Key Clinical Message
A 70-year-old patient having massive refractory ascites in the course of idiopathic myelofibrosis was diagnosed of peritoneal extramedullary hematopoiesis and developed an overwhelming nontuberculous mycobacterial infection. The case describes this unusual infection and highlights the need for additional studies to confirm the etiology of ascites in primary myelofibrosis.
Keywords: Extramedullary, hematopoiesis, mycobacteria, myelofibrosis, peritoneal
Introduction
Primary myelofibrosis (PM) is a myeloproliferative disorder characterized by unexplained fibrosis in the bone marrow and osteosclerosis, extramedullary hematopoiesis with marked splenomegaly, and a leukoerythroblastic anemia. Other manifestations include abdominal fullness associated with the mass effect of splenomegaly, esophageal varices, and ascites caused by portal hypertension in 2–10% of patients 1,2. More rarely, ascites may result due to peritoneal extramedullary hematopoiesis 3,4. We describe an unusual case of myelofibrosis in a splenectomized patient presenting with massive ascites, and peritoneal extramedullary hematopoiesis; that developed nontuberculous mycobacterium infection.
Case Report
An 70-year-old Moroccan man was admitted to our hospital with fever, abdominal fullness, and general fatigue. He gave a previous history of splenectomy, diabetes, and hypertension. He had otherwise been well until 2–3 months before entry, when he began to note fever, poor appetite, and progressive painful abdominal fullness. He was treated in his country for suspected myelofibrosis with hydroxyurea. Because his symptoms responded poorly to therapy he decided to ask for a second opinion. On physical examination, he appeared pale and cachectic. A distended abdomen with an obvious fluid wave was noted. Pretibial ulcer was present. No leg edema, icterus, or lymphadenopathy was noted.
Initial laboratory data were as follows: white-cell count 3.6 × 103/μL; hemoglobin 10.1 g/dL, platelet count 108 × 103/μL. Lactate dehydrogenase was 1092 U/L. The results of all liver function test, including bilirubin, serum aspartate aminotransferase, serum alanine aminotransferase, alkaline phosphatase, and ammonium were within normal limits. Serologic tests for hepatitis B surface antigen, hepatitis C antibody, and HIV ELISA were negative. Tuberculin skin test was negative. JAK-2V617F negative.
Diagnostic paracentesis yielded yellow-colored ascites that contained 824 cells/mm3, with 70% lymphocytes. The serum protein was 3.5 g/dL, and adenosine-deaminase was 9.6 U/L. Smear and cultures for bacteria, and tuberculosis were all negative.
A computed tomographic (CT) scan of the abdomen showed massive ascites, hepatomegaly, with no evidence of the thrombosis of the portal vein, and nodular thickening and enhancement of the peritoneum (Fig.1). A right lower lobe consolidation on chest CT scan was observed. Urinary antigens for Streptococcus pneumoniae and Legionella pneumophila were negatives. Bronchoscopy with bronchoalveolar lavage was performed. Although the lavage fluid results were unrevealing, smear and cultures for bacteria, nocardia/actinomyces, and fungi were all negative. Mycobacterium culture was positive for Mycobacterium avium complex (MAC) in bronchoalveolar lavage and urine.
Figure 1.
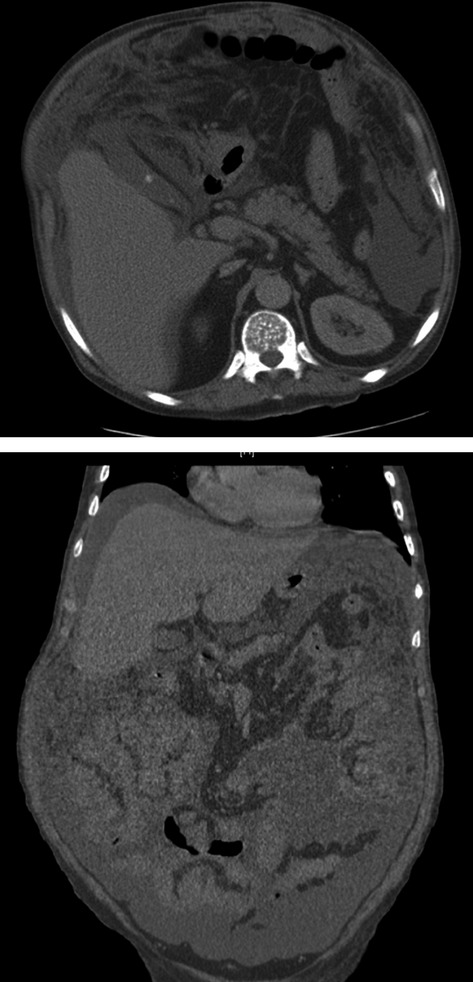
CT scan (sagital and coronal views): nodular thickening and enhancement of the peritoneum. Peritoneal effusion is also present.
A biopsy specimen of the peritoneum showed foci of extramedullary hematopoiesis and fibrous tissue. Most of the cells were described as megakaryocytic.
A biopsy specimen of the bone marrow showed decreased hematopoietic cells, fibrosis, and thickening and distortion of the bony trabeculae (osteosclerosis). Flow cytometry of bone marrow aspirate showed 6% of myeloid blasts (CD45+low, CD13+, CD133+low, CD34+, HLA-DR+, CD5+low, CD38+, CD117low, CD71+low) compatible with a chronic myeloproliferative neoplasm and transformation to Acute Myeloid Leukemia.
We administered hydroxyurea and corticosteroid to reduce extramedullary hematopoiesis, but he was refractory to the treatment. He received treatment with Azithromycin, Rifampicin, Ethambutol, and decided to go back to his country where he was admitted to a hospital. Finally he died after 10 days of progression of his disease. An autopsy was not performed.
Discussion
Extramedullary hematopoiesis refers to the production of blood cells outside the bone marrow and is a compensatory mechanism for bone marrow dysfunction. Because the bone marrow reserve does not necessarily correlate with its metastatic potential, a presentation resembling metastasis of unknown origin in an otherwise asymptomatic patient with PM is possible 5.
Patients with symptomatic massive ascites caused by peritoneal extramedullary hematopoiesis have been reported previously, but are limited in number 5–10.
Symptomatic ascites in PM is rare and is often attributed to portal hypertension accompanied by ectopic hematopoiesis in the liver and spleen, and in some cases thrombosis of the portal vein 11. In the previously reported cases, ascites occurred after splenectomy, as initial manifestation, or from 2 months to 17 years after the diagnosis of myelofibrosis was established 1,5,9.
Peritoneal extramedullary hematopoiesis as the cause of ascites remains uncommon 12–14. We have not found more than nine case reports in MEDLINE (See Table1).
Table 1.
Case reports of ascitis related to EMH on peritoneum confirmed by peritoneal biopsy
| References | Year | Age (years) | Sites of EMH | Ascites with megakaryocytes |
|---|---|---|---|---|
| 12 | 1969 | 45 | Peritoneum | Yes |
| 13 | 1983 | 60 | Peritoneum | Yes |
| 10 | 1985 | 45 | Spleen, liver, heart, lungs, peritoneum, and pleura | Yes |
| 1 | 1991 | 61 | Peritoneum | Yes |
| 14 | 1991 | 66 | Liver and peritoneum | No |
| 9 | 1993 | 44 | Spleen, liver, peritoneum | Yes |
| 5 | 1999 | 83 | Peritoneum | Yes |
| 8 | 1999 | 33 | Peritoneum and pleura | Yes |
| 4 | 2003 | 55 | Peritoneum | Yes |
EMH, extramedullary hematopoiesis.
A CT scan sometimes shows the mass of extramedullary hematopoiesis 15. Peritoneal extramedullary hematopoiesis is usually proven by peritoneal biopsy. Peritoneal biospy is the gold standard method as a diagonostic procedure for the peritoneal hematopoiesis, but additional cytogenetic analysis of the cells in the ascites might be useful. Megakaryocytes are rarely found in peritoneal fluid and it seems to be suggestive of peritoneal extramedullary hematopoiesis 8.
There is no effective treatment for refractory ascites in patients with PM. There is a report about the use of busulfan and hydroxyurea for treating ascites 9. Corticosteroid has been used for pleural effusion 16. TIPS may be considered as rescue management for refractory ascites secondary to portal hypertension 17.
Leukemic transformation occurs in approximately 20% of patients during the first 10 years after the onset of the disease, as our case 18.
Nontuberculous mycobacterial infection can cause four clinical syndromes: pulmonary disease, superficial lymphadenitis, skin/soft tissue infection, and disseminated disease in severely immunocompromised patients like our case. Disseminated MAC disease may complicate MAC pulmonary disease through local multiplication and entry into the bloodstream with seeding of other organs and tissues 19. Isolation in urine culture is rare and should be specifically collected in special media, different from the media used in standard bacterial blood cultures. In a series of 15 patients with genitourinary infections by nontuberculous mycobacteria, only two patients had disseminated infection not related to hematologic malignancy 20. To the best of our knowledge, this is the first report of MAC associated to PM. Tuberculous peritonitis and extramedullary peritoneal hematopoiesis has been reported previously 21. The treatment regimen for M. avium complex pulmonary and disseminated infection includes simultaneous use of three oral antibiotics: a macrolide (clarithromycin or azithromycin), ethambutol, and a rifamycin (rifampin or rifabutin), for at least 1 year 22,23. Monotherapy is never advised in the treatment of MAC because of the concern for developing resistance. Successful treatment of disseminated MAC relies on recovery of the immune system, in case of HIV patients 24,25.
Conclusion
This case demonstrates that refractory ascites caused by peritoneal implants of myeloid tissues may be a manifestation of myelofibrosis. Extramedullary hematopoiesis should be included in the differential diagnosis of ascites. Nontuberculous mycobacterial disseminated infection should be included in the differential diagnosis of fever in PM patients.
Acknowledgments
We express our gratitude to Eulalia Grifol and Tedec-Meiji Laboratory for help us with bibliographic support.
Conflict of Interest
None declared.
References
- Liote F, Yeni P, Teillet-Thiebaud F, Barge J, Devars Du Mayne JF, Flamant Y, et al. Ascites revealing peritoneal and hepatic extramedullary hematopoiesis with peliosis in agnogenic myeloid metaplasia: case report and review of the literature. Am. J. Med. 1991;90:111–117. doi: 10.1016/0002-9343(91)90513-w. [DOI] [PubMed] [Google Scholar]
- Pitcock JA, Reinhard EH, Justus BW. Mendelsohn RS. A clinical and pathological study of seventy cases of myelofibrosis. Ann. Intern. Med. 1962;57:73–84. doi: 10.7326/0003-4819-57-1-73. [DOI] [PubMed] [Google Scholar]
- Stephenson RW, Britt DA. Schumann GB. Primary cytodiagnosis of peritoneal extramedullary hematopoiesis. Diagn. Cytopathol. 1986;2:241–243. doi: 10.1002/dc.2840020316. [DOI] [PubMed] [Google Scholar]
- Yotsumoto M, Ishida F, Ito T, Ueno M, Kitano K. Kiyosawa K. Idiopathic myelofibrosis with refractory massive ascites. Intern. Med. 2003;42:525–528. doi: 10.2169/internalmedicine.42.525. [DOI] [PubMed] [Google Scholar]
- Hung SC, Huang ML, Liu SM. Hsu HC. Massive ascites caused by peritoneal extramedullary hematopoiesis as the initial manifestation of myelofibrosis. Am. J. Med. Sci. 1999;318:198–200. doi: 10.1097/00000441-199909000-00017. [DOI] [PubMed] [Google Scholar]
- Kumar NB. Naylor B. Megakaryocytes in pleural and peritoneal fluids: prevalence, significance, morphology, and cytohistological correlation. J. Clin. Pathol. 1980;33:1153–1159. doi: 10.1136/jcp.33.12.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NM. Kurtides ES. Ascites in agnogenic myeloid metaplasia. Cancer. 1982;50:1189–1190. doi: 10.1002/1097-0142(19820915)50:6<1189::aid-cncr2820500627>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Oren I, Goldman A, Haddad N, Azzam Z, Krivoy N. Alroy G. Ascites and pleural effusion secondary to extramedullary hematopoiesis. Am. J. Med. Sci. 1999;318:286–288. doi: 10.1097/00000441-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Knobel B, Melamud E, Virag I. Meytes D. Ectopic medullary hematopoiesis as a cause of ascites in agnogenic myeloid metaplasia. Acta Haematol. 1993;89:104–107. doi: 10.1159/000204498. [DOI] [PubMed] [Google Scholar]
- Silverman JF. Extramedullary hematopoietic ascitic fluid cytology in myelofibrosis. Am. J. Clin. Pathol. 1985;84:125–128. doi: 10.1093/ajcp/84.1.125. [DOI] [PubMed] [Google Scholar]
- Silverstein MN, Wollaeger EE. Baggenstoss AH. Gastrointestinal and abdominal manifestations of agnogenic myeloid metaplasia. Arch. Intern. Med. 1973;131:532–537. [PubMed] [Google Scholar]
- Gorshein D. Brauer MJ. Ascites in myeloid metaplasia due to ectopic peritoneal implantation. Cancer. 1969;23:1408–1412. doi: 10.1002/1097-0142(196906)23:6<1408::aid-cncr2820230623>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Marshall JB, Burnett DA, Anderson JC, et al. Ascites progressing to an abdominal mass due to extramedullary hematopoiesis in a patient with agnogenic myeloid metaplasia. Dig. Dis. Sci. 1983;28:912–917. doi: 10.1007/BF01317043. [DOI] [PubMed] [Google Scholar]
- Jacobs P, Wood L. Robson S. Refractory ascites in the chronic myeloproliferative syndrome: a case report. Am. J. Hematol. 1991;37:128–129. doi: 10.1002/ajh.2830370213. [DOI] [PubMed] [Google Scholar]
- Bartlett RP, Greipp PR, Tefferi A, Cupps RE, Mullan BP. Trastek VF. Extramedullary hematopoiesis manifesting as a symptomatic pleural effusion. Mayo Clin. Proc. 1995;70:1161–1164. doi: 10.4065/70.12.1161. [DOI] [PubMed] [Google Scholar]
- Hirayama Y, Koyama R, Nagai T, et al. A pleural effusion caused by fibrous hematopoietic tumor successfully treated with prednisolone in a patient with agnogenic myeloid metaplasia with myelofibrosis. Int. J. Hematol. 2002;75:305–308. doi: 10.1007/BF02982047. [DOI] [PubMed] [Google Scholar]
- Wiest R, Strauch U, Wagner H, Strotzer M, Woenckhaus M, Schröder G, et al. A patient with myelofibrosis complicated by refractory ascites and portal hypertension: to tips or not to tips? A case report with discussion of the mechanism of ascites formation. Scand. J. Gastroenterol. 2004;39:389–394. doi: 10.1080/00365520310007521. [DOI] [PubMed] [Google Scholar]
- Tefferi A. Myelofibrosis with myeloid metaplasia. N. Engl. J. Med. 2000;342:1255–1265. doi: 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- Horsburgh C, Mason U, Farhi D. Iseman M. Disseminated infection with Mycobacterium avium-intracellulare. A report of 13 cases and a review of the literature. Medicine. 1985;64:36. doi: 10.1097/00005792-198501000-00003. [DOI] [PubMed] [Google Scholar]
- Huang C, Chen C, Chen H, Chou C, Ruan S, Lai C, et al. Genitourinary infections caused by nontuberculous mycobactreia at a university hospital in Taiwan, 1996–2008. Clin. Microbiol. Infect. 2010;16:1585–1590. doi: 10.1111/j.1469-0691.2010.03180.x. [DOI] [PubMed] [Google Scholar]
- Su-Jung K, Lee Y, Kim S, Kim H, Park K, Bang S, et al. Extramedullary peritoneal hematopoiesis combined with tuberculosis in a patient with primary myelofibrosis. Med. Oncol. 2009;26:238–241. doi: 10.1007/s12032-008-9099-2. [DOI] [PubMed] [Google Scholar]
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- Zheng C. Fanta C. Non-tuberculous mycobacterial pulmonary infection in the immunocompetent host. Q. J. Med. 2013;106:307–315. doi: 10.1093/qjmed/hct022. [DOI] [PubMed] [Google Scholar]
- Benson CA, Williams PL, Currier JS, Holland F, Mahon LF, MacGregor RR, et al. A prospective, randomized trial examining the efficacy and safety of clarithromycin in combination with ethambutol, rifabutin, or both for the treatment of disseminated Mycobacterium avium complex disease in persons with acquired immunodeficiency syndrome. Clin. Infect. Dis. 2003;37:1234–1243. doi: 10.1086/378807. [DOI] [PubMed] [Google Scholar]
- Kasperbauer S. Daley C. Diagnosis and treatment of infections due to Mycobacterium avium complex. Semin. Respir. Crit. Care Med. 2008;29:569–576. doi: 10.1055/s-0028-1085708. [DOI] [PubMed] [Google Scholar]


