Key Clinical Message
Herpes simplex virus 2 caused a genital ulcer, and a secondary herpetic whitlow appeared during acyclovir therapy. The secondary and recurrent whitlow isolates were acyclovir-resistant and temperature-sensitive in contrast to a genital isolate. We identified the ribonucleotide reductase mutation responsible for temperature-sensitivity by deep-sequencing analysis.
Keywords: Herpes simplex virus, high-throughput DNA sequencing, reactivation, ribonucleotide reductase, temperature-sensitive (Ts), thymidine kinase deficient
Introduction
A genital herpetic lesion progressed in size despite a 13-week course of oral acyclovir, and a herpetic whitlow appeared on the patient's right thumb. The whitlow recurred 6 months later without recurrence of the genital lesion. We obtained isolates from the genital and secondary and recurrent whitlow lesions. The isolate from the genital lesion was acyclovir sensitive but not temperature-sensitive (ts), whereas the isolates from the secondary and recurrent whitlow were acyclovir resistant/ts. As a marker of acyclovir resistance/thymidine kinase (TK) deficiency, the secondary and recurrent whitlow isolates had a frameshift mutation, c.819delG (p.Pro274 fs), of the TK gene 1. However, we could not identify the mutation causing the temperature-sensitivity of whitlow isolates. This is a follow-up study on the characterization of isolated viruses with temperature-sensitivity.
Ts mutants of herpes simplex virus (HSV) have been generated or found in the laboratory and reported to have a mutation in UL36 of HSV-1 2, ICP4 of HSV-1 3–8, UL15 of HSV-1 9, HSV-1 protease of HSV-1 10, UL9 of HSV-1 11, UL28 of HSV-1 12, gB of HSV-1 3,13, ribonucleotide reductase (RR) large subunit of HSV-1 14,15 and HSV-2 15 and small subunit of HSV-1 14,16, UL11 of HSV-1 17, Vmw65 of HSV-1 18, ICP27 of HSV-1 19, ICP8 of HSV-1 6, DNA polymerase 6, and virion-associated host shutoff protein 20. Thus, ts mutants have been analyzed to understand their gene functions, but all mutants examined were laboratory strains, making it difficult to predict the location of the mutated gene among various genes responsible for temperature-sensitivity of clinical isolates in the large HSV genome. However, deep sequencing revealed a frameshift mutation in the UL13 kinase in a strain F isolate 21. We applied the deep-sequencing strategy to identify the mutation responsible for the temperature-sensitivity of the clinical HSV-2 isolates.
In this study two clinical isolates from the whitlow had two phenotypic mutations, ACV resistance and temperature-sensitivity, compared with the parent wild-type isolate in these three closely related strains. This was a good test of the feasibility of deep sequencing to identify two mutations in the large HSV genome. We successfully identified the substitution mutation, c.566C>T (p.Ala189Val), in UL40 of the small subunit of RR in addition to TK by comparative deep sequencing of these three isolates using Illumina high-throughput sequencing, followed by confirmation by sequencing using the ABI capillary sequencer. This is the first identification of the TK and RR mutations in acyclovir-resistant and temperature-sensitive isolates from the whitlow of a patient by deep sequencing.
Clinical History
The clinical history and virus isolation (Fig. 1) have been reported previously 1. Briefly, a 40-year-old man with acute myelogenous leukemia received induction therapy in November 1998, resulting in complete remission. He had a relapse in the bone marrow in December 1999, and a peripheral blood stem cell transplant from a matched related donor was performed in April 2000. A genital ulcer appeared 1 year later, and he developed both a genital ulcer and herpetic whitlow on his right thumb in October 2002; in June 2003, the whitlow recurred. HSV strains were isolated from swabs of the genital lesion and secondary whitlow in October 2002 and the recurrent whitlow in June 2003.
Figure 1.
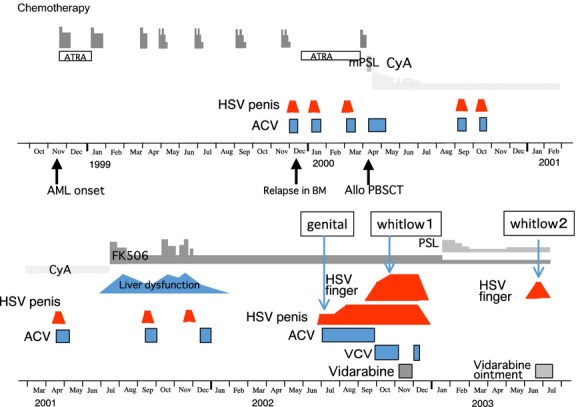
Clinical course of treatment of the genital herpes and secondary and recurrent herpetic whitlows. Viruses were isolated from both the genital lesion and secondary whitlow in October 2002 and from the recurrent whitlow in June 2003. The clinical course and virus isolation have been reported previously 1 and isolated viruses were characterized for temperature-sensitivity and the whole genome sequence in this study. AML: acute myelogenous leukemia, BM: bone marrow, Allo PBSCT: allogenic peripheral blood stem cell transplant, ATRA: all-trans retinoic acid, mPSL: methylprednisolone sodium succinate, PSL: prednisolone acetate, CyA: cyclosporine, FK506: tacrolimus hydrate, ACV: acyclovir, VCV: valacyclovir hydrochloride.
Examination
Swabs of the three clinical specimens were suspended separately in 1 ml of phosphate-buffered saline, and 0.2 mL was inoculated into Vero cell cultures in 25 cm2 plastic flasks; the remaining suspensions were frozen at −85°C. To characterize the virus populations of the genital and secondary whitlow lesions, an aliquot of 0.7 mL from the rest of the inoculation suspension was infected into Vero cell cultures at the adjusted density of several plaques per 60 mm Petri dish from the initial isolation condition, and the dishes were overlaid with 0.8% nutrient methylcellulose. All plaques were isolated independently as virus clones for analysis of susceptibility to ACV, temperature-sensitivity, and sequence analysis.
The temperature-sensitivity of the viruses was determined by culturing infected Vero cells with 100 plaque forming units (PFU)/0.2 mL of isolates; after 1 h incubation at room temperature for virus adsorption, the cells were overlaid with prewarmed 0.8% nutrient methylcellulose medium in 60 mm Petri dishes at 33°C, 37°C, and 39°C in water-jacket CO2 incubators. The number of plaques formed at each temperature was counted to determine the temperature-sensitivity 1.
Nucleocapsid DNA was isolated as previously described 1,22,23. Briefly, infected cells were lysed with 20 mM Tris-HCl (pH 8.0) containing 0.5% Triton X-100 and 10 mmol/L EDTA, and the lysate was centrifuged twice at 1500 g for 15 min. The supernatants were centrifuged at 100,000 g for 1 h at 4°C, and viral DNA was purified from the resultant pellets 1.
Library preparation was performed using a genomic DNA sample preparation (Illumina, San Diego, CA), and DNA clusters were generated on a slide using the cluster generation kit (v.2) on an Illumina cluster station (Illumina) according to the manufacturer's instructions. All sequencing runs were performed with the GA II using the Illumina sequencing kit (v.3). Fluorescent images were analyzed with the Illumina base-calling pipeline v.1.3.2 to obtain FASTQ-formatted sequence data of 81-mer paired-end short reads. The short reads obtained were subjected to read-mapping using bwa-sw mapping script against the HSV-2 reference genome sequence (NC_001798). To characterize notable genetic alterations for temperature dependency of HSV2, the read-mapping was visualized and confirmed by Genome Jack viewer software (MSSbio, Japan).
Comparative analysis of the deep sequencing of the three strains was used to identify the mutation in the whitlow isolates. The sequences of RL2, UL6, UL16, UL22, UL23 (TK), intergenic region of UL24-25, intergenic region of UL26-UL27, UL36, intergenic region of RL1-RS1, UL40 (RR), and US5 of the three isolates as candidates for the mutated genes were determined directly from the purified HSV genome according to the manufacturer's procedures (ABI Prism 3130 DNA sequencer) and compared 1. The amplified fragments of RL2, UL6, UL16, UL22, UL23 (TK), intergenic region of UL24-25, intergenic region of UL26-UL27, UL36, intergenic region of RL1-RS1, UL40 (RR), and US5 by PCR were sequenced by using the ABI Prism 3130 DNA sequencer according to the manufacture's procedures. The determined HSV-2 UL40 sequence was compared with the HSV-2 HG52 strain and the nucleotide differences of the isolate common to the both were identified as the significant nucleotide changes. PCR cyclic sequencing was carried out with the purified DNA material in a reaction mixture containing the chosen primer and the Dye Terminator Cycle Sequencing mixture (ABI). The PCR cyclic-sequencing program consisted of 25 cycles of 30 seconds at 96°C, 15 seconds at 50°C, and 4 min at 60°C. The sequencing reaction was carried out in both the sense and antisense directions for confirmation. After cyclic sequencing, the products were precipitated with ethanol, dissolved in HiDi formamide, and then denatured. The sequencing was carried out on the ABI Prism 3130 DNA sequencer. Heterogeneities of the sequences in the strain were examined by 32 clones derived from each strain to confirm the presence of heterogeneity and mutation in each strain.
The nucleotide sequences determined in this study have been deposited in the GenBank/DDBJ/EMBL database. The accession numbers of the TK and RR genes from the genital lesion, secondary whitlow (whitlow 1), and recurrent whitlow (whitlow 2) are AB178228, AB178229, and AB178230 1 for the TK gene and AB860125, AB860126, and AB860127 for the RR gene.
The temperature-sensitivity was expressed as means ± standard deviations of five independent experiments. The comparisons among groups were done with 2-way factorial analysis of variance with Bonferroni/Dunn post hoc tests. The differences were considered significant at P < 0.05 levels.
As illustrated in Figure1, genital lesions appeared frequently, and a whitlow appeared on the patient's right thumb during ACV treatment. Two isolates were obtained simultaneously from the genital ulcer and whitlow during ACV treatment and one from the recurrent whitlow 6 months later.
The genital isolate was ACV sensitive, and both whitlow isolates were TK deficient/ACV resistant 1. Because the temperature of the thumb is 33/34°C or lower 24,25, the whitlow isolates were examined for the temperature-adapted nature of plaque formation at 33°C and 39°C. Two whitlow isolates were similarly and significantly more ts than the genital isolate and wild-type HSV-2 strain, indicating that the whitlow isolates grew better at 33°C than at 39°C by adapting the thumb temperature (Fig.2).
Figure 2.
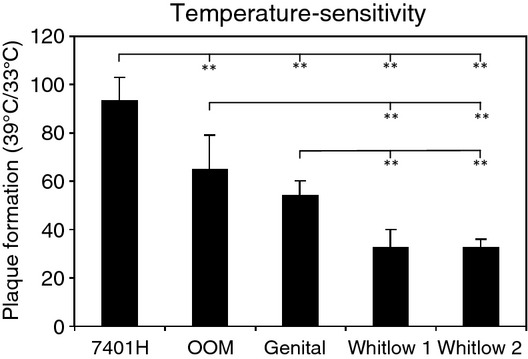
Comparison of temperature-sensitivity of genital and two whitlow isolates. Temperature-sensitivity of three isolates and an unrelated clinical isolate of the HSV-2 strain was assessed by the ratio of plaque formation at 39°C and 33°C in Vero cells; the columns and error bars of the HSV strains indicate the mean ± SD (%) of five independent experiments 1,40. **P < 0.01 by two-way factorial analysis of variance with Bonferroni/Dunn post hoc tests.
Deep sequencing revealed a single remarkable nonsynonymous mutation on UL40 throughout the whole genome sequence of HSV-2 between the genital and two whitlow isolates (Fig.3). Although heterogeneity of nucleotides have been observed in RL2, UL6, UL16, UL22, UL23, intergenic region of UL24-25, intergenic region of UL26-UL27, UL36, intergenic region of RL1-RS1, and US5 sequence, no notable nucleotide variation has been identified except UL40.
Figure 3.
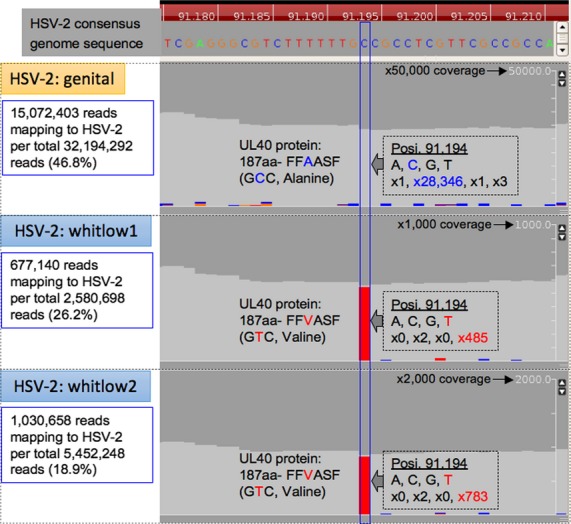
Detection of nonsynonymous mutation in UL40 gene of HSV-2 strains of genital and whitlow 1 and 2 isolates relative to HSV2-consensus genome sequence. Read depth at the 91,194 nt position was evaluated with a threshold of Phred score ≥15.
The genital and two whitlow isolates were subjected to the Illumina sequencing and there were polymorphism including substitutions and deletions in UL6, UL16, UL22, UL23, and UL40 of the whitlow isolates. Thirty-two clones of secondary whitlow isolates were used to examine the heterogeneity in the sequences of UL6, UL16, and UL22 detected in the deep sequencing using the ABI Prism 3130 DNA sequencer, Heterogeneity in the sequences of UL6, UL16, UL22, and UL36 was common as sequence variations in the three strains; mixtures of A and C at the 1795th nucleotide of UL6 from genital and whitlow isolates, T and G at the 213th nucleotide of UL16, and G and C at the 62nd nucleotide of UL22 from whitlow isolates. Thus the sequence of UL6, UL16, and UL22 were identical to the genital isolate but the all clones from the whitlow isolates had the deletion, c.819delG (p.Pro274 fs), in the TK gene (UL23) and the substitution, c.566C>T (p.Ala189Val), in the small subunit of the RR gene (UL40). Thus, we confirmed the mutation of the whitlow isolates by mutation screening by the sequence by the Illimina and followed by sequence of their clones by the ABI Prism 3130 DNA sequencer. The heterogeneity of the sequences observed in the deep sequencing was not confirmed and no heterogeneity was found in the indicated nucleotides by the sequencing of clones, indicating a misreading by the machine or an error of analysis software. It was not clear whether this problem was generated by the analysis software or reading analysis of the machine.
Genomic sequencing revealed mutations in UL23 and UL40 in secondary and recurrent whitlow isolates compared with a genital isolate. The mutation in UL23 was the same mutation of the TK gene previously reported 1. Sequencing of UL40 confirmed the substitution mutation c.566C >T (p.Ala189Val) in the small subunit of RR.
Discussion
Both the genital lesion and secondary whitlow continued for 3 months without cure by ACV. This ACV-resistant HSV became latent in the innervating sensory ganglia, and the whitlow alone recurred as a whitlow 6 months later without recurrence in the genital region. ACV resistance was due to the frameshift mutation, c.819delG (p.Pro274 fs), of the TK gene as identified in our previous study 1.
Although the TK-deficient/ts isolates of the whitlows did not exhibit cutaneous pathogenicity in a mouse midflank, in contrast to the genital isolate, the TK-deficient/ts isolates of the whitlows, as well as the genital isolates, replicated similarly and caused cutaneous lesions in the ear pinna skin, which has a lower temperature, indicating the preservation of pathogenicity of whitlow isolates at a lower temperature 1. The difference in the level of temperature-sensitivity between genital and whitlow isolates was similar to that between wild HSV-1 and 2 28–30,26,27 (Fig.2) but the difference between genital and whitlow isolates caused that in the skin pathogenicity. The difference in temperature-sensitivity was found to be due to mutation in the RR gene but the enzyme activity of RR was failed by the difficulty in separating rCDP and dCDP. This indicated that the temperature-sensitivity was not the suitable surrogate marker in vitro for representing the pathogenicity in vivo caused by the mutation in the RR gene. However, the difference in the temperature-sensitivity was a clue to identify the mutation in the gene by the literatures reporting the relationship among temperature-sensitivity, the RR mutation, and attenuation of pathogenicity.
The relationship between the temperature-sensitivity and the differential pathogenicity of genital and whitlow isolates were characterized in this study using deep-sequencing analysis of the isolates. The adaptability of HSV to the local temperature by mutation of the RR gene may be an important element of pathogenicity in causing the herpetic whitlows. This deep-sequencing analysis successfully identified mutations in the TK and RR genes as mutation markers for TK deficiency and temperature-sensitivity, respectively.
RR catalyzes the reduction in nucleoside diphosphates to deoxynucleoside diphosphates and is essential for de novo synthesis of deoxyribonucleotides required for DNA replication and repair. The RR holoenzymes are composed of two subunits called RR1 and RR2. Both RR1 (α2) and RR2 (β2) are homodimers. HSV RR is the complex between the large (RR1) and small (RR2) subunits for enzyme function 14,31–33. Mutation in the RR1 or RR2 subunit is responsible for temperature-sensitivity 14–16,32,34,35. A mutation at 189 of the RR2 subunit locates between the Fe1 ligand and the Fe1 pocket of RR2, and this mutation might render the complex unstable at high temperature, resulting in possible loss of the RR function.
Mutations in the TK gene are principally found in long homopolymer runs of guanosines or cytosines 22,36–39, and the mutations found in the whitlow isolates were located in a run of four cytosines in the TK gene and in the six consecutive G and C sequences in the small subunit of the RR gene, which suggested that the mutations in the TK and RR genes might be related to acyclovir treatment.
Two clinical isolates in addition to the parent wild-type isolate had two phenotypic mutations, ACV resistance and temperature-sensitivity, in the three closely related strains, and this was an excellent opportunity to identify two mutations by comparing the nucleotide sequences of the three strains. In order to investigate the populations of virus variation in the clinical samples and determine the genetic mutations associated with phenotype, it should be direct deep-sequencing analysis from clinical specimens by the next-generation sequencing. This study successfully identified the two mutations in the clinical HSV isolates by their deep sequencing and demonstrated that the next-generation sequencing technology is a powerful tool to identify mutation in clinical isolates of HSV.
Acknowledgments
We thank Mr. Yoshihiro Yoshida for their technical assistance and Ms. Katherine Ono for editing the manuscript. This work was partly supported by a Grant-in-Aid (25293108, 25461508) from Japan Society for the Promotion of Science.
Conflict of Interest
None declared.
References
- Shimada Y, Suzuki M, Shirasaki F, E Saito, K Sogo, Hasegawa M, et al. Genital herpes due to acyclovir-sensitive herpes simplex virus caused secondary and recurrent herpetic whitlows due to thymidine kinase-deficient/temperature-sensitive virus. J. Med. Virol. 2007;79:1731–1740. doi: 10.1002/jmv.20990. [DOI] [PubMed] [Google Scholar]
- Abaitua F, Daikoku T, Crump CM, Bolstad M. O'Hare P. A single mutation responsible for temperature-sensitive entry and assembly defects in the VP1-2 protein of herpes simplex virus. J. Virol. 2011;85:2024–2036. doi: 10.1128/JVI.01895-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N, Person S, Bzik DJ. Snipes W. Genome locations of temperature-sensitive mutants in glycoprotein gB of herpes simplex virus type 1. Virology. 1984;137:382–389. doi: 10.1016/0042-6822(84)90230-7. [DOI] [PubMed] [Google Scholar]
- DeLuca NA, McCarthy AM. Schaffer PA. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber SW. Wilcox KW. Characterization of a herpes simplex virus regulatory protein: aggregation and phosphorylation of a temperature-sensitive variant of ICP 4. Arch. Virol. 1986;91:297–312. doi: 10.1007/BF01314289. [DOI] [PubMed] [Google Scholar]
- Hafner J, Mohammad F. Farber FE. Alkaline nuclease activity in cells infected with herpes simplex virus type 1 (HSV-1) and HSV-1 temperature-sensitive mutants. Biochim. Biophys. Acta. 1987;910:85–88. doi: 10.1016/0167-4781(87)90097-2. [DOI] [PubMed] [Google Scholar]
- Leopardi R. Roizman B. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc. Natl Acad. Sci. U. S. A. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston VG. Fine-structure mapping of herpes simplex virus type 1 temperature-sensitive mutations within the short repeat region of the genome. J. Virol. 1981;39:150–161. doi: 10.1128/jvi.39.1.150-161.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PL, Ogle WO. Roizman B. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J. Virol. 1996;70:4623–4631. doi: 10.1128/jvi.70.7.4623-4631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register RB. Shafer JA. A facile system for construction of HSV-1 variants: site directed mutation of the UL26 protease gene in HSV-1. J. Virol. Methods. 1996;57:181–193. doi: 10.1016/0166-0934(95)01984-7. [DOI] [PubMed] [Google Scholar]
- Blumel J. Matz B. Thermosensitive UL9 gene function is required for early stages of herpes simplex virus type 1 DNA synthesis. J. Gen. Virol. 1995;76:3119–3124. doi: 10.1099/0022-1317-76-12-3119. [DOI] [PubMed] [Google Scholar]
- Cavalcoli JD, Baghian A, Homa FL. Kousoulas KG. Resolution of genotypic and phenotypic properties of herpes simplex virus type 1 temperature-sensitive mutant (KOS) tsZ47: evidence for allelic complementation in the UL28 gene. Virology. 1993;197:23–34. doi: 10.1006/viro.1993.1563. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Sauer B, Levine M. Glorioso JC. A cell-free recombination system for site-specific integration of multigenic shuttle plasmids into the herpes simplex virus type 1 genome. J. Virol. 1992;66:5509–5515. doi: 10.1128/jvi.66.9.5509-5515.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikas I, Darling AJ, Lankinen HM, Cross AM, Marsden HS. Clements JB. A single amino acid substitution in the large subunit of herpes simplex virus type 1 ribonucleotide reductase which prevents subunit association. J. Gen. Virol. 1990;71:2369–2376. doi: 10.1099/0022-1317-71-10-2369. [DOI] [PubMed] [Google Scholar]
- Smith CC, Kulka M, Wymer JP, Chung TD. Aurelian L. Expression of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) is required for virus growth and neoplastic transformation. J. Gen. Virol. 1992;73:1417–1428. doi: 10.1099/0022-1317-73-6-1417. [DOI] [PubMed] [Google Scholar]
- Preston VG, Darling AJ. McDougall IM. The herpes simplex virus type 1 temperature-sensitive mutant ts1222 has a single base pair deletion in the small subunit of ribonucleotide reductase. Virology. 1988;167:458–467. [PubMed] [Google Scholar]
- MacLean CA, Dolan A, Jamieson FE. McGeoch DJ. The myristylated virion proteins of herpes simplex virus type 1: investigation of their role in the virus life cycle. J. Gen. Virol. 1992;73:539–547. doi: 10.1099/0022-1317-73-3-539. [DOI] [PubMed] [Google Scholar]
- Ace CI, Dalrymple MA, Ramsay FH, Preston VG. Preston CM. Mutational analysis of the herpes simplex virus type 1 trans-inducing factor Vmw65. J. Gen. Virol. 1988;69:2595–2605. doi: 10.1099/0022-1317-69-10-2595. [DOI] [PubMed] [Google Scholar]
- Rice SA. Knipe DM. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 1988;62:3814–3823. doi: 10.1128/jvi.62.10.3814-3823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read GS. Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 1983;46:498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpara ML, Parsons L. Enquist LW. Sequence variability in clinical and laboratory isolates of herpes simplex virus 1 reveals new mutations. J. Virol. 2010;84:5303–5313. doi: 10.1128/JVI.00312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida M, Kageyama S, Sato H, T Kamiyama, J Yamamura, Kurokawa M, et al. Emergence of resistance to acyclovir and penciclovir in varicella-zoster virus and genetic analysis of acyclovir-resistant variants. Antiviral Res. 1999;40:155–166. doi: 10.1016/s0166-3542(98)00057-6. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Li Z, Kurokawa M, Kawana T, Imakita M. Shiraki K. Growth of herpes simplex virus in epidermal keratinocytes determines cutaneous pathogenicity in mice. J. Med. Virol. 2005;75:421–426. doi: 10.1002/jmv.20284. [DOI] [PubMed] [Google Scholar]
- Daanen HA, Van de Linde FJ, Romet TT. Ducharme MB. The effect of body temperature on the hunting response of the middle finger skin temperature. Eur. J. Appl. Physiol. Occup. Physiol. 1997;76:538–543. doi: 10.1007/s004210050287. [DOI] [PubMed] [Google Scholar]
- Oerlemans HM, Graff MJ, Dijkstra-Hekkink JB, de Boo T, Goris RJ. Oostendorp RA. Reliability and normal values for measuring the skin temperature of the hand with an infrared tympanic thermometer: a pilot study. J. Hand Ther. 1999;12:284–290. doi: 10.1016/s0894-1130(99)80065-9. [DOI] [PubMed] [Google Scholar]
- Ejercito PM, Kieff ED. Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Longson M. A temperature marker test for the differentiation of strains of Herpesvirus hominis. Ann. Inst. Pasteur. (Paris) 1971;120:699–708. [PubMed] [Google Scholar]
- Ratcliffe H. The differentiation of herpes simplex virus type 1 and type 2 by temperature markers. J. Gen. Virol. 1971;13:181–183. doi: 10.1099/0022-1317-13-1-181. [DOI] [PubMed] [Google Scholar]
- Wheeler CE. The effect of temperature upon the production of herpes simplex virus in tissue culture. J. Immunol. 1958;81:98–106. [PubMed] [Google Scholar]
- Wheeler CE. Canby CM. Effect of temperature on the growth curves of herpes simplex virus in tissue cultures. J. Immunol. 1959;83:392–396. [PubMed] [Google Scholar]
- Darling AJ, McKay EM, Ingemarson R. Booth B. Herpes simplex virus-encoded ribonucleotide reductase: evidence for the dissociation/reassociation of the holoenzyme. Virus Genes. 1990;3:367–372. doi: 10.1007/BF00569043. [DOI] [PubMed] [Google Scholar]
- Darling AJ, McKay EM, Ingemarson R. Preston VG. Reconstitution of herpes simplex virus type 1 ribonucleotide reductase activity from the large and small subunits. Virus Genes. 1989;2:187–194. doi: 10.1007/BF00315262. [DOI] [PubMed] [Google Scholar]
- Frame MC, Marsden HS. Dutia BM. The ribonucleotide reductase induced by herpes simplex virus type 1 involves minimally a complex of two polypeptides (136K and 38K) J. Gen. Virol. 1985;66:1581–1587. doi: 10.1099/0022-1317-66-7-1581. [DOI] [PubMed] [Google Scholar]
- Dutia BM. Ribonucleotide reductase induced by herpes simplex virus has a virus-specified constituent. J. Gen. Virol. 1983;64:513–521. doi: 10.1099/0022-1317-64-3-513. [DOI] [PubMed] [Google Scholar]
- Preston VG, Palfreyman JW. Dutia BM. Identification of a herpes simplex virus type 1 polypeptide which is a component of the virus-induced ribonucleotide reductase. J. Gen. Virol. 1984;65:1457–1466. doi: 10.1099/0022-1317-65-9-1457. [DOI] [PubMed] [Google Scholar]
- Gaudreau A, Hill E, Erice HH, Balfour A., Jr Boivin G. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J. Infect. Dis. 1998;178:297–303. doi: 10.1086/515626. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Bestman-Smith J. Boivin G. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist. Updat. 2002;5:88–114. doi: 10.1016/s1368-7646(02)00021-3. [DOI] [PubMed] [Google Scholar]
- Morfin F, Souillet G, Bilger K, Ooka T, Aymard M. Thouvenot D. Genetic characterization of thymidine kinase from acyclovir-resistant and -susceptible herpes simplex virus type 1 isolated from bone marrow transplant recipients. J. Infect. Dis. 2000;182:290–293. doi: 10.1086/315696. [DOI] [PubMed] [Google Scholar]
- Sasadeusz JJ, Tufaro F, Safrin S, K Schubert, M. M Hubinette, Cheung PK, et al. Homopolymer mutational hot spots mediate herpes simplex virus resistance to acyclovir. J. Virol. 1997;71:3872–3878. doi: 10.1128/jvi.71.5.3872-3878.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Kurokawa M, Matsuo K, Honda M, Niimura M. Shiraki K. Suppression of generation and replication of acyclovir-resistant herpes simplex virus by a sensitive virus. J. Med. Virol. 2004;72:112–120. doi: 10.1002/jmv.10562. [DOI] [PubMed] [Google Scholar]


