Abstract
Introduction
The question of whether removal of sensory receptors in the prepuce by circumcision affects sensitivity and/or sexual pleasure is often debated.
Aims
To examine histological correlates relevant to penile sensitivity and sexual pleasure.
Methods
Systematic review of the scientific literature on penile structures that might affect sensitivity and sexual sensation. Articles were included if they contained original data on human male penile histology or anatomy. Individual articles, including reference lists, were evaluated. They were then considered in relation to physiological data from articles retrieved by a previous systematic review.
Results
We retrieved 41 publications on penile structure. Considered in the light of 12 reporting physiological measurements, our evaluation finds that sexual response is unlikely to involve Meissner’s corpuscles, whose density in the prepuce diminishes at the time of life when male sexual activity is increasing. Free nerve endings also show no correlation with sexual response. Because tactile sensitivity of the glans decreases with sexual arousal, it is unrelated to sexual sensation. Thermal sensitivity seems part of the reward mechanism of intercourse. Vibrational sensitivity is not related to circumcision status. Observations that penile sexual sensation is higher post circumcision are consistent with greater access of genital corpuscles to sexual stimuli after removal of the prepuce. This is based on the distribution of these corpuscles (which are located in the glans) and, in uncircumcised men, the position of the retracted prepuce during intercourse, rather than any change in the number of genital corpuscles. The scientific literature suggests that any sexual effect of circumcised men may depend solely on exposure of the glans and not on the absence of the prepuce.
Conclusion
Based on histological findings and correlates of sexual function, loss of the prepuce by circumcision would appear to have no adverse effect on sexual pleasure. Our evaluation supports overall findings from physiological measurements and survey data.
Keywords: Circumcision, Prepuce, Glans Penis, Sexual Satisfaction, Penile Sensation, Genital Corpuscles, Meissner’s Corpuscles, Free Nerve Endings, Neurophysiology, Orgasm, Male Sexual Pleasure
Introduction
Male circumcision is one of the most ancient and most common surgical procedures [1]. Although the practice of circumcision is often a topic of debate, scientific evidence documenting the health benefits and low risks has led to several evidence-based statements by professional bodies advocating male circumcision 2–4.
The positive medical benefits of circumcision need to be considered in the context of any potential adverse effects on reproductive potential and sexual satisfaction. In the past, attention was focused on the sensitivity of the glans penis. It was generally held as axiomatic that glans exposure would reduce the sensitivity of the organ and thereby prolong intercourse, to the greater satisfaction of the female partner. Whether this led to greater satisfaction for the man, by prolonging intercourse, or less satisfaction because of reduced sensitivity, was a matter for debate [5]. Later, the possibility was raised that the prepuce itself might function in sexual response [6].
This question has been addressed in a number of ways. First, population surveys of sexual function, practices, and satisfaction have been undertaken involving thousands of participants in the United States [7] and Australia [8]. Survey studies can provide only indirect information about sexual sensitivity and satisfaction. Second, many studies (often prompted by the expansion of male circumcision programs in Africa to combat HIV) have addressed the sexual function and satisfaction of men circumcised after commencement of sexual activity. One study involved a randomized controlled trial of men well matched for sexual activities in which men in the circumcised and uncircumcised arms of the trial were followed for 24 months [9]. These surveys provide an opportunity (not always taken) to ask about sexual sensation before and after circumcision. Third, many studies, beginning with Masters and Johnson [10], have attempted to measure penile sensitivity of circumcised and uncircumcised men via a range of techniques (see recent review: [11]). Fourth, several studies have examined the histology of the prepuce and penis sometimes with a view to evaluating the effect of circumcision.
These lines of research have been conducted in isolation. No previous study has set out to consider structural aspects in drawing these disparate strands together.
Aims
This study aims to correlate what histology can tell us about the different nerve endings in the penis with experimental measures of sexual response and satisfaction in circumcised and uncircumcised men. Our ultimate objective was to determine if there is any structural basis for claims of a potential effect of loss of the foreskin on sexual sensation and pleasure.
Retrieval of References
A systematic literature review was undertaken through February 2015 by searching the PubMed, EMBASE, and Scopus databases using keywords shown in Table 1. The searches retrieved 26 references 12–37. We reviewed the abstracts, then the text of appropriate articles. Articles that did not contain original data or histological or anatomical information relevant to human penile sensory sensation were not included in our evaluation. The searches led to the identification of 30 unique articles.
Table 1.
Search terms, strategy, and articles retrieved from PubMed, EMBASE, and Scopus*
| Search term | Total† | Found‡ | References |
|---|---|---|---|
| PubMed | |||
| Circumcision sexual function | 230 | 2 | [12],[13] |
| Circumcision sexual satisfaction | 73 | 0 | |
| Penis histology nerves sensitivity | 30 | 1 | [14] |
| Penis sexual sensation | 158 | 1 | [15] |
| Penis Meissner’s | 4 | 2 | [6],[16] |
| Penis Meissner | 8 | 2 | [17],[18] |
| Penile nerve structure | 77 | 2 | [19],[20] |
| Penis histology circumcision | 515 | 1 | [21] |
| Meissner’s corpuscles human | 58 | 0 | |
| Prepuce sensory sensation | 6 | 0 | |
| Prepuce innervation | 44 | 2 | [20],[23] |
| Penis nerve human anatomy | 1,014 | 1 | [24] |
| Sensory genital | 369 | 1 | [25] |
| Mucocutaneous end-organ | 6 | 2 | [26],[27] |
| EMBASE | |||
| “Circumcision” and “sexual function” | 125 | 0 | |
| “Circumcision” and “sexual satisfaction” | 70 | 0 | |
| “Penis” and “histology” and “nerves” and “sensitivity” | 3 | 1 | [28] |
| “Penis” and “sexual sensation” | 9 | 0 | |
| “Penis” and “Meissner” | 15 | 0 | |
| “Penile nerve” and “structure” | 135 | 1 | [29] |
| “Penis histology” and “circumcision” | 14 | 0 | |
| “Penis” and “histology” and “circumcision” | 173 | 0 | |
| “Meissner corpuscle” and “human” | 94 | 0 | |
| “Prepuce” and “sensory sensation” | 0 | 0 | |
| “Prepuce” and “sensation” | 0 | 0 | |
| “Prepuce” and “innervation” | 23 | 3 | 30–32 |
| “Penis” and “nerve” and “human” and “anatomy” | 435 | 5 | 33–35 |
| “Penis” and “glans” and “epithelium” | 171 | 0 | |
| “Sensory” and “ corpuscle” and “genital” | 29 | 0 | |
| Scopus | |||
| “Penis” and “sensitivity” | 848 | 3 | [36],[37] |
| “Genital corpuscles” | 11 | 0 | |
| “Genital end bulbs” | 4 | 0 | |
Searches were conducted in the order listed. Any article already retrieved was not included if it appeared again during any subsequent search. Thus, each search was intended to identify only articles not found already
“Total” is the total number of articles that appeared for each search
“Found” is the number of articles that fulfilled the search criteria of containing structural information
A further eight relevant publications were identified from the reference lists of the articles retrieved: 38–45. The authors’ own reference collections were also examined, finding three further publications on structural aspects: 46–48. Major textbooks of medical histology revealed relevant structural information in Rhodin [49]. Anatomical and histological studies prior to 1930 have been excluded—they are adequately reviewed by Winkelmann [26].
In total, we identified a total of 41 unique publications on penile structures containing information that may be relevant to sensory properties of the penis, not necessarily all of which concern sexual sensation.
We then evaluated this information in relation to publications on physiological measurements of sensation in circumcised and uncircumcised men retrieved in a previous systematic review [11]. These references were: 10,50–62. Because data in two of those retrieved [58],[61] have been found to be seriously flawed [11], these two articles were excluded.
The Prepuce
There have now been many studies on the innervation of the prepuce. This may in part reflect the ready availability of samples, as circumcision is common. The first such study we identified was that of Bazett et al. [38], and most subsequent studies have used the same source of material. A few studies did not use prepuces removed during circumcision. For example, Taylor et al. studied 22 uncircumcised adult penises obtained at autopsy [6]. These were selected for “long” or “short” prepuce and therefore were not a random sample. The authors identified a “ridged band” at the junction of the inner and outer preputial layers. The “ridged band” seems to be a name used for the concertinaed distal skin that becomes stretched for retraction over the glans. We consider that such a conformation is merely a matter of individual idiosyncrasy and not a universal feature. Furthermore, different illustrations of the so-called “ridged band” do not appear to show the same structure—both illustrations in the subsequent publication by Cold and Taylor appear to label the entire inner layer of the prepuce (naturally wrinkled after retraction behind the glans) as a “ridged band” [21].
That there can be a “tight ring” at the tip of the prepuce is not in doubt, but this is not a band, rather it is a very narrow ring. This is prominent in infants and generally disappears at puberty. Kayaba et al. showed that it was present in 84% of neonates but in only 8.6% of boys aged 11–15 [46].
Meissner’s Corpuscles
Meissner’s corpuscles are mechanoreceptors involved in fine-touch sensitivity. They are the most complex cutaneous receptors, having two to six afferent nerve fibers that branch into a complex system of nerve endings arranged spirally, and are sandwiched between Schwann cells and collagen fibers, surrounded by a capsule of collagen and fibrocytes [39],[40],[49]. The bigger their size and greater their complexity, the higher their likely sensitivity to touch.
Meissner’s corpuscles are the most studied nerve endings in the prepuce. Bazett et al. found only two per square centimeter, which they considered insufficient to account for the demonstrable fine-touch sensitivity of the prepuce [38]. Taylor et al. reported that Meissner’s corpuscles were most numerous on the ridges of the purported “ridged band” and least numerous in the smooth inner layer of prepuce but gave no quantitative results [6].
Workers at Futian Hospital, Guandong, China, published several papers on Meissner’s corpuscles in the prepuce, including three 41–43 with reasonable sample sizes. Their largest and most significant paper looked at 204 excised prepuces and tracked corpuscle density against age for both phimosis and redundant prepuce (the most common reasons for circumcision in their population). Their results (Figure 1) all referred to the outer preputial layer.
Figure 1.
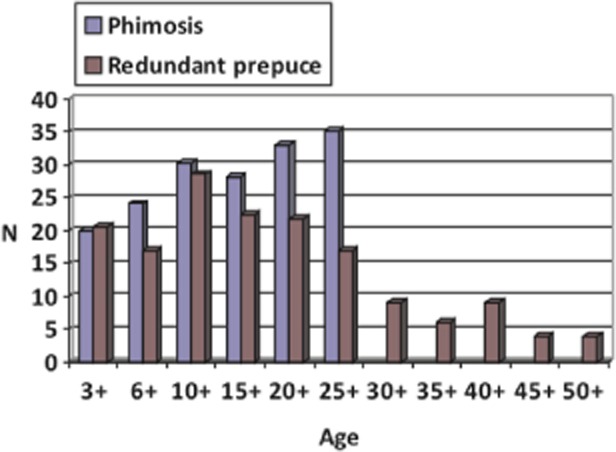
Density of Meissner’s corpuscles in the prepuce as a function of age in patients circumcised for phimosis and redundant prepuce. (Data shown were derived from Jiang et al. [42].)
The density of Meissner’s corpuscles in that study was much higher than reported by Bazett et al. but was quite variable, and in some of the other papers from the Guandong group, no Meissner’s corpuscles at all were found [41]. No data were provided for males younger than the age of 3 years, apparently because circumcision is not carried out prior to this age in China [62]. As can be seen in Figure 1, Meissner’s corpuscle density shows a variable, nonsignificant increase up to age 10–14 years in the prepuce of males circumcised for redundant prepuce, followed by a steady, statistically significant decline of 90% by age 45–50. In the case of phimosis, the density continued to increase, but not significantly, to age 25–30 years. There were no data after this age in the phimosis group. Nevertheless, other papers from the Guandong group do show a decline in density with age in prepuces from men circumcised for phimosis [43]. The latter study also investigated the density of Meissner’s corpuscles in the inner prepuce and the so-called “ridged band” in some subjects finding that the “ridged band” had more Meissner’s corpuscles, whereas in others, Meissner’s corpuscle density was substantially lower than adjacent inner preputial tissue [44]. This supports our contention that the “ridged band” is not a defined structure but merely a matter of interindividual idiosyncrasy. Recently, Martín-Alguacil et al. also described Meissner’s corpuscles in the prepuce but gave no numerical estimate [16].
To understand the importance of these results one should relate the observations to skin in other body areas. In a histological study of eight glabrous (hairless) skin locations, Meissner’s index (number of Meissner’s cells divided by number of epidermal ridges) was highest in the finger tip (0.96) and lowest in the prepuce (0.28), as was the size of the Meissner’s corpuscles in each part of the body: 120–260 × 64–84 μm for finger tip compared with 66–84 × 38–52 μm for the prepuce [45]. The latter study concluded that the prepuce is the least sensitive glabrous tissue of the body.
These findings suggest that the prepuce has fewer Meissner’s corpuscles than any other glabrous skin and that the number of these nerve endings decreases significantly after the teenage to young adult years when sexual activity begins. This makes it very difficult to propose any sexual function for Meissner’s corpuscles. A more feasible hypothesis is to regard them as a juvenile phenomenon, perhaps serving to protect the penis until the onset of puberty reveals its sexual function.
Free Nerve Endings
Bazett et al found that free, nonbranched, nerve endings were by far the most common nerve termination in the prepuce, with 115 per square centimeter [38]. Free nerve endings are implicated in the detection of a wide range of sensation, including nociception, touch, and temperature. Martín-Alguacil et al. also demonstrated free nerve endings but did not quantify them [16]. Malkoc et al. showed that free, nonbranched, nerve endings were least common in the proximal region (outer layer) of the prepuce and most common in the distal region (inner layer) [13]. These authors correlated their number with sexual function, as discussed below.
Other Nerve Endings
Krause’s end bulbs were reported by Bazett et al., at a density of 15 per square centimeter of preputial skin [38]. These are mechanoreceptors and might, therefore, be involved in sexual sensation. The density of Krause’s end bulbs in the prepuce was much higher than Meissner’s corpuscles. However, Cold and Taylor reported that Meissner’s corpuscles were the most abundant encapsulated receptors, without providing any numerical data to support their claim. Two other nerve terminations were reported in significant numbers by Bazett et al.: Type 2—a small, nonencapsulated bulb made up of very fine, richly anastomosing branches (density 52 cm−2)—and Type 3, made up of many branches, each ending in a number of smaller branches with small plate-like terminations (23 cm−2) [38]. Martín-Alguacil et al. also reported Pacinian corpuscules—encapsulated terminals with a lamellate structure and that respond to pressure and vibration. This appears to be the only such report, which seems surprising if they are normally present as their structure is quite distinctive. The foreskins used in their study were all from juvenile patients (age 1–9 years). Could it be that Pacininian corpuscules are lost later? More studies are needed.
There is likely to be some degree of confusion in the classification of the various kinds of nerve endings. Possibly, some of the Type 2 bulbs of Bazett et al. were scored as Meissner’s corpuscles by others, or vice versa. Likewise, Bazett et al.’s Type 3 matches many descriptions of free nerve endings. Bazett et al. only allowed unbranched filaments under this heading. Further research will be needed to resolve these apparent discrepancies.
Does the Prepuce Have a Sexual Function?
Ascribing any special erogenous nature to the prepuce is rather difficult despite numerous claims and counter-claims. The question of sexual response before and after circumcision is dealt with in the following section, where it should be clear that isolating any specific contribution of the prepuce is fraught with difficulties.
Two studies provide direct evidence pertaining to this question. Malkoc et al. carried out a prospective experiment on young adult volunteers who wished to be circumcised [13]. In the 3 weeks prior to circumcision, each recorded his ejaculatory latency time (ELT; time from intromission to ejaculation) with a stopwatch, three times at least 24 hours apart. The density of free nerve endings on the excised prepuce showed no correlation with their ELT values recorded precircumcision. The density of free nerve endings (presumably per square centimeter, although this is not stated in the paper)—7.5 ± 3.1, 6.2 ± 2.5, and 4.5 ± 6.0 for distal, middle, and proximal prepuce—differed significantly between the proximal site and the middle and distal sites but this had no relationship with ELT.
Hosseini et al. studied the effect of the so-called “mucosal cuff”—the amount of inner prepuce remaining after circumcision—on ELT [12]. (We question the term “mucosal cuff” as there is no basis for calling it “mucosal” because no goblet cells or other mucus-secreting structures have ever been demonstrated in it.) Different circumcision methods can leave anything from a substantial amount of this skin to virtually none [1]. Hosseini et al. found no correlation between the amount of residual “cuff” and ELT [12]. Bodacki et al. found the same [48].
The conclusion we draw from both histological and experimental results is that there is no biological basis for ascribing any sexual function to the prepuce.
The Glans
Meissner’s Corpuscles
Although some have said that no Meissner’s corpuscles are present in the glans penis [17], others report the presence of some Meissner’s corpuscles [6]. Histologists may be ascribing the identification of similar structures differently.
Free Nerve Endings
Free nerve endings, which are characterized by incomplete Schwann cell investment and irregularly scattered neurofilaments and neurotubules, comprise 80–90% of axon terminals in the glans penis and exceed corpuscular receptors by 10-fold [63]. These free nerve endings confer high temperature-sensitivity upon the glans [17]. Every male will have discovered that a shower that runs cold, or a bath that is a too hot, is felt far more keenly on the glans penis than other skin surfaces. In extreme cases this can be excruciatingly painful [64].
The function of such temperature-sensitivity is interesting to consider. It cannot be purely protective, as the penis has a copious blood supply and is much less likely to be affected by external temperatures than other extremities. Furthermore, the glans is normally covered by the foreskin, thereby insulating it. Vaginal temperature does change during the menstrual cycle, but sensing this after intromission would seem to be irrelevant. We speculate that exquisite temperature-sensitivity might represent part of the “reward” system that encourages sexual congress. A sexually naïve youth, upon intromission, will experience a “perfect” temperature that his penis, dangling loose, could never otherwise sense. This reward mechanism could thereby encourage him to complete the sexual act.
Free nerve endings can also mediate sensitivity to touch [49] and, in view of the dispute over the presence of Meissner’s corpuscles (above), free nerve endings are probably the major touch sensors in the glans.
Temperature sensing is mediated by small nerve fibers, as are the autonomic functions controlling potency and micturition. This led to the hypothesis that measuring penile temperature sensitivity could indicate whether autonomic failures such as impotence are due to a general neuropathy affecting the small nerves. This does indeed seem to be the case [51],[55]. Subsequent work by Bleustein has shown that other sensory measurements such as tactile sensation, pressure, and vibration (discussed below) also correlate with dysfunction, but temperature sensation has the strongest correlation [65]. None of these studies relate directly to sexual sensitivity, but they do provide a baseline of penile somatosensory measurements.
Genital Corpuscles
The glans has a unique corpuscular receptor, consisting of axon terminals that resemble the tangled skein of free nerve endings and that are probably derived from Krause’s end bulbs [49]. Genital corpuscles of the glans are most abundant in the corona and near the frenulum [17]. Confusion has been pointed out between the genital corpuscle and Meissner’s corpuscle [22]. The genital corpuscles are the only mediators of sexual response. They are connected to a unique innervation system, which is quite separate from that of the prepuce, but does involve the meatus [24]. Chouchkov dismissed genital corpuscles in 1978 as being just Krause end bulbs [66]. However, Halata and Munger identified a population of genital end bulbs that was distinct from Krause end bulbs and unique to the glans (see p. 225 of Halata and Munger’s study [17]). Although lay commentators claim there is no evidence that genital corpuscles are related to sexual sensations because they are present in other body areas such as around the nipples, the latter are, nevertheless, somewhat erogenous.
Keratinization of the Glans Following Circumcision
It has often been claimed that exposure of the glans by circumcision causes the epithelium to cornify. Histological examination of cadaver penises from circumcised and uncircumcised men has, however, revealed there is no difference in keratinization [47].
Measurement of Glans Sensitivity
A recent systematic review concluded that sexual function, sensitivity, sexual sensation, and satisfaction were not adversely affected by circumcision [11]. Halata and Munger [17] describe a 19th-century test of fine touch and pain sensitivity carried out by von Frey, using a calibrated hair. He found the glans to be very insensitive to fine touch but very sensitive to pain, with only a small difference in applied force separating the two thresholds. Masters and Johnson compared the tactile sensitivity of the glans in circumcised and uncircumcised men [10] and found no difference. It was admittedly a crude test, and no statistics were given. A much more substantial study was conducted by Payne et al. in 2007 [57]. These authors determined tactile and pain sensitivity thresholds measured using a monofilament applied with calibrated pressures. They also studied the effect of sexual arousal by exposing subjects either to an erotic movie or to a documentary. Thermal imaging was used to monitor the effects of arousal on the penis. The test site was the penile shaft just behind the corona glandis and was thus intended to be equivalent on both circumcised and uncircumcised men. Interestingly, although the penile temperature increased with arousal to the same value in both groups, the circumcised group had a significantly higher baseline temperature. These findings suggest that exposure of the glans may confer slight, but continuous, arousal in circumcised men. The glans proved less sensitive to touch than the forearm, but more sensitive to pain. There was no difference in glans sensitivity at any time between circumcised and uncircumcised men, whether aroused or not. However, the tactile sensitivity of the glans, in all men, decreased significantly with sexual arousal. Intriguingly, circumcised men also had more tactile sensitivity on their forearms.
The key messages from this small case-control study (20 circumcised and 20 uncircumcised men) are that penile tactile sensitivity is not affected by circumcision and that sensitivity decreases with sexual arousal. As tactile sensitivity decreases with arousal, it seems unlikely that it plays any part in sexual response.
Sensitivity to vibration might seem a more relevant measure as vibrators are used by both males and females as a means of achieving arousal and thence orgasm. This is the only stimulus that has shown a positive correlation with sexual response, implying that it directly stimulates genital corpuscles. Xin et al. showed that sensitivity to vibration correlated strongly with likelihood of premature ejaculation (PE), which might appear to argue against the view that PE is a purely psychological problem and demonstrating the existence of a measurable stimulus that actually reflects sexual responsiveness [50].
The situation is, however, rather more complex than this. In normally functional men, vibration sensitivity decreased substantially with age, whereas in PE sufferers, it did not [50], implying some deeper difference. Furthermore, when vibrational sensitivity was compared with ELT—the time from commencement of activity to climax in normal, non-PE men, there was no correlation [59]. This study involved 58 uncircumcised men aged between 20 and 40 and measured vibrotactile sensitivity with two different instruments to minimize instrumental bias. ELT was measured in three situations—vaginal intercourse, laboratory stimulation with a vibrator applied to the frenulum, and masturbation at home. ELT was consistent for each individual over multiple measures with each technique. ELT was longest in intercourse, shortest in masturbation, and in-between in the laboratory simulation. However, they cited results from two studies (not involving sensitivity measurements) of PE sufferers, which found that ELT was longest during masturbation and shortest during intercourse, again suggesting a real difference between normally functional men and PE sufferers, though the difference here could easily be psychological.
Vibratory stimulation of the glans can induce ejaculation, this being how semen samples are collected from patients with spinal cord injury. The favored areas to accomplish this are the corona [67] and frenulum/underside of the glans [68]. This coincides with locations where the concentration of genital corpuscles is highest and is achieved irrespective of the presence of a foreskin.
One of the problems with these studies is that comparison is difficult when each uses different instrumentation, and Rowland [54] attempted to draw parallels between them. His conclusion was that decline in vibrotactile sensitivity with age is real, as is the difference between premature ejaculators and sexually normal men. He also expressed surprise at the findings, from several studies, that vibrational sensitivity decreased with sexual arousal. Yet this may be no more than geometry—with erection the surface area of the glans increases so that receptors are more widely spaced. This will be significant when measuring with instruments that typically contact only a small part of the penis but not during intercourse when the whole glans is involved.
Bleustein et al. compared both vibration and pressure sensitivity in neonatally circumcised and uncircumcised men [56]. Although raw data showed that circumcised men were more sensitive to vibration and less sensitive to pressure, controlling for age and other factors showed that there was no significant difference. In other words, circumcised men were, if anything, more sexually sensitive than uncircumcised men, though the difference was not statistically significant. Because the two groups used by Bleustein et al. were not well matched, it would be interesting to replicate this experiment with larger and better-matched samples. Yang et al. measured vibrotactile sensitivity by taking a ratio between the sensitivity of the index finger and that of the glans penis [62]. They had 73 uncircumcised controls and 96 patients with redundant prepuce who were measured before and 1, 2, and 3 months after circumcision. There was no significant difference between control and experimental groups, but the circumcised group showed a marginally significant decrease in sensitivity postoperation. The rationale for using ratiometric rather than direct measurement is obscure, as finger sensitivity could be affected by occupational influences, such as callus formation. Until there are better data, the null hypothesis remains that circumcision has no effect on vibration or pressure sensitivity.
Surveys of Circumcised and Uncircumcised Men
The topic of circumcision and sexual function was comprehensively evaluated in a meta-analysis by Tian et al. in 2013 [69] and in an extensive systematic review by Morris and Krieger the same year [11]. Both studies concluded that there were no significant differences in sexual function between circumcised and uncircumcised men.
Here we will only touch on points that relate to the histology-related topics addressed above. Two different types of study are represented—case-control studies that look at the sexual functioning of circumcised and uncircumcised men, and before and after studies looking at men’s sexual functioning before and after circumcision. In the former, the larger studies showed that circumcision protected against erectile dysfunction at older ages [7],[8], but there is no clear histologic basis for this. In the latter studies, all reported no significant difference. Here we will focus on survey studies that asked about penile sensitivity before and after circumcision. Krieger et al. reported on a large sample of young Kenyan men, in a controlled trial in which uncircumcised men were randomly assigned to circumcision or no circumcision groups [9]. By the end of the 2-year follow-up period, 64.0% of men in the circumcision group reported that “penile sensitivity” (sexual sensation) was “much more” and 7.8% that it was “somewhat more” than before circumcision. Only 1.8% reported penile sensitivity being “much less” and 5.3% “somewhat less.” Masood et al., in a much smaller, uncontrolled study, found that 38% of men reported improved penile sensation after circumcision, 18% reported worse penile sensation, whereas 44% reported no change [70]. (Curiously, they interpreted this as grounds for counseling men about adverse consequences of getting circumcised despite the finding that a large majority of men found the consequences positive.)
An increase in penile sensitivity post circumcision is contrary to much current dogma and to what has been the received opinion for centuries. We identified no histological studies comparing density of genital corpuscles in circumcised and uncircumcised penises, but one would regard it as improbable that this would differ in young adults of each status. Rather, the answer would appear to lie in increased accessibility of these terminals to a stimulus. The density of genital corpuscles is highest in the corona and frenular areas (above), and it can be surmised that even a retracted prepuce would tend to reduce the stimulus to these regions, particularly on the outward stroke of intercourse.
Conclusions
The present systematic review, correlating histological studies of penile structures with studies of sexual response, identified no basis for ascribing any sexual function to the prepuce. Meissner’s corpuscles in the prepuce diminish at the time of life when male sexual activity is increasing, and free nerve endings show no correlation with sexual response. Therefore, any sexual effect of circumcision must depend solely on the exposure of the glans and not on the absence of the prepuce.
Studies of tactile sensitivity of the glans seem to be unrelated to sexual sensation as tactile sensation decreases with sexual arousal. The thermal sensitivity, on the other hand, is probably part of the reward mechanism of intercourse. Vibration sensitivity clearly relates to sexual response, but no significant relationship to circumcision has been demonstrated.
The two studies that examined penile sensitivity before and after circumcision both found higher penile sensitivity post circumcision. This probably relates to greater access of genital corpuscles to sexual stimuli, based on the distribution of these corpuscles and the position of the retracted prepuce in intercourse, rather than any change in the number of genital corpuscles.
Our conclusions are generally consistent with findings from a recent systematic review [11] and a meta-analysis [69] that each concluded that male circumcision has no adverse effect on parameters relevant to sexual function, sensation, sensitivity, satisfaction, or pleasure. An evidence-based consensus based on this detailed evaluation of the literature is that if there is any effect of circumcision on these various sexual parameters, then the effect of circumcision is likely to be perceived as beneficial. Nevertheless, psychological factors may also influence the sexual experience of some men.
Acknowledgments
We thank Dr. Pu (Paul) Xu for translation of articles in Chinese.
Conflict of Interest
The authors report no conflicts of interest.
References
- Cox G, Morris BJ. Why circumcision: From pre-history to the twenty-first century. In: Bolnick DA, Koyle MA, Yosha A, editors. Surgical guide to circumcision. London: Springer; 2012. pp. 243–259. [Google Scholar]
- American Academy of Pediatrics. Circumcision policy statement. Task Force on Circumcision. Pediatrics. 2012;130:e756–785. [Google Scholar]
- Morris BJ, Wodak AD, Mindel A, Schrieber L, Duggan KA, Dilly A, Willcourt RJ, Cooper DA, Lumbers ER, Russell CT, Leeder SR. Infant male circumcision: An evidence-based policy statement. Open J Prev Med. 2012;2:79–82. doi: 10.1111/j.1445-5994.2012.02823.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2014. Recommendations for providers counseling male patients and parents regarding male circumcision and the prevention of HIV infection, STIs, and other health outcomes. Docket No. CDC-2014-0012.. Available at: http://www.gpo.gov/fdsys/pkg/FR-2014-12-02/pdf/2014-27814.pdf (accessed January 27, 2015)
- Van de Velde TH. Ideal marriage. Its physiology and technique. London: William Heinemann Medical Books; 1926. [Google Scholar]
- Taylor JR, Lockwood AP, Taylor AJ. The prepuce: Specialized mucosa of the penis and its loss to circumcision. Br J Urol. 1996;77:291–295. doi: 10.1046/j.1464-410x.1996.85023.x. [DOI] [PubMed] [Google Scholar]
- Laumann EO, Masi CM, Zuckerman EW. Circumcision in the United States. Prevalence, prophyactic effects, and sexual practice. J Am Med Assoc. 1997;277:1052–1057. [PubMed] [Google Scholar]
- Richters J, Smith AM, de Visser RO, Grulich AE, Rissel CE. Circumcision in Australia: Prevalence and effects on sexual health. Int J STD AIDS. 2006;17:547–554. doi: 10.1258/095646206778145730. [DOI] [PubMed] [Google Scholar]
- Krieger JN, Mehta SD, Bailey RC, Agot K, Ndinya-Achola JO, Parker C, Moses S. Adult male circumcision: Effects on sexual function and sexual satisfaction in Kisumu, Kenya. J Sex Med. 2008;5:2610–2622. doi: 10.1111/j.1743-6109.2008.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters WH, Johnson VE. Human Sexual Response. Boston: Little Brown; 1966. [Google Scholar]
- Morris BJ, Krieger JN. Does male circumcision affect sexual function, sensitivity or satisfaction?—A systematic review. J Sex Med. 2013;10:2644–2657. doi: 10.1111/jsm.12293. [DOI] [PubMed] [Google Scholar]
- Hosseini SR, Khazaeli MH, Atharikia D. Role of postcircumcision mucosal cuff length in lifelong premature ejaculation: A pilot study. J Sex Med. 2008;5:206–209. doi: 10.1111/j.1743-6109.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- Malkoc E, Ates F, Tekeli H, Kurt B, Turker T, Basal S. Free nerve ending density on skin extracted by circumcision and its relation to premature ejaculation. J Androl. 2012;33:1263–1267. doi: 10.2164/jandrol.112.016709. [DOI] [PubMed] [Google Scholar]
- Zhang HF, Zhang CY, Li XH, Fu ZZ, Chen ZY. Dorsal penile nerves and primary premature ejaculation. Chin Med J. 2009;122:3017–3019. [PubMed] [Google Scholar]
- Robert R, Labat JJ, Riant T, Louppe JM, Hamel O. [The pudendal nerve: Clinical and therapeutic morphogenesis, anatomy, and physiopathology] Neurochirurgie. 2009;55:463–469. doi: 10.1016/j.neuchi.2009.07.004. (in French) [DOI] [PubMed] [Google Scholar]
- Martin-Alguacil N, Cooper RS, Aardsma N, Mayoglou L, Pfaff D, Schober J. Terminal innervation of the male genitalia, cutaneous sensory receptors of the male foreskin. Clin Anat. 2015;28:385–391. doi: 10.1002/ca.22501. [DOI] [PubMed] [Google Scholar]
- Halata Z, Munger BL. The neuroanatomical basis for the protopathic sensibility of the human glans penis. Brain Res. 1986;37:205–230. doi: 10.1016/0006-8993(86)90357-4. [DOI] [PubMed] [Google Scholar]
- Munger BL, Ide C. The structure and function of cutaneous sensory receptors. Arch Histol Cytol. 1988;51:1–34. doi: 10.1679/aohc.51.1. [DOI] [PubMed] [Google Scholar]
- Yang CC, Bradley WE. Neuroanatomy of the penile portion of the human dorsal nerve of the penis. Br J Urol. 1998;82:109–113. doi: 10.1046/j.1464-410x.1998.00669.x. [DOI] [PubMed] [Google Scholar]
- Yang CC, Bradley WE. Innervation of the human glans penis. J Urol. 1999;161:97–102. [PubMed] [Google Scholar]
- Cold CJ, Taylor JR. The prepuce. BJU Int. 1999;83:34–44. doi: 10.1046/j.1464-410x.1999.0830s1034.x. [DOI] [PubMed] [Google Scholar]
- Winkelmann RK. The cutaneous innervation of human newborn prepuce. J Invest Dermatol. 1956;26:53–67. doi: 10.1038/jid.1956.5. [DOI] [PubMed] [Google Scholar]
- Shih C, Cold CJ, Yang CC. Cutaneous corpuscular receptors of the human glans clitoris: Descriptive characteristics and comparison with the glans penis. J Sex Med. 2013;10:1783–1789. doi: 10.1111/jsm.12191. [DOI] [PubMed] [Google Scholar]
- Yang CC, Bradley WE. Peripheral distribution of the human dorsal nerve of the penis. J Urol. 1998;159:1912–1916. doi: 10.1016/S0022-5347(01)63194-X. [DOI] [PubMed] [Google Scholar]
- Tammaro A, Parisella FR, Cavallotti C, Persechino S, Cavallotti C. Ultrastructural age-related changes in the sensory corpuscles of the human genital skin. J Biol Regul Homeost Agents. 2013;27:241–245. [PubMed] [Google Scholar]
- Winkelmann RK. The mucocutaneous end-organ. The primary organized sensory ending in human skin. Arch Dermatol. 1957;76:225–235. doi: 10.1001/archderm.1957.01550200069015. [DOI] [PubMed] [Google Scholar]
- MacDonald DM, Schmitt D. Ultrastructure of the human mucocutaneous end organ. J Invest Dermatol. 1979;72:181–186. doi: 10.1111/1523-1747.ep12676374. [DOI] [PubMed] [Google Scholar]
- Montagna W. Morphology of cutaneous sensory receptors. J Invest Dermatol. 1977;69:4–7. doi: 10.1111/1523-1747.ep12497855. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Li XH, Yuan T, Zhang HF, Liu JH, Ye ZQ. Regional anatomy of the dorsal penile nerve and its clinical significance. Zhonghua Nan Ke Xue. 2009;15:130–133. [.] (in Chinese) [PubMed] [Google Scholar]
- Bourlond A, Winkelmann RK. The innervation of the prepuce of the newborn infant. Arch Belges Dermatol Syphiligraphie. 1965;21:139–153. [PubMed] [Google Scholar]
- De Girolamo A, Cecio A. [Contribution to the knowledge of sensory innervation of the prepuce in man.] Boll Soc Ital Biol Sper. 1968;44:1521–1522. (in Italian) [PubMed] [Google Scholar]
- Pérez Casas A, Vega Alvarez JA, López Muñiz A, Romo Hidalgo E, Suárez Garnacho S, Bengoechea González E. [Microscopic innervation of the penis. I. Prepuce and glans penis] Arch Esp Urol. 1988;41:1–7. (in Spanish) [PubMed] [Google Scholar]
- Hauser-Kronberger C, Hacker GW, Graf A-H, Mack D, Sundler F, Dietze O, Frick J. Neuropeptides in the human penis: An immunohistochemical study. J Androl. 1994;15:510–520. [PubMed] [Google Scholar]
- Baskin LS, Lee YT, Cunha GR. Neuroanatomical ontogeny of the human fetal penis. Br J Urol. 1997;79:628–640. doi: 10.1046/j.1464-410x.1997.00119.x. [DOI] [PubMed] [Google Scholar]
- Wu Z-M, Ling S-C, Zhu X, Wu S-J. Difference of distributive density of calcitonin gene-related peptide immunoreactive positive nerve terminals in the prepuce of penis and frenulum of prepuce and convection of perceptive information in adult human. Chin J Clin Rehabil. 2005;9:82–83. [Google Scholar]
- Colombel M, Droupy S, Paradis V, Lassau JP, Beniot G. Caverno-pudendal nervous communicating branches in the penile hilum. Surg Radiol Anat. 1999;21:273–276. doi: 10.1007/BF01631399. [DOI] [PubMed] [Google Scholar]
- Akman Y, Liu W, Li YW, Baskin LS. Penile anatomy under the pubic arch: reconstructive implications. J Urol. 2001;166:225–230. [PubMed] [Google Scholar]
- Bazett HC, McGlone B, Williams RG, Lufkin HM. Depth, distribution and probable identification in the prepuce of sensory end-organs concerned in sensations of temperature and touch; thermometric conductivity. Arch Neurol Psychiatry. 1932;27:490–517. [Google Scholar]
- Cauna N. Structure and origin of the capsule of Meissner’s corpuscle. Anat Rec. 1956;124:77–93. doi: 10.1002/ar.1091240106. [DOI] [PubMed] [Google Scholar]
- Castano P, Rumio C, Morini M, Miani A, Jr, Castano SM. Three-dimensional reconstruction of the Meissner corpuscle of man, after silver impregnation and immunofluorescence with PGP 9.5 antibodies using confocal scanning laser microscopy. J Anat. 1995;186:261–270. [PMC free article] [PubMed] [Google Scholar]
- Jiang H-Y, Wang G-X, Guo D, Tan M-B, Xu S-M. Observation of Meissner’s corpuscle in abundant prepuce and phimosis. J Med Urol. 2005;4:219–224. , (in Chinese) [Google Scholar]
- Jiang H-Y, Guo D, Tan M-B, Xu S-M, Wang G-X. Observations on Meissner’s corpuscle in prepuces of different ages. Chin J Urol. 2006;27:707–709. [Google Scholar]
- Guo D, Xu S-M, Jiang H-Y, Tan M-B, Luan H. Observation of Meissner’s corpuscle on fused phimosis. J Guangdong Med Coll. 2007;20:15–16. [Google Scholar]
- Tan M-B, Jiang H-Y, Wang G-X, Luan H, Xu S-M, Guo D. Observation of Meissner’s corpuscle in entire-revealing prepuce. J Mod Urol. 2007;2007:Article 7. [Google Scholar]
- Bhat GH, Bhat MA, Kour K, Shah BA. Density and structural variations of Meissner’s corpuscles at different sites in human glaborous skin. J Anat Soc India. 2008;57:30–33. [Google Scholar]
- Kayaba H, Tamura H, Kitajima S, Fujiwara Y, Kato T, Kato T. Analysis of shape and retractability of the prepuce in 603 Japanese boys. J Urol. 1996;156:1813–1815. [PubMed] [Google Scholar]
- Szabo R, Short RV. How does male circumcision protect against HIV infection? BMJ. 2000;320:1592–1594. doi: 10.1136/bmj.320.7249.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodakçi MN, Bozkurt Y, Söylemez H, Hatipoglu NK, Penbegül N, Atar M, Sancaktutar AA. Relationship between premature ejaculation and postcircumcisional mucosal cuff length. Scand J Urol. 2014;47:399–403. doi: 10.3109/21681805.2013.764350. [DOI] [PubMed] [Google Scholar]
- Rhodin JAG. Histology. Histology. A Text and Atlas. London: Oxford University Press; 1974. p. 893. [Google Scholar]
- Xin ZC, Chung WS, Choi YD, Seong DH, Choi YJ, Choi HK. Penile sensitivity in patients with primary premature ejaculation. J Urol. 1996;156:979–981. [PubMed] [Google Scholar]
- Yarnitsky D, Sprecher E, Vardi Y. Penile thermal sensation. J Urol. 1996;156(2 Pt 1):391–393. doi: 10.1097/00005392-199608000-00014. [DOI] [PubMed] [Google Scholar]
- Bradley WE, Farrell DF, Ojemann GA. Human cerebrocortical potentials evoked by stimulation of the dorsal nerve of the penis. Somatosens Mot Res. 1998;15:118–127. doi: 10.1080/08990229870844. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Namima T, Aizawa M, Uchi K, Kaiho Y, Yoshikawa K, Orikasa S, Nakasato N. Somatosensory evoked magnetic fields elicited by dorsal penile, posterior tibial and median nerve stimulation. Electroencephalogr Clin Neurophysiol. 1998;108:57–61. doi: 10.1016/s0168-5597(97)00093-2. [DOI] [PubMed] [Google Scholar]
- Rowland DL. Penile sensitivity in men: A composite of recent findings. Urology. 1998;52:1101–1105. doi: 10.1016/s0090-4295(98)00413-0. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Yiou R, Colombel M, Chopin DK, Abbou CC. Relationship between penile thermal sensory threshold measurement and electrophysiologic tests to assess neurogenic impotence. Urology. 2001;57:306–309. doi: 10.1016/s0090-4295(00)00906-7. [DOI] [PubMed] [Google Scholar]
- Bleustein CB, Fogarty JD, Eckholdt H, Arezzo JC, Melman A. Effect of neonatal circumcision on penile neurological sensation. Urology. 2005;65:773–777. doi: 10.1016/j.urology.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Payne K, Thaler L, Kukkonen T, Carrier S, Binik Y. Sensation and sexual arousal in circumcised and uncircumcised men. J Sex Med. 2007;4:667–674. doi: 10.1111/j.1743-6109.2007.00471.x. [DOI] [PubMed] [Google Scholar]
- Sorrells ML, Snyder JL, Reiss MD, Eden C, Milos MF, Wilcox N, Van Howe RS. Fine-touch pressure thresholds in the adult penis. BJU Int. 2007;99:864–869. doi: 10.1111/j.1464-410X.2006.06685.x. [DOI] [PubMed] [Google Scholar]
- Vanden Broucke H, Everaert K, Peersman W, Claes H, Vanderschueren D, Van Kampen M. Ejaculation latency times and their relationship to penile sensitivity in men with normal sexual function. J Urol. 2007;177:237–240. doi: 10.1016/j.juro.2006.08.071. [DOI] [PubMed] [Google Scholar]
- Senol MG, Sen B, Karademir K, Sen H, Saraçoğlu M. The effect of male circumcision on pudendal evoked potentials and sexual satisfaction. Acta Neurol Belg. 2008;108:90–93. [PubMed] [Google Scholar]
- Podnar S. Clinical elicitation of the penilo-cavernosus reflex in circumcised men. BJU Int. 2012;109:582–585. doi: 10.1111/j.1464-410X.2011.10364.x. [DOI] [PubMed] [Google Scholar]
- Yang DM, Lin H, Zhang B, Guo W. [Circumcision affects glans penis vibration perception threshold] Zhonghua Nan Ke Xue. 2008;14:328–330. (in Chinese) [PubMed] [Google Scholar]
- Halata Z, Spaethe A. Sensory innervation of the human penis. Adv Exp Med Biol. 1997;424:265–266. doi: 10.1007/978-1-4615-5913-9_48. [DOI] [PubMed] [Google Scholar]
- Hershkowitz M. Penile frostbite, an unforeseen hazard of jogging. N Engl J Med. 1977;296:178. doi: 10.1056/NEJM197701202960321. [DOI] [PubMed] [Google Scholar]
- Bleustein CB, Eckholdt H, Arezzo JC, Melman A. Quantitative somatosensory testing of the penis: Optimizing the clinical neurological examination. J Urol. 2003;169:2266–2269. doi: 10.1097/01.ju.0000065824.35996.c8. [DOI] [PubMed] [Google Scholar]
- Chouchkov C. Cutaneous receptors. Adv Anat Embryol Cell Biol. 1978;54:3–61. doi: 10.1007/978-3-642-66992-7. [DOI] [PubMed] [Google Scholar]
- Szasz G, Carpenter C. Clinical observations in vibratory stimulation of the penis of men with spinal cord injury. Arch Sex Behav. 1989;18:461–474. doi: 10.1007/BF01541673. [DOI] [PubMed] [Google Scholar]
- Sarkarati M, Rossier AB, Fam BA. Experience in vibratory and electro-ejaculation techniques in spinal cord injury patients: A preliminary report. J Urol. 1987;138:59–62. doi: 10.1016/s0022-5347(17)42988-0. [DOI] [PubMed] [Google Scholar]
- Tian Y, Liu W, Wang JZ, Wazir R, Yue X, Wang KJ. Effects of circumcision on male sexual functions: A systematic review and meta-analysis. Asian J Androl. 2013;15:662–666. doi: 10.1038/aja.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood S, Patel HRH, Himpson RC, Palmer JH, Mufti GR, Sheriff MKM. Penile sensitivity and sexual satisfaction after circumcision: Are we informing men correctly? Urol Int. 2005;75:62–66. doi: 10.1159/000085930. [DOI] [PubMed] [Google Scholar]


