Abstract
Introduction
Time spent viewing visual sexual stimuli (VSS) has the potential to habituate the sexual response and generalize to the partner context.
Aim
The aim of this study was to examine whether the time spent viewing VSS is related to sexual responsiveness felt in the laboratory or with a sexual partner.
Methods
Nontreatment-seeking men (N = 280) reported their weekly average VSS viewing in hours. VSS hours were examined in relation to the sexual arousal experienced while viewing a standardized sexual film in the laboratory and erectile problems experienced with a sexual partner.
Main Outcome Measures
Self-reported sexual arousal in response to sexual films and erectile problems on the International Index of Erectile Function were the main outcome measures.
Results
More hours viewing VSS was related to stronger experienced sexual responses to VSS in the laboratory, was unrelated to erectile functioning with a partner, and was related to stronger desire for sex with a partner.
Conclusions
VSS use within the range of hours tested is unlikely to negatively impact sexual functioning, given that responses actually were stronger in those who viewed more VSS.
Keywords: Erectile Dysfunction, Sex Stimuli, Erotica, Sexual Arousal
Introduction
The introduction of VHS tapes allowed many people to access erotic films in the privacy of their own homes, which appears to have increased the viewing of sexual films [1]. Many speculate that the Internet has increased viewing of visual sexual stimuli (VSS) [2], although data have not yet supported this viewing change [3]. People do increase the breadth of their interests in visual erotica over time [4]. Although some activists (e.g., Dines [5]) and clinicians (e.g., Bronner and Ben-Zion [6]) have voiced concerns that watching VSS causes erectile dysfunction (ED), others report using VSS to overcome erectile problems [7],[8]. Surprisingly, data have not yet tested the relationship between the amount of VSS viewing and erectile functioning.
Studies of very high frequency viewing have thus far not found a relationship between VSS consumption and erectile problems. In one study of 24 sexually “compulsive” individuals, just 4 reported erectile problems [9]. A small study of 19 “hypersexual” men reported that 11 had “diminished libido or erectile function specifically in physical relationships with women” (p. 4), leaving the specific erectile problems, and their magnitude, unclear [10]. The reported problems in this study of 19 men could have been elevated because erectile problems may have been used to meet the impairment criteria in recruitment. In a different study of 78 men who were described by therapists as having sexual addiction problems, 18 also were diagnosed with some type of sexual dysfunction [11].
In contrast, published case reports deny the presence of erectile problems in those reporting addiction problems to VSS [12]. Another study reported no relationship between sexual inhibition and impaired erectile function in a sample of self-identified sex addicts [13]. Moreover, another study of 161 men with a primary complaint of hypersexuality found no elevation in erectile problems [14]. Mudry and colleagues [15] noted a lack of empirical studies on hypersexuality, suggesting the required data to link hypersexuality and erectile problems may simply not exist yet.
Some “hypersexuals” report difficulty finding a new sex partner, so it may not be reasonable to expect this group as a whole to report erectile problems if they do not have a partner [16]. However, an overwhelming majority of those who self-report hypersexuality endorse problems with VSS use [17]. Accordingly, hypersexual patients may represent a special subset of VSS consumers. For example, many of those who label themselves as sex addicts also report a stronger religious background [18]. For these reasons, it would be useful to examine whether erectile functioning is dependent on the level of VSS consumed by those who are not receiving treatment for hypersexual problems.
VSS viewing may affect erectile functioning through at least two mechanisms. More VSS viewing may result in tolerance or desensitization. For example, those who started viewing VSS at earlier ages report more breadth (termed “deviant” in the study) in their current VSS viewing [4]. Thus, erectile problems may occur when real-life sexual stimulation does not match the broad content accessed through VSS. There is evidence that gross anatomical differences exist in the average brain of those who view more VSS [19], but it is unclear whether the differences precede or follow VSS consumption. Secondly, erections may become conditioned to aspects of VSS that do not transition easily to real-life partner situations. Sexual arousal may be conditioned to novel stimuli (for review, see Brom et al. [20]), including particular sexual images [21], specific sexual films [22], or even nonsexual images [23],[24]. It is conceivable that experiencing the majority of sexual arousal within the context of VSS may result in a diminished erectile response during partnered sexual interactions. Similarly, young men who view VSS expect that partnered sex will occur with themes similar to what they view in VSS [25]. Accordingly, when high stimulation expectations are not met, partnered sexual stimulation is ineffective.
Confounding variables also may link VSS consumption to erectile problems, thereby creating an association between the two that is not actually causal. Perhaps the most likely of these confounding variables is anxiety. Anxiety has been clearly and repeatedly linked to erectile problems (e.g., Beck and Barlow [26]). VSS lacks many of the features of partnered sex that could induce anxiety during sex, such as concerns about the partner’s sexually transmitted disease status, relationship expectations, and concerns about one’s own attractiveness or penis size. In sum, there are direct and indirect reasons why VSS may be associated with erectile problems.
The generation of an erection in response to sexual stimulation requires a relatively complex integration of information. When responding to VSS with an erection, men must attend to the VSS, recognize it as possessing sexual content, be motivated by the particular content presented, and experience general autonomic activation in addition to the very specific activation of penile motor cortex [27]. Men with erectile problems show similar areas of activity in their brain when viewing VSS to men without erectile problems, and apomorphine further enhances response in the lateral superior frontal cortex associated with erections [28],[29]. In studies that do suggest some brain response differences between these men, these appear to reflect primarily frontal areas of control coming online [28],[30]. This appears more consistent with problems due to a third variable, such as anxiety, rather than a downregulation of areas associated with sexual rewards.
Some neuro-structural differences have been suggested in men with erectile problems. Differences in white matter tracts in the splenium of the corpus callosum in patients with erectile problems have been noted, which the authors interpreted as likely problems in the communication of sensory information and conduction of visual signals [31]. Others have investigated gray matter. Of seven subcortical structures investigated, the nucleus accumbens specifically was smaller in men reporting erectile problems [32]. This finding is particularly interesting in light of a very recent publication associating the hours of VSS viewing per week with a smaller ventral striatum [19]. The latter acknowledged an association between the amount of VSS consumed with depression and alcohol consumption, which were not controlled and might provide an alternative interpretation of the results. Whereas fMRI studies are more suggestive of active inhibition contributing to erectile problems, neuro-structural studies point a bit more toward reward sensitivity problems. These are hardly completely separable processes, and either could be sufficient to link VSS use and erectile problems.
Aims
In sum, VSS use could be related to erectile functioning, but this has never been investigated in non-patient samples. When described in hypersexual patients, there appears to be no remarkable elevations of erectile problems relative to the general population. The current study investigated erectile difficulties in a non-patient sample using a secondary data analysis approach. Specifically, these studies included 280 men who reported the average number of hours they consumed VSS per week. First, their experienced sexual arousal to standardized VSS was examined in relation to the number of hours they consumed VSS in the average week. Second, self-reported erectile problems with a partner were compared with VSS consumption in a subset of these men in relationships. These tests provide information on erectile functioning in the context of both solo and partnered sexual stimulation. In the case of solo activity, it is predicted that men who view more VSS will report lower sexual arousal to the VSS, because they are expected to be desensitized (or less rewarded by) these relatively weak VSS. It is further predicted that men who consume more VSS will report more erectile difficulties with sexual partners.
Methods
Participants
Two hundred eighty men participated over four different studies conducted by the first author. These data have been published or are under review 33–36, and all men provided informed consent for the study in which they participated. These tended to be younger (see Table 1), white (60.6%) men. While exclusion criteria varied for each study, no study screened participants on the basis of the number of hours they viewed VSS, relationship status, or erectile problems. All reported attraction to women, so that the VSS would be relevant for their orientation [37]. All were aware that they would be asked to view VSS as a part of the study.
Table 1.
Demographic information
| Variable | n* | Mean | SD |
|---|---|---|---|
| Age | 262 | 23.3 | 6.1 |
| Intercourse partners (last year) | 280 | 4.5 | 10.0 |
| International Index of Erectile Function | |||
| Total† | 127 | 52.2 | 19.8 |
| Erection subscale‡ | 127 | 21.4 | 9.8 |
| Sexual Desire Inventory | |||
| Dyadic§ | 257 | 47.3 | 12.9 |
| Solitary¶ | 259 | 9.8 | 5.9 |
| n | % | ||
| Relationship status | 259 | ||
| Monogamous | 123 | 47.5 | |
| Non-monogamous | 30 | 11.6 | |
| No relationship | 106 | 40.9 | |
| Ethnicity | 212 | ||
| White | 113 | 53.3 | |
| Hispanic | 49 | 23.1 | |
| Black | 34 | 16.0 |
Studies varied in the information requested and participants were not forced to respond to all questions
Range 5 to 75
Range 1 to 30
Range 8 to 70
Range 3 to 26
SD = standard deviation.
Self-Report
Sexual Arousal
Men reported their level of sexual arousal after each sexual film (see below). Specifically, men were asked to indicate their level of “sexual arousal” ranging from 1 “not at all” to 9 “extremely.” This item has been shown to converge strongly with men’s erectile responses across many studies [38]. This relationship between erection and reported sexual arousal may be strong in men because they tend to monitor their physiological cues as indicators of their experienced sexual arousal [39] more than women [40]. Questions after sexual films were used instead of continuous lever ratings, which can decrease the sexual arousal experienced in the moment [41].
International Index of Erectile Function
The International Index of Erectile Function (IIEF) [42] is a 19-item questionnaire that requires men to provide Likert-scaled ratings of their experience with erectile function, intercourse satisfaction, orgasmic function, overall satisfaction, and sexual desire. The scales converge with clinical interviews, have high test–retest reliability, and do not covary with measures of social desirability [42]. While not a useful tool for differentiating vascular causes of erectile problems [43], it has been used in thousands of studies to quantify clinical improvement (e.g., Martin-Morales et al. [44]) and general erectile functioning [45]. A recent critique suggested that the IIEF only appeared useful when the domain of interest was sexual arousal and the sample was sexually experienced [46]. The current study is precisely interested in sexual arousal. Specifically, the total scale score and the erection subscale were examined for their relationship with the amount of VSS viewed weekly.
Sexual Desire Inventory
Some participants completed the Sexual Desire Inventory (SDI; see Table 1). The SDI is a 14-item, Likert-style questionnaire that measures levels of trait sexual desire. One-month test–retest reliability was r = 0.76 [47]. SDI scores also have been related to activity in areas of the brain associated with rewards in general [48]. The SDI is typically calculated as two subscales [47],[49]. The three-item Solitary Sexual Desire scale measures an individual’s desire for autoerotic sexual activity. The eight-item Dyadic Desire scale measures an individual’s desire for sex with a partner and is commonly used as an index of trait sexual desire level 50–52. Both subscales are related to impulsive sexual behaviors and intentions to engage in risky sexual behaviors, including anal intercourse, but only the dyadic scale was related to actual sex acts, including with uncommitted partners [53]. The results of both scales are reported for completeness, although the dyadic scale is of greater interest from the perspective of likely impact on partnered interactions.
Stimuli
The VSS presented in the studies were all films. They varied in length from 20 seconds to 3 minutes. All films portrayed one man and one woman engaging in consensual vaginal intercourse. They excluded low base rate sexual behaviors (e.g., bondage, anal sex). Films were drawn from a standardized film set [54] or were demonstrated to evoke similar levels of sexual arousal to those films. As some studies included several longer sexual films, the first sexual film was used. In the case of the sexual regulation study [36], only the 20-second sexual films in the watch-only condition were averaged across. The sexual arousal reported did not differ by film length, so data were collapsed across studies for this analysis.
Protocol
VSS Viewing
Participants were solicited by flyers in the community and from psychology courses in Pocatello, Idaho and Albuquerque, New Mexico. All participants were required to be age 18 or over and were informed that they would be viewing sexual films as a part of the study. Men contacted the laboratory by phone or e-mail to volunteer. On arrival to the laboratory, they provided informed consent. The institutional review boards at Idaho State University or the University of New Mexico approved all protocols.
Participants entered a private room with a computer. They were reassured that they could not be viewed during the study. After receiving instructions about the particular study in which they were participating, the experimenter informed the men that they would not reenter the testing room without first requesting their permission to reenter, then the experimenter left the room, closing the door. Subsequent communication occurred with the experimenter via intercom.
Participants viewed neutral films. Neutral films were the same length as the sexual film in each study. Neutral films did not include any violence, sexual content, or other highly evocative interactions. Neutral films are primarily used in sex studies to provide a period for genital responses to return to pre-stimulation levels. Unlike brain responses, which are relatively rapid and transient, detumescence in the genitals requires a more extended period of quiescence to return to baseline. This ensured that any sexual arousal at the start of the study, or in response to a recent sexual stimulus, had sufficient time to dissipate before the start of the (next) sexual film.
Main Outcome Measures
Hours of VSS viewing per week were expected to be strongly positively skewed (cp. Kühn and Gallinat [19]) and were in these data as well. Transformations were not sufficient to support a Gaussian distribution, so data were binned. These bins represented watching 0 (n = 25), up to 2 (n = 56), or more than 2 (n = 55) hours of VSS in the average week.
The amount of VSS viewed during the week was used to predict the level of sexual arousal reported in response to the VSS in the laboratory using a Kruskal–Wallis test. (Correlations are included for interested readers.) Analyses on the IIEF were conducted in several ways. First, analyses were conducted on the full sample with IIEF scores (n = 133). Then analyses were conducted including only the participants who reported being currently in a sexual relationship (n = 59). This is a notable challenge of research in this area, as men are likely to increase their VSS use when a partner is not available. Many have mentioned the need to characterize the impact of relationship status on VSS use [55]. To partially address this challenge, hours of VSS consumed in the average week were also used to predict the level of sexual desire reported for sex with a partner. While a less direct test, the desire for partnered sex is not dependent on the availability of a sexual partner like current erectile function.
Results
Hours of VSS and Self-Reported Sexual Response to VSS
Men (n = 136) reported an average sexual arousal rating of 5.1 (standard deviation = 2.0) to the sexual films. The hours of VSS viewed in the typical week significantly predicted the sexual arousal reported to the sexual film (Kruskal–Wallis χ2(2) = 9.1, P = 0.01, Φ = 0.26; r = 0.28, P < 0.0001). The group viewing the most VSS also reported the highest sexual arousal in response to the sexual film (see Figure 1).
Figure 1.
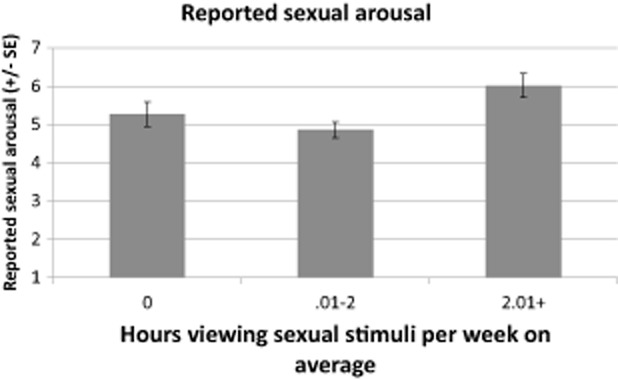
Stronger sexual arousal reported to standardized sexual films with more hours of viewing sexual films at home. SE = standard error.
Hours of VSS and Sexual Response with a Partner
Men (n = 127) reported relatively good erectile functioning (see Table 1). Neither the total scale score nor the erectile subscale score on the IIEF was related to the hours of VSS viewed in the average week. There was no association whether the total sample or the subset of individuals who were currently in relationships was included in analyses. Erectile functioning with a partner appeared unrelated to the hours of VSS being consumed in the average week.
Hours of VSS and Desire for Sex
Men reported their desire for sex with a partner and desire for solitary sex. Both were significantly related to the hours of VSS viewed in the average week. Specifically, higher desire for sex with a partner (n = 257, Kruskal–Wallis χ2(2) = 59.4, P < 0.0001, Φ = 0.48; r(255) = 0.29, P < 0.0001) or for solo sexual activity (n = 259, Kruskal–Wallis χ2(2) = 77.2, P < 0.0001, Φ = 0.55; r(257) = 0.38, P < 0.0001) was associated with more VSS use (see Figure 2).
Figure 2.
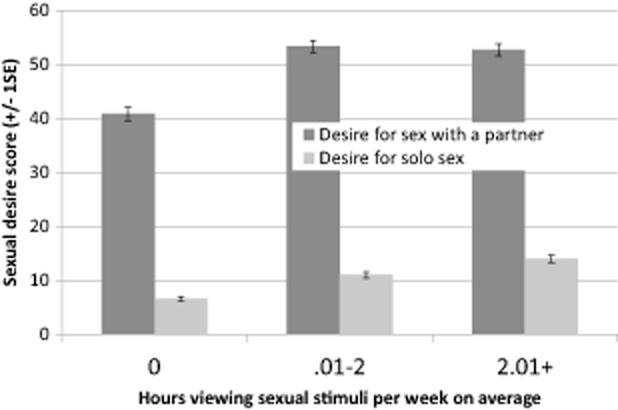
More hours viewing sexual stimuli associated with stronger sexual desire both for a partner and for masturbation. SE = standard error.
Conclusions
Data from a large sample of men (N = 280) across similar studies were aggregated to test the hypothesis that consuming more VSS was related to erectile problems. These men answered questions about their sexual behaviors and feelings, including their consumption of VSS, and viewed sexual films in the laboratory. Those who reported viewing more VSS in their own life reported higher sexual arousal to films in the laboratory. Self-reported erectile functioning with a partner was not related to the hours of VSS viewed weekly. Finally, those who viewed VSS more also reported higher desire for both partnered sexual behaviors and solo sexual behaviors. This pattern suggests that those who view more VSS likely have a higher sexual drive and experience a stronger sexual response to standardized VSS than those who view less VSS. Sexual arousal responsivity may not be impaired by viewing more VSS at home, as it actually was related to stronger desire and sexual arousal in two of the three relationships tested.
Greater time spent viewing VSS has been related to many negative mental health outcomes [56], so the mechanism for VSS to promote positive outcomes is unclear. One explanation may be that those who view more VSS report more positive sexual attitudes in general [56]. Also, consumers tend to view VSS that they find sexually arousing [57], contributing to ego-syntonic, positive affect. In the laboratory, VSS tend to promote both positive and negative feelings, although feelings are generally strongly skewed toward more positive feelings [58], including in hypersexuals [35]. In other words, it may be the case that VSS viewing promotes both positive and negative feelings and outcomes. Many studies simply do not ask about positive effects, which may contribute to poor identification of the possible benefits of VSS use. These data further suggest that the relationship of VSS with higher sexual drive might explain why viewing VSS appears to relate positively with self-reported sexual arousal. The ultimate impact of these mixed states might be determined by individual predispositions.
The positive relationships between VSS viewing and many indices of sexual responsiveness suggest that VSS viewing might even improve erectile functioning. VSS might provide a greater variety of sexual stimulation. In a Drosophila model, males raised with access to multiple partners were more successful in securing later partners than males that had been raised monogamously [59]. While the comparison is hardly direct, VSS may broaden male’s ability to respond to different sexual stimuli types. This could facilitate their sexual responses to females who may vary tremendously in their sexual behavior preferences, such as those who may experience more clitoral or vaginal sensations as contributing to their orgasm experience.
Rumors linking VSS use and ED may arise from clinical lore. A systematic literature review identified lack of data as a pervasive problem of publications in the domain of excessive sexual behaviors [15]. To determine whether this might still be the case, we repeated this systematic literature search1 of peer-reviewed articles. Two raters trained by the first author independently classified each article by its approach to the topic. The categories were “clinical,” “review,” and “empirical.” “Clinical” was used to describe articles that were written by clinicians presenting their opinions or individual cases that did not include any quantitative data. “Review” was used to describe articles that discussed existing published data, whether systematic or nonsystematic, while not presenting any new quantitative data from new study participants. “Empirical” was used to describe articles that presented new quantitative data. Additional subcategories were included for descriptive purposes only (see Figure 3). Cohen’s kappa [60] calculated using R library irr [61] was high (k = 0.90, z = 14.4, P < 0.0001) [61]. There was a significant relationship between the publication year and publication type, χ2(4) = 10.1, P = 0.04, Φ = 0.26 (see Figure 4). While the number of publications on hypersexuality generally rose from year to year, the proportion of articles that was empirical relative to the proportion of articles that was review articles fell between 2011 to 2012 (χ2(1) = 5.0, P = 0.04, Φ = 0.18) and 2012 to 2013 (χ2(1) = 4.8, P = 0.03, Φ = 0.18). The experimental portion of those empirical articles was very small (see Figure 3). Taken together, the oft-repeated link between ED and VSS might be perpetuated by data-poor literature.
Figure 3.
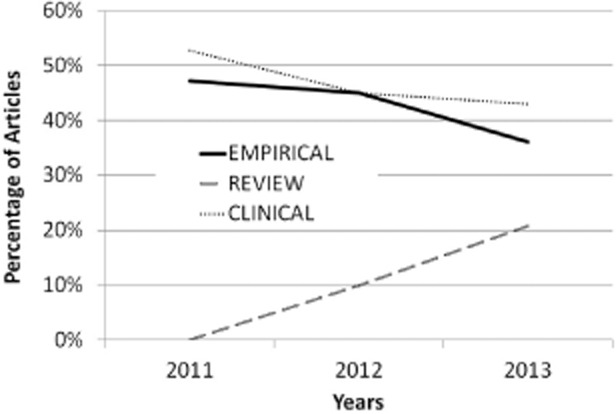
Types of publications in hypersexuality over time.
Figure 4.
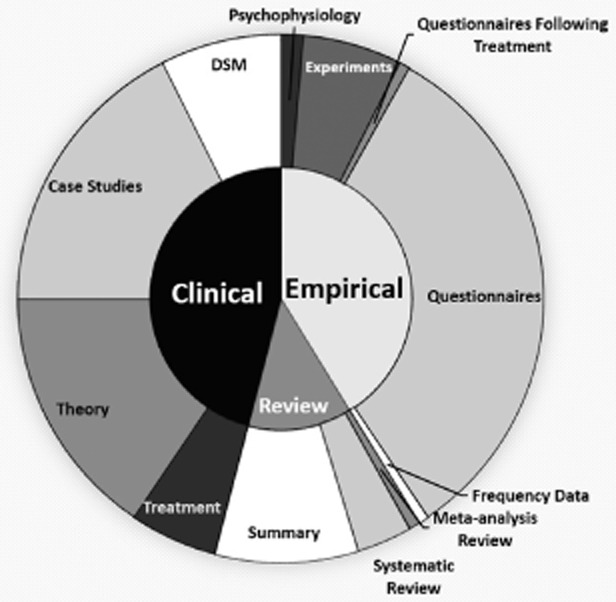
Types of publications addressing hypersexuality from 2011 to 2013. DSM = Diagnostic and Statistical Manual of Mental Disorders.
A secondary analysis approach was taken to provide the first data directly investigating the potential impacts of VSS, and limitations are inherent in such an approach. There were relatively fewer individuals with data on the measure of sexual functioning with a partner. This raises the possibility of a statistical error, where a small effect may be undetectable. The 127 men included in this analyses were sufficient to detect an effect, conservatively estimated using analysis of variance assumptions, down to f = 0.28 at 1-β = 0.8 and α = 0.05. Examination of these raw data suggested that current data pattern actually was suggestive of patterns of lesser erectile problems with more VSS use, so it appears a larger sample would be very unlikely to switch the direction of this effect. Also, these data are consistent with clinical reports that men reporting problems with hypersexual behaviors deny ED [14]. Another limitation of these data is that no physiological genital response data were included to support men’s self-reported experience. While men’s genital and self-reported sexual arousal typically correspond strongly [62], measures of genital response would offer additional support of the patterns reported by the men. Additionally, it is unclear to what extent viewing sexual films in a laboratory that were not self-selected and were time-limited reflects VSS viewing behaviors in the home environment. Such a strategy should have decreased sexual arousal, because the VSS used had a small breadth of behaviors, lower novelty, and did not progress to very high arousal states such as orgasm. While it would be worthwhile to characterize more naturalistic viewing behaviors, the pattern observed in this laboratory study was opposite of the issues (e.g., high stimulus novelty) those data might test. Finally, it is worth reiterating that these data did not include hypersexual patients. Results are probably best interpreted as limited to men with normal, regular VSS use. It would be useful to recruit men stratified on VSS use to very high ranges to characterize whether this benefit of VSS actually may be curvilinear (i.e., become detrimental at very high use).
The type of erectile problem(s) associated with VSS has not been specified. Getting versus maintaining an erection appears to be dissociable processes [63]. It may be that some aspect of erection is affected that was not surveyed. These data may reflect erection attainment (i.e., sexual arousal in response to brief sexual films) and maintenance (i.e., IIEF item “During sexual arousal, how difficult was it to maintain your erection to the completion of intercourse?”). However, perhaps the erections took longer to reach maximum rigidity or were less rigid, or self-reported sexual arousal does not reflect erections as strongly in those who view more VSS. It would be useful in the future to assess erection maintenance directly.
Regular VSS use could facilitate erectile functioning in a number of ways. Regular VSS use may prime sexual thoughts and, hence, sexual response. For example, viewing VSS led to men in one study recalling more of the physical features of the female experimenter than those men who had viewed a neutral film [64]. VSS also may serve an education function that promotes sexual responses. For example, men who view VSS more also engage in more oral and anal sex [65]. VSS may suggest or normalize sexual behaviors, providing a wider repertoire of stimuli for which men may experience desire. Finally, VSS use has been associated with more positive attitudes about sex [66]. If this is causal from VSS use, VSS use might be reducing some anxieties about sexual interactions that are a common cause of erectile problems.
Acknowledgments
The authors thank Peter Mingjui Lee and Melissa Conklin for their assistance coding article content and Whitney R. Cale for editorial assistance.
Footnotes
Search terms “hypersexu*” OR “sex addiction” OR “sexual addiction” OR “sex addict” OR “sexual addict” OR “porn addiction” OR “pornography addiction” OR “compulsive sexual” was entered in PubMed and PsychInfo databases for 2011, 2012, and 2013. One hundred fifty-one unique articles were identified.
Conflict of Interest
The authors report no conflicts of interest.
References
- Buzzell T. Demographic characteristics of persons using pornography in three technological contexts. Sex Cult. 2005;9:28–48. [Google Scholar]
- Cooper A, Boies S, Maheu M, Greenfield D. Sexuality and the Internet: The next sexual revolution. In: Szuchman LT, Muscarella F, editors. Psychological perspectives on human sexuality. New York, NY: John Wiley & Sons, Inc; 2000. pp. 519–545. [Google Scholar]
- Ley D, Prause N, Finn P. The emperor has no clothes: A review of the “pornography addiction” model. Curr Sex Health Rep. 2014;6:1–12. [Google Scholar]
- Seigfried-Spellar KC, Rogers MK. Does deviant pornography use follow a Guttman-like progression? Comput Human Behav. 2013;29:1997–2003. [Google Scholar]
- Dines G. Pornland: How porn has hijacked our sexuality. Boston: Beacon Press Books; 2010. [Google Scholar]
- Bronner G, Ben-Zion IZ. Unusual masturbatory practice as an etiological factor in the diagnosis and treatment of sexual dysfunction in young men. J Sex Med. 2014;1:1798–1806. doi: 10.1111/jsm.12501. [DOI] [PubMed] [Google Scholar]
- Tan H-M, Low WY, Ng CJ, Chen KK, Sugita M, Ishii N, Marumo K, Lee SW, Fisher W, Sand M. Prevalence and correlates of Erectile Dysfunction (ED) and treatment seeking for ED in Asian men: The Asian Men’s Attitudes to Life Events and Sexuality (MALES) study. J Sex Med. 2007;4:1582–1592. doi: 10.1111/j.1743-6109.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- Kalra S, Balhara YP, Baruah M, Saxena A, Makker G, Jumani D, Kochhar K, Majumdar S, Agrawal N, Zaveri H. Consensus guidelines on male sexual dysfunction. J Med Nutr Nutraceut. 2013;2:5–18. [Google Scholar]
- Raymond NC, Coleman E, Miner MH. Psychiatric comorbidity and compulsive/impulsive traits in compulsive sexual behavior. Compr Psychiatry. 2003;44:370–380. doi: 10.1016/S0010-440X(03)00110-X. [DOI] [PubMed] [Google Scholar]
- Voon V, Mole TB, Banca P, Porter L, Morris L, Mitchell S, Lapa TR, Karr J, Harrison NA, Potenza MN, Irvine M. Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours. PLoS ONE. 2014;9:e102419. doi: 10.1371/journal.pone.0102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briken P, Habermann N, Berner W, Hill A. Diagnosis and treatment of sexual addiction: A survey among German sex therapists. Sex Addict Compulsivity. 2007;14:131–143. [Google Scholar]
- Fong TW, Reid RC, Parhami I. Behavioral addictions: Where to draw the lines? Psychiatr Clin North Am. 2012;35:279–296. doi: 10.1016/j.psc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Vukadinovic Z. Sexual addiction, sexual compulsivity, sexual impulsivity, or what? Toward a theoretical model. J Sex Res. 2004;41:225–234. doi: 10.1080/00224490409552230. [DOI] [PubMed] [Google Scholar]
- Sutton KS, Stratton N, Pytyck J, Kolla NJ, Cantor JM. Patient characteristics by type of hypersexuality referral: A quantitative chart review of 115 consecutive male cases. J Sex Marital Ther. 2015 doi: 10.1080/0092623X.2014.935539. . Manuscript in press. [DOI] [PubMed] [Google Scholar]
- Mudry TE, Hodgins DC, el Guebaly N, Wild TC, Colman I, Patten SB, Schopflocher D. Conceptualizing excessive behaviour syndromes: A systematic review. Curr Psychiatry Rev. 2011;7:138–151. [Google Scholar]
- Quadland MC. Compulsive sexual behavior: Definition of a problem and an approach to treatment. J Sex Marital Ther. 1985;11:121–132. doi: 10.1080/00926238508406078. [DOI] [PubMed] [Google Scholar]
- Reid RC, Carpenter BN, Hook JN, Garos S, Manning JC, Gilliland R, Cooper EB, McKittrick H, Davtian M, Fong T. Report of findings in a DSM-5 field trial for hypersexual disorder. J Sex Med. 2012;9:2868–2877. doi: 10.1111/j.1743-6109.2012.02936.x. [DOI] [PubMed] [Google Scholar]
- Grubbs J, Exline J, Pargament K, Hook J, Carlisle R. Transgression as addiction: Religiosity and moral disapproval as predictors of perceived addiction to pornography. Arch Sex Behav. 2015;44:125–136. doi: 10.1007/s10508-013-0257-z. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. Brain structure and functional connectivity associated with pornography consumption: The brain on porn. JAMA Psychiatry. 2014;71:827–834. doi: 10.1001/jamapsychiatry.2014.93. [DOI] [PubMed] [Google Scholar]
- Brom M, Both S, Laan E, Everaerd W, Spinhoven P. The role of conditioning, learning and dopamine in sexual behavior: A narrative review of animal and human studies. Neurosci Biobehav Rev. 2014;38:38–59. doi: 10.1016/j.neubiorev.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Both S, Laan E, Spiering M, Nilsson T, Oomens S, Everaerd W. Appetitive and aversive classical conditioning of female sexual response. J Sex Med. 2008;5:1386–1401. doi: 10.1111/j.1743-6109.2008.00815.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann H, Janssen E, Turner SL. Classical conditioning of sexual arousal in women and men: Effects of varying awareness and biological relevance of the conditioned stimulus. Arch Sex Behav. 2004;33:43–53. doi: 10.1023/B:ASEB.0000007461.59019.d3. [DOI] [PubMed] [Google Scholar]
- Plaud JJ, Martini JR. The respondent conditioning of male sexual arousal. Behav Modif. 1999;23:254–268. doi: 10.1177/0145445599232004. [DOI] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Merz CJ, Tabbert K, Walter B, Kagerer S, Vaitl D, Stark R. Neural activations of the acquisition of conditioned sexual arousal: Effects of contingency awareness and sex. J Sex Med. 2009;6:3071–3085. doi: 10.1111/j.1743-6109.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- Štulhofer A, Buško V, Landripet I. Pornography, sexual socialization, and satisfaction among young men. Arch Sex Behav. 2010;39:168–178. doi: 10.1007/s10508-008-9387-0. [DOI] [PubMed] [Google Scholar]
- Beck JG, Barlow DH. The effects of anxiety and attentional focus on sexual responding: I. Physiological patterns in erectile dysfunction. Behav Res Ther. 1986;24:9–17. doi: 10.1016/0005-7967(86)90144-0. [DOI] [PubMed] [Google Scholar]
- Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F, Pizzella V, Pompa P, Rigatti P, Rossini P, Salonia A, Tartaro A, Romani G. Dynamics of male sexual arousal: Distinct components of brain activation revealed by fMRI. Neuroimage. 2005;26:1086–1096. doi: 10.1016/j.neuroimage.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Montorsi F, Perani D, Anchisi D, Salonia A, Scifo P, Rigiroli P, Zanoni M, Heaton JPW, Rigatti P, Fazio F. Apomorphine-induced brain modulation during sexual stimulation: A new look at central phenomena related to erectile dysfunction. Int J Impot Res. 2003;15:203–209. doi: 10.1038/sj.ijir.3900999. [DOI] [PubMed] [Google Scholar]
- Hagemann JH, Berding G, Bergh S, Sleep DJ, Knapp WH, Jonas U, Stief CG. Effects of visual sexual stimuli and apomorphine SL on cerebral activity in men with erectile dysfunction. Eur Urol. 2003;43:412–420. doi: 10.1016/s0302-2838(03)00002-2. [DOI] [PubMed] [Google Scholar]
- Cera N, Di Pierro ED, Sepede G, Gambi F, Perrucci MG, Merla A, Tartaro A, Del Gratta C, Galatioto Paradiso G, Vicentini C, Romani GL, Ferretti A. The role of left superior parietal lobe in male sexual behavior: Dynamics of distinct components revealed by fMRI. J Sex Med. 2012;9:1602–1612. doi: 10.1111/j.1743-6109.2012.02719.x. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liu J, Li G, Pan J, Li Z, Liu Q, Qin W, Dong M, Sun J, Huang X, Wu T, Chang D. White matter microstructural changes in psychogenic erectile dysfunction patients. Andrology. 2014;2:379–385. doi: 10.1111/j.2047-2927.2014.00191.x. [DOI] [PubMed] [Google Scholar]
- Cera N, Delli Pizzi S, Di Pierro ED, Gambi F, Tartaro A, Vicentini C, Paradiso Galatioto G, Romani GL, Ferretti A. Macrostructural alterations of subcortical grey matter in psychogenic erectile dysfunction. PLoS ONE. 2012;7:e39118. doi: 10.1371/journal.pone.0039118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prause N, Moholy M, Staley C. Biases for affective vs. sexual content in multidimensional scaling analysis: An individual difference perspective. Arch Sex Behav. 2013;43:463–472. doi: 10.1007/s10508-013-0128-7. [DOI] [PubMed] [Google Scholar]
- Prause N, Staley C, Roberts V. Frontal alpha asymmetry and sexually motivated states. Psychophysiology. 2014;51:226–235. doi: 10.1111/psyp.12173. [DOI] [PubMed] [Google Scholar]
- Prause N, Staley C, Fong TW. No evidence of emotion dysregulation in “hypersexuals” reporting their emotions to a sexual film. Sex Addict Compulsivity. 2013;20:106–126. [Google Scholar]
- Moholy M, Prause N, Proudfit GH, Rahman A, Fong T. Sexual desire, not hypersexuality, predicts self-regulation of sexual arousal. Cogn Emot. 2015;6:1–12. doi: 10.1080/02699931.2014.993595. [DOI] [PubMed] [Google Scholar]
- Rosenthal AM, Sylva D, Safron A, Bailey JM. Sexual arousal patterns of bisexual men revisited. Biol Psychol. 2011;88:112–115. doi: 10.1016/j.biopsycho.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Chivers ML, Seto MC, Lalumière ML, Laan E, Grimbos T. Agreement of self-reported and genital measures of sexual arousal in men and women: A meta-analysis. Arch Sex Behav. 2010;39:5–56. doi: 10.1007/s10508-009-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook KS, Hammen CL. A cognitive perspective on the experience of sexual arousal. J Soc Issues. 1977;33:7–29. [Google Scholar]
- Graham C, Sanders S, Milhausen R, McBride K. Turning on and turning off: A focus group study of the factors that affect women’s sexual arousal. Arch Sex Behav. 2004;33:527–538. doi: 10.1023/B:ASEB.0000044737.62561.fd. [DOI] [PubMed] [Google Scholar]
- Wincze JP, Venditti E, Barlow D, Mavissakalian M. The effects of a subjective monitoring task in the physiological measure of genital response to erotic stimulation. Arch Sex Behav. 1980;9:533–545. doi: 10.1007/BF01542157. [DOI] [PubMed] [Google Scholar]
- Rosen R, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- Kassouf W, Carrier S. A comparison of the International Index of Erectile Function and erectile dysfunction studies. BJU Int. 2003;91:667–669. doi: 10.1046/j.1464-410x.2003.04174.x. [DOI] [PubMed] [Google Scholar]
- Martin-Morales A, Gutiérrez-Hernández P, Romero-Otero J, Romero-Martín JA Vadeopen Study Group. Duration of erection: Does it really matter? A randomized, double-blind clinical trial to assess the impact of vardenafil ODT on duration of erection and its correlation with patients’ and partners’ sexual quality of life and duration of intercourse: The VADEOPEN Study. J Sex Med. 2014;11:1527–1538. doi: 10.1111/jsm.12496. [DOI] [PubMed] [Google Scholar]
- Janssen E, Sanders SA, Hill BJ, Amick E, Oversen D, Kvam P, Ingelhart K. Patterns of sexual arousal in young, heterosexual men who experience Condom-Associated Erection Problems (CAEP) J Sex Med. 2014;11:2285–2291. doi: 10.1111/jsm.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes MK, Baillie AJ, Schniering CA. Critical flaws in the female sexual function index and the international index of erectile function. J Sex Res. 2014;51:485–491. doi: 10.1080/00224499.2013.876607. [DOI] [PubMed] [Google Scholar]
- Spector I, Carey M, Steinberg L. The sexual desire inventory: Development, factor structure, and evidence of reliability. J Sex Marital Ther. 1996;22:175–190. doi: 10.1080/00926239608414655. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters J. Hypersexual disorder: A more cautious approach. Arch Sex Behav. 2010;39:594–596. doi: 10.1007/s10508-010-9607-2. [DOI] [PubMed] [Google Scholar]
- Giargiari TD, Mahaffey AL, Craighead WE, Hutchison KE. Appetitive responses to sexual stimuli are attenuated in individuals with low levels of sexual desire. Arch Sex Behav. 2005;34:547–556. doi: 10.1007/s10508-005-6280-y. [DOI] [PubMed] [Google Scholar]
- Prause N, Janssen E, Hetrick W. Attention and emotional responses to sexual stimuli and their relationship to sexual desire. Arch Sex Behav. 2008;37:934–959. doi: 10.1007/s10508-007-9236-6. [DOI] [PubMed] [Google Scholar]
- Goldey KL, van Anders SM. Sexual arousal and desire: Interrelations and responses to three modalities of sexual stimuli. J Sex Med. 2012;9:2315–2329. doi: 10.1111/j.1743-6109.2012.02845.x. [DOI] [PubMed] [Google Scholar]
- Turchik JA, Garske JP. Measurement of sexual risk taking among college students. Arch Sex Behav. 2009;38:936–948. doi: 10.1007/s10508-008-9388-z. [DOI] [PubMed] [Google Scholar]
- Janssen E, Carpenter D, Graham CA. Selecting films for sex research: Gender differences in erotic film preference. Arch Sex Behav. 2003;32:243–251. doi: 10.1023/a:1023413617648. [DOI] [PubMed] [Google Scholar]
- Paul B, Kobach M. Male-female reactions to variations in sexual explicitness in pornography: An empirical test of predictions of intra- and inter-gender differences. Sex Cult. 2014;18:56–75. [Google Scholar]
- Levin ME, Lillis J, Hayes SC. When is online pornography viewing problematic among college males? Examining the moderating role of experiential avoidance. Sex Addict Compulsivity. 2012;19:168–180. [Google Scholar]
- Paul B. Predicting Internet pornography use and arousal: The role of individual difference variables. J Sex Res. 2009;46:344–357. doi: 10.1080/00224490902754152. [DOI] [PubMed] [Google Scholar]
- Peterson Z, Janssen E. Ambivalent affect and sexual response: The impact of co-occurring positive and negative emotions on subjective and physiological sexual responses to erotic stimuli. Arch Sex Behav. 2007;36:793–807. doi: 10.1007/s10508-006-9145-0. [DOI] [PubMed] [Google Scholar]
- Hollis B, Kawecki TJ. Male cognitive performance declines in the absence of sexual selection. Proc Biol Sci. 2014;281:1781. doi: 10.1098/rspb.2013.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA. Computing inter-rater reliability for observational data: An overview and tutorial. Tutor Quant Methods Psychol. 2012;8:23–34. doi: 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Lemon J, Fellows I, Singh P. 2012. irr: Various coefficients of interrater reliability and agreement.
- Chivers ML, Seto MC, Lalumière ML, Laan E, Grimbos T. Agreement of self-reported and genital measures of sexual arousal among men and women: A meta-analysis. Arch Sex Behav. 2010;39:5–56. doi: 10.1007/s10508-009-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa Y, Tsujimura A, Fujita K, Matsuoka Y, Takahashi T, Takao T, Takada S, Matsumiya K, Osaki Y, Takasawa M, Oku N, Hatazawa J, Kaneko S, Okuyama A. Differential brain processing of audiovisual sexual stimuli in men: Comparative positron emission tomography study of the initiation and maintenance of penile erection during sexual arousal. Neuroimage. 2007;36:830–842. doi: 10.1016/j.neuroimage.2007.03.055. [DOI] [PubMed] [Google Scholar]
- McKenzie-Mohr D, Zanna MP. Treating women as sexual objects: Look to the (gender schematic) Male who has viewed pornography. Pers Soc Psychol Bull. 1990;16:296–308. [Google Scholar]
- Häggström-Nordin E, Hanson U, Tydén T. Associations between pornography consumption and sexual practices among adolescents in Sweden. Int J STD AIDS. 2005;16:102–107. doi: 10.1258/0956462053057512. [DOI] [PubMed] [Google Scholar]
- Watson MA, Smith RD. Positive porn: Educational, medical, and clinical uses. Am J Sex Educ. 2012;7:122–145. [Google Scholar]


