performance in SZ participants. After baseline fMRI, SZ participants were randomly assigned to either computerized cognitive training or the computer games control condition.
3.2. N-back Performance after the Intervention
After 16 weeks in which SZ participants performed either 80 hours of active training (SZ-AT) or computer games (SZ-CG), all participants participated in a second fMRI session. A repeated-measures ANOVA (HC, SZ-CG, SZ-AT) revealed a group-by-session effect in d-prime scores for the 2-back task during the second session compared to the first (F=3.37, df=2, p=.045) (Fig. 4A), and a marginal group-by-session effect for the 1-back (F=3.17, df=2, p=.054). The significant group-by-session effect for the 2-back was driven by the SZ-AT participants, who performed significantly better after cognitive training versus baseline when compared to SZ-CG participants (F=5.91, df=1, p=.022), and when compared to HC participants (F=4.33, df=1, p=.047). There were no differences between sessions for SZ-CG and HC participants on 2-back performance (F=.05, df=1, p=.83). Within-group paired t-tests confirmed that the SZ-AT participants performed significantly better on the 2-back after training versus baseline (t=2.6, df=14, p=.02) as well as on the 1-back after training versus baseline (t=2.11, df=14, p=.05). After 16 weeks, one-way ANOVAs revealed that there was no significant difference between the SZ-AT and HC subjects on either 1-back or 2-back accuracy (1-back: F=.01, df=1, p=.94; 2-back: F=.26, df=1, p=.62). Neither SZ-CG nor HC participants showed significant improvement on either the 1-back (SZ-CG: t=.36, df=12, p=.73; HC: t=.10, df=12, p=.92) or the 2-back (SZ-CG: t=.71, df=12, p=.49; HC: t=.63, df=12, p=.54) in the second session, suggesting that the improvements observed in the SZ-AT group were specific to the training and extended beyond practice effects on the task (Table 4). Together, these data indicate that cognitive training of auditory, verbal, visual, and social cognitive processes generalized to improved performance on an untrained N-back working memory task in SZ participants such that they began to “normalize” to the performance level of HC subjects.
Fig. 4.
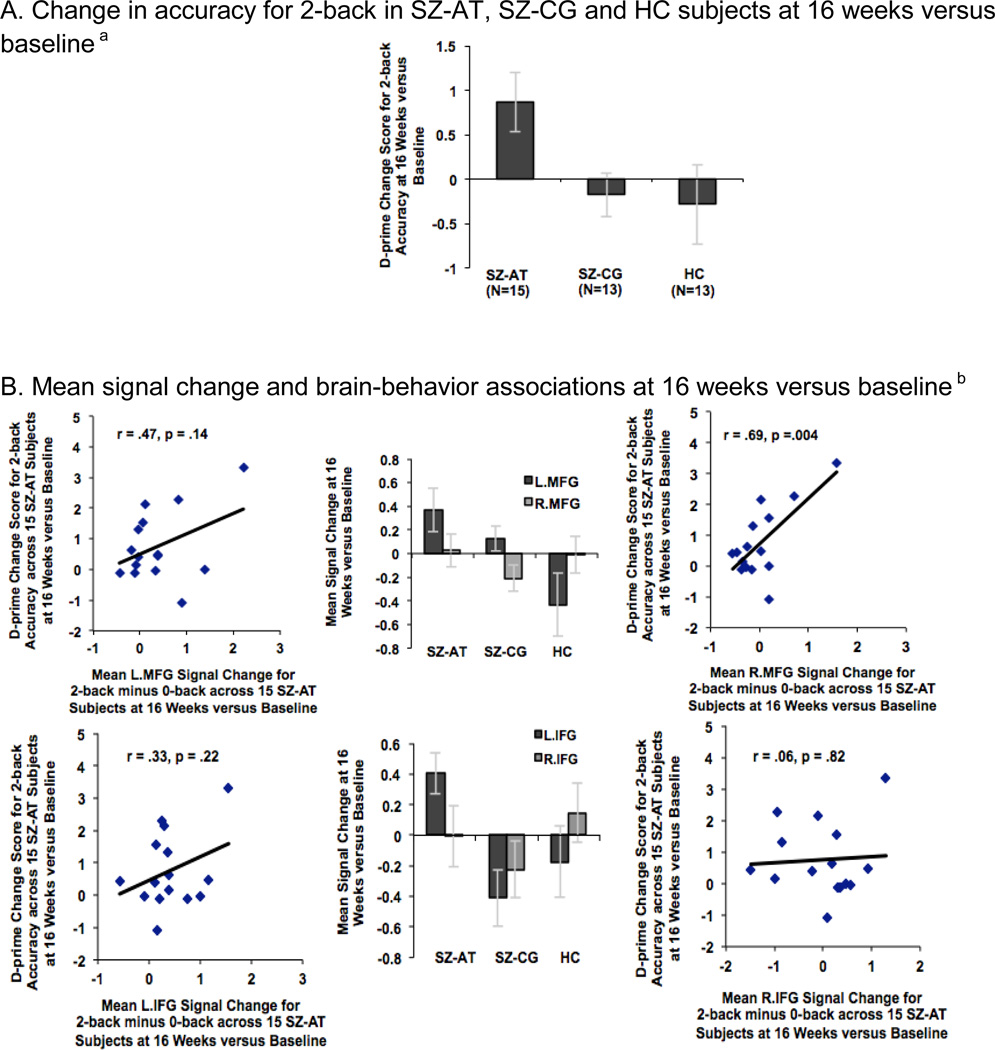
Cognitive training effects: performance and brain signal change at 16 weeks versus baseline between Active Training Schizophrenia subjects (SZ-AT), Computer Games Control Schizophrenia subjects (SZ-CG), and Healthy Comparison subjects (HC). A. Change in accuracy for 2-back in SZ-AT, SZ-CG and HC subjects at 16 weeks versus baselinea B. Mean signal change and brain-behavior associations at 16 weeks versus baselineb
aRepeated-measures ANOVA revealed a group-by-session interaction in d-prime scores for the 2-back task at 16 weeks compared to baseline, driven by the SZ-AT subjects (F=3.37, df=2, p=.045).
bMean signal change in R.MFG correlated with change in 2-back accuracy (R.MFG: r=.69, df=13, p=.004) in SZ-AT subjects at 16 weeks versus baseline.
Table 4.
Performance on the 1-back and 2-back tasks (mean, SD) of Healthy Control Subjects (HC), Control Condition Computer Games Schizophrenia Subjects (SZ-CG), and Active Training Schizophrenia Subjects (SZ-AT) who Completed the Intervention.
| 1-back Accuracy at Baseline |
1-back Accuracy at 16 Weeks |
2-back Accuracy at Baseline |
2-back Accuracy at 16 Weeks |
|
|---|---|---|---|---|
| HC | 3.20 (SD=.57) | 3.23 (SD=.85) | 3.30 (SD=1.65) | 3.02 (SD=1.73) |
| SZ-CG | 2.89 (SD=.81) | 2.86 (SD=.69) | 2.22 (SD=1.15) | 2.04 (SD=1.05) |
| SZ-AT | 2.01 (SD=1.69) | 3.21 (SD=.81) | 1.88 (SD=.93) | 2.75 (SD=1.02) |
3.3. fMRI Findings after the Intervention
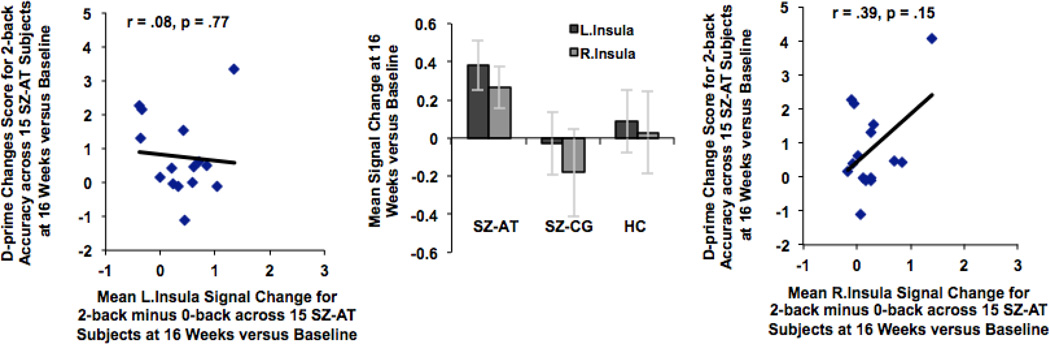
In order to investigate between-group differences at 16 weeks versus baseline, mean beta weights from the 2-back versus 0-back contrast were extracted across all the voxels within each ROI for each group and for each session (i.e., baseline, and 16 weeks), and submitted to repeated-measures ANOVAs in SPSS. There was a significant group-by-session effect in only two regions, the L.MFG and L.IFG, driven by the SZ-AT participants during the second session versus the first (LMFG: F=4.23, df=2, p=0.02; L.IFG: F=5.69, df=2, p=0.007). There was no group-by-session increase in signal observed in any of the other ROIs (all p values > 0.15). Assessments of repeatability for the control groups at baseline and at 16 weeks, assessed with intraclass correlation coefficients (ICCs), were greater than 0.90 for each of the ROIs tested in the HC group and were greater than 0.80 for each of the regions in the SZ-CG group.
Interestingly, in SZ-AT participants, the improvement in 2-back performance showed a strong association with the change in R.MFG signal (r=.69, df=13, p=.004), similar to HC participants at baseline. Additionally, Fisher r-to-z transformation indicated that the strength of the correlation between R.MFG signal with 2-back performance in SZ-AT subjects at 16 weeks was significantly different from the correlation at baseline (z=1.69, p=.04, 1-tailed), indicating enhanced right prefrontal efficiency as a result of training. Interestingly, signal change in R.MFG was correlated with signal gains in two other regions: L.IFG (r=.61, p=.01) and L.MFG (r=.82, p=.0002). Thus, it is possible that improved 2-back performance is related to increased connectivity between R.MFG with L.MFG and L.IFG. Signal change in the remaining ROIs did not correlate with the increase in 2-back performance in SZ-AT participants (Fig. 4B). SZ-CG participants did not show any association between change in 2-back performance and mean signal change in any of the ROIs after 80 hours of computer games versus baseline (all p values > 0.15). These findings suggest that training-enhanced efficiency of R.MFG signal may be associated with increased functional connectivity between bilateral MFG regions, which may help to support better 2-back performance in in SZ-AT participants.
3.4. Association of Behavioral and fMRI Findings with Clinical Measures after Cognitive Training
Overall, symptom ratings were low in this clinically stable group of SZ participants (average rating slightly over 2, mild) at baseline and at 16 weeks. There was no change in positive or negative symptom ratings at 16 weeks compared to baseline in either the SZ-AT group or in the SZ-CG group (all p values > 0.20). However, after training, SZ-AT participants had lower disorganized symptom ratings compared to baseline (t=2.43, df=14, p=0.03). Furthermore, lower disorganized symptom severity levels after training in SZ-AT participants was correlated with improved 2-back performance levels (r=−.57, df=13, p=.03). Two-back performance after training was not correlated with positive or negative symptoms (p values > 0.40). There was no association between severity of disorganized symptoms and mean beta signal in any of the ROIs in SZ-AT participants after training (all p values > 0.15). There was no association between 2-back performance or mean beta signal in any of the 6 ROIs with disorganized symptoms in SZ-CG participants (all p values > 0.60). These data indicate that SZ participants who made greater improvements on the 2-back task also had lower disorganized symptoms after training.
3.5. Findings Six Months after the Intervention
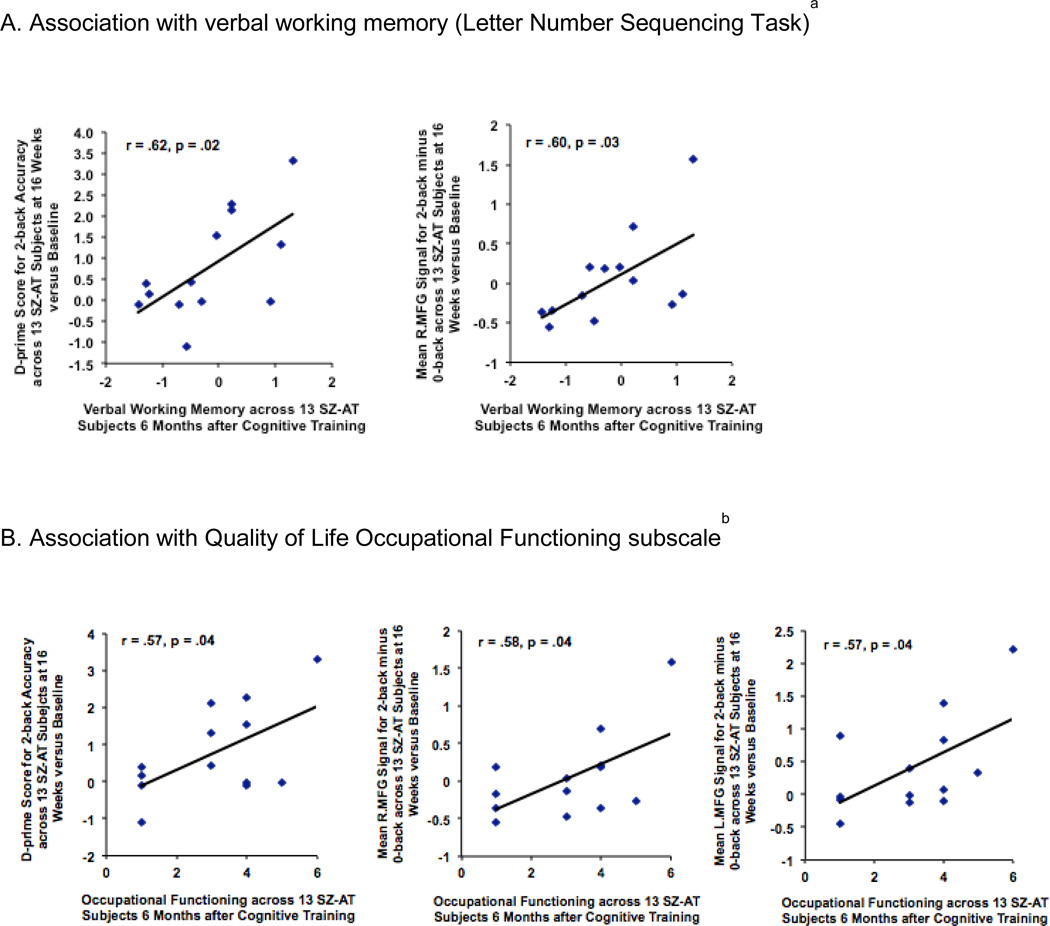
Working memory gains as assessed by the Letter-Number Sequencing task were sustained in SZ-AT participants at 6-months follow-up as compared to baseline (t=2.98, df=12, p=.01). In addition, improved 2-back performance and mean beta signal in the R.MFG ROI immediately after training showed a significant correlation with better Letter-Number Sequencing performance at 6 months (2-back d-prime: r=0.62, df=11, p=0.02; R.MFG: r=0.60, df=11, p=0.03) (Fig. 5A). Improved 2-back performance after training were also associated with lower disorganized symptoms at 6 months (r=−.55, df=11, p=.05), even though disorganized symptom severity overall was not significantly lower at 6 months when compared to baseline (t=.78, df=12, p=.40). In the 12 SZ-CG participants, there was no increase in working memory performance at 6 months compared to baseline; further 2-back performance after 16 weeks of computer games did not correlate with better working memory or lower disorganized symptoms (all p values > 0.30) at 6 months.
Fig. 5.
Association of 2-back performance and brain signal change after training with cognitive and clinical measures in Active Training Schizophrenia subjects (SZ-AT) assessed 6 months after cognitive training was completed. A. Association with verbal working memory (Letter Number Sequencing Task)a B. Association with Quality of Life Occupational Functioning subscaleb
aBetter performance on the 2-back and R.MFG signal in SZ-AT subjects immediately after 16 weeks of cognitive training were associated with better verbal working memory assessed 6 months after cognitive training was completed (2-back: r=0.62, df=11, p=0.02; R.MFG: r=0.60, df=11, p=0.03).
bBetter performance on the 2-back and bilateral MFG signal in SZ-AT subjects immediately after 16 weeks of cognitive training were associated with better occupational functioning assessed 6 months after cognitive training was completed (2-back: r= .57, df=11, p=.04; R.MFG: r= .58, df=11, p= .04; L.MFG: r= .57, df=11, p= .04)
There was an increase in QLS Occupational Functioning ratings at 6 months compared to baseline in the SZ-AT (t=2.52, df=12, p=0.03) but not the SZ-CG group (t=0.57, df=11, p=0.58). Two-back performance and mean beta signal in R.MFG and L.MFG ROIs after training were correlated with these better QLS Occupational Functioning ratings at 6 months in the SZ-AT group (Fig. 5B) but not in the SZ-CG group (all p values > 0.20).
4. Discussion
We performed an fMRI study of verbal working memory using the letter N-back task before and after a double-blind randomized controlled trial of 80 hours of intensive computerized training of component auditory/verbal, visual, and social cognitive processes in adults with schizophrenia compared to patients in a computer games control condition. We found that the SZ participants who underwent training showed:
Significant improvement in accuracy during the untrained 2-back working memory task.
“Restoration” of significant brain-behavior associations between R.MFG signal and 2-back accuracy, similar to that seen in healthy participants at baseline.
Significant associations between 2-back accuracy and R.MFG signal after training, with improved Letter-Number Sequencing performance 6 months later.
Significant associations between 2-back accuracy and in R.MFG and L.MFG signal after training, with better occupational functioning 6 months later.
No such improvements or associations were seen in the schizophrenia participants who underwent the control computer games condition, indicating that these findings were not due to practice effects, nor to non-specific effects of attention, motivation, computer exposure, or social engagement.
To our knowledge, this is the first study to demonstrate improved working memory function and increased neural system efficiency in individuals with schizophrenia after intensive cognitive training as compared to a rigorously-matched control condition, and to relate those improvements to better working memory and occupational functioning at 6 month follow up. These data are consistent with our prior findings of training-induced increases in medial prefrontal cortical signal associated with better reality monitoring performance and with better social functioning at 6 month follow up (Subramaniam et al 2012). In the present experiment, we show that the effects of training occur in lateral as well as medial prefrontal systems with slightly different long-term effects.
At baseline, schizophrenia participants showed both reduced prefrontal capacity as well as prefrontal inefficiency on the 2-back task. At a low processing load, on the 0-back task, schizophrenia participants showed no impairments in performance when compared to healthy participants, but revealed reduced accuracy during the higher-load 2-back task. Interestingly, consistent with the Minzenberg et al. (2009) meta-analysis, we found that although schizophrenia patients revealed a similar qualitative pattern of cortical-subcortical activation (extending from bilateral MFG to insula) to that of healthy participants at baseline, they revealed quantitative activation differences within L. MFG. Specifically, we found that individuals with schizophrenia showed reduced activation in L. MFG on the 2-back task, which may indicate that they had hit MFG “ceiling” capacity on the higher-load 2-back task. Intensive cognitive training enhanced both 2-back performance and L.MFG activation such that there was no longer any quantitative difference in activation levels between healthy comparison subjects and schizophrenia participants, suggesting that training may have enhanced prefrontal capacity.
Schizophrenia subjects also showed no association between 2-back accuracy and MFG signal at baseline, unlike what was seen in the healthy comparison subjects, consistent with a picture of prefrontal cortical inefficiency during increased working memory demands (Deserno et al., 2012; Karlsgodt et al., 2007; Minzenberg et al., 2009; Potkin et al., 2009). This is in agreement with the conclusions of Minzenberg et al. (2009) that if prefrontal activation does not support task performance, this likely indicates disrupted or inefficient processing. We found that 16 weeks of intensive training enhanced R.MFG efficiency such that signal became correlated with improved 2-back performance; the strength of this correlation was significantly greater than the correlation at baseline, suggesting enhanced right prefrontal efficiency. We also found that signal change in R.MFG was correlated with signal gains in two other regions: L.IFG and L.MFG. Thus, it may be possible that improved 2-back performance and enhanced R.MFG efficiency is related to increased connectivity within prefrontal neural networks. Moreover, increases in both 2-back performance and R.MFG signal immediately after training predicted better verbal working memory performance on the Letter Number Sequencing test 6 months later, as well as better occupational functioning. Together, these findings suggest that the improved efficiency in the R.MFG circuitry we observed in the training group represents a robust and adaptive plastic change that generalizes to enduring behavioral gains.
Perhaps not surprisingly, better 2-back working memory performance after training was associated with lower disorganized symptoms, consistent with prior studies which have shown a strong association between impaired working memory in schizophrenia and disorganization (Sanz et al 2009; Yoon et al 2008). Further, our finding that training-induced improvements in 2-back performance and bilateral MFG signal levels were associated with better occupational functioning at 6-months is consistent with prior research indicating that verbal working memory is a strong predictor of functional outcome in schizophrenia (e.g., Green et al 1996). It is also an interesting counterpoint to our prior report of improved reality monitoring performance and increased signal in medial PFC as associated with better ratings on QLS Social Functioning at 6 months (Subramaniam et al., 2012). It appears that intensive cognitive training of auditory/verbal, visual, and social cognitive processes induces improved efficiency in several critical and perhaps partially dissociable higher-order prefrontal circuits, each with distinct implications for long-term real-world functioning.
We do not know which specific components of the cognitive training contributed most to the improvements we observed. However, the auditory/verbal learning exercises we employed for 50 hours of training (the first module) did drive significant improvements in verbal working memory and verbal learning and memory, assessed with MATRICS measures, as reported previously, which endured at the 6 month follow up (Fisher et al., 2009, Fisher et al., 2010). It thus appears likely that the heavy emphasis on training in auditory/verbal processing contributed significantly to behavioral improvement on the 2-back task as well as improvement in R.MFG signal efficiency that supports 2-back performance; however changes in visual working memory were not assessed with MATRICS measures after the second module, and therefore at present, the contribution of this form of visual training alone (or in sequence) to visual working memory changes remains unknown. Additional limitations of the study include the modest sample sizes and the unrepresentative nature of the study sample, which included schizophrenia participants who were higher-functioning than the typical clinical sample. We may have been underpowered with the current sample sizes to detect significant improvements in L.MFG signal efficiency supporting improved 2-back performance, although current results revealed marginally significant correlation effects. Participants were paid for their participation and were motivated to engage in training, which was intensive in nature and occurred in a well-controlled laboratory environment. It is not known whether similar results can be generated in real-world treatment settings.
We acknowledge that while our data show considerable potential, additional research is needed to establish the necessary and sufficient elements of training, the critical neural mechanisms that support long-term adaptive behavioral change, and the optimal dissemination approaches that will lead to widespread and enduring improvements in quality of life and functional outcome.
Supplementary Material
Highlights.
Computerized cognitive training improves working-memory (WM) in schizophrenia (SZ)
Better WM is supported by enhanced prefrontal signal efficiency in SZ
Training-induced prefrontal signal efficiency predicts better long-term functioning
Training-induced WM improvements generalize beyond the trained tasks
Training-induced WM improvements are sustained 6 months after the intervention
Acknowledgments
This work was supported by NIMH grant R01MH081051, which was administered by the Northern California Institute for Research and Education, and with resources of the Department of Veterans Affairs Medical Center, San Francisco, California. Its contents are solely the responsibility of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
A portion of the cognitive training software used in this study was supplied to the senior author free of charge by Posit Science Inc. Dr. Vinogradov is a consultant to Posit Science Inc., a company with a commercial interest in cognitive training software. None of the other authors have any financial interest in Posit Science. Drs. Subramaniam, Luks, Chung, Garrett, Fisher and Nagarajan report no competing interests. Drs. Vinogradov, Luks, and Nagarajan have received grants or research support from the National Institute of Mental Health.
References
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, Nagarajan S, Vinogradov S. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull. 2008;35:1132–1141. doi: 10.1093/schbul/sbp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Hill J, Wheelwright S. Mind Reading: the interactive guide to emotions. London and New York: University of Cambridge, Kingsley Publishers; 2003. [Google Scholar]
- Bilker WB, Brensinger C, Kurtz MM, Kohler C, Gur RC, Siegel SJ, Gur RE. Development of an abbreviated schizophrenia quality of life scale using a new method. Neuropsychopharmacology. 2003;28:773–777. doi: 10.1038/sj.npp.1300093. [DOI] [PubMed] [Google Scholar]
- Bor J, Brunelin J, d'Amato T, Costes N, Suaud-Chagny MF, Saoud M, Poulet E. How can cognitive remediation therapy modulate brain activations in schizophrenia? An fMRI study. Psychiatry Res. 2011;192:160–166. doi: 10.1016/j.pscychresns.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Davis M, Goff D, Green MF, Keefe RS, Leon AC, Nuechterlein KH, Laughren T, Levin R, Stover E, Fenton W, Marder SR. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31:5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Delahunt P, Hardy JL, Brenner DF, Chan SC, Dewey JA, Mahncke HW, Wade TW, Merzenich MM. InSight. Scientific principles of a brain-plasticity based visual training program. Posit Science Corporation; 2008. [Google Scholar]
- Deserno L, Sterzer P, Wustenberg T, Heinz A, Schlagenhauf F. Reduced prefrontal-parietal effective connectivity and working memory deficits in schizophrenia. J Neurosci. 2012;32:12–20. doi: 10.1523/JNEUROSCI.3405-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman P. Micro Expressions Training Tool and The Subtle Expressions Training Tool (METT AND SETT) Venice, CA: MOZGO Media; 2003. [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using Neuroplasticity-Based Auditory Training to Improve Verbal Memory in Schizophrenia. Am J Psychiat. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity- Based Cognitive Training in Schizophrenia: An Interim Report on the Effects 6 Months Later. Schizophr Bulletin. 2010;36:869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia Human brain mapping. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Haut KM, Lim KO, Macdonald A., 3rd Prefrontal Cortical Changes Following Cognitive Training in Patients with Chronic Schizophrenia: Effects of Practice, Generalization, and Specificity. Neuropsychopharmacology. 2010;35:1850–1859. doi: 10.1038/npp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111:1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M, Kaprio J, Cohen MS, Lonnqvist J, Cannon TD. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control participants. Schizophr Res. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (Panss) for Schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Fox KH, Harvey PD, Cucchiaro J, Siu C, Loebel A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr Res. 2011;125:161–168. doi: 10.1016/j.schres.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Koch K, Wagner G, von Consbruch K, Nenadic I, Schultz C, Ehle C, et al. Temporal changes in neural activation during practice of information retrieval from short-term memory: an fMRI study. Brain Res. 2006;1107:140–150. doi: 10.1016/j.brainres.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–124. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- Mishara AL, Goldberg TE. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia: opening a closed book. Biol Psychiatry. 2004;55:1013–1022. doi: 10.1016/j.biopsych.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Penades R, Catalan R, Salamero M, Boget T, Puig O, Guarch J, Gasto C. Cognitive Remediation Therapy for outpatients with chronic schizophrenia: A controlled and randomized study. Schizophr Res. 2006;87:323–331. doi: 10.1016/j.schres.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol.Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer A, Schneider FC, Grosselin A, Pellet J, Barral FG, Laurent B, et al. Brain activation during executive processes in schizophrenia. Psychiatry Res. 2009;173:170–176. doi: 10.1016/j.pscychresns.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Sanz JH, Karlsgodt KH, Bearden CE, van Erp TG, Nandy RR, Ventura J, et al. Symptomatic and functional correlates of regional brain physiology during working memory processing in patients with recent onset schizophrenia. Psychiatry Res. 2009;173:177–182. doi: 10.1016/j.pscychresns.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M, Gabrieli JD, Stebbins GT, Sullivan EV. Working and strategic memory deficits in schizophrenia. Neuropsychology. 1998;12:278–288. doi: 10.1037//0894-4105.12.2.278. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73:842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Iwase M, Nakahachi T, Sekiyama R, Tabushi K, Kajimoto O, et al. Spatial working memory deficit correlates with disorganization symptoms and social functioning in schizophrenia. Psychiatry Clin Neurosci. 2005;59:453–460. doi: 10.1111/j.1440-1819.2005.01398.x. [DOI] [PubMed] [Google Scholar]
- Tan BL. Profile of cognitive problems in schizophrenia and implications for vocational functioning. Aust Occup Ther J. 2009;56:220–228. doi: 10.1111/j.1440-1630.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon S. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry. 2009;66:549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Nagarajan S. Cognitive training in schizophrenia: golden age or wild west? Biol Psychiatry. 2013;73:935–937. doi: 10.1016/j.biopsych.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF. Prefrontal function in schizophrenia: Confounds and controversies. Philos T R Soc B. 1996;351:1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Anderson M, Fulbright RK, Gore JC. Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am.J.Psychiatry. 2000;157:1694–1697. doi: 10.1176/appi.ajp.157.10.1694. [DOI] [PubMed] [Google Scholar]
- Wilcox R. Trimming and Winsorization. In: Armitage P, Colton T, editors. Encyclopedia of biostatistics. 2nd ed. Chichester, UK: Wiley; 2005. [Google Scholar]
- Wykes T, Brammer M, Mellers J, Bray P, Reeder C, Williams C, et al. Effects on the brain of a psychological treatment: cognitive remediation therapy -Functional magnetic resonance imaging in schizophrenia. Brit J Psychiat 1. 2002;81:144–152. doi: 10.1017/s0007125000161872. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Walters R, Wendelken C, Ragland JD, et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: Relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiat. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.